Introduction
Ischemic heart disease (IHD) remains the leading
cause of morbidity and mortality worldwide (1,2).
Ischemic injury to the heart muscle often results in irreversible
loss of myocardial tissue, with ensuing impairment of left
ventricular (LV) function. In addition to medical treatment and
surgical or interventional revascularization methods, there is
currently a considerable number of studies on autologous bone
marrow stem cell (BMSC) therapy in combination with coronary artery
bypass grafting (CABG) in the treatment of IHD (3–16). BMSC
therapy is aimed at repairing the damaged myocardium, preventing
ventricular remodeling and improving overall cardiac function
(17).
In clinical studies, the use of BMSCs is the most
popular cardiac cell-based therapy. This may be due to the fact
that BMSCs are easier to obtain compared with other stem cells
(e.g., circulating stem cells and cardiac stem cells), and their
preparation does not require prolonged ex vivo manipulation
(18). Although recent studies
demonstrated that catheter-based cell delivery (e.g., NOGA™
mapping) enables increased myocardial retention of cells, this
method may not be feasible in certain patients with peripheral
vascular disease (19). Therefore,
injection of BMSCs is a good option for patients undergoing
CABG.
However, the efficacy of CABG in combination with
BMSC therapy remains controversial. It has been demonstrated that
CABG combined with BMSC therapy is beneficial for cardiac function,
without any adverse effects, and is therefore a safe and feasible
adjunct therapy in clinical practice (3,4,13,16).
However, other studies reported that CABG combined with BMSC
therapy had no effect on global LV function and clinical symptoms
(5,7).
Several previous meta-analyses on CABG combined with
BMSC therapy either had certain methodological limitations or
included an insufficient number of studies (20–22). In
addition, since the publication of those meta-analyses, several new
randomized controlled trials (RCTs) have been published (3,13,15).
Hence, the present meta-analysis was performed to re-evaluate the
effectiveness of CABG combined with BMSC therapy.
Materials and methods
Trial search
The PubMed, Cochrane Library, EMBASE and Web of
Science databases were searched from inception to November 22,
2017, using the key words ‘bone marrow cells OR s'tem cells’ OR
‘cell’ OR ‘progenitor cell’ OR ‘stem cell transplantation’ OR ‘cell
transplantation’ OR ‘bone marrow transplantation’ OR ‘stromal
cells’ and ‘coronary artery bypass’ OR ‘coronary artery bypass
grafting’ OR ‘Myocardial Revascularization’. There were no language
restrictions.
Inclusion criteria
Studies were included based on the following
criteria: i) Participants with a clinical diagnosis of chronic IHD;
ii) RCTs comparing CABG in combination with BMSC therapy and CABG
alone for chronic IHD; iii) follow-up for at least 3 months after
stem cell therapy.
Exclusion criteria
The exclusion criteria were as follows: i) Non-RCTs;
ii) catheter-based stem cell injection methods; iii) stem cells
derived from sources other than the bone marrow (e.g.,
c-kit+ cardiac stem cells); iv) participants with a
clinical diagnosis of acute myocardial infarction; v) stem cell
injection without CABG; and vi) studies with incomplete LV function
data.
Risk of bias assessment
The methodological quality of the selected RCTs was
independently assessed by 2 researchers (SW and LY) based on the
Cochrane risk of bias criteria (23), and each quality item was rated as
low-risk, high-risk or unclear-risk. The 7 items used to evaluate
bias in each trial included random sequence generation, allocation
concealment, blinding of participants and personnel, blinding of
outcome assessment, incomplete outcome data and selective
reporting.
Data extraction
Two reviewers (SW and LY) independently extracted
the following relevant data from each study: First author; year of
publication; country of origin; study population, including
treatment and control group; participant characteristics, including
age and sex; follow-up time; type of stem cells; dose of stem
cells; route of stem cell administration; outcome measurement
method; LV ejection fraction (LVEF), including baseline
(LVEFbaseline), follow-up (LVEFfollow-up),
and LVEF change from baseline to follow-up for the treatment
(LVEFBMSC change) and control groups (LVEFcontrol
change); LV end-diastolic volume (LVEDV), including baseline
(LVEDVbaseline), follow-up (LVEDVfollow-up),
and LVEDV change from baseline to follow-up for the treatment
(LVEDVBMSC change) and control groups (LVEDVcontrol
change); LV end-systolic volume (LVESV), including baseline
(LVESVbaseline), follow-up (LVESVfollow-up),
and LVESV change from baseline to follow-up for the treatment
(LVESVBMSC change) and control groups (LVESVcontrol
change); LV end-diastolic volume index (LVEDVI), including
baseline (LVEDVIbaseline), follow-up
(LVEDVIfollow-up), and LVEDVI change from baseline to
follow-up for the treatment (LVEDVIBMSC change) and
control groups (LVEDVIcontrol change); and LV
end-systolic volume index (LVESVI), including baseline
(LVESVIbaseline) and follow-up
(LVESVIfollow-up), and LVESVI change from baseline to
follow-up for the treatment (LVESVIBMSC change) and
control groups (LVESVIcontrol change). Any disagreements
between the reviewers were resolved by reaching a consensus.
Statistical analysis
The statistical analysis software R, version 3.4.2
was used to analyze the data. A meta-analysis was performed to
calculate the mean difference (MD) LVEFchange (MD
LVEFchange=LVEFBMSC change-LVEFcontrol
change, LVEFBMSC change=LVEFBMSC
follow-up-LVEFBMSC baseline, LVEFcontrol
change=LVEFcontrol follow-up-LVEFcontrol
baseline), and similarly, the MD LVEDVchange, MD
LVEDVIchange, MD LVESVchange, and MD
LVESVIchange, as well as their 95% confidence intervals
(CIs). The majority of the studies reported the mean and standard
deviation (SD). In one study (3), LV
volume and ejection fraction values were expressed as mean and
standard error (SE). The SE was converted into the SD by appling
the formula SD=SE n,
where n is the sample size. In two studies (4,7), the LV
volume and ejection fraction values were expressed as the median
and interquartile range. Median and interquartile range were
converted into the mean and SD using the method introduced by Hozo
et al (24).
In addition, the mean and SD of the LVEFBMSC
change and LVEFcontrol change were not
directly reported by certain studies (3,5,6,9,10,12,13,15,16). The
mean of the LVEFBMSC change and LVEFcontrol
change may be easily obtained by calculating the difference
between the means of the LVEFbaseline and
LVEFfollow-up. However, the SD of the LVEFBMSC
change and LVEFcontrol change may only be
effectively calculated from the LVEFbaseline and
LVEFfollow-up values if the value of the correlation
coefficient (Corr) is known. Therefore, the SD of LVEFBMSC
change and LVEFcontrol change in the study by
Hendrikx et al (11) were
used to calculate the Corr values by using the following
formula:
Corr=SDbaseline2+SDfollow -
up2-SDchange22xSDbaselinexSDfollow - up
The calculation yielded Corr=0.6 for the BMSC and
the control groups. The SD of LVEFBMSC change and
LVEFcontrol change was calculated by inputting these
values in the following formula:
SDchange=SDbaseline2+SDfollow -
up2-2xCorrxSDbaselinexSDfollow - up.
The mean and SD of the LV volume change values were
calculated in the same manner.
A random-effects model was used to pool the data,
and statistical heterogeneity between summary data was evaluated
using I2 statistics. Egger's test was applied to examine
publication bias. All tests were two-tailed and P<0.05 was
considered to indicate a statistically significant difference.
Subgroup analysis
To evaluate whether the effectiveness of CABG
combined with BMSC therapy in ischemic heart disease patients was
influenced by the clinical characteristics, subgroup analyses were
performed based on i) follow-up time (>6 or ≤6 months); ii)
method to determine the outcome measure [echocardiography or
cardiac magnetic resonance imaging (cMRI)]; iii) type of stem cells
[bone marrow mononuclear cells (BMMNCs) or other selected cell
populations (CD133+ and CD34+ cells)]; iv)
route of injection [intramyocardial (IM) or intracoronary (IC)]; v)
dose of stem cells [≥108 or <108 cells
(108 was the median number of BMSCs injected)]; vi)
baseline LVEF ≤35 or >35% (35% was the median LVEF at baseline
in the included studies). Analyses were performed to evaluate
whether the differences between the subgroups were statistically
significant.
Sensitivity analysis
Sensitivity analysis was performed by excluding
low-quality studies, trials recruiting participants with particular
conditions or trials with characteristics different from the
others. A sensitivity analysis of the primary outcome LVEF was
performed.
Results
Search results
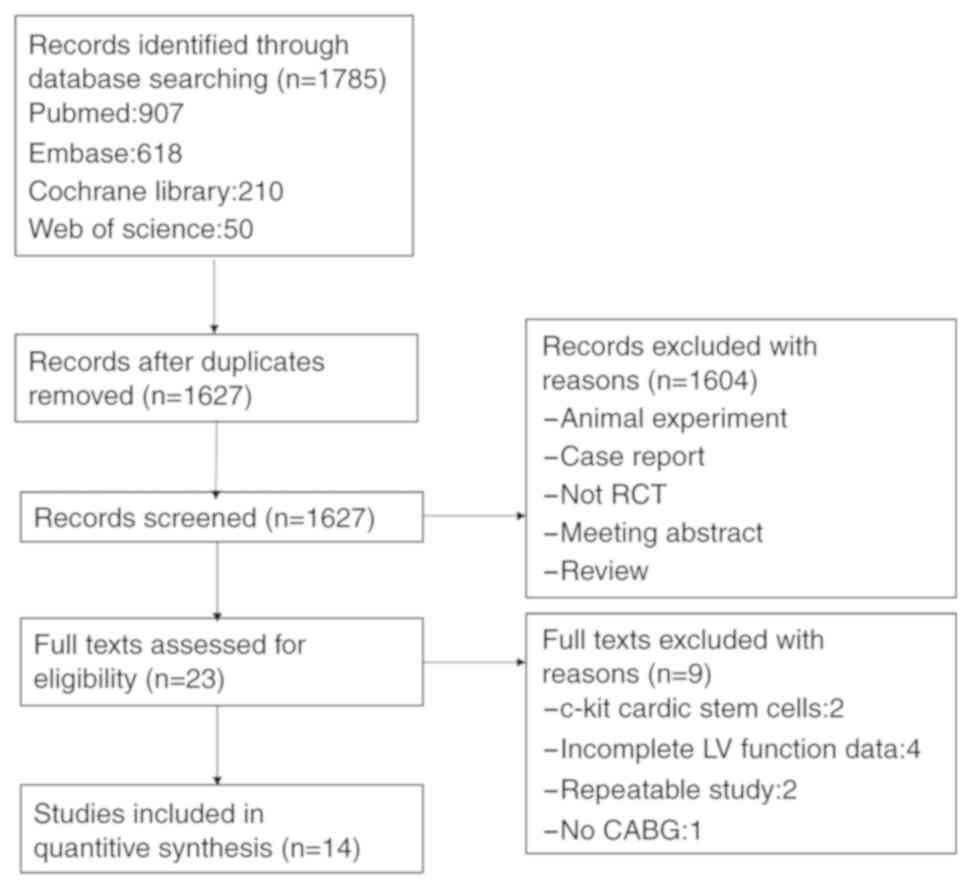
A total of 1,785 studies were identified from the
electronic database search. Deduplication and removal of all
clearly irrelevant studies excluded 151 articles. Initial screening
of the remaining 1,627 studies against the inclusion criteria
excluded a further 1,602 studies (animal experiments, case reports,
meeting abstracts, insufficient data and reviews). In the remaining
23 studies, the full text was assessed for eligibility,
subsequently excluding 9 studies: 2 studies used c-kit+
cardiac stem cells rather than BMSCs, 4 studies did not provide
complete LV function data, 2 studies were replicated and no CABG
was performed in 1 study. The final analysis included 14
independent RCTs. A flow chart depicting the study selection
process is presented in Fig. 1.
Study characteristics
A total of 14 studies met the inclusion criteria for
the present meta-analysis, including a total of 596 participants
who were assessed for the primary outcomes of the study. The
‘treatment group’ (n=316) included participants who had received
CABG combined with BMSC therapy, while the ‘control group’ (n=280)
included patients who had only received CABG. The mean follow-up
period was 11 months. The mean age of the participants ranged from
53.8 to 66.8 years, and the percentage of male patients ranged from
70 to 100%. A total of 5 studies were performed in China, 2 in
Germany and 1 each in the USA, UK, Canada, Serbia, Finland, France
and Belgium; the Canadian study was a multicenter trial (15
patients in Montreal and 18 in Toronto) (3). The baseline characteristics of the
included studies are summarized in Table
I.
 | Table I.Information on included studies,
including cohorts, treatment and outcome measures. |
Table I.
Information on included studies,
including cohorts, treatment and outcome measures.
|
|
|
|
|
|
| Age (years) | Sex, male, n
(%) | Treatment |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| First author
(year) | Country | Sample size | T (n) | C (n) | Follow-up
(months) | Treatment | Control | Treatment | Control | Type of stem
cells | Dose of stem
cells | Route of cell
admin-istration | Treatment of
control group | Method for
determining outcome measure | (Refs.) |
|---|
| Qi (2016) | China | 42 | 24 | 18 | 12 | 57.88±8.52 | 56.56±9.09 | 23 (95.8%) | 17 (94.4%) | CABG+BMMNC |
13.28±9.41×107 | IC | CABG only |
Echocardiography | (15) |
| Noiseux (2016) | Canada | 41 | 19 | 14 | 6 | 66.40±6.50 | 63.1±7.2 | 17 (89.5%) | 13 (92.9%) |
CABG+CD133+ |
6.5±3.1×106 | IM | CABG+Placebo | cMRI | (3) |
| Wang (2015) | China | 90 | 45 | 45 | 6 | 61.4±7.45 | 62.9±6.93 | 37 (82.0%) | 35 (78.0%) | CABG+BMC |
5.21±0.44×108 | IM | CABG+saline |
Echocardiography | (13) |
| Trifunović
(2015) | Serbia | 30 | 15 | 15 | 60 | 53.8±10.1 | 60±6.8 | 14 (93.3%) | 14 (93.3%) | CABG+BMMNC |
70.7±32.4×106 | IM | CABG only |
Echocardiography | (6) |
| Pätilä (2014) | Finland | 39 | 20 | 19 | 12 | 65±4 | 64±3 | 19 (95.0%) | 18 (94.7%) | CABG+BMMNC |
8.4±2.08×108 | IM | CABG+Placebo | cMRI | (7) |
| Nasseri (2014) | Germany | 60 | 30 | 30 | 6 | 61.9±7.3 | 62.7±10.6 | 28 (93.3%) | 29 (96.7%) |
CABG+CD133+ |
5.1±1.017×106 | IM | CABG+Placebo | cMRI | (5) |
| Lu (2013) | China | 50 | 25 | 25 | 12 | 58.0±7.8 | 57.0±8.3 | 22 (88.0%) | 24 (96.0%) | CABG+BMMNC |
13.38±8.14×107 | IM | CABG only | cMRI | (16) |
| Maureira
(2012) | France | 14 | 7 | 7 | 6 | 58±10 | 57±10 | 7 (100.0%) | 6 (85.7%) | CABG+BMMNC |
3.42±0.41×108 | IM | CABG only | cMRI | (8) |
| Hu (2011) | China | 60 | 31 | 29 | 6 | 56.61±9.72 | 58.27±8.86 | None reported | CABG+BMMNC |
13.17±10.66×107 | IC | CABG only | cMRI | (4) |
| Zhao (2008) | China | 36 | 18 | 18 | 6 | 60.3±10.4 | 59.1±15.7 | 15 (83.3%) | 15 (83.3%) | CABG+BMMNC |
6.59±5.12×108 | IM | CABG only |
Echocardiography | (14) |
| Ang (2008) | UK | 62 | 42 | 20 | 6 | 63.4±8.69 | 61.3±8.3 | 34 (81.0%) | 18 (90.0%) | CABG+ BMC |
9.95±6.61×107 | IM+IC | CABG only | cMRI | (9) |
| Stamm (2007) | Germany | 40 | 20 | 20 | 6 | 62±10.2 | 63.5±8.4 | 15 (75.0%) | 16 (80.0%) |
CABG+CD133+ |
5.8±20.6×106 | IM | CABG only |
Echocardiography | (10) |
| Hendrikx
(2006) | Belgium | 20 | 10 | 10 | 4 | 63.2±8.5 | 66.8±9.2 | 10 (100.0%) | 7 (70.0%) | CABG+BMC |
60.25±31.35×106 | IM | CABG only | cMRI | (11) |
| Patel (2005) | USA | 20 | 10 | 10 | 6 | 64.8±7.1 | 63.6±5.2 | 8 (80.0%) | 8 (80.0%) |
CABG+CD34+ | Median
22×106 | IM | CABG only |
Echocardiography | (12) |
Risk of bias assessment
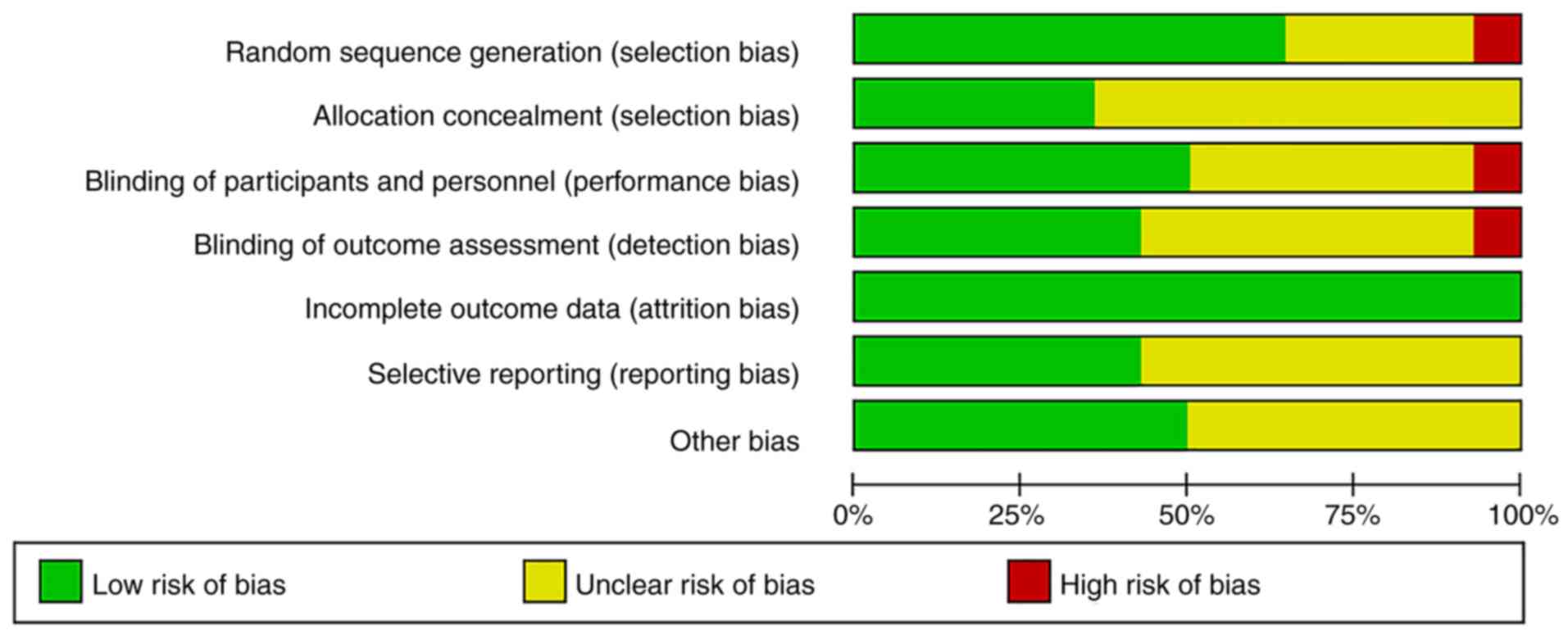
Of the 14 studies, 9 (64.2%) adequately generated
their randomisation sequence, 5 (35%) concealed allocation, 7 (50%)
blinded participants and personnel, 6 (42.9%) blinded outcome
assessment and 6 studies had a low risk of bias regarding selective
reporting. All of the studies had a low risk of bias regarding
missing outcome data. The detailed information on risk of bias is
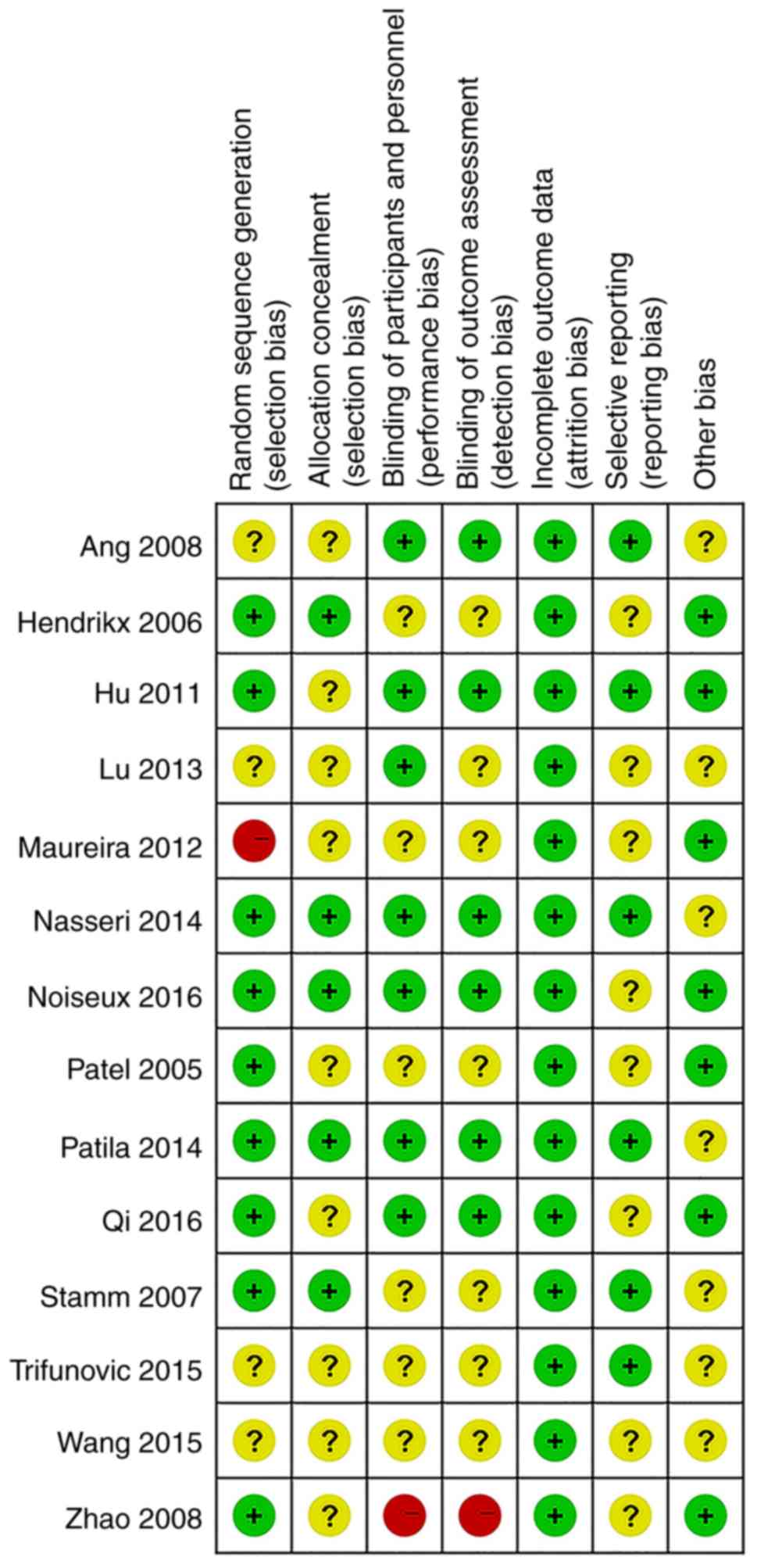
provided in Figs. 2 and 3.
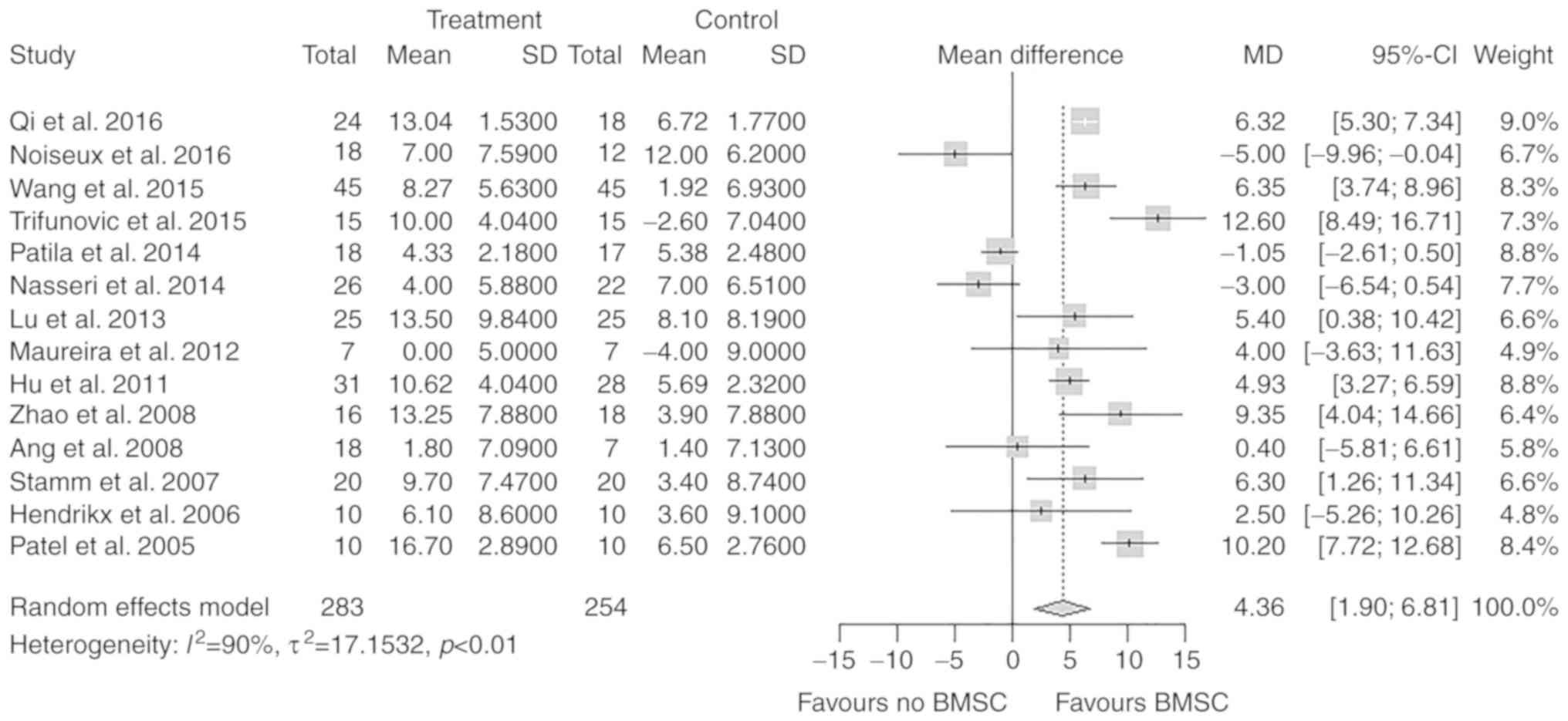
LVEFchange
All 14 studies, including a total of 537
participants, reported on the change in LVEF after the treatment.
In the treatment group, the mean change in the LVEF from baseline
to follow-up was 8.46%. In the control group, the mean change in
the LVEF from baseline to follow-up was 4.22%. The difference in
the change of the LVEF between the treatment and control groups was
statistically significant (MD=4.36%; 95% CI: 1.90–6.81%; P<0.01;
Fig. 4).
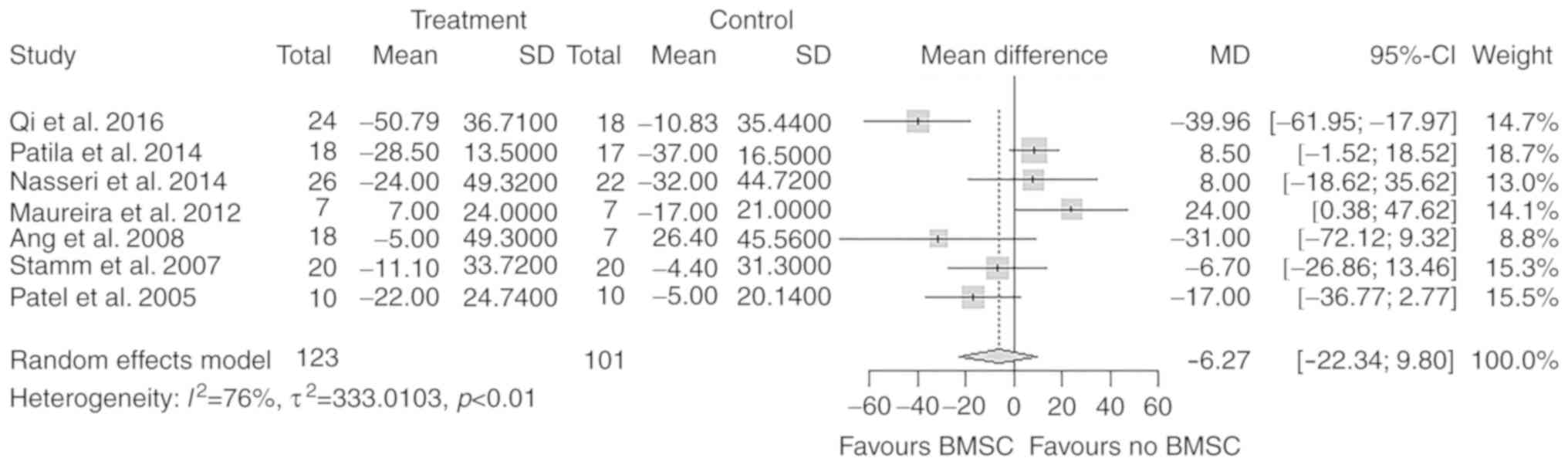
LVEDVchange
A total of 7 studies with 224 participants reported
on the change in LVEDV after the treatment. There was no
significant difference in the overall change of LVEDV from baseline
to follow-up between the treatment and control groups (MD=−6.27 ml;
95% CI: −22.34 to 9.80 ml; P=0.44; Fig.
5).
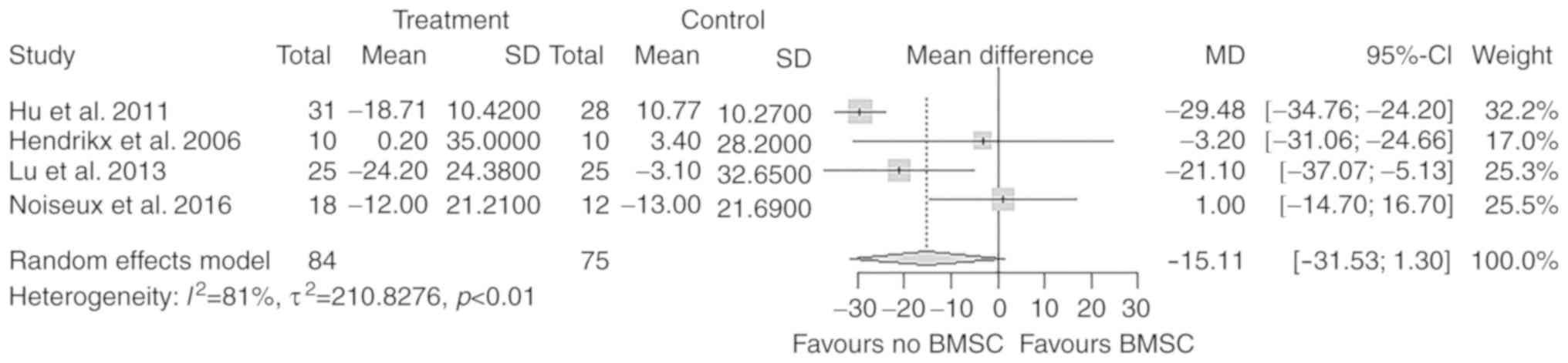
LVEDVIchange
A total of 4 studies with 159 participants reported
on the change in LVEDVI after the treatment. There was no
significant difference in the overall change of LVEDVI from
baseline to follow-up between the treatment and control groups
(MD=−15.11 ml/m2; 95% CI: −31.53 to 1.30
ml/m2; P=0.07; Fig.
6).
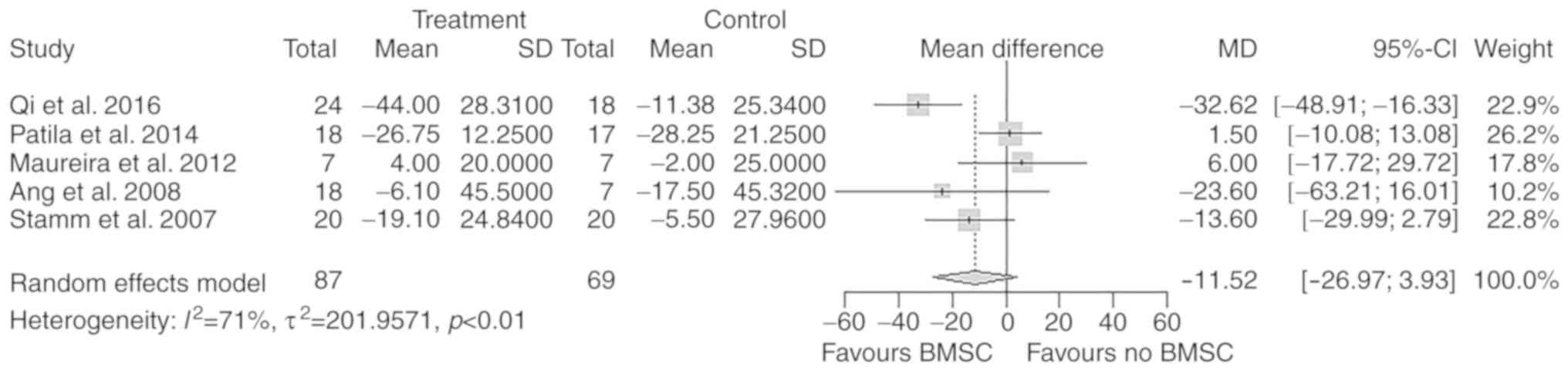
LVESVchange
A total of 5 studies with 156 participants reported
a change in LVESV after the treatment. There was no significant
difference in the overall change of LVESV from baseline to
follow-up between the treatment and control groups (MD=−11.52 ml;
95% CI: −26.97 to 3.93 ml; P=0.14; Fig.
7).
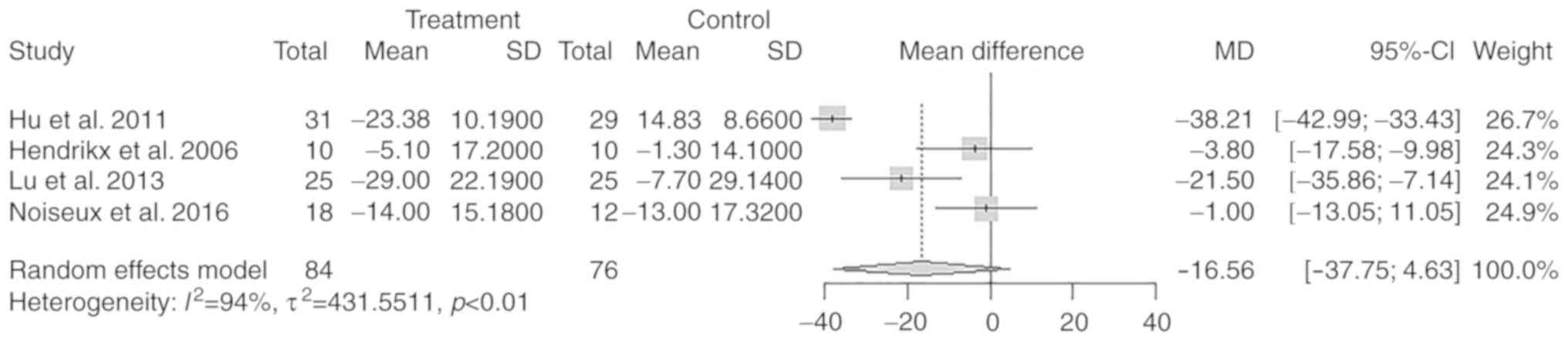
LVESVIchange
A total of 4 studies with 159 participants reported
a change in LVESVI after the treatment. There was no significant
difference in the overall change of LVESVI from baseline to
follow-up between the treatment and control groups (MD=−16.56
ml/m2; 95% CI: −37.75 to 4.63 ml/m2; P=0.13;
Fig. 8).
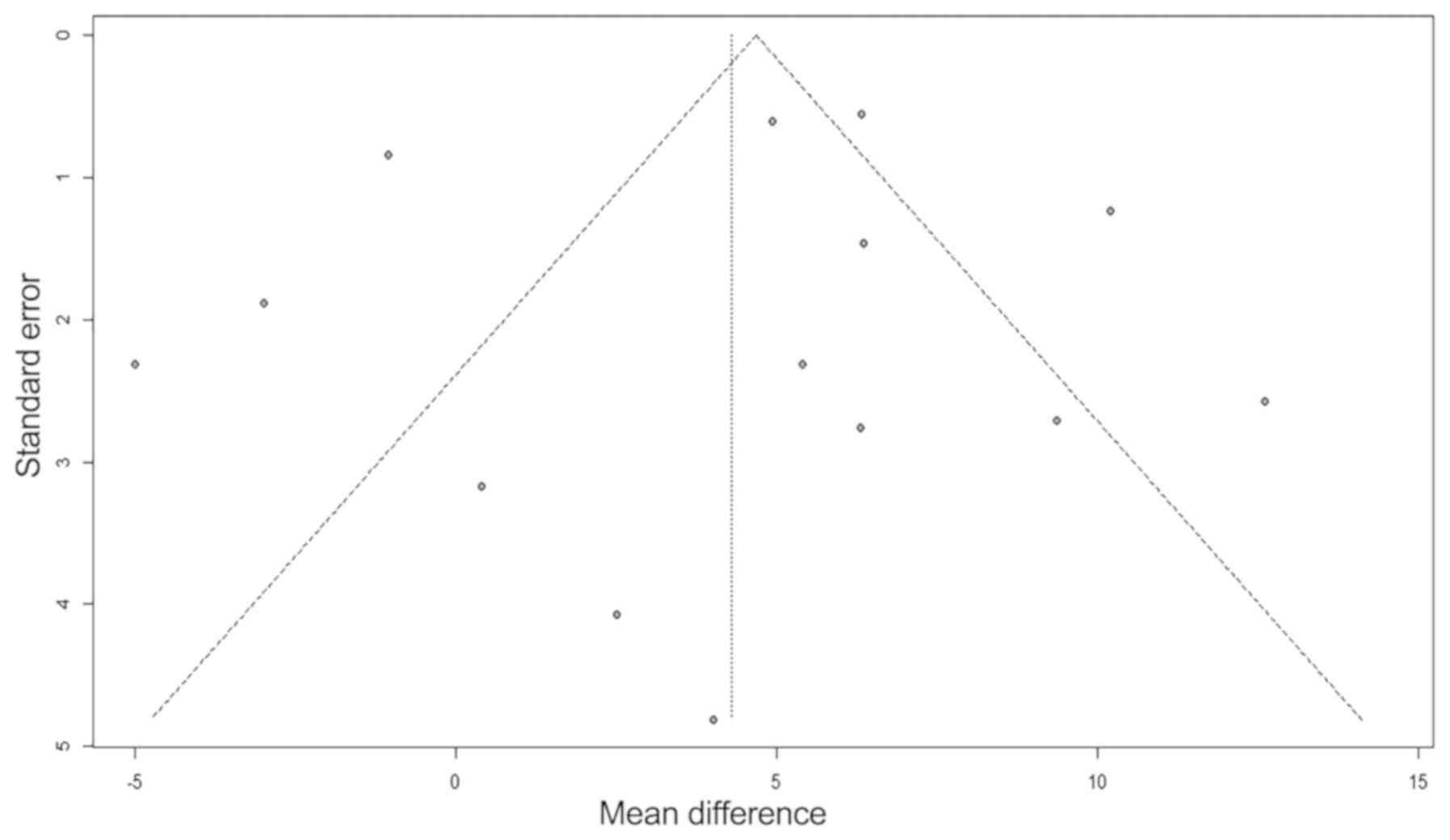
Publication bias
To exclude potential publication bias, funnel plots
(Fig. 9) and Egger's test for
publication bias was performed. No publication bias was evident for
the 14 studies included in the LVEF meta-analysis (Egger's test
P=0.48 for the MD of LVEFchange).
Subgroup analysis
The subgroup analysis did not reveal any significant
differences within subgroups based on follow-up period, type of
stem cells, route of cell administration, dose of stem cells and
baseline LVEF (Table II). However,
the measurement method for the LVEF (echocardiography or cMRI)
affected the effectiveness of CABG combined with BMSC injection in
IHD patients (P<0.01; Table
II).
 | Table II.Subgroup analysis of LVEF change for
each variable. |
Table II.
Subgroup analysis of LVEF change for
each variable.
| Variable | No. of trials | MD (95% CI) | P-value |
|---|
| Follow-up for
examining LVEF (months) |
|
|
|
|
>6 | 4 | 5.61
(0.34–10.89) | 0.56 |
| ≤6 | 10 | 3.81
(0.78–6.83) |
|
| Method of
measurement |
|
|
|
|
Echocardiography | 6 | 8.26
(6.15–10.36) | <0.0001 |
|
cMRI | 8 | 0.86
(−2.19–3.90) |
|
| Type of stem
cells |
|
|
|
|
BMMNC | 7 | 5.73
(2.46–9.01) | 0.42 |
|
CD133+/CD34+ | 4 | 2.21
(−5.69–10.12) |
|
| Route of cell
administration |
|
|
|
| IC | 2 | 5.79
(4.46–7.11) | 0.47 |
| IM | 11 | 4.35
(0.67–8.03) |
|
| Amount of stem
cells administered |
|
|
|
|
≥108 | 7 | 4.84
(1.95–7.73) | 0.69 |
|
<108 | 7 | 3.56
(−1.96–9.08) |
|
| Baseline LVEF
(%) |
|
|
|
|
≤35 | 6 | 4.49 (1.65;
7.34) | 0.97 |
|
>35 | 8 | 4.39 (0.10;
8.68) |
|
Sensitivity analysis
A sensitivity analysis, in which the trials with
relatively low-quality data by Maureira et al (8) and Zhao et al (14) were excluded, indicated that the
results were not markedly affected by the exclusion
[LVEFchange (MD=4.01%; 95% CI: 1.47–6.56%;
P<0.01)].
Discussion
The present meta-analysis demonstrated that BMSC
therapy may improve cardiac function during CABG in patients with
IHD. The change of LVEF from baseline to follow-up in the treatment
group (CABG + BMSCs) increased by 4.36% compared with that in the
control group (CABG alone). The LVESVchange and
LVEDVchange were reduced in the treatment group, but the
difference from the control group was not statistically
significant. Sensitivity analyses that excluded low-quality studies
and studies that only included patients with particular medical
conditions did not alter these results. Furthermore, these results
were generally consistent, regardless of the follow-up time, type
of stem cells, route of cell injection (IM or IC), dose of stem
cells and baseline LVEF. However, the difference in the measurement
method of LVEF (echocardiography or cMRI) affected the results
(P<0.0001).
At present, the mechanisms of the efficacy of BMSC
therapy in patients undergoing CABG remains elusive, and it may be
multifactorial. Certain studies suggested that BMSCs may exert
their beneficial effect by paracrine stimulation, cell fusion and
transdifferentiation (25–28). Rota et al (29) demonstrated in rats that
c-kit+ BMSCs engraft in proximity to the infarcted
myocardium and differentiate into cells of the cardiogenic lineage,
forming functionally competent cardiomyocytes and vascular
structures. In addition, the effect of certain cytokines, including
vascular endothelial growth factor (VEGF), has been indicated to
restore coronary vessels and myocytes via angiogenesis following
experimental infarction. BMSCs express a number of cytokines,
including VEGF, insulin-like growth factor and platelet-derived
growth factor, which stimulate the regeneration and proliferation
of residual normal myocytes and intrinsic myocardial stem cells
(endogenous stem cells) for cell regeneration and fusion (30,31).
In the present meta-analysis, 10 studies provided
short-term follow-up (≤6 months) and 4 studies reported long-term
follow-up (>6 months) data, but only 1 study provided 5-year
follow-up data. There was no significant difference regarding the
improvement in the LVEF between the short-term and long-term
follow-up. A systematic review and meta-analysis by Jeevanantham
et al (32) indicated that
adult BMSC therapy improves the LVEF in patients with IHD compared
with standard treatment, and these benefits persist at least beyond
24 months (32). Similarly, Nesteruk
et al (33) indicated that
the LVEF in the stem cell therapy group improved at 5 years
compared with that in the CABG alone group. These data suggest that
the benefits of BMSC therapy on cardiac function are not
short-lived.
With regard to the methods for measuring the LVEF
(echocardiography or cMRI), the subgroup analysis demonstrated that
the choice of method affected the determined effectiveness of CABG
combined with BMSC injection in IHD patients (P<0.0001). cMRI
and echocardiography have important diagnostic value in assessing
cardiac function, and perform similarly regarding the method of
calculaton in the measurement of the cardiac ejection fraction.
However, echocardiographic measurements may be affected by the
ultrasonographer, whereas MRI is more reliable and accurate for
measuring cardiac function.
Regarding the type of BMSC therapy, 2 of the 4
studies that included CD133+ or CD34+ cells
in the meta-analysis had an unfavorable MD. However, only 1 of the
10 studies using BMMNCs/BMCs had an unfavorable MD. These results
suggest that using BMMNCs/BMCs may lead to a more noticeable
improvement in the LVEF compared with CD133+ or
CD34+ cells. However, this result may be limited by the
small sample size of the cohort treated with CD133+ or
CD34+ cells, and accordingly, the conclusions may only
be preliminary. Autologous cell preparations are medical products
characterized by complexity in terms of differences in cell
isolation protocols and storage of cell products, and the methods
for assessing outcome may be inhomogeneous. These factors may
affect the role of CD133+ or CD34+ cell
therapy in improving cardiac function. Therefore, the function of
CD133+ or CD34+ cells in improving LVEF and
the underlying mechanisms and remain to be further elucidated.
A total of 3 previous meta-analyses (20–22) have
analyzed the effect of CABG in combination with BMSC therapy in
patients with IHD. The present results differ from those of the
previous meta-analyses in several aspects. In the meta-analysis
study by Donndorf et al (22)
reported that the improvement in the LVEF (MD of
LVEFchange of 5.40%) tended to be more prominent;
however, their study only included 6 trials (4 RCTs and 2 cohorts;
Table III presents the studies
that were included in previous meta-analyses) with a total of 179
patients, whereas the present study included 14 RCTs with a total
of 596 participants. Therefore, the present results may be more
reliable. The meta-analysis by Qin et al (20) indicated that BMSC therapy
significantly improved the LVEF and reduced the LVEDV and LVESV;
however, their study only indicated the MD of the post-treatment
LVEF values between the BMSC and the CABG alone groups, whereas in
the present meta-analysis, the MD of LVEF change from follow-up to
baseline between the BMSC and CABG groups was calculated.
Therefore, the present results may be more reliable.
Ali-Hassan-Sayegh et al (21), who included 9 studies (6 RTCs and 3
cohorts) with a total of 335 patients, obtained similar results
compared with the present meta-analysis for the
LVEFchange (MD=4.06%, 95% CI: 0.41 to 7.72%; P<0.01)
and LVEDVchange (MD=7.06 ml, 95% CI: 8.58 to 22.7 ml;
P=0.30). Our study demonstrated, with markedly narrower CIs, that
CABG in combination with BMSC therapy may improve cardiac function.
Compared with the previous meta-analysis studies, a large number of
additional studies was included and more thorough analyses were
performed. The addition of large RCTs provided more reliable
estimates of the effects of CABG combined with BMSC therapy.
Furthermore, the LVEDVI and LVESVI were only determined in 4 trials
included in the present study (3,4,11,16).
Although this is a small sample, these indexes are more reflective
regarding the heart function compared with LVEDV and LVESV, as each
individual's body surface area is different. In addition, the
majority of the studies reported on the LVEDV and LVESV as clinical
outcomes, so their data were extracted separately. This may be one
of the reasons for the difference in LVEDV and LVESV not being
statistically significant. Therefore, future meta-analyses must
include more studies to obtain significant results.
 | Table III.Comparison of studies included in the
previous meta-analyses. |
Table III.
Comparison of studies included in the
previous meta-analyses.
| Study (years) | Meta-analysis by
Qin et al (20) | Meta-analysis by
Donndorf et al (22) | Meta-analysis by
Ali-Hassan-Sayegh et al (21) | (Refs.) |
|---|
| Qi et al
(2016) | No | No | No | (15) |
| Noiseux et
al (2016) | No | No | No | (3) |
| Wang et al
(2015) | No | No | No | (13) |
| Trifunović et
al (2015) | No | No | No | (6) |
| Pätilä et al
(2014) | No | No | No | (7) |
| Nasseri et
al (2014) | No | No | Yes | (5) |
| Lu et al
(2013) | Yes | No | No | (16) |
| Maureira et
al (2012) | Yes | No | No | (8) |
| Hu et al
(2011) | Yes | No | No | (4) |
| Zhao et al
(2008) | Yes | Yes | Yes | (14) |
| Ang et al
(2008) | Yes | No | Yes | (9) |
| Stamm et al
(2007) | No | Yes | Yes | (10) |
| Hendrikx et
al (2006) | Yes | Yes | Yes | (11) |
| Patel et al
(2005) | No | Yes | Yes | (12) |
The present meta-analysis was based on a
comprehensive search strategy, including a systematic rigorous
approach to the evaluation of RCTs investigating the effectiveness
of BMSC therapy in combination with CABG in IHD patients. A
detailed subgroup analysis was performed to explore differences in
LVEFchange. Although the results of the present study
appear promising regarding the efficacy of BMSC therapy, there were
also certain limitations: First, there was significant
heterogeneity in the present meta-analysis, which may be
attributable to the dose and type of BMSC therapy, the timing of
CABG combined with BMSC therapy after myocardial ischemia, the
baseline LVEF, method of cell processing and outcome measurement
methods, and these factors may affect the efficacy of BMSC therapy.
Furthermore, the CIs were relatively wide, most likely due to the
small number of studies and the relatively sparse subjects in all
outcomes. Finally, the follow-up was relatively short in most
studies, and the sustained efficacy of BMSC therapy for patients
undergoing CABG remains to be further demonstrated. The results of
the present meta-analysis should be confirmed in large, adequately
powered RCTs assessing the efficacy of BMSC therapy, and outcome
measures should be standardised (e.g. LVEF, LVEDV and LVESV).
Future research should also focus on the mechanisms of action of
BMSC therapy to further confirm the results of meta-analyses in IHD
patients.
In conclusion, based on the present evidence,
autologous BMSC therapy for patients undergoing CABG appears to be
associated with an improvement in LV function. This improvement is
beyond that achieved by CABG alone. Therefore, BMSC therapy may be
beneficial as an adjuvant therapy for patients undergoing CABG.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81770300), the International
Cooperation Exchange Project of Gansu province (grant no.
18YF1WA046), the Science and Technology Projects of Lanzhou, China
(grant no. 2016-2-57) and CAS ‘Light of West China’ Program granted
to MZ.
Availability of data and materials
All the datasets generated and analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
SW and LY analyzed the patient data and were the
major contributors in the preparation of the manuscript. QB and AW
analyzed part of the patient data. XS and YD performed the
literature search and extracted the data. XD and XL were
responsible for the statistical analysis. YZ, PY and KY made
substantial contributions to the conception of the study. MZ and YC
drafted the manuscript. All the authors have read and approved the
final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Townsend N, Nichols M, Scarborough P and
Rayner M: Cardiovascular disease in Europe-epidemiological update
2015. Eur Heart J. 36:2696–2705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Writing Group Members, ; Mozaffarian D,
Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de
Ferranti S, Després JP, et al: Heart Disease and Stroke
Statistics-2016 update: A report from the American Heart
Association. Circulation. 133:e38–e360. 2016.PubMed/NCBI
|
|
3
|
Noiseux N, Mansour S, Weisel R, Stevens
LM, Der Sarkissian S, Tsang K, Crean AM, Larose E, Li SH,
Wintersperger B, et al: The IMPACT-CABG trial: A multicenter,
randomized clinical trial of CD133+ stem cell therapy
during coronary artery bypass grafting for ischemic cardiomyopathy.
J Thorac Cardiovasc Surg. 152:1582–1588. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu S, Liu S, Zheng Z, Yuan X, Li L, Lu M,
Shen R, Duan F, Zhang X, Li J, et al: Isolated coronary artery
bypass graft combined with bone marrow mononuclear cells delivered
through a graft vessel for patients with previous myocardial
infarction and chronic heart failure: A single-center, randomized,
double-blind, placebo-controlled clinical trial. J Am Coll Cardiol.
57:2409–2415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nasseri BA, Ebell W, Dandel M, Kukucka M,
Gebker R, Doltra A, Knosalla C, Choi YH, Hetzer R and Stamm C:
Autologous CD133+ bone marrow cells and bypass grafting
for regeneration of ischaemic myocardium: The Cardio133 trial. Eur
Heart J. 35:1263–1274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trifunović Z, Obradović S, Balint B, Ilić
R, Vukić Z, Šišić M, Kostić J, Rusović S, Dobrić M and Ostojić G:
Functional recovery of patients with ischemic cardiomyopathy
treated with coronary artery bypass surgery and concomitant
intramyocardial bone marrow mononuclear cell implantation-a
long-term follow-up study. Vojnosanit Pregl. 72:225–232. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pätilä T, Lehtinen M, Vento A, Schildt J,
Sinisalo J, Laine M, Hämmäinen P, Nihtinen A, Alitalo R, Nikkinen
P, et al: Autologous bone marrow mononuclear cell transplantation
in ischemic heart failure: A prospective, controlled, randomized,
double-blind study of cell transplantation combined with coronary
bypass. J Heart Lung Transplant. 33:567–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maureira P, Tran N, Djaballah W, Angioï M,
Bensoussan D, Didot N, Fay R, Sadoul N, Villemot JP and Marie PY:
Residual viability is a predictor of the perfusion enhancement
obtained with the cell therapy of chronic myocardial infarction: A
pilot multimodal imaging study. Clin Nucl Med. 37:738–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ang KL, Chin D, Leyva F, Foley P, Kubal C,
Chalil S, Srinivasan L, Bernhardt L, Stevens S, Shenje LT and
Galiñanes M: Randomized, controlled trial of intramuscular or
intracoronary injection of autologous bone marrow cells into
scarred myocardium during CABG versus CABG alone. Nat Clin Pract
Cardiovasc Med. 5:663–670. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stamm C, Kleine HD, Choi YH, Dunkelmann S,
Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski
D, et al: Intramyocardial delivery of CD133+ bone marrow
cells and coronary artery bypass grafting for chronic ischemic
heart disease: Safety and efficacy studies. J Thorac Cardiovasc
Surg. 133:717–725. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hendrikx M, Hensen K, Clijsters C, Jongen
H, Koninckx R, Bijnens E, Ingels M, Jacobs A, Geukens R, Dendale P,
et al: Recovery of regional but not global contractile function by
the direct intramyocardial autologous bone marrow transplantation:
Results from a randomized controlled clinical trial. Circulation.
114 Suppl 1:I101–I107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel AN, Geffner L, Vina RF, Saslavsky J,
Urschel HC Jr, Kormos R and Benetti F: Surgical treatment for
congestive heart failure with autologous adult stem cell
transplantation: A prospective randomized study. J Thorac
Cardiovasc Surg. 130:1631–1638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Wang Z, Jiang H, Ma D, Zhou W,
Zhang G, Chen W, Huang J and Liu Y: Effect of autologous bone
marrow cell transplantation combined with off-pump coronary artery
bypass grafting on cardiac function in patients with chronic
myocardial infarction. Cardiology. 130:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Q, Sun Y, Xia L, Chen A and Wang Z:
Randomized study of mononuclear bone marrow cell transplantation in
patients with coronary surgery. Ann Thorac Surg. 86:1833–1840.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qi Z, Liu S, Lv X, Duan F, Wang H, Gao Y
and Wang J: Effects of bone marrow mononuclear cells delivered
through a graft vessel for patients with previous myocardial
infarction and chronic heart failure: An echocardiographic study of
left atrium function. Echocardiography. 33:1835–1843. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu M, Liu S, Zheng Z, Yin G, Song L, Chen
H, Chen X, Chen Q, Jiang S, Tian L, et al: A pilot trial of
autologous bone marrow mononuclear cell transplantation through
grafting artery: A sub-study focused on segmental left ventricular
function recovery and scar reduction. Int J Cardiol. 168:2221–2227.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strauer BE and Steinhoff G: 10 years of
intracoronary and intramyocardial bone marrow stem cell therapy of
the heart: From the methodological origin to clinical practice. J
Am Coll Cardiol. 58:1095–1104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tongers J, Losordo DW and Landmesser U:
Stem and progenitor cell-based therapy in ischaemic heart disease:
Promise, uncertainties, and challenges. Eur Heart J. 32:1197–1206.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Banovic M, Ostojic MC, Bartunek J,
Nedeljkovic M, Beleslin B and Terzic A: Brachial approach to
NOGA-guided procedures: Electromechanical mapping and
transendocardial stem-cell injections. Tex Heart Inst J.
38:179–182. 2011.PubMed/NCBI
|
|
20
|
Qin SL, He CY, Xu JS, Lai XY, Liu SS and
He WP: Meta-analysis of coronary artery bypass graft surgery
combined with stem cell transplantation in the treatment of
ischemic heart diseases. Coron Artery Dis. 26:170–175. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali-Hassan-Sayegh S, Mirhosseini SJ,
Lotfaliani MR, Dehghan HR, Sedaghat-Hamedani F, Kayvanpour E,
Rezaeisadrabadi M, Ghaffