Introduction
Cardiovascular diseases represent the primary cause
of mortality in Western countries, and numerous risk factors,
including the reduced glomerular filtration rate, have been
associated with greater incidence in myocardial infarction, stroke
and sudden cardiac mortality (1–6).
Ischemia is a primary pathogenetic factor leading to these events,
which represent the final stages of morphofunctional cardiovascular
alterations that develop over a long period of time, mostly
asymptomatically (7).
Numerous efforts have been made in recent years to
search for markers, globally termed subclinical organ damage, to
early identify those asymptomatic patients, who already presented a
variable combination of vascular, cardiac or renal alterations,
thus being at enhanced risk for cardiovascular diseases.
Recently, intrarenal haemodynamics were identified
as a potential marker of systemic vascular damage (8) and a useful tool to better stratify the
short and long-term cardiovascular risk of patients in different
clinical subsets. In particular, renal resistance index (RI) and
renal pulsatility index (PI), non-invasively assessed by Duplex
ultrasound, were closely associated with well-known markers of
subclinical organ damage, including carotid intima-media thickness
(cIMT) (9–11), arterial stiffness (9,12) and
left ventricular mass (13,14). Furthermore, recent studies detected
the direct role of intrarenal haemodynamic impairment as an
independent predictor of adverse cardiovascular events and
mortality in different populations, particularly in the elderly
(15) and in patients with chronic
kidney disease (CKD), hypertension or heart failure (16–18).
The association between carotid and coronary
atherosclerosis has been previously identified (19–23). In
the present study, it was hypothesized that the atherosclerotic
process additionally affects the renal district and that renal
haemodynamics are correlated with coronary atherosclerosis.
Therefore, the association between intrarenal vascular alterations
with coronary atherosclerotic burden, as well as with carotid
atherosclerotic disease, in patients with hypertension was
investigated.
Materials and methods
Study population
The present study involved 130 Caucasian patients,
who attended the Department of Cardiology of the University
Hospital of Palermo (Palermo, Italy). All subjects aged between 30
and 80 years with a history of hypertension, who were referred for
elective coronary angiography, were consecutively enrolled for 18
months from September 2015.
The exclusion criteria included heart failure (New
York Heart Association Class IV), permanent atrial fibrillation,
heart rate >100 bpm or <50 bpm, current or previous acute
coronary syndrome, previous coronary artery bypass graft, moderate
to severe aortic or mitral valve disease, stenosis of renal
arteries as assessed by Doppler ultrasound criteria (24), endocrine or malignant hypertension,
severe obesity [body mass index (BMI) ≥40 Kg/m2],
previous surgery or percutaneous interventions on carotid arteries,
renal replacement therapy (patients who had a transplant or
dialysis), and principal non-cardiovascular diseases. This
information had been gathered from medical records and outpatient
medical examinations. Written informed consent was obtained from
each subject, and the local review board of the University of
Palermo approved the study protocol, which conformed to the ethical
guidelines of the Helsinki declaration. The datasets used and/or
analysed during the current study are available from the
corresponding author on reasonable request.
Study design
In all subjects, a comprehensive evaluation of
clinical history and physical examination were performed. Patients
who reported smoking cigarettes regularly during the past year were
considered current smokers. The body weight and height of the
patients were measured by a nurse. Clinic blood pressure (BP) was
recorded by a doctor, following the recommendations of the 2013
European Society of Hypertension/European Society of Cardiology
guidelines (25).
Fasting blood samples were collected to assess
routine blood chemistry. A B-mode and Duplex-Doppler
ultrasonographic examination of intrarenal vasculature was
performed to evaluate RI, PI, and renal acceleration time (AT).
Carotid atherosclerotic disease was additionally assessed by Duplex
Doppler ultrasound as a model of vascular damage associated with
renal parameters, and echocardiography was conducted to prevent
bias due to interference of the left ventricular ejection fraction
(LVEF), that was calculated using the modified biplane Simpson's
rule, according to the American Society of Echocardiography
recommendations (26). Later in the
same day, a coronary angiography was performed to assess
atherosclerotic burden using the Gensini Score (GS) (27).
Measurements
Clinical BP was considered the mean of three
consecutive measurements obtained, at 2-min intervals, using an
electronic oscillometric validated device (Microlife AG Swiss
Corporation, Widnau, Switzerland) (28), after 5 min of rest in the sitting
position.
Routine biochemical parameter determination was
performed with standard techniques using an autoanalyser
(Boehringer Mannheim for Hitachi system 911; Mannheim, Germany).
Low-density lipoprotein cholesterol was calculated using the
Friedwald formula. Definition and classification of CKD followed
the Kidney Disease Improving Global Outcomes guidelines, and the
estimated glomerular filtration rate (eGFR) was estimated using the
Chronic Kidney Disease-Epidemiology Collaboration equation
(29).
Ultrasound evaluation of renal
vasculature
The intrarenal Duplex ultrasonography was performed
using a GE Logiq P5 PRO instrument (GE Healthcare, Chicago, IL,
USA), with a 4 MHz transducer operating at 2.5 MHz for Doppler
analysis. The Doppler signal was obtained from the interlobar
arteries by placing the sample volume at the level of the
cortico-medullary junction (10,24).
Peak systolic velocity (PSV), telediastolic velocity (TDV) and mean
velocity (MV) were measured. RI was calculated using the formula:
RI=(PSV-TDV)/PSV, whereas, PI was obtained using the formula:
PI=(PSV-TDV)/MV. The AT was measured as the time distance (msec)
between the onset of systolic upstroke with the first systolic
peak. The Doppler angle selected was <60°, and special care was
taken not to compress the kidney and to prevent the patient
performing a Valsalva maneuver, because these factors may affect
measurements. The values were calculated as the average of six
measurements from the upper, middle and lower thirds of the two
kidneys. Values of RI, PI and AT were ≥0.70, ≥1.20 and ≥100 ms,
respectively, and were considered abnormal in agreement with
existing literature (10,24,30,31).
Ultrasound evaluation of carotid
arteries
The carotid ultrasonographic investigation was
performed [using the same GE Logiq P5 PRO instrument (GE
Healthcare)] with a 10 MHz linear-array transducer operating at 5
MHz for Doppler analysis. The examination was obtained following
the recommendations of the Mannheim Carotid Intima-Media Thickness
Consensus (32).
cIMT was defined as the distance between the
vascular lumen-intima interface (internal measurement site) and the
media-adventitia transition (external limit). It was obtained on
the common carotid artery through the examination of a freezed
longitudinal section of 10 mm at 1 cm from the bifurcation, at the
end-diastolic state, in the proximal and far wall, using lateral,
anterior and posterior projections. A total of six measurements was
obtained for the right carotid artery and six for the left carotid
using the average values (average cIMT). cIMT was not obtained in
the presence of a carotid plaque; however, its measurement was
shifted proximally to the plaque-free site. According to the 2013
European Society of Hypertension/European Society of Cardiology
guidelines for the management of arterial hypertension, cIMT
>0.90 mm was considered abnormal (25,32).
Plaques, whose focal structures encroached into the
arterial lumen of ≤0.5 mm or 50% of the surrounding cIMT value, or
demonstrating a cIMT >1.5 mm, were considered (32). The severity of the carotid stenosis
was evaluated according to the European Carotid Surgery Trial
criteria (33). A 25% cut-off was
selected to distinguish between subjects with higher or lower
carotid atherosclerotic burden (34,35).
Angiographic evaluation of coronary
arteries
Selective coronary angiography was obtained by
applying Judkins technique in all patients (36). Angiograms were recorded in multiple
projections with the biplanar digital cardiac imaging floor-mounted
system Artis Zee (Siemens AG, Munich, Germany). Coronary angiograms
were assessed by two expert interventional cardiologists (DB and
DP) who were blinded to the clinical characteristics of the
patients and the Doppler ultrasonographic results. According to the
American College of Cardiolgy/American Heart Association guidelines
for percutaneous coronary intervention (36), an haemodynamically significant
coronary artery stenosis was assumed for a value at least equal to
the 50% of the lumen in any of the principal epicardial vessels,
including the left primary coronary artery, left anterior
descending artery, left circumflex artery, right coronary artery or
their principal branches.
The atherosclerotic burden of coronary arteries was
evaluated using the Gensini Score (GS) (27). According to this method, a severity
coefficient was assigned for each vessel, according to the degree
of stenosis (0, 1, 2, 4, 8, 16 or 32) and the importance of the
segment itself (5 for the left primary trunk to 0.5 for the most
distal segments). Subsequently, total GS was obtained by the sum of
the partial results as a global severity index of coronary
atherosclerotic disease (27).
Statistical analysis
Statistical analysis was firstly conducted on the
entire population (n=130) and on the population divided in
quintiles based on GS cut-offs as follows: ≤9.0 (GS I; n=26); >9
and ≤17 (GS II; n=27); >17 and ≤30 (GS III; n=25); >30 and
≤44 (GS IV; n=26); and >44 (GS V; n=26). Subsequently, data were
analysed in three groups based on the Atherosclerotic-Score (AS) as
follows: Absence of plaques (AS I; n=23); carotid stenosis <25%
(AS II; n=64); and carotid stenosis ≥25% (AS III; n=43).
GS values were further evaluated in the population
divided into tertiles of PI (I tertile: PI <1.30; II tertile: PI
≥1.30; and <1.57; III tertile: PI ≥1.57) and RI (I tertile: RI
<0.715; II tertile: RI ≥0.715 and <0.755; and III tertile: RI
≥0.755).
The distribution of variables was analysed by the
Kolmogorov-Smirnov test. Continuous variables are presented as the
mean ± standard deviation, and were compared by Students' t-test or
analysis of variance with Dunnett's post-hoc test for multiple
comparisons, with ‘GS I’ considered the control group. Categorical
variables were presented as numbers (and percentages) and compared
with χ2 test and Monte Carlo correction. Variables with
non-Gaussian distribution (GS and triglycerides) were expressed as
the median and interquartile range because of their skewed
distribution, and they were log-transformed to better satisfy
distributional assumptions prior to using parametric tests.
The univariate and multivariate associations were
tested by simple and multiple linear regression analyses. The
strength of the associations among the variables was expressed by
the Pearson correlation coefficients (r) and the unstandardised (B)
and standardised (β) multiple regression coefficients. The
differences between the Pearson coefficients were assessed by
r-to-z Fisher transformation. The association between PI (or
alternately RI) and GS was further analysed with the correlation
curve using different mathematical models (linear, exponential,
logarithmic, quadratic, cubic and sigmoidal). The stepwise
multivariate regression analysis was performed considering GS
(log-transformed) as an outcome variable, and independent
variables, including age, sex, smoking habit, BMI, eGFR, PI (or
alternatively RI), serum uric acid, serum glucose levels (or
diabetes as a dichotomous variable), high density lipoprotein
(HDL)-cholesterol, triglycerides (log-transformed), clinic mean BP,
clinic pulse pressure, statin treatment (0=no treatment,
1=treatment) and antidiabetic treatment (0=no treatment, 1=
treatment), were included in the models.
Univariate and multivariate analyses were first
conducted on the entire population and subsequently in the two
groups; low (GS ≤30; n=78) or high (GS >30; n=52) coronary
atherosclerotic burden. The cut-off of GS=30 was selected according
to existing literature (36). These
analyses were additionally conducted using similar multivariate
models, as appropriate, in the subgroup of subjects with cIMT ≤0.90
mm (n=81) and in the small group of subjects with no carotid
plaques (n=23).
In all multiple regression analyses, a backward
stepwise procedure was used, with α=0.15 as the cut-off for entry
or removal of variables. For all analyses, the null hypothesis was
rejected at a two-tailed P≤0.05. SPSS software (version 22.0; IBM
Corp., Armonk, NY, USA) was used.
Results
Higher GS quintiles are associated
with greater cIMT, a larger percentage of carotid plaques and
coronary involvement
Vascular and haemodynamic characteristics are
summarised in Table I. Subjects in
the higher GS quintiles had a greater cIMT (P=0.001), a larger
percentage of carotid plaques (P=0.001) and coronary involvement
(P<0.001) compared with those in lower ones. A weak significant
difference in PI was identified among quintiles (P=0.041), whereas,
RI and AT did not differ significantly. Furthermore, the
percentages of subjects with PI ≥1.20 or RI ≥0.70; however, not
with AT ≥100 ms, were significantly higher with the higher GS
quintiles (P=0.033 and P=0.024, respectively).
 | Table I.Vascular and haemodynamic data of the
overall study population and of the population divided into
quintiles based on Gensini Score (GS). |
Table I.
