Introduction
Ulcerative colitis (UC) is a definite risk factor
for the development of many colon cancers (1,2).
Inflammatory substances released from the inflamed tissue can
promote the tumor growth by inducing oncogene deoxyribonucleic acid
(DNA) replication or reducing tumor suppressor genes, and increase
the mutant cell proliferation (3,4). Among
various substances in the inflammatory environment, changes in
inflammatory factors have significant differences, which are the
most obvious in inflammatory factors, such as interleukin-6 (IL-6),
tumor necrosis factor-α (TNF-α), IL-10 and IL-4 in related cancers.
It is reported in the literature that IL-6 has been proven to play
a basic role in experimental colitis, and high-level IL-6 is a
major risk factor for the development of human colorectal tumor and
hepatocellular carcinoma (5). Such a
close relationship between IL-6 and cancer suggests that the
effects of IL-6 on promoting cell proliferation and reducing
apoptosis may not be the only tumor-inducing mechanism controlled
by cytokines, but it should be assumed that the additional IL-6
effect may lead to tumorigenesis.
Recent studies have demonstrated that IL-6
downregulates the p53 expression and activity through stimulation
of the upregulation of ribosomal ribonucleic acid (rRNA)
transcription in proliferation factors and enhancing mouse double
minute 2 homolog (MDM2)-mediated p53 proteasome digestion. It is
hypothesized that stimulating factors of cell proliferation are
produced under pathological conditions of human body, such as
chronic inflammatory process, and the similar mechanism may be
positive, thus contributing to tumor transformation. In this study,
therefore, whether IL-6 could stimulate rRNA transcription and
downregulate p53 transcription and p53 expression and activity in
untransformed cell lines was investigated first. It was found that
IL-6 enhanced the rRNA transcription, and reduced p53 expression
and function through activation of ribonucleoprotein-MDM2
degradation pathway of p53 and upregulating rRNA synthesis. In
addition, it was observed that p53 downregulation leads to
epithelial-mesenchymal transition (EMT) characterized by phenotypic
and functional changes in cells exposed to IL-6. Considering that
the onset of colon cancer in UC patients is a highly representative
example of the occurrence of chronic inflammation-related tumors
(6), whether changes induced by IL-6
in human cell lines also existed in chronic ulcerative colonic
mucosa in human epithelial cells was studied.
The results revealed that there was significant
nucleolus hypertrophy (a morphological marker of rRNA upregulation)
in epithelial cells of all UC cases examined (7), and the p53 immunostaining was
decreased. Moreover, major EMT phenotypic changes and decreased
E-cadherin expression could also be seen in epithelial cells in
colonic mucosa in UC patients. These data provide a new pathway of
IL-6-induced stimulation of rRNA transcription, and link
inflammation to cancer.
Materials and methods
Patient data
A total of 25 UC patients, including 14 males and 11
females, aged 49.8±10.3 years on average, and 25 healthy subjects,
including 12 males and 13 females, aged 45.6±9.6 years on average,
were enrolled in this study. After routine clinical nursing and
monitoring were conducted, and informed consent was obtained,
biopsy specimens were obtained via colonoscopy. The diagnosis of UC
was based on the routine clinical, endoscopic and histopathological
criteria described by Lennard-Jones (8). The biopsy tissues of patients without
gross lesions or history of any gastrointestinal disease were used
as the control group. The patients had suffered from UC for >5
years. In all UC cases, the specimens were obtained from the
transverse colon and ascending colon. All the UC patients underwent
initial colonoscopy during the active disease and then received a
second endoscopy during the clinical remission which was confirmed
histologically. The specimens collected were fixed in formalin and
embedded in paraffin for histological examination. The study was
approved by the Ethics Committee of PLA Army General Hospital
(Beijing, China).
Cell lines and chemotherapy
HepG2, SW1990 and LS174T cell lines were obtained
from the China Center for Type Culture Collection (Wuhan, China),
and the NCM460 cell line was purchased from INCELL Corporation (San
Antonio, TX, USA). All cell lines were p53 wild-type. The HCT116
p53−/− cell line was generously donated by Professor
Bert Vogelstein.
Experimental materials
Cytokine enzyme-linked immunosorbent assay (ELISA)
kit (Genzyme, Cambridge, MA, USA), recombinant human IL-6 at a
final concentration of 50 ng/ml (Sigma-Aldrich, Milan, Italy),
proteasome inhibitor MG-132 at a final concentration of 10 mM
(Calbiochem; Merck Chemicals Ltd., Nottingham, UK), cycloheximide
at a final concentration of 20 µg/ml (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), hydroxyurea (Sigma-Aldrich; Merck KGaA), 5-FU
(Fluorouracil; Teva Pharma Italia, Milan, Italy), Nutlin-3
(Sigma-Aldrich; Merck KGaA), anti-E-cadherin (clone 32A8, Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-IL-6
(Sigma-Aldrich; Merck KGaA), anti-p53 (Novocastra, Laboratories,
Ltd., Newcastle upon Tyne, UK), and p53 Taqman gene expression
quantitative assay kit (Applied Biosystems, Foster City, CA, USA)
were used in the present study.
