Introduction
Fecal microbiota transplantation (FMT) is a
treatment method that transfers the functional bacteria in human
feces into the gastrointestinal tract of patients through different
routes of administration, so as to remodel the intestinal flora
structure, thereby achieving the purpose of treating
intestinal-associated diseases (1,2).
Inflammatory bowel disease (IBD) is a disease characterized by
recurrent episodes of chronic inflammation of the intestine, and
the term is predominantly used in reference to two conditions:
Ulcerative colitis (UC) and Crohn's disease. At present, the
pathological mechanism of IBD remains to be fully elucidated,
although the important role of intestinal symbiotic bacteria and
flora disorders in the pathogenesis of IBD has been widely
investigated (3). Numerous studies
have demonstrated that the numbers of intestinal dominant bacteria,
or anti-inflammatory bacteria (including firmicutes, lactobacilli
and bacteroides) of patients with IBD are reduced, whereas those of
harmful bacteria, including enterococcus and proteobacteria, are
increased, and the intestinal microbial diversity is markedly
reduced (4). Therefore, adjusting
intestinal flora disturbances and restoring the homeostasis between
host and intestinal microorganisms has become a novel strategy for
the treatment of IBD.
A large number of clinical trials have confirmed
that FMT is a safe and effective method for the treatment of
recurrent Clostridium difficile infections, and a recent
review of IBS by El-Salhy and Mazzawi (5) revealed that FMT in patients with IBS
led to a reversion of the gut microbiota dysbiosis to normalcy,
reduced the symptoms of IBS in ~70% of patients and was not
associated with any serious adverse events. FMT was therefore
demonstrated to be a promising tool for managing IBS. It appears to
be an effective, easy and inexpensive procedure (5); however, studies on the treatment of IBD
have been limited to partial case reports and studies, and the
specific mechanism of the anti-inflammatory effect remains elusive
(6). A previous study that
established an experimental UC model in mice with dextran sulfate
sodium (DSS) revealed an intestinal flora disturbance similar to
that observed in human IBD (7). The
animal model of FMT is an invaluable complement to the clinical
study of the efficacy of FMT due to the controllability of various
variables.
Therefore, the aim of the present study was to
investigate the effect of FMT on the acute inflammatory response in
a murine model of DSS-induced colitis, and to attempt to elucidate
the possible underlying mechanism(s). The results obtained indicate
that FMT may exert a therapeutic effect on experimental colitis in
mice via reshaping the intestinal flora and regulating intestinal
T-cell immunity homeostasis.
Materials and methods
Experimental animals
A total of 32 BALB/c mice (age, 8 weeks) were used
for the present study (other characteristics: Female; average
weight, 21.0±3.0 g). The study also employed, as FMT donor animals,
3 Sprague Dawley (SD) rats (female; weight, 200–220 g; age, 8
weeks) and 3 C57/BL6 mice (female; age, 8 weeks).
All of the animals were purchased from the
Experimental Animal Center of Wuhan University (Wuhan, China) and
raised in the specific pathogen-free-grade animal room of the
Stomatological Hospital of Wuhan University (Wuhan, China;
certificate no. 2800360003056) with adaptive feeding for 1 week
prior to the start of the experiment. The room temperature was
maintained at 20±2°C, with a relative humidity of 50±10% and
artificial lighting (12-h light/dark cycle). All procedures for the
care and handling of animals in the present study were approved by
the University of Wuhan Animal Care Committee [certificate no. SYXK
(E) 2009–0027].
Reagents
DSS (molecular weight, 36,000–50,000 Da) was
purchased from MP Biomedicals, LLC (Santa Ana, CA, USA),
5-aminosalicylic acid (5-ASA) was from Maya Reagent Co., Ltd.
(Jiaxing, China); and sodium carboxymethylcellulose was obtained
from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The
stool occult blood test kit (cat. no. 32365192910) and the kit
(cat. no. 30365193913) for the determination of myeloperoxidase
(MPO) activity were purchased from the Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). An ELISA kit (cat. no.
35790106219) for detecting the levels of tumor necrosis factor-α
(TNF-α), interleukin (IL)-1β and IL-10 was obtained from XinBosheng
Biotechnology Co., Ltd. (Xi'an, China).
Animal groups and treatment
The mice were randomly divided into 4 groups by
using the numerical table method, with 8 mice in each group, namely
the i) normal group; ii) DSS group; iii) 5-ASA group; and iv) FMT
group. From the first day of the experiment, with the exception of
the normal group, the other 3 groups were administered a 3%
solution of DSS provided as their freely provided drinking water
for 7 days, in order to induce the acute UC model, and the
DSS-containing water was replaced daily. On days 1, 3, 5 and 7, the
mice in the DSS, 5-ASA and FMT groups were respectively given 200
µl 0.5% carboxymethylcellulose sodium, 5-ASA in suspension (100
mg/kg) (8) or 200 µl fecal
suspension by enema. By contrast, the normal group was left
untreated and provided with normal food and water ad
libitum. On the eighth day of the experiment, the mice were
sacrificed by cervical dislocation following anesthesia, and the
abdominal cavity was subsequently dissected, the colon was isolated
and its length was measured.
Intestinal cavities were cut along the vertical axis
of the mesentery, and after rinsing with ice-cold PBS, three
portions of the tissue were retained from an identical part for
each mouse, of which the lesioned sections of ~0.4 cm were fixed
with 4% paraformaldehyde, embedded with paraffin, sliced and
subjected to H&E staining. The two remaining parts of the colon
tissue were immediately placed in liquid nitrogen and subsequently
transferred to a refrigerator at −80°C prior to biochemical
testing.