Vascular and haemodynamic data of the
overall study population and of the population divided into
quintiles based on Gensini Score (GS).
| Variables | Overall study
population (n=130) | GS I (≤9)
(n=26) | GS II (9–17)
(n=27) | GS III (17-0)
(n=25) | GS IV (30–44)
(n=26) | GS V (>44)
(n=26) | P-value |
|---|
| Gensini Score | 23 (10–38) | 6 (3–7) | 11
(10–12)b | 22
(19–26)c | 35
(31–38)c | 53
(46–60)c | <0.001 |
| CAD$, n
(%) | 74
(57) | 0 (0) | 3 (11)a | 19
(76)c | 26
(100)c | 26
(100)c | <0.001 |
| LVEF, % |
52±11 |
53±13 | 53±8 | 55±8 |
51±11 |
50±12 | 0.709 |
| PI |
1.48±0.31 |
1.32±0.26 |
1.52±0.35a |
1.57±0.25b |
1.46±0.36 |
1.51±0.27a | 0.041 |
| RI |
0.74±0.06 |
0.71±0.06 |
0.75±0.06 |
0.75±0.05 |
0.73±0.06 |
0.75±0.07 | 0.090 |
| AT (ms) | 117±48 | 122±48 | 123±45 | 107±46 | 118±63 | 115±38 | 0.843 |
| PI ≥1.20, n
(%) | 110 (85) | 16 (62) | 23 (85) | 24
(96)a | 22 (85) | 25
(96)a | 0.033 |
| RI ≥0.70, n
(%) | 97
(75) | 11 (42) | 24
(89)a | 22
(88)a | 19
(73)NS | 22 (85) | 0.024 |
| AT ≥100 ms, n
(%) | 80
(62) | 18 (69) | 17 (63) | 14 (56) | 14 (54) | 17 (65) | 0.894 |
| cIMT, mm |
0.87±0.17 |
0.76±0.13 |
0.83±0.16 |
0.92±0.15c |
0.91±0.18b |
0.92±0.18c | 0.001 |
| cIMT >0.90 mm, n
(%) | 49
(38) | 2 (8) | 9 (33)
NS | 14
(56)c | 12
(46)a | 12
(46)a | 0.009 |
| Carotid plaques, n
(%) | 107 (82) | 18 (69) | 21 (78)
NS | 18 (72) | 24
(92)a | 26
(100)b | 0.007 |
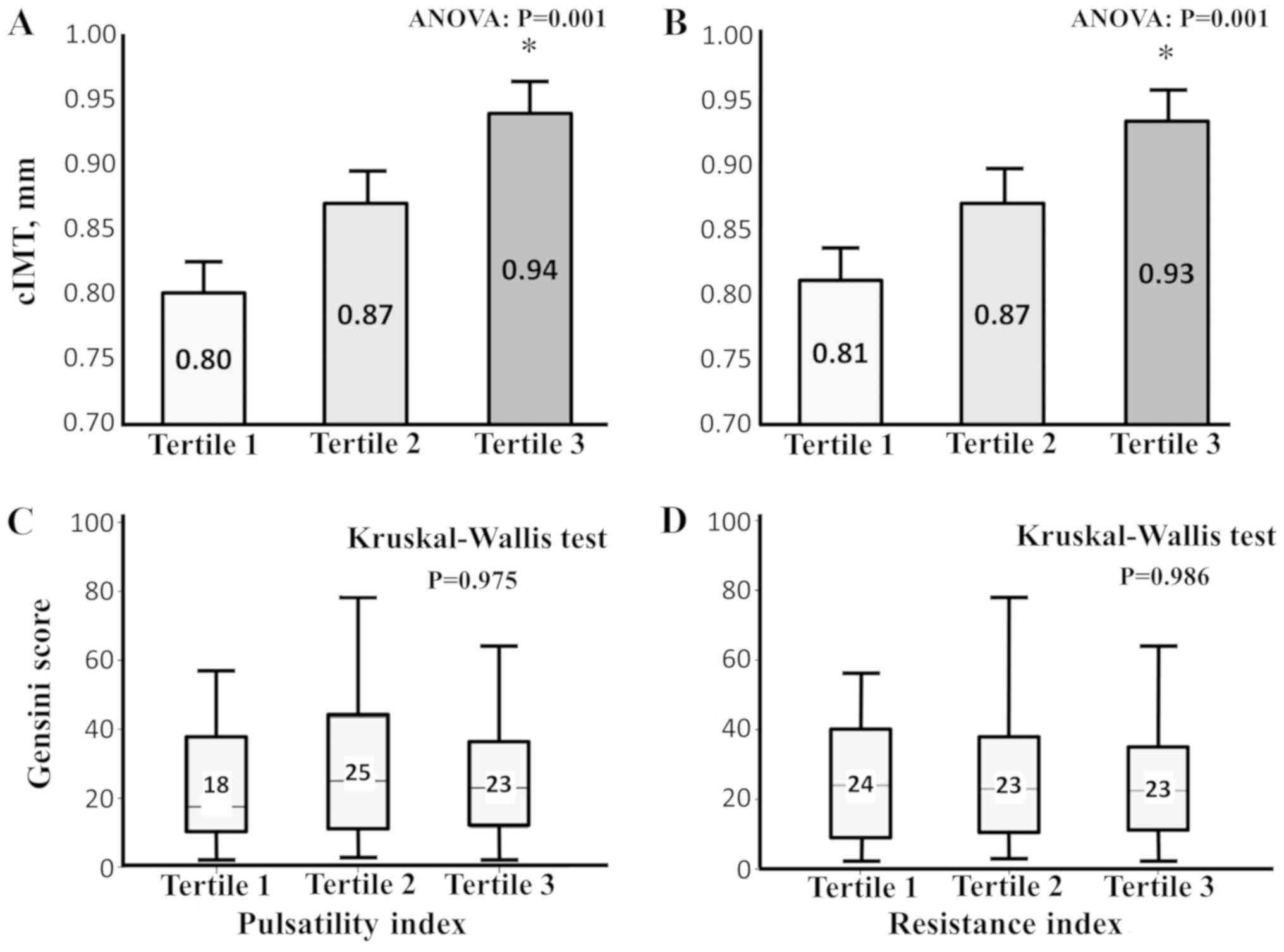
Higher tertiles of PI and RI are
associated with greater cIMT values
When the entire population was divided into tertiles
of PI, greater cIMT values were observed in subjects with higher PI
(Fig. 1; Panel A; P=0.001). Similar
results were observed by dividing the population into tertiles
according to RI values (Fig. 1;
Panel B; P=0.006). However, no statistically significant difference
in GS existed among the groups (Fig.