Immunohistochemical evaluation
Antigens in tissue sections were displayed by using
the non-biotin amplification method. The sections were then
incubated with primary anti-human p53 monoclonal antibody (1:200;
cat. no. 2527, Cell Signaling Technology, Inc.) diluted with 1%
bovine serum albumin in Tris-buffer at 4°C overnight. The sections
were counterstained with hematoxylin, and dehydrated and covered
with the cover glass. The nuclear p53 immunostaining was evaluated
via image cytometry by using the Cytometrica program. According to
the study of Faccioli et al (9), staining in the section labeling index
was presented as the percentage of the labeled nuclear area in the
total nuclear area of epithelial cells.
Detection of cytokine levels (IL-6,
TNF-α, IL-10 and IL-4) in human peripheral blood via ELISA
After 5 ml peripheral venous blood was drawn from
patients and anti-coagulated with ethylene diamine tetraacetic acid
(EDTA), and the serum was isolated and stored in a refrigerator at
−70°C to be detected. The pro-inflammatory factors (IL-6, TNF-α,
IL-10 and IL-4) (cat. nos. KE00007, 17590-1-AP, KE00012, KE00016,
respectively; ProteinTech Group, Inc.; Wuhan, China) in serum of
patients were detected via ELISA.
Taqman fluorescence quantitative
polymerase chain reaction (PCR)
Total RNA was extracted using TRIzol reagent, and
the whole cell RNA was quantified via spectrophotometry (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). RNA (2 µg) was reversely
transcribed using a high-capacity complementary DNA (cDNA) reverse
transcription kit according to the manufacturer's protocol.
Quantiative PCR was performed on ABI Prism 7000 instrument, and the
average ΔCq value of control specimens was used in each experiment
to calculate the replicated ΔΔCq value of specimens (10). Primers and probes were selected by
using the Roche online primer design tool, and primers used for
Taqman real-time PCR analysis of human PUMA and p53 were designed.
p53 and C-myc were quantitatively detected by using the Taqman gene
expression assay primer and probe kits (11). p53: Forward,
GACGGTGACACGCTTCCCTGGATT; reverse, GGGAACAAGAAGTGGAGAATGTCA; c-MYC:
Forward, GAGAGGCAGAGGGAGCGAGCGGGC; reverse,
TGTCGTTGAGAGGGTAGGGGAAGA.
Isolation of polysome messenger RNA
(mRNA)
Cells were washed in phosphate-buffered saline (PBS)
at 4°C, and the cell sediment was lysed in 10 mmol/l Tris-HCl (pH
7.4), 10 mmol/l NaCl, 3 mmol/l MgCl2 and 0.5% NP40 at
4°C for 10 min. The lysate was centrifuged at 14,000 × g at 4°C for
10 min, and the polysome was isolated from supernatant. The lysate
was layered into 15–50% sucrose gradient by using 30 mmol/l
HEPES/KOH (pH 7.5), 80 mmol/l KCl and 1.8 mmol/l Mg-acetate,
followed by centrifugation at 14,000 × g at 4°C for 15 h. Then, 1
ml lysate was collected from each gradient, and the absorbance was
read at 260 nm on a microplate reader (Bio-Rad Laboratories, Inc.).
The polyribosome was integrated and centrifuged at 100,000 × g and
4°C for 15 h, and RNA was extracted using TRIzol reagent.
Western blot analysis
Whole cell protein extraction, and subsequent sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and western blot
analysis were performed as previously described (12). Briefly, the total cell protein was
extracted in lysis buffer [KH2PO4 0.1 M pH
7.5, NP-40 1%, added with complete protease inhibitor cocktail
(Roche Diagnostics, Basel, Switzerland) and 0.1 mM
β-glycerophosphate], and detected via Bio-Rad protein assay.
Nucleoprotein used for western blot analysis was obtained. After
being fixed on the nitrocellulose membrane, the protein was
incubated with the primary mouse anti-human c-myc, p53, β-actin
monoclonal antibodies (1:500; cat. nos. sc-42, sc-81168 and
sc-70319, respectively) [Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA)] at 4°C overnight. Horseradish peroxidase-conjugated
secondary goat anti-mouse IgG-HRP polyclonal antibody (1:1,000;
cat. no. sc-2005) was purchased from Santa Cruz Biotechnology, Inc.