Pharmaceutical preparations
In order to prepare the 5-ASA suspension, 5-ASA was
added to 0.5% sodium carboxymethyl cellulose (5 ml), followed by
vortexing, to obtain a 20 mg/ml suspension for subsequent use. This
mixing step was performed prior to performing each enema.
For the preparation of the fecal bacteria liquid
(9), SD rats and C57/BL6 mice were
sacrificed by cervical dislocation following anesthesia, and the
cecum contents were extracted from the 6 donor animals. After
weighing the extracted cecum contents in 3 ml/g sodium
carboxymethyl cellulose solution, stirring on ice and
vortex-mixing, the contents were placed in a clean centrifuge tube
and centrifuged at 5,000 × g for 20 min at 20°C. The supernatant
was then taken and placed in a refrigerator at −80°C for
cryopreservation.
Mice enema method
A solution of 4% chloral hydrate (0.4 mg/kg body
weight) was injected intraperitoneally into the mice to induce mild
anesthesia. The mice were placed into the prone position, followed
by placement of a plastic tube (inner diameter, 2 mm). After
applying paraffin oil onto its surface, the tube was gently
inserted into the colon through the anus of the mice up to ~4 cm
from the top of the tube to the anus, when the suspension was
warmed to body temperature and the fecal bacteria solution (200 µl)
was slowly injected. Other suspensions were similarly administered.
Subsequently, the tube was withdrawn and a cotton swab was pressed
onto the anus. By clasping their tails, the mice were held upside
down for 1 min, prior to placing the mice back into the cage. Mice
in the normal group received no treatment.
Observation indexes
Normal functions of the mice, including feeding and
activity, weight, fecal composition and fecal occult blood of mice,
were monitored daily, and the disease activity index (DAI) was
determined. The DAI score was calculated as follows: 0 points for
no weight loss, 1 point for a 1–5% reduction in weight; 2 points
for a decrease in weight of 5–10%; 3 points for a loss in weight of
10–15%; and 4 points for a decrease of >15% in weight. Scores
for the condition of the stools were as follows: 0 points, normal
(formalized) stools; 2 points, loose stools (mushy, semi-formed
stools that did not adhere to the anus); 4 points, loose stools
(may adhere to the anus in the water sample). In addition, 0 points
were assigned if no fecal occult blood or gross blood feces were
identified; and a positive fecal occult blood test scored 2 points,
whereas gross blood count was credited with 4 points. The score for