1; Panel C, D).
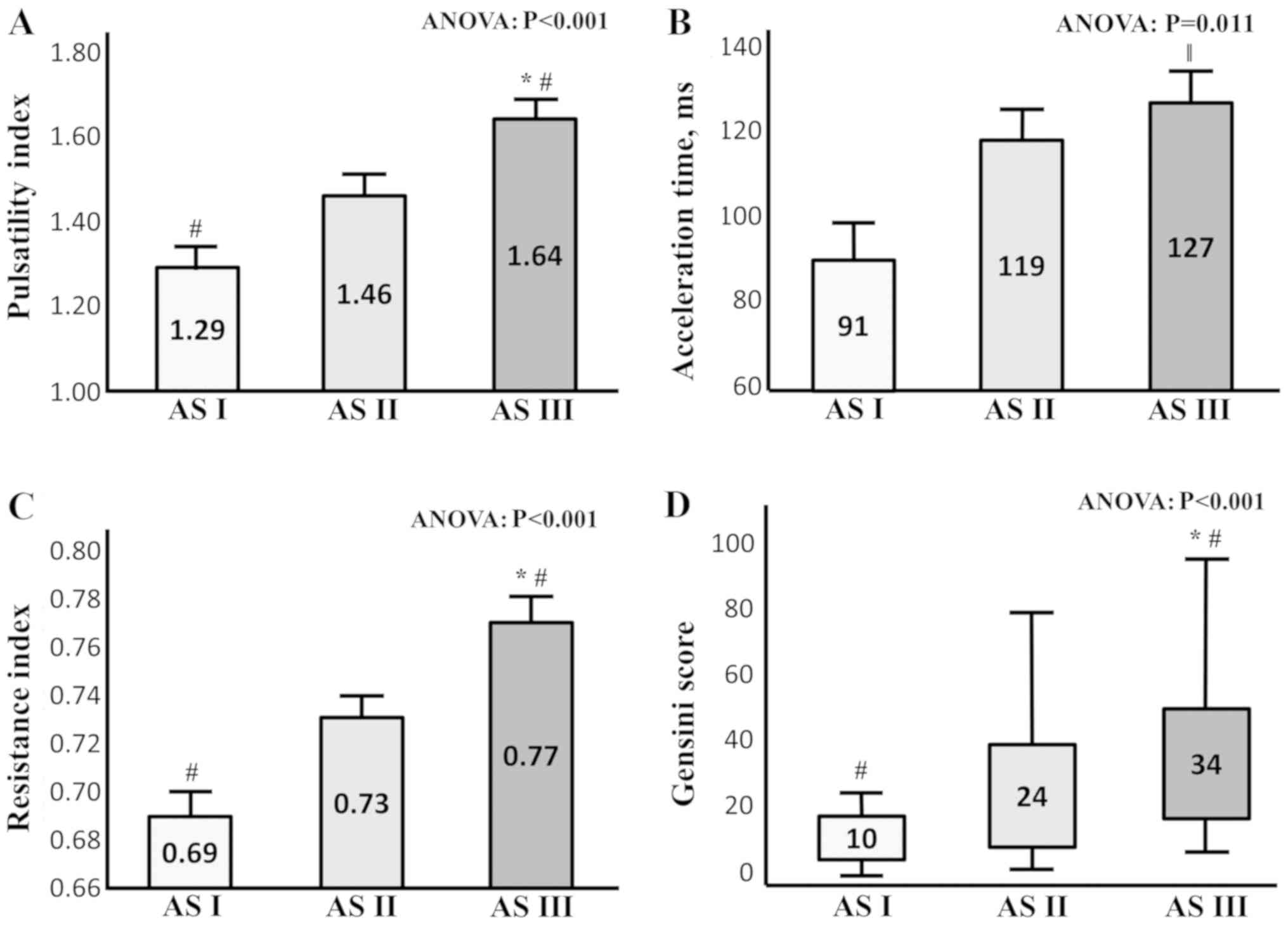
Higher values of PI, RI, AT and GS are
associated with severe carotid artery disease
Higher values of PI, RI and AT, in addition to an
increased GS, were additionally observed in patients with severe
carotid artery disease (AS III) compared with subjects belonging to
AS I and AS II (Fig. 2; Panel A-D;
P<0.001 for PI; RI and GS; P=0.011 for AT).
Low GS is positively correlated with
uric acid and triglyceris, and negatively correlated with HDL
Table II
demonstrated the correlations between GS with other variables in
the whole study population and in the groups, based on severity of
coronary (GS ≤30 or GS >30) or carotid (subjects without or with
carotid plaques) atherosclerotic disease; uric acid and
triglycerides were positively associated with were correlated with
GS in patients with low GS, and HDL was negatively correlated;
however, these correlations were not observed in patients with high
GS. PI and RI (in addition to AT) did not significantly correlate
with GS in entire population by linear univariate analysis, even
following adjustment for age and LVEF, and no differences were
identified when these associations were alternately evaluated in
patients divided for eGFR (≥ or <60 ml/min/1.73 m2)
or diabetes (absence or presence). On the contrary, cIMT was
significantly associated with PI (r=0.294; P<0.001), RI
(r=0.338; P<0.001) and GS (r=0.310; P<0.001); however, not
with AT.
 | Table II.Main correlations in the entire study
population, in the groups with GS <30 or GS ≥30, and in the 2
groups without or with carotid plaques. |
Table II.