The densitometry was analyzed using Image J (National Institutes of
Health, Bethesda, MD, USA).
mRNA transfection
The capped mRNA was transcribed in the linearized
pR-C-myc-IRES-F (donated by Professor R.J. Schneider) and the
control pRF plasmid by using the mMessage mMachine T3 kit (Ambion;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). According to the
manufacturer's instructions, cells were transfected with 0.4 µg RNA
in each specimen by using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). After transfection for 8 h, the cells
were collected and analyzed by using the dual-luciferase assay kit
(Promega Corporation, Madison, WI, USA) in accordance with the
manufacturer's protocol.
Statistical analysis
Chi-square test or Mann-Whitney U test was used for
the intergroup comparison if appropriate. The consistency among
scores was evaluated via K statistics. Statistical Product and
Service Solutions (SPSS) software package was used for all
statistical data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Detection of changes in inflammatory
factors via ELISA
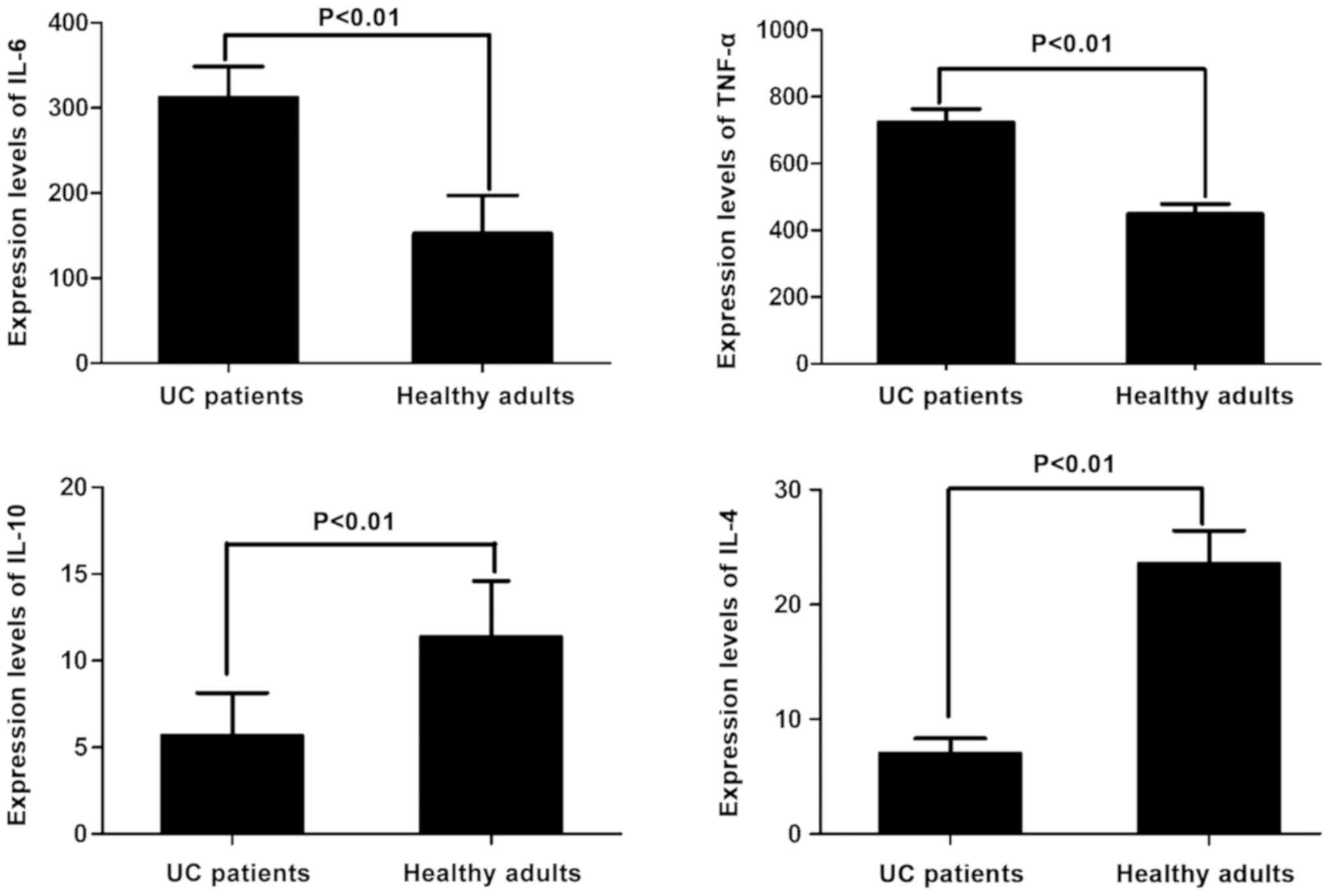
The level of IL-6 in 25 patients with UC
(312.16±36.34 ng/l) was significantly higher than that in healthy
adult controls (152.66±44.42 ng/l), which was nearly 2-fold higher
than that in healthy adults (P<0.01). The level of TNF-α in 25
patients with UC (723.56±40.45 ng/l) was also significantly higher
than that in healthy adult controls (448.45±30.13 ng/l), which was
approximately 1.5-fold higher than that in healthy adults
(P<0.01). The level of IL-10 in 25 patients with UC (5.68±2.46
ng/l) was significantly lower than that in healthy adult controls
(11.37±3.24 ng/l), which was approximately 50% of that in healthy
adults (P<0.01). The level of IL-4 in 25 patients with UC
(7.01±1.32 ng/l) was also significantly lower than that in healthy
adult controls (23.56±2.89 ng/l), which was <30% of that in
healthy adults (P<0.01). All the differences were statistically
significant (Fig. 1).