the above three characteristics were added together to obtain the
DAI for each mouse, and the severity of colitis was thereby
determined (10).
The degree of histological damage was assessed by
determining the product of the scores of inflammation, lesion
depth, crypt destruction and lesion range. The average value was
used as the score of histological damage of the colon (11).
Determination of the MPO activity in
colon tissue
The tissue weight was accurately determined, and the
colon tissue homogenate medium was added at a weight-to-volume
ratio of 1:19. The mixture was fully homogenized on ice to obtain a
5% homogenate, and the remainder of the steps were performed
according to the instructions of the kit (obtained from the Nanjing
Jiancheng Bioengineering Institute).
ELISA assay for determination of
TNF-α, IL-1β and IL-10
The tissue weight was accurately determined and
pre-cooled PBS was added at a weight-to-volume ratio of 1:9. A
sufficient amount of homogenate was placed on ice and subjected to
ultrasonic crushing in parallel. Finally, the slurry was
centrifuged at 10,000 × g at 4°C for 5 min, and the supernatant was
retained for detection of the contents of the various cytokines,
according to the instructions provided by the ELISA kit.
Statistical methods
SPSS version 17 software (SPSS, Inc., Chicago, IL,
USA) was used for data analysis. Values are expressed as the mean ±
standard deviation. Comparisons between multiple groups were
performed by analysis of variance and the least-significance
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
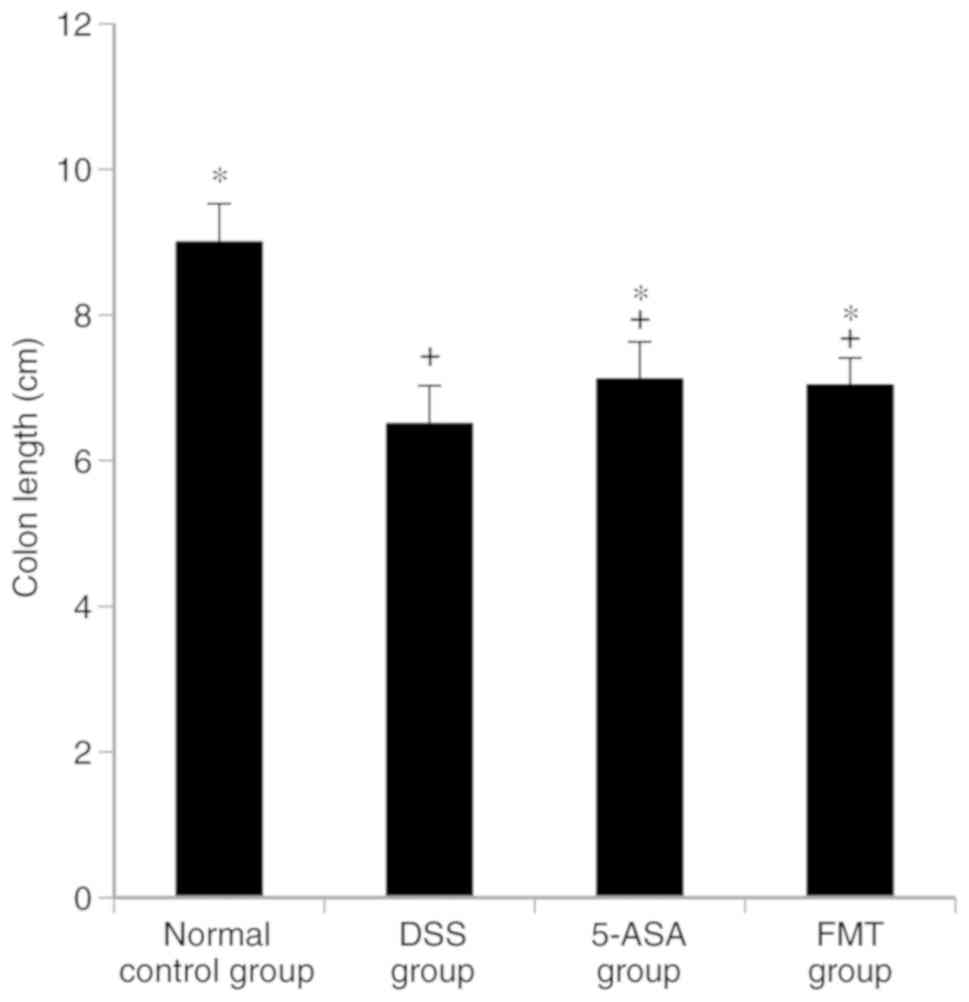
FMT attenuates UC-induced reduction in
colonic length
The colonic length in the DSS, 5-ASA and FMT groups
was identified to be shorter compared with that in the normal group
(P<0.05), the length in the 5-ASA and the FMT groups was greater
than that in the DSS group (P<0.05). No significant difference
in colonic length was identified between the 5-ASA and the FMT
groups (P>0.05, Fig. 1).
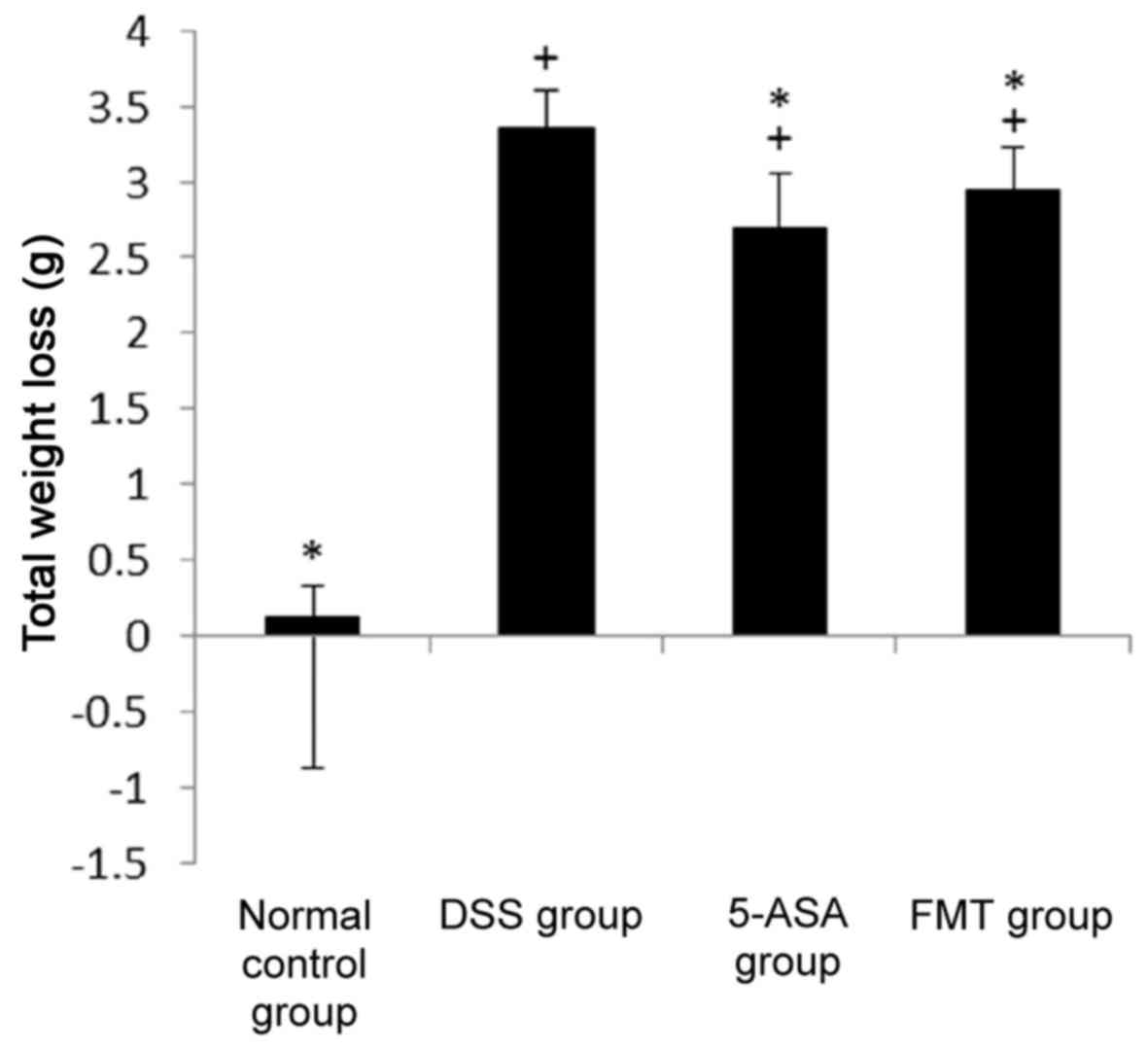
Weight changes in each group
Prior to the experiment, no significant differences
in body weight were observed between the groups (P>0.05). At the
end of the experiment, however, the weight of the mice in the DSS,
5-ASA and FMT groups had clearly decreased. Furthermore, the total
weight loss in these 3 groups (difference between the start and end
of the experiment) was significantly different compared with that
in the normal group (P<0.05). Compared with that in the DSS
group, the weight loss in the 5-ASA group and FMT group was
significantly decreased (P<0.05, Fig.