Main correlations in the entire study
population, in the groups with GS <30 or GS ≥30, and in the 2
groups without or with carotid plaques.
|
| Gensini score
(GS) |
|---|
|
|
|
|---|
| Variables | Overall population
(n=130) | GS ≤30 (n=78) | GS >30
(n=52) | Subjects without
carotid plaques (n=23) | Subjects with
carotid plaques (n=107) |
|---|
| Age | −0.046 | 0.017 | 0.215 | 0.410 | −0.189 |
| Height | 0.154 | 0.176 | −0.152 | 0.114 | 0.187 |
| Weight | 0.074 | 0.032 | 0.019 | 0.201 | 0.187 |
| BMI | 0.004 | −0.094 | 0.171 | 0.235 | 0.097 |
| Glucose | 0.060 | −0.175 | 0.076 | 0.222 | 0.227a |
| Uric acid | 0.022 | 0.276a | 0.004 | 0.403 | 0.054 |
| Total
cholesterol | −0.182a | −0.207 | −0.109 | −0.070 | −0.161 |
| LDL-c | −0.037 | −0.075 | −0.202 | −0.056 | 0.007 |
| HDL-c | −0.37c | −0.573c | 0.040 | −0.339 | −0.330c |
| Tryglicerides | 0.176a | 0.356b | 0.077 | −0.047 | 0.125 |
| Serum
creatinine | −0.097 | −0.111 | 0.003 | 0.248 | 0.100 |
| eGFR | 0.079 | 0.067 | −0.156 | −0.130 | −0.023 |
| Hemoglobin | 0.133 | 0.236a | −0.083 | 0.102 | 0.179 |
| Systolic BP | 0.043 | 0.166 | −0.059 | 0.318 | −0.130 |
| Diastolic BP | 0.182a | 0.280a | −0.062 | 0.586b | 0.079 |
| Mean BP | 0.122 | 0.241a | −0.063 | 0.513a | −0.026 |
| Pulse Pressure | −0.080 | 0.019 | −0.043 | −0.147 | −0.248b |
| Heart Rate | −0.048 | −0.120 | 0.098 | 0.254 | −0.085 |
| LVEF | −0.168 | −0.086 | 0.007 | −0.163 | −0.106 |
| PI | 0.136 | 0.226a | 0.108 | 0.511a | 0.084 |
| RI | 0.138 | 0.201 | 0.133 | 0.380 | 0.053 |
| AT | −0.031 | −0.137 | 0.088 | −0.343 | −0.090 |
| cIMT | 0.310c | 0.345b | −0.097 | 0.371 | 0.244a |
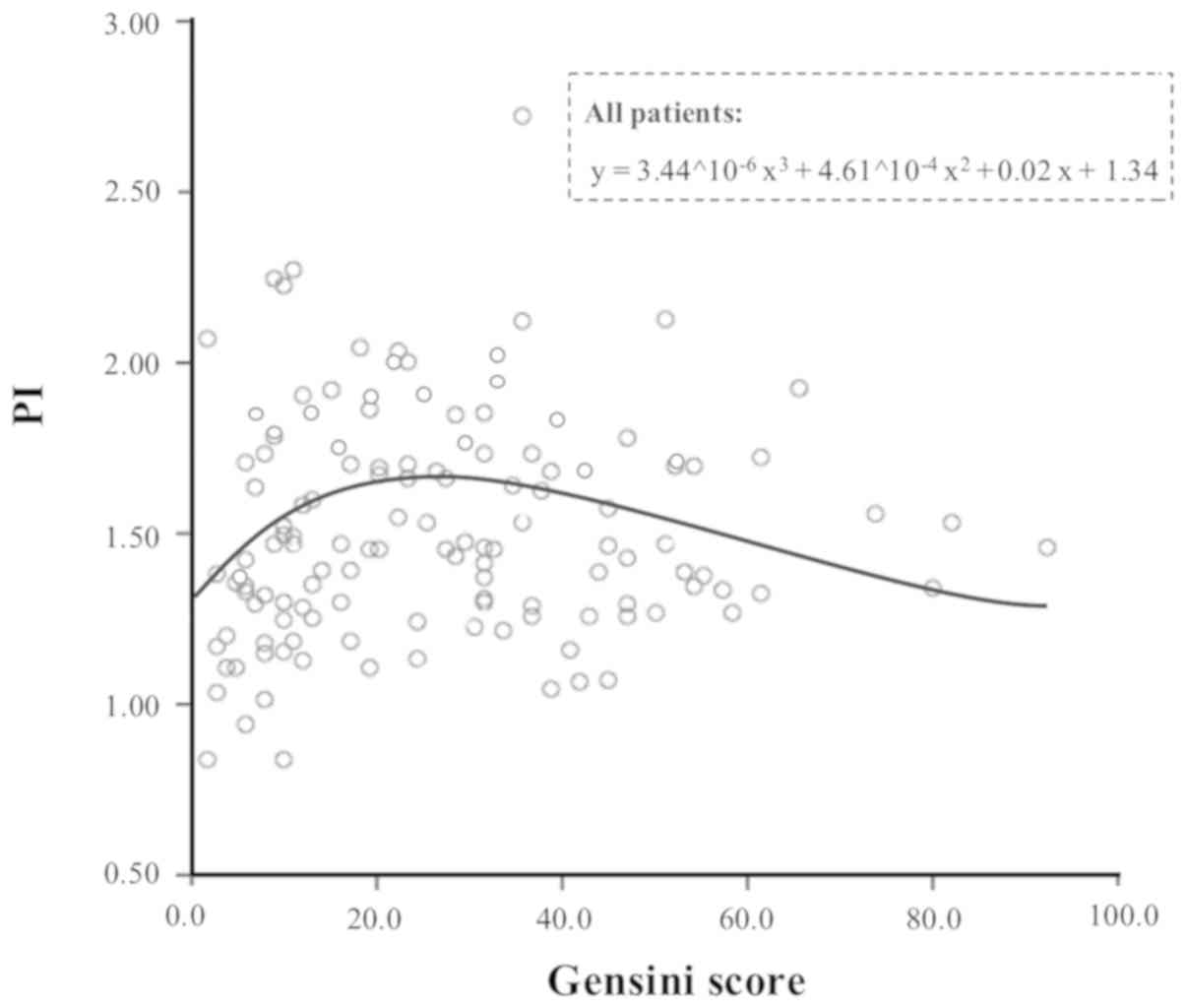
GS is associated with PI in patients
with low coronary atherosclerotic burden
When the association between GS and renal
haemodynamic indices was tested using different mathematical
models, the best resulting model was the one between GS and PI, as
presented in Fig. 3. Due to the
shape of the curve, this association in the two groups at low (GS
≤30) or high (GS >30) coronary atherosclerotic burden was
separately analysed. GS was demonstrated to be statistically
associated with PI (however, not to RI or AT) only in the group
with GS ≤30 (r=0.226; P=0.047). This association was more
significant in subjects with cIMT ≤0.90 mm (r=0.249; P=0.025), and
it was additionally maintained in the small subgroup of subjects
with no carotid plaques (r=0.511; P=0.013).