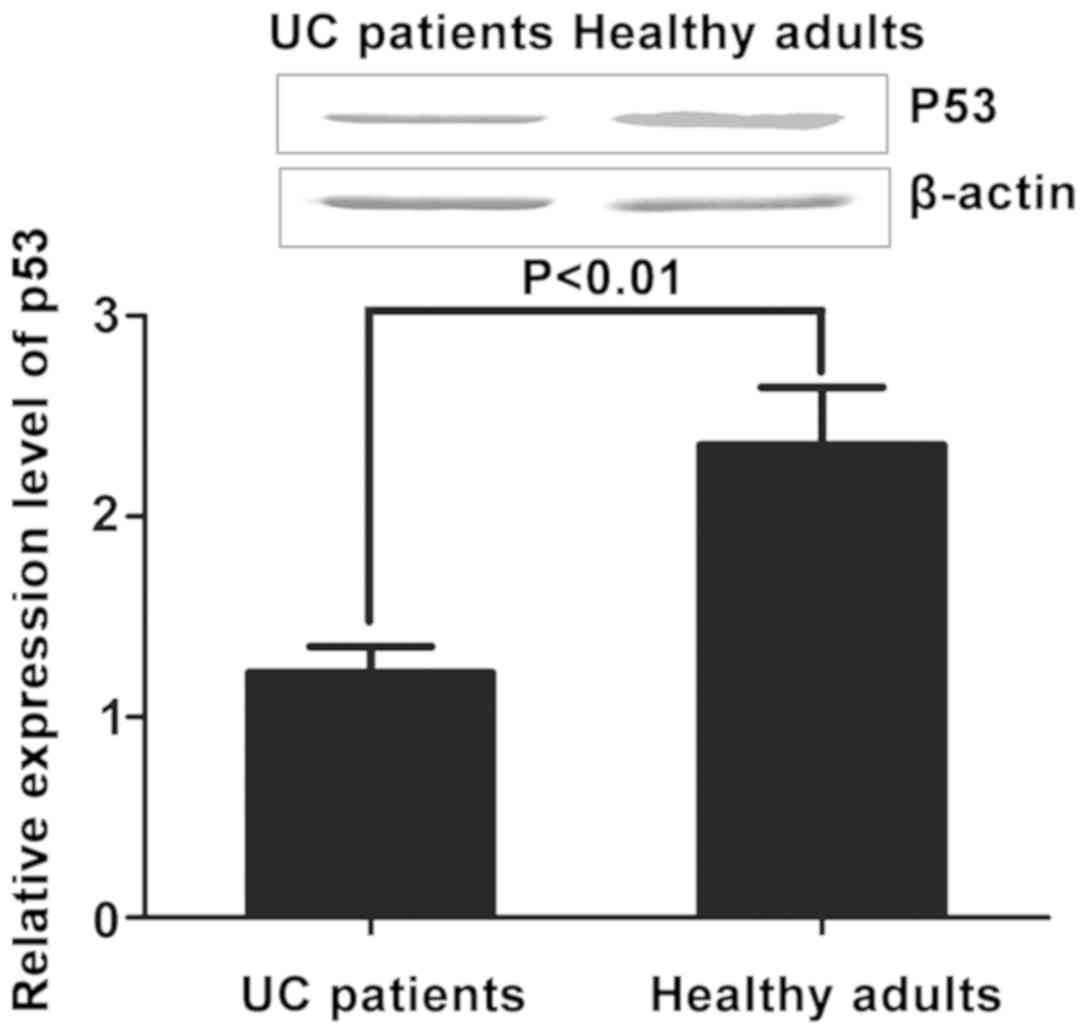
Detection of p53 expression levels in
UC patients and healthy adults
The relative expression levels of p53 in peripheral
blood of UC patients and healthy adults were detected via
quantitative PCR and western blotting. The results revealed that
the expression level of p53 in peripheral blood of UC patients
(312.16±36.34 ng/l) was obviously decreased compared with that in
healthy adults (152.66±44.42 ng/l) (P<0.01) (Fig. 2).