2).
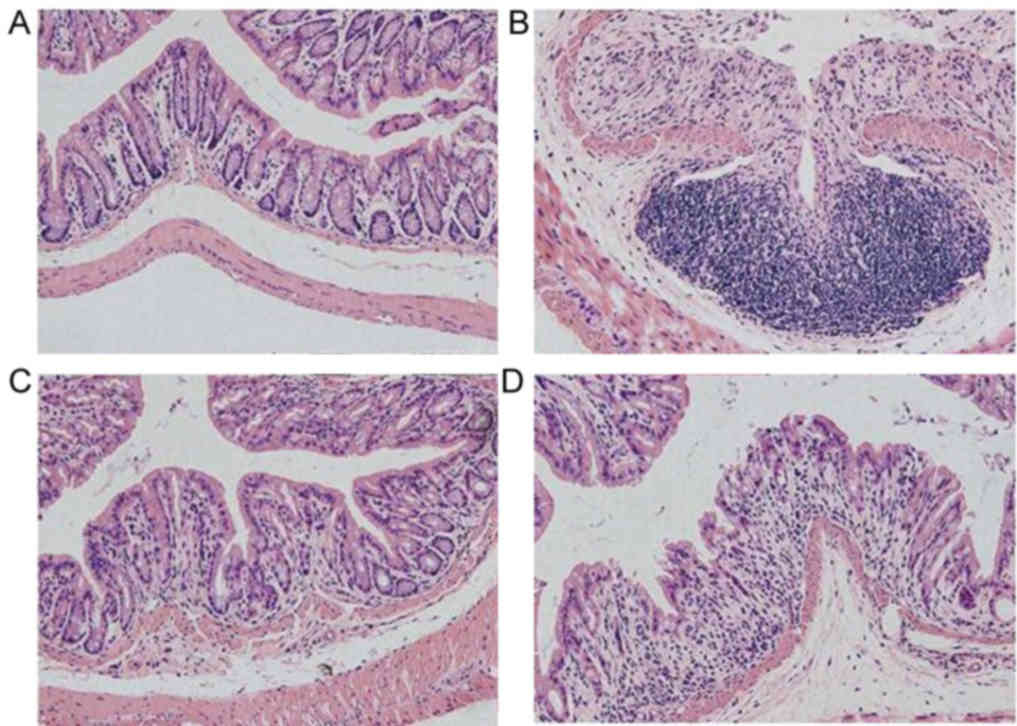
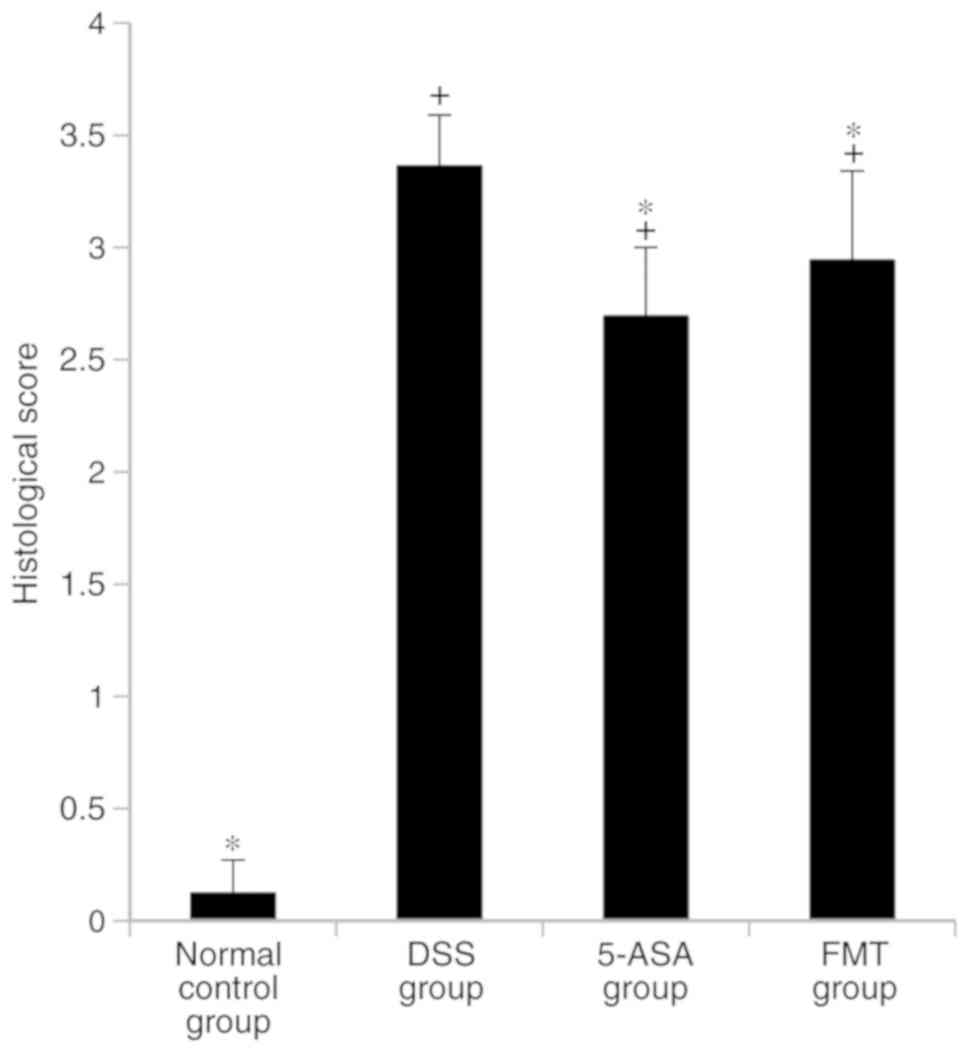
Histological injury score of mice in
each group
In the DSS, 5-ASA and FMT groups, colonic mucosal
epithelial cells were widely absent, glands had lost their
integrity, an extensive infiltration of inflammatory cells was
apparent and typical inflammatory reactions were observed (Fig. 3). The histological scores for these 3
groups were higher compared with those in the normal control group,
and the difference was statistically significant (P<0.05). The
histological score in the FMT group was lower compared with that in
the DSS group (P<0.05), but this difference was less pronounced
than that observed between the 5-ASA and the DSS groups (Fig. 4).