PI does not independently correlate
with GS; however, is associated with GS
From the multivariate analysis, PI (in addition to
RI) did not independently correlate with GS in the overall study
(Table III); however, it was
independently associated with GS when multivariate analyses were
performed alternatively in the group with GS ≤30 (Table III) or in subjects with cIMT ≤0.90
mm (Table III), even if the
standardized regression coefficients of PI were not particularly
high.
 | Table III.Independent multivariate correlates
of Gensini Score (log-transformed) in the overall study population
(A), in the group of patients with GS ≤30(B) and in subjects with
cIMT ≤0.90 mm (C).a |
Table III.
Independent multivariate correlates
of Gensini Score (log-transformed) in the overall study population
(A), in the group of patients with GS ≤30(B) and in subjects with
cIMT ≤0.90 mm (C).a
| A, Model
(R2=0.245) |
|---|
|
|---|
|
| Regression
coefficients |
|
|---|
|
|
|
|
|---|
|
| Not
standardised | Standardised |
|
|---|
|
|
|
|
|
|---|
| Outcome variable:
Gensini score | B | SE | β | P-value |
|---|
| Clinic systolic
BP | 0.005 | 0.002 | 0.251 | 0.002 |
| Gender | 0.188 | 0.066 | 0.236 | 0.005 |
| Statins | 0.164 | 0.059 | 0.224 | 0.006 |
|
| B, Model
(R2=0.454) |
|
|
| Regression
coefficients |
|
|
|
|
|
|
| Not
standardised |
Standardised |
|
|
|
|
|
|
| Outcome
variable: Gensini score | B | SE | β | P-value |
|
| HDL-c | −0.009 | 0.002 | −0.425 | <0.001 |
| Statins | 0.182 | 0.054 | 0.321 | 0.001 |
| Smoke | −0.222 | 0.072 | −0.304 | 0.003 |
| Serum glucose | −0.002 | 0.001 | −0.273 | 0.007 |
| PI | 0.206 | 0.090 | 0.222 | 0.025 |
|
| C, Model
(R2=0.205) |
|
|
| Regression
coefficients |
|
|
|
|
|
|
| Not
standardised |
Standardised |
|
|
|
|
|
|
| Outcome
variable: Gensini score | B | SE | β | P-value |
|
| Sex | 0.207 | 0.083 | 0.258 | 0.014 |
| PI | 0.274 | 0.124 | 0.226 | 0.030 |
| Statins | 0.173 | 0.081 | 0.225 | 0.035 |
Discussion
The primary result of the present study is that the
intrarenal vascular alterations were significantly correlated with
coronary artery disease in patients with low atherosclerotic burden
(GS ≤30 or cIMT ≤0.90 mm), whereas, this association was not
identified in those with severe coronary artery disease (GS
>30).
A number of previous studies demonstrated that renal
haemodynamic alterations may represent the local epiphenomena of
early damage of the systemic vascular bed in patients with
hypertension; however, renal haemodynamic impairment was able to
promote early atherosclerotic damage itself, thus, resulting in a
self-perpetuating process (9,10). In
356 asymptomatic patients with hypertension, with or without
impairment in renal function, it was previously demonstrated that
RI strongly correlated with subclinical carotid atherosclerosis
(10), and numerous previous studies
demonstrated that intrarenal vascular alterations were associated
with other early markers of organ damage (including left
ventricular mass, aortic pulse wave velocity or microalbuminuria),
in the absence of overt clinical involvement (9,11,12,14).
Furthermore, renal haemodynamic alterations may reflect systemic
vascular damage (38,39), even regardless of hypertension
(40), and they may occur at a very
early stage in the temporal evolution of atherosclerotic damage. In
this regard, Florczak et al (41) observed an independent association
between RI and cIMT in healthy subjects, and a limited value of RI
in differentiating patients with uncomplicated hypertension from
healthy controls. In the present study, PI and RI correlated with
cIMT in the overall study population (in agreement with existing
literature); however, this association was not observed in the
subgroup of subjects with carotid plaques determining stenosis
≥25%. Similarly, in the present study, a significant association
between PI (or RI) and GS was only identified in the group of
patients with lower coronary atherosclerotic burden (and with an
earlier vascular damage), and this may additionally explain why the
independent association between PI and GS is weak in subjects with
early vascular damage, as demonstrated by not particularly high
values of standardised regression coefficients of PI at
multivariate analyses.
In this regard, it is of interest that the enrolled
subjects were referred for elective coronary angiography, and thus
they represent patients with overt vascular disease and with high
or very high cardiovascular risk. Consequently, a number of factors
may have been implicated in the pathogenesis of coronary artery
plaques. The present results suggested that the renal and coronary
haemodynamic impairment proceeds in parallel in the early steps of
atherosclerotic process, whereas, other factors have a pivotal role
in more advanced coronary artery disease. Alternatively, the
greatest variations of the intrarenal haemodynamic indices, even
within the normal ranges, may occur prior to the development of a
significant atherosclerotic coronary burden and may represent an
early step of cardiorenal syndrome type 4, and chronic
abnormalities in renal function may lead to cardiac disease
(42). In support of this
hypothesis, a PI ≥1.20 (or a RI ≥0.70) was already present in 61.5%
(or 42.3%) of the subjects belonging to the first quintile of GS
(GS ≤9), and the majority of the subjects with GS ≤30 had renal
haemodynamic indices above the normal values (PI: 80.8%; RI:
73.1%). Furthermore, patients with GS ≤30 had a large percentage of
carotid plaques (73.1%), whereas, a low atherosclerotic coronary
involvement (28.2%) was present.
The present study has certain potential limitations.
Unlike carotid ultrasound, GS does not allow identification of
early coronary alterations, and the smallest coronary damage that
may be detected through GS is a lumen stenosis of 25% (27). Furthermore, identical structural
damage (or identical degrees of stenosis) may result in different
scores when located in different areas of the coronary district
(27) and this may distort the
results and partly explain the lack of correlation between
intrarenal haemodynamics and coronary atherosclerosis burden in
patients with high GS. However, when the population was divided
into groups only based on the number of coronary arteries involved,
regardless of the degree and location of the stenosis, results were
confirmed. The cross-sectional design of the present study does not
allow complete clarification of the association between the
intrarenal vascular alterations and coronary atherosclerotic
damage. Therefore, an ongoing longitudinal study is required to
address this issue. Finally, renal angiography was not performed in
the patients because it was considered unethical to perform a
procedure with an increased risk for acute kidney injury in a
population with a low prevalence of renal artery stenosis (43). Consequently, renal artery stenosis
was excluded in the patients enrolled in the present study with a
non-invasive Doppler ultrasound examination (24).
In conclusion, renal vascular alterations were
associated with coronary atherosclerotic burden in patients with
hypertension with mild coronary disease.
Acknowledgements
None.
Funding
No funding was received.
Availability of data and materials
The datasets generated/analyzed in the current study
are available on reasonable request from the corresponding
author.
Authors' contributions
GG, DB and LZ have contributed substantially to the
conception and design of the study, analysis and interpretation of
data and drafting the paper; AG, SS, PF, SC and GM have contributed
substantially to the design of the study, interpretation of data,
revising the work critically for important intellectual content;
KDN, MMZ, EN, CG, EM, GZ, DP and GA have contributed substantially
to the acquisition of data and revising the work critically for
important intellectual content. All Authors have read and approved
the manuscript and agree to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from each
subject, and a local review board of the University of Palermo
approved the study protocol, conformed to the ethical guidelines of
Helsinki declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y,
Wu Y, Tang H and Xu D: Association of all-cause and cardiovascular
mortality with prehypertension: A meta-analysis. Am Heart J.
167:160–168.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li G, Zhang P, Wang J, An Y, Gong Q, Gregg
EW, Yang W, Zhang B, Shuai Y, Hong J, et al: Cardiovascular
mortality, all-cause mortality, and diabetes incidence after
lifestyle intervention for people with impaired glucose tolerance
in the da qing diabetes prevention study: A 23-year follow-up
study. Lancet Diabetes Endocrinol. 2:474–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakamura K, Nakagawa H, Murakami Y,
Kitamura A, Kiyama M, Sakata K, Tsuji I, Miura K, Ueshima H and
Okamura T; EPOCH-JAPAN research group, : Smoking increases the risk
of all-cause and cardiovascular mortality in patients with chronic
kidney disease. Kidney Int. 88:1144–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nedkoff L, Knuiman M, Hung J and Briffa
TG: Long-term all-cause and cardiovascular mortality following
incident myocardial infarction in men and women with and without
diabetes: Temporal trends from 1998 to 2009. Eur J Prev Cardiol.
23:1273–1281. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia X, He F, Wu X, Peng F, Huang F and Yu
X: Relationship between serum uric acid and all-cause and
cardiovascular mortality in patients treated with peritoneal
dialysis. Am J Kidney Dis. 64:257–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Capodanno D, Marcantoni C, Ministeri M,
Dipasqua F, Zanoli L, Rastelli S, Mangiafico S, Sanfilippo M,
Romano G and Tamburino C: Incorporating glomerular filtration rate
or creatinine clearance by the modification of diet in renal
disease equation or the cockcroft-gault equations to improve the
global accuracy of the age, creatinine, ejection fraction [ACEF]
score in patients undergoing percutaneous coronary intervention.
Int J Cardiol. 168:396–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosei EA and Muiesan ML: Early target
organ damage and its reversibility: The heart. Clin Exp Hypertens.
26:673–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tublin ME, Bude RO and Platt JF: Review.
The resistive index in renal Doppler sonography: where do we stand?
AJR Am J Roentgenol. 180:885–892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Geraci G, Mule G, Costanza G, Mogavero M,
Geraci C and Cottone S: Relationship between carotid
atherosclerosis and pulse pressure with renal hemodynamics in
hypertensive patients. Am J Hypertens. 29:519–527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Geraci G, Mule G, Mogavero M, Geraci C,
D'Ignoti D, Guglielmo C and Cottone S: Renal haemodynamics and
severity of carotid atherosclerosis in hypertensive patients with
and without impaired renal function. Nutr Metab Cardiovasc Dis.
25:160–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tedesco MA, Natale F, Mocerino R,
Tassinario G and Calabrò R: Renal resistive index and
cardiovascular organ damage in a large population of hypertensive
patients. J Hum Hypertens. 21:291–296. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geraci G, Mule G, Geraci C, Mogavero M,
D'Ignoto F, Morreale M, Foraci AC and Cottone S: Association of
renal resistive index with aortic pulse wave velocity in
hypertensive patients. Eur J Prev Cardiol. 22:415–422. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mule G, Geraci G, Geraci C, Morreale M and
Cottone S: The renal resistive index: is it a misnomer? Intern
Emerg Med. 10:889–891. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Doi Y, Iwashima Y, Yoshihara F, Kamide K,
Takata H, Fujii T, Kubota Y, Nakamura S, Horio T and Kawano Y:
Association of renal resistive index with target organ damage in
essential hypertension. Am J Hypertens. 25:1292–1298.
2012.PubMed/NCBI
|
|
15
|
Pearce JD, Craven TE, Edwards MS, Corriere
MA, Crutchley TA, Fleming SH and Hansen KJ: Associations between
renal duplex parameters and adverse cardiovascular events in the
elderly: A prospective cohort study. Am J Kidney Dis. 55:281–290.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toledo C, Thomas G, Schold JD, Arrigain S,
Gornik HL, Nally JV and Navaneethan SD: Renal resistive index and
mortality in chronic kidney disease. Hypertension. 66:382–388.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doi Y, Iwashima Y, Yoshihara F, Kamide K,
Hayashi S, Kubota Y, Nakamura S, Horio T and Kawano Y: Renal
resistive index and cardiovascular and renal outcomes in essential
hypertension. Hypertension. 60:770–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ennezat PV, Maréchaux S, Six-Carpentier M,
Pinçon C, Sediri I, Delsart P, Gras M, Mounier-Véhier C, Gautier C,
Montaigne D, et al: Renal resistance index and its prognostic
significance in patients with heart failure with preserved ejection
fraction. Nephrol Dial Transplant. 26:3908–3913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jashari F, Ibrahimi P, Nicoll R,
Bajraktari G, Wester P and Henein MY: Coronary and carotid
atherosclerosis: Similarities and differences. Atherosclerosis.
227:193–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inaba Y, Chen JA and Bergmann SR: Carotid
plaque, compared with carotid intima-media thickness, more
accurately predicts coronary artery disease events: A
meta-analysis. Atherosclerosis. 220:128–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spence JD: Ultrasound measurement of
carotid plaque as a surrogate outcome for coronary artery disease.
Am J Cardiol. 89:10B–15B; discussion 15B-16B. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sinha AK, Eigenbrodt M and Mehta JL: Does
carotid intima media thickness indicate coronary atherosclerosis?
Curr Opin Cardiol. 17:526–530. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komorovsky R and Desideri A: Carotid
ultrasound assessment of patients with coronary artery disease: A
useful index for risk stratification. Vasc Health Risk Manag.
1:131–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Granata A, Fiorini F, Andrulli S, Logias
F, Gallieni M, Romano G, Sicurezza E and Fiore CE: Doppler
ultrasound and renal artery stenosis: An overview. J Ultrasound.
12:133–143. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mancia G, Fagard R, Narkiewicz K, Redon J,
Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G,
Dominiczak A, et al: 2013 ESH/ESC guidelines for the management of
arterial hypertension: the task force for the management of
arterial hypertension of the european society of hypertension (ESH)
and of the european society of cardiology (ESC). Eur Heart J.
34:2159–2219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lang RM, Badano LP, Mor-Avi V, Afilalo J,
Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA,
Kuznetsova T, et al: Recommendations for cardiac chamber
quantification by echocardiography in adults: An update from the
american society of echocardiography and the european association
of cardiovascular imaging. Eur Hear J Cardiovasc Imaging.
16:233–270. 2015. View Article : Google Scholar
|
|
27
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51:6061983. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stergiou GS, Tzamouranis D, Protogerou A,
Nasothimiou E and Kapralos C: Validation of the microlife watch BP
office professional device for office blood pressure measurement
according to the International protocol. Blood Press Monit.
13:299–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Levey AS, Stevens LA, Schmid CH, Zhang YL,
Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene
T, et al: A new equation to estimate glomerular filtration rate.
Ann Intern Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nazzal MM, Hoballah JJ, Miller EV, Sharp
WJ, Kresowik TF and Corson J: Renal hilar doppler analysis is of
value in the management of patients with renovascular disease. Am J
Surg. 174:164–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Granata A, Zanoli L, Clementi S, Fatuzzo
P, Di Nicolò P and Fiorini F: Resistive intrarenal index: Myth or
reality? Br J Radiol. 87:201400042014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Touboul PJ, Hennerici MG, Meairs S, Adams
H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S,
Hernandez Hernandez R, et al: Mannheim carotid intima-media
thickness and plaque consensus (2004-2006-2011). An update on
behalf of the advisory board of the 3rd, 4th and 5th watching the
risk symposia, at the 13th, 15th and 20th European stroke
conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and
Hamburg, Germany, 2011. Cerebrovasc Dis. 34:290–296. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Neale ML, Chambers JL, Kelly AT, Connard
S, Lawton MA, Roche J and Appleberg M: Reappraisal of duplex
criteria to assess significant carotid stenosis with special
reference to reports from the North American symptomatic carotid
endarterectomy trial and the European carotid surgery trial. J Vasc
Surg. 20:642–649. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Polak JF, Szklo M, Kronmal RA, Burke GL,
Shea S, Zavodni AE and O'Leary DH: The value of carotid artery
plaque and intima-media thickness for incident cardiovascular
disease: The multi-ethnic study of atherosclerosis. J Am Heart
Assoc. 2:e0000872013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilson PW, Hoeg JM, D'Agostino RB,
Silbershatz H, Belanger AM, Poehlmann H, O'Leary D and Wolf PA:
Cumulative effects of high cholesterol levels, high blood pressure,
and cigarette smoking on carotid stenosis. N Engl J Med.
337:516–522. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith SC Jr, Dove JT, Jacobs AK, Kennedy
JW, Kereiakes D, Kern MJ, Kuntz RE, Popma JJ, Schaff HV, Williams
DO, et al: ACC/AHA guidelines for percutaneous coronary
intervention (revision of the 1993 PTCA guidelines)-executive
summary: A report of the American college of cardiology/American
Heart Association task force on practice guidelines (committee to
revise the 1993 guidelines for percutaneous transluminal coronary
angioplasty) endorsed by the society for cardiac angiography and
interventions. Circulation. 103:3019–3041. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Krämer B, Brill M, Brühn A and Kübler W:
Relationship between the degree of coronary artery disease and of
left ventricular function and the duration of the QT-interval in
ECG. Eur Heart J. 7:14–24. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zanoli L, Rastelli S, Marcantoni C,
Tamburino C, Laurent S, Boutouyrie P and Castellino P: Renal artery
diameter, renal function and resistant hypertension in patients
with low-to-moderate renal artery stenosis. J Hypertens.
30:600–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zanoli L, Rastelli S, Marcantoni C,
Capodanno D, Blanco J, Tamburino C, Laurent S, Boutouyrie P and
Castellino P: Non-hemodynamically significant renal artery stenosis
predicts cardiovascular events in persons with ischemic heart
disease. Am J Nephrol. 40:468–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bigé N, Lévy PP, Callard P, Faintuch JM,
Chigot V, Jousselin V, Ronco P and Boffa JJ: Renal arterial
resistive index is associated with severe histological changes and
poor renal outcome during chronic kidney disease. BMC Nephrol.
13:1392012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Florczak E, Januszewicz M, Januszewicz A,
Prejbisz A, Kaczmarska M, Michałowska I, Kabat M, Rywik T, Rynkun
D, Zieliński T, et al: Relationship between renal resistive index
and early target organ damage in patients with never-treated
essential hypertension. Blood Press. 18:55–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Granata A, Clementi A, Virzì GM, Brocca A,
de Cal M, Scarfia VR, Zanoli L, Ronco C, Corrao S and Malatino L:
Cardiorenal syndrome type 4: From chronic kidney disease to
cardiovascular impairment. Eur J Intern Med. 30:1–6. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marcantoni C, Rastelli S, Zanoli L,
Tripepi G, Di Salvo M, Monaco S, Sgroi C, Capodanno D, Tamburino C
and Castellino P: Prevalence of renal artery stenosis in patients
undergoing cardiac catheterization. Intern Emerg Med. 8:401–408.
2013. View Article : Google Scholar : PubMed/NCBI
|