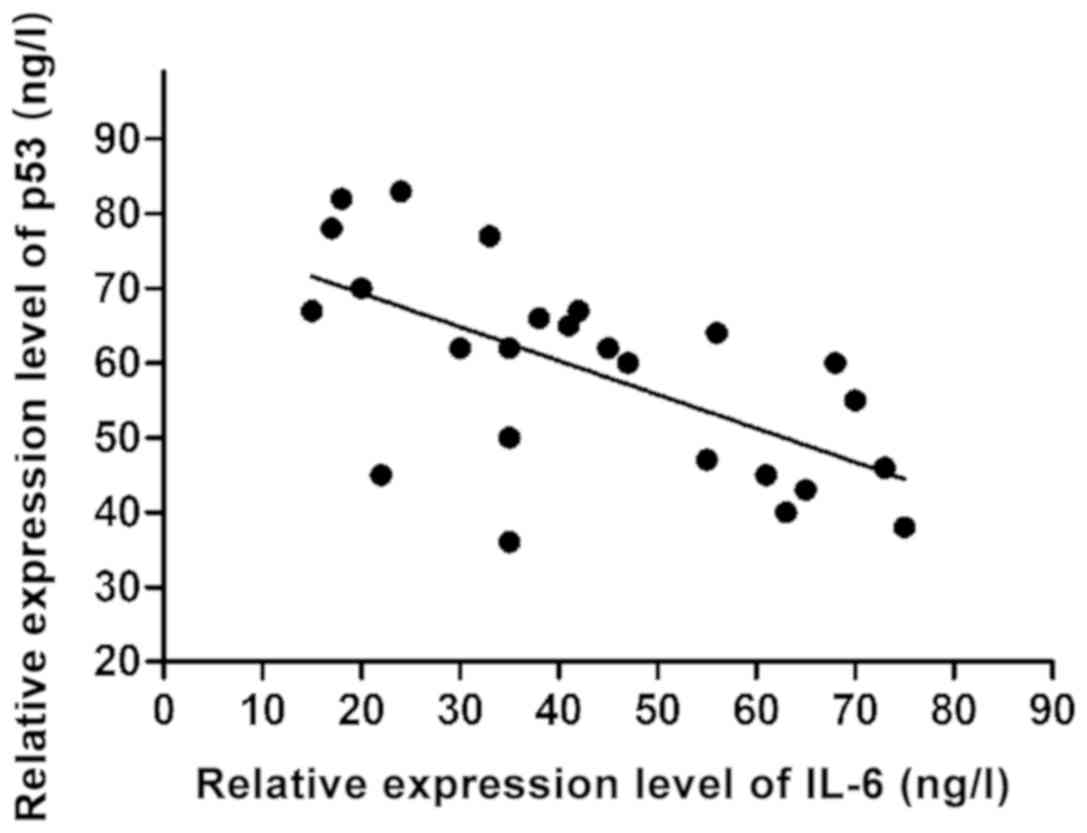
Correlation analyses of p53 expression
with inflammatory factors
The relative expression level of p53 in peripheral
blood of 25 UC patients and the relative expression level of
cytokines were analyzed. According to results of linear correlation
analyses, only the expression of IL-6 had a significant negative
linear correlation with the p53 expression (r=−0.437, P<0.001)
(Fig. 3).
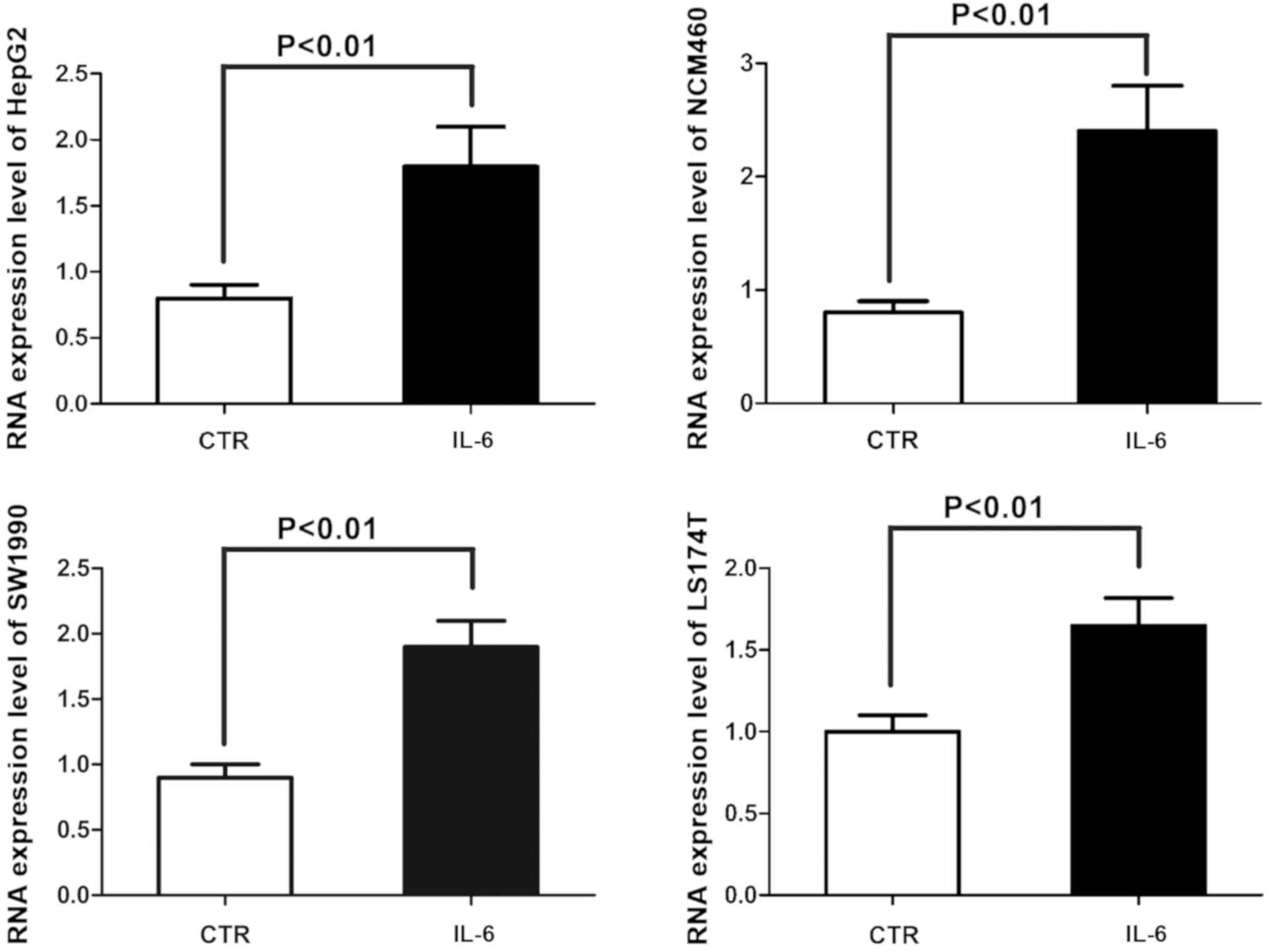
Analysis of 45S preribosomal RNA
(preRNA) expression via qPCR
The effects of IL-6 on the ribosomal biosynthesis of
4 kinds of human epithelial cell lines (normal colon epithelial
NCM460 cell line, colon cancer LS174T cell line, hepatocellular
carcinoma HepG2 cell line and pancreatic cancer SW1990 cell line)
were analyzed. The 45S preRNA expression was analyzed via real-time
PCR to confirm changes in rRNA transcriptional activity in the four
kinds of cell lines exposed to IL-6, indicating that IL-6 greatly
enhances the transcription of rRNA (Fig.
4).
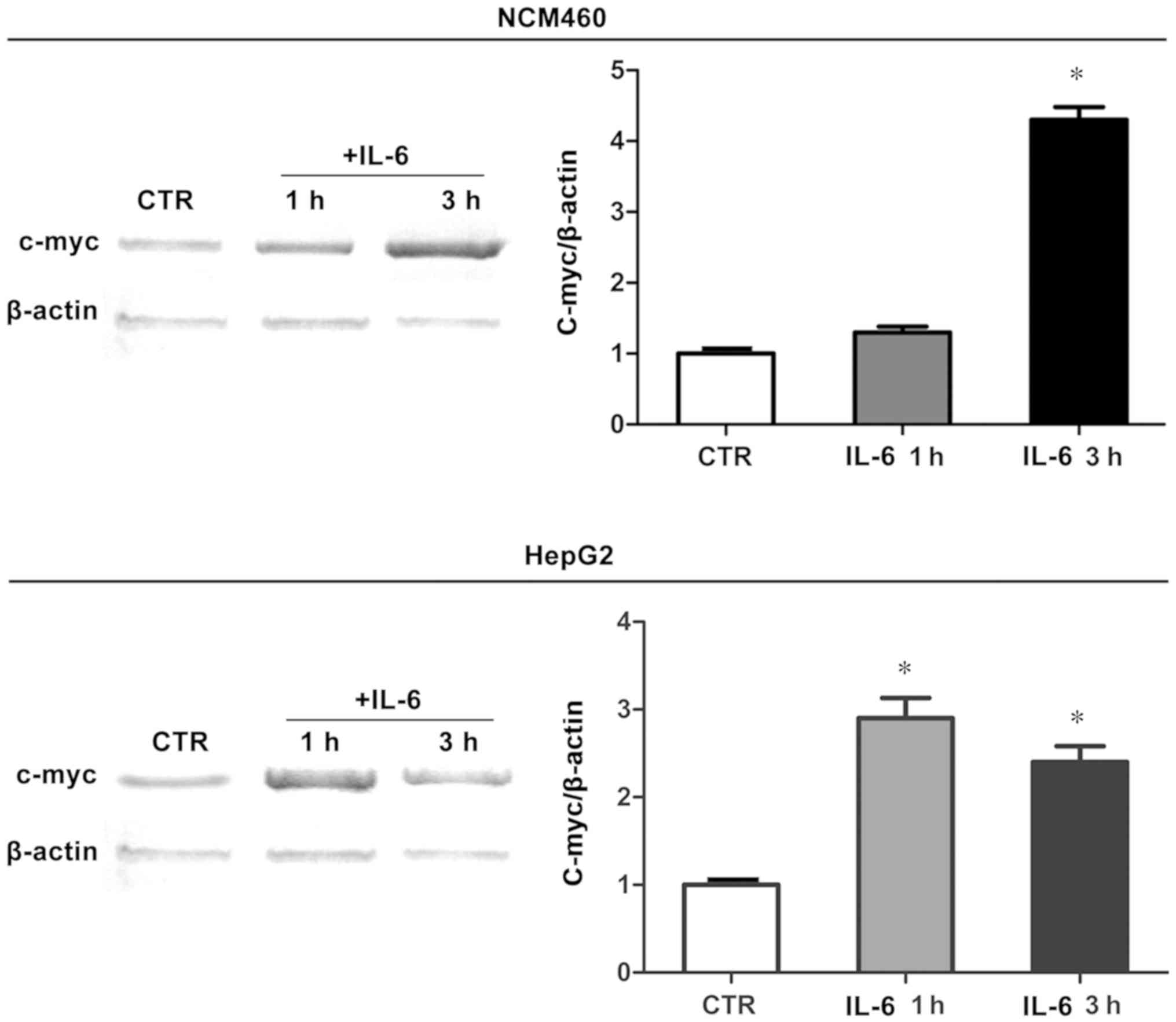
C-myc protein expression in human
epithelial cells
To study the mechanism of IL-6 in stimulating rRNA
transcription, whether IL-6 also enhanced the C-myc protein
expression in human epithelial cells was investigated. Therefore,
NCM460 and HepG2 cell lines were exposed to IL-6. Western blot
analysis revealed that, as evaluated, C-myc protein expression in
the two cell lines were significantly increased at 1 h after IL-6
stimulation. Results showed that IL-6 activated the transcriptional
mechanism and increased the C-myc protein expression under
experimental conditions in this study (Fig. 5).
In conclusion, these results strongly suggest that
the decrease in p53 expression after treatment with IL-6 is the
result of increased C-myc protein translation induced by IL-6,
thereby facilitating the p53 protein digestion.
Discussion
By studying the expression of p53 protein and
related inflammatory factors in UC patients and healthy adults, it
was manifested that there was a significant negative correlation
between IL-6 and p53 protein expressions. Therefore, its intrinsic