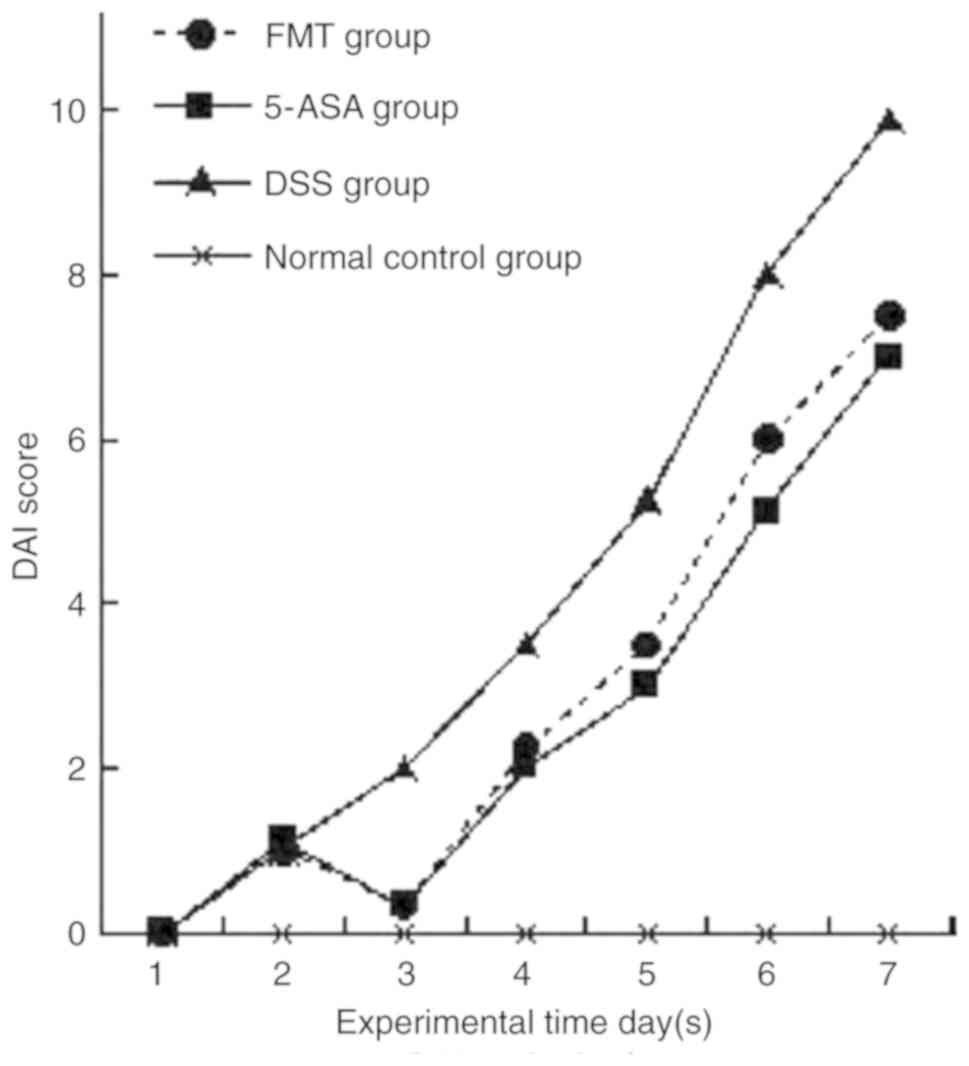
DAI score in each group
In the group subjected to FMT treatment, the body
weight loss, fecal consistency and occult blood status were
improved in the FMT group compared with those in the DSS group
(P<0.05). Furthermore, the DAI score was higher compared with
that in the 5-ASA group, although the difference was revealed not
to be statistically significant (P>0.05, Fig. 5). It is noteworthy that the DAI score
recorded for the experiment on day 3 was lower than that determined
on day 2. The possible explanation for this was that no enema
stress occurred on the second day, and the mice gained weight after
having had a 24-h opportunity to feed undisturbed.