mechanism was investigated. It was demonstrated by using human cell
lines that IL-6 stimulated the expression of C-myc. Consequently,
the increased C-myc protein expression stimulated the upregulation
of rRNA transcription, and enhanced rRNA transcription mediated the
p53 degradation (13,14). The decreased p53 protein level was
associated with the decline in p53 function, which activated the
EMT and increased the invasive potential of cells exposed to IL-6
(15). Downregulation of p53 protein
was entirely due to the upregulated ribosomal biosynthesis.
Furthermore, it has been reported that the stimulation of IL-6 on
rRNA transcription can be counteracted by C-myc silencing. IL-6
exposure, as a result of RNA, does not stimulate the polymerase I
depletion of p53 protein level in rRNA transcription cells.
Evidence suggests that signal transducer and activator of
transcription 3 (STAT3) plays a key role in the occurrence of
inflammation-related tumors, such as colon and liver cancer
(16). IL-6 is a major activator of
STAT3, whose stimulation products increase the transcription of
target genes and cell proliferation and survival (17–20). On
the other hand, results in this study displayed that the IL-6
effect did not depend on the activation of STAT3 pathway. It was
observed that IL-6 induced the expression and transcription of
C-myc protein via stimulating C-myc mRNA IRES translation without
modifying C-myc mRNA at 1 h after exposure, which, in fact, is
consistent with the reported data on myeloma cell lines (21). Therefore, the possibility that the
elevated C-myc protein level may be the result of changes in C-myc
mRNA expression and transcription due to activation of STAT3 by
IL-6 is ruled out.
The downregulation of p53 expression may be the most
important in the transformation process of inflamed tissues and
cells. In fact, inflammatory cells, cytokines, chemotactic factors
and growth factors that stimulate proliferation and inhibit
apoptosis produce reactive oxygen and nitrogen substances, causing
DNA oxidative damage, which may no longer be sufficiently repaired
as it downregulates the function of p53 (22). As a result, epigenetic changes in
oncogenes and other tumor suppressor genes responsible for tumor
transformation and progression may be produced. This mechanism may
play a role in long-term inflamed tissues, such as human UC colonic
mucosa. Actually, the histochemical and immunohistochemical results
obtained by using biopsy specimens of colon from UC patients in
this study were highly consistent with the results of research
conducted in human cell lines. In fact, it has been found that
epithelial cells of UC patients were characterized by nucleolus
hypertrophy, suggesting the upregulation of rRNA and downregulation
of p53 expression (23,24).
Acknowledgements
Not applicable.
Funding
This work was supported by Capital Health
Development Special Fund (2018–5091).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS wrote the manuscript and was responsible for
colonoscopy detection. QK and HW helped with immunohistochemical
evaluation. HY performed ELISA and LD, YL and RF contributed to
isolation of polysome messenger RNA. All authors read and approved
the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
PLA Army General Hospital (Beijing, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakagawa H, Maeda S, Yoshida H, Tateishi
R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N,
et al: Serum IL-6 levels and the risk for hepatocarcinogenesis in
chronic hepatitis C patients: An analysis based on gender
differences. Int J Cancer. 125:2264–2269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Terzić J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Derenzini M, Trerè D, Pession A, Montanaro
L, Sirri V and Ochs RL: Nucleolar function and size in cancer
cells. Am J Pathol. 152:1291–1297. 1998.PubMed/NCBI
|
|
8
|
Lennard-Jones JE: Classification of
inflammatory bowel disease. Scand J Gastroenterol. Suppl 170
(sup170):2 6, discussion. 16–19. 1989.PubMed/NCBI
|
|
9
|
Faccioli S, Chieco P, Gramantieri L,
Stecca BA and Bolondi L: Cytometric measurement of cell
proliferation in echo-guided biopsies from focal lesions of the
liver. Mod Pathol. 9:120–125. 1996.PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Canales RD, Luo Y, Willey JC, Austermiller
B, Barbacioru CC, Boysen C, Hunkapiller K, Jensen RV, Knight CR,
Lee KY, et al: Evaluation of DNA microarray results with
quantitative gene expression platforms. Nat Biotechnol.
24:1115–1122. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurien BT and Scofield RH: Western
blotting. Methods. 38:283–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y and Lu H: Signaling to p53:
Ribosomal proteins find their way. Cancer Cell. 16:369–377. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deisenroth C and Zhang Y: Ribosome
biogenesis surveillance: Probing the ribosomal protein-Mdm2-p53
pathway. Oncogene. 29:4253–4260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montanaro L, Treré D and Derenzini M:
Nucleolus, ribosomes, and cancer. Am J Pathol. 173:301–310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao C, Wang W, Yu W, Jou D, Wang Y, Ma H,
Xiao H, Qin H, Zhang C, Lü J, et al: A novel small molecule STAT3
inhibitor, LY5, inhibits cell viability, colony formation, and
migration of colon and liver cancer cells. Oncotarget.
7:12917–12926. 2016.PubMed/NCBI
|
|
17
|
Bollrath J, Phesse TJ, von Burstin VA,
Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T,
Canli O, Schwitalla S, et al: gp130-mediated Stat3 activation in
enterocytes regulates cell survival and cell-cycle progression
during colitis-associated tumorigenesis. Cancer Cell. 15:91–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L, et al: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park EJ, Lee JH, Yu GY, He G, Ali SR,
Holzer RG, Österreicher CH, Takahashi H and Karin M: Dietary and
genetic obesity promote liver inflammation and tumorigenesis by
enhancing IL-6 and TNF expression. Cell. 140:197–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He G and Karin M: NF-κB and STAT3 - key
players in liver inflammation and cancer. Cell Res. 21:159–168.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Risques RA, Lai LA, Himmetoglu C, Ebaee A,
Li L, Feng Z, Bronner MP, Al-Lahham B, Kowdley KV, Lindor KD, et
al: Ulcerative colitis-associated colorectal cancer arises in a
field of short telomeres, senescence, and inflammation. Cancer Res.
71:1669–1679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim S, Keku TO, Martin C, Galanko J,
Woosley JT, Schroeder JC, Satia JA, Halabi S and Sandler RS:
Circulating levels of inflammatory cytokines and risk of colorectal
adenomas. Cancer Res. 68:323–328. 2008. View Article : Google Scholar : PubMed/NCBI
|