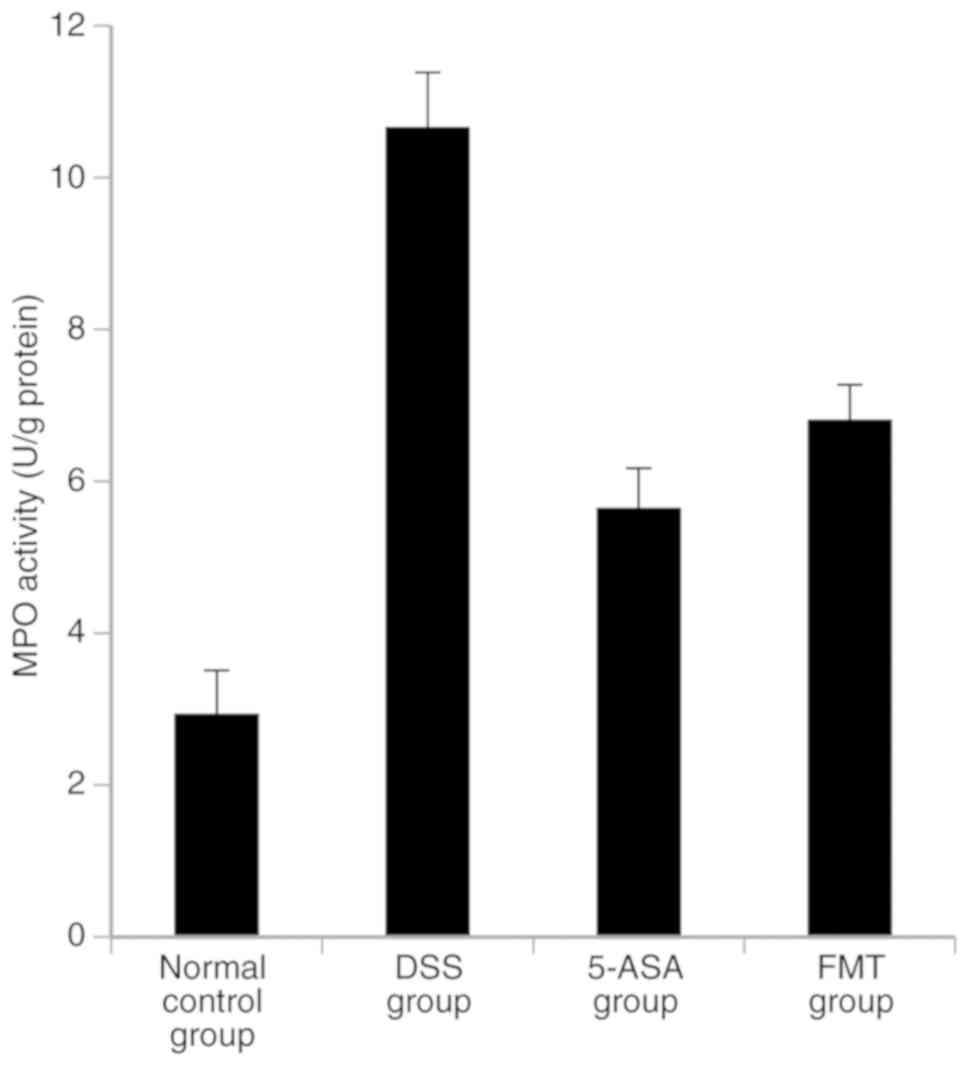
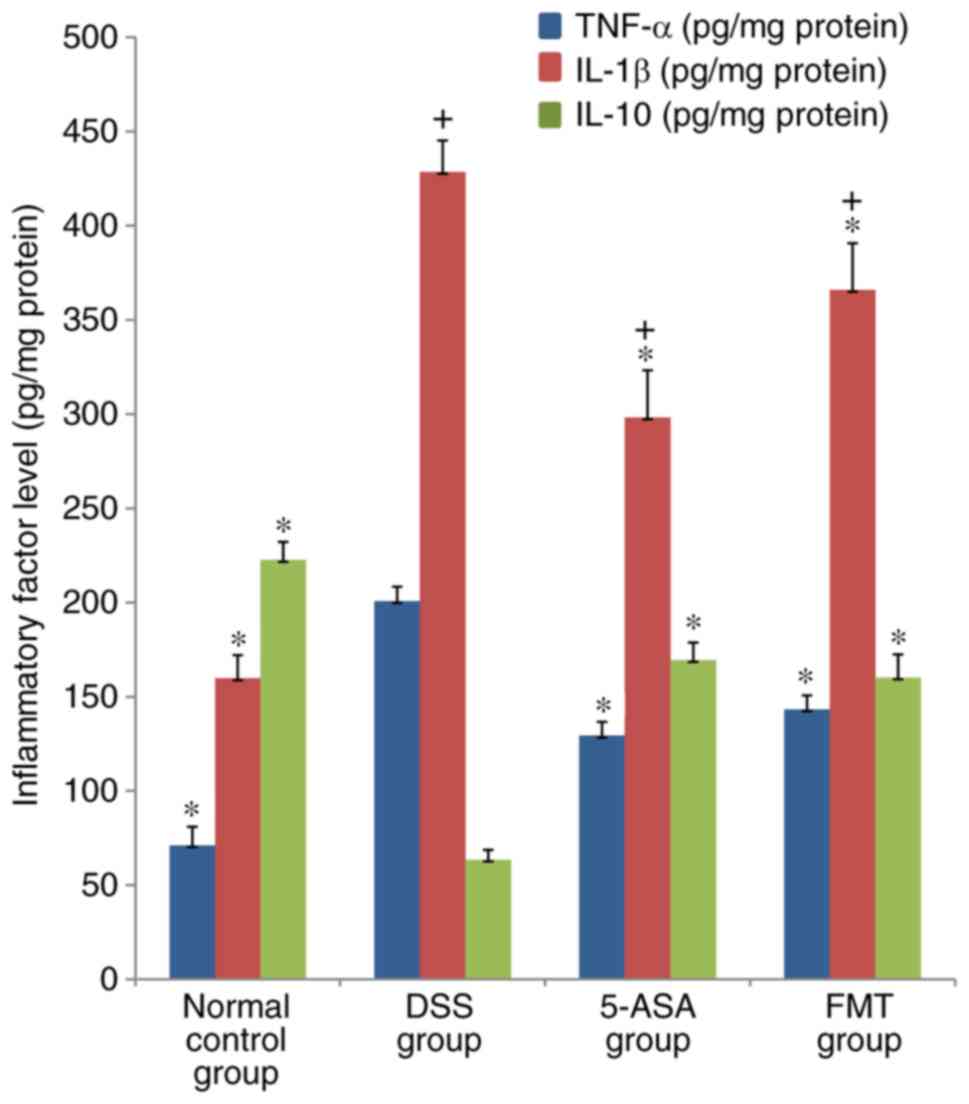
Colonic MPO activity, and the levels
of TNF-α, IL-1β and IL-10
MPO activity, and the levels of TNF-α and IL-1β in
the DSS, 5-ASA and FMT groups were significantly higher compared
with those in the normal group (P<0.05), while the level of
IL-10 was lower than that in the normal group (P<0.05). MPO
activity, and the levels of TNF-α and IL-1β in the 5-ASA and FMT
group were lower compared with those in the DSS group, and these
differences were shown to be statistically significant (P<0.05);
in addition, the level of IL-10 was significantly higher compared
with that in the DSS group (P<0.05; Figs. 6 and 7).
Discussion
In the present study, a murine model of acute UC was
induced by the administration of 3% DSS solution, and the efficacy
of FMT to reduce intestinal inflammation in the constructed UC
model was preliminarily assessed. It was revealed that the weight
loss, stool consistency, fecal occult blood, colon length change
and intestinal mucosal injury in mice treated with FMT were all
improved compared with those in the model group. In the FMT group,
the MPO activity in colon tissues was decreased, and the levels of
the inflammatory factors TNF-α and IL-1β were also decreased,
whereas the level of the immunomodulator IL-10 was increased
compared with that in the DSS group, although this was not as
effective as treatment with 5-ASA.
Germ-free mice are more likely to develop
experimental UC upon DSS induction, and this phenomenon was
previously demonstrated to be reversed following fecal bacteria
transplantation from healthy wild mice, indicating that intestinal
symbiotic bacteria are able to slow down the formation of
DSS-induced UC to a certain extent (12). In addition, an increasing number of
studies suggest that an imbalance in the number and function of
type 1 T-helper cells (Th1)/Th17 and forkhead box P3+
regulatory T cells (Treg) may cause intestinal immune dysfunction
and immunopathological damage, thereby leading to IBD (13). In the intestinal tract, Th1 cells
predominantly secrete the pro-inflammatory cytokines interferon-γ
and TNF-α. Th17 cells, a subgroup of T cells, secrete the
pro-inflammatory cytokine IL-17. Treg cells secrete the
anti-inflammatory factor IL-10 and exert a protective role in IBD
(14). Once the symbiotic balance
between host-intestinal microbes and the immune system is broken,
inflammatory diseases including IBD are able to arise. The
occurrence of intestinal inflammation is caused by the overly
strong immune response in susceptible individuals induced by an
imbalance of the intestinal flora. Certain studies have indicated
that FMT is able to reconstruct the composition of the intestinal
flora in animal models (15).
Recently, a number of studies have focused on
determining which types of symbiotic bacteria inhibit inflammation
and investigating the associated, underlying mechanisms. It has
been indicated that certain intestinal symbiotic bacteria and their
metabolites are able to promote the immune balance between Th cells
and Treg cells in mammals, thereby preventing, or effectively
treating, IBD. Fragile Pseudomonas bacilli and its
polysaccharide A are able to inhibit the infiltration of
neutrophils in the intestinal tract of experimental animals with
IBD by stimulating Treg cells to produce IL-10, which leads to a
reduction in the release of cytokines including TNF-α, IL-1β and
IL-23 (16). It was also confirmed
that Sporogenous sclerenchyma of fusobacteria IV and XIVa
may allay DSS-induced UC in mice, where the action of the bacteria
was facilitated by inducing Treg cells (17). Fragile Clostridium is able to
reduce 2,4,6-trinitrobenzene sulfonic acid-induced colitis. In
addition, short-chain fatty acids, produced by intestinal symbiotic
bacteria, are able to regulate the homeostasis of Treg cells in the
colon, which is another anti-inflammatory mechanism occurring in
the gut microbiota (18). In the
present study, UC was induced in healthy mice by DSS provided with
drinking water, which was alleviated by enema administration of
FMT, and a mixture of fecal bacteria from rats and mice was used to
increase the microbial diversity. This led to a remodeling of the
flora diversity, and it is possible to hypothesize that, to a
certain extent, various microbes and associated metabolites may
adjust the intestinal mucosal immune status, including the balance
between Treg and Th cells, based on the promotion of secretion of
anti-inflammatory cytokines, e.g. IL-10, and inhibition of the
secretion of the pro-inflammatory cytokines TNF-α, IL-1β and IL-17,
such as to reduce inflammation and repair the intestinal mucosal
barrier function.
In the present study, FMT was applied to the lower
gastrointestinal tract by enema, which led to certain therapeutic
effects. However, various issues remain to be addressed. For
instance, the identity of the specific microorganism(s) or
metabolite(s) that are able to exert the anti-inflammatory effects.
Furthermore, it remains elusive whether the intestinal flora
imbalance is the cause or the result of IBD, and the most effective
viscosity and transplantation volume of the fecal bacteria solution
remains to be determined. The effects of the administration of FMT
in animal models may be further analyzed with the application of
16S ribosomal RNA gene detection technology to investigate the
intestinal microorganism macrogenome, in order to elucidate the
relevant mechanism(s) underlying the effects of FMT in the
treatment of IBD. The production of microbiotic preparations for
individualized treatment may hold great promise for the clinical
treatment of IBD. However, one limitation of the present study was
that mice in the normal group received no treatment.
In conclusion, the present study suggested that FMT
was efficient in treating DSS-induced colitis in mice. DSS is one
of the most common pharmacological drugs that is used to induce
experimental colitis and closely simulates the clinical
pathological manifestations of human UC. Of note, no significant
differences in the therapeutic effects between 5-ASA and FMT in UC
were identified in the present study. However, 5-ASA was slightly
more efficient regarding the improvement of certain parameters,
such as reducing the levels of inflammatory factors and
upregulation of anti-inflammatory factors. Furthermore, by
remodelling the intestinal flora, FMT may restore the immune
balance that depends on the interaction between the host and
symbiotic microorganisms, to inhibit the release of
pro-inflammatory cytokines and promote the production of
anti-inflammatory cytokines, so as to reduce the inflammation in
the colon.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
and Technological Project of Hubei Province (grant no. 2015BKB013)
and the Natural Science Foundation of Hubei Province in China
(grant no. 2013CHB025).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ conceived and designed the experiments. PJ
performed the experiments. MM, SJ and JG were responsible for
analysis and interpretation of data, statistical analysis and
manuscript drafting, and provided critical revisions of the
manuscript for important intellectual content. JZ was responsible
for the research creation and design, analysis and interpretation
of data, and manuscript drafting, and provided critical revision of
the manuscript for important intellectual content. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
All procedures for the care and handling of animals
used in the present study were approved by the University of Wuhan
Animal Care Committee [Wunah, China; certificate no. SYXK (E)
2009–0027].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li D, Wang P, Wang P, Hu X and Chen F: The
gut microbiota: A treasure for human health. Biotechnol Adv.
34:1210–1224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yadav V, Varum F, Bravo R, Furrer E, Bojic
D and Basit AW: Inflammatory bowel disease: Exploring gut
pathophysiology for novel therapeutic targets. Transl Res.
176:38–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miyoshi J and Chang EB: The gut microbiota
and inflammatory bowel diseases. Transl Res. 179:38–48. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morgan XC, Tickle TL, Sokol H, Gevers D,
Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, et
al: Dysfunction of the intestinal microbiome in inflammatory bowel
disease and treatment. Genome Biol. 13:R792012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El-Salhy M and Mazzawi T: Fecal microbiota
transplantation for managing irritable bowel syndrome. Expert Rev
Gastroenterol Hepatol. 12:439–445. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wohlgemuth S, Haller D, Blaut M and Loh G:
Reduced microbial diversity and high numbers of one single
Escherichia coli strain in the intestine of colitic mice. Environ
Microbiol. 11:1562–1571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaur R, Thakur S, Rastogi P and Kaushal N:
Resolution of Cox mediated inflammation by Se supplementation in
mouse experimental model of colitis. PLoS One. 13:e02013562018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moulari B, Pertuit D, Pellequer Y and
Lamprecht A: The targeting of surface modified silica nanoparticles
to inflamed tissue in experimental colitis. Biomaterials.
29:4554–4560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamilton MJ, Weingarden AR, Sadowsky MJ
and Khoruts A: Standardized frozen preparation for transplantation
of fecal microbiota for recurrent Clostridium difficile infection.
Am J Gastroenterol. 107:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
11
|
Murano M, Maemura K, Hirata I, Toshina K,
Nishikawa T, Hamamoto N, Sasaki S, Saitoh O and Katsu K:
Therapeutic effect of intracolonically administered nuclear factor
kappa B (p65) antisense oligonucleotide on mouse dextran sulphate
sodium (DSS)-induced colitis. Clin Exp Immunol. 120:51–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maslowski KM, Vieira AT, Ng A, Kranich J,
Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al:
Regulation of inflammatory responses by gut microbiota and
chemoattractant receptor GPR43. Nature. 461:1282–1286. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maloy KJ and Powrie F: Intestinal
homeostasis and its breakdown in inflammatory bowel disease.
Nature. 474:298–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shale M, Schiering C and Powrie F: CD4(+)
T-cell subsets in intestinal inflammation. Immunol Rev.
252:164–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Manichanh C, Reeder J, Gibert P, Varela E,
Llopis M, Antolin M, Guigo R, Knight R and Guarner F: Reshaping the
gut microbiome with bacterial transplantation and antibiotic
intake. Genome Res. 20:1411–1419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazmanian SK, Round JL and Kasper DL: A
microbial symbiosis factor prevents intestinal inflammatory
disease. Nature. 453:620–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Atarashi K, Tanoue T, Shima T, Imaoka A,
Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al:
Induction of colonic regulatory T cells by indigenous clostridium
species. Science. 331:337–341. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smith PM, Howitt MR, Panikov N, Michaud M,
Gallini CA, Bohlooly-Y M, Glickman JN and Garrett WS: The microbial
metabolites, short-chain fatty acids, regulate colonic Treg cell
homeostasis. Science. 341:569–573. 2013. View Article : Google Scholar : PubMed/NCBI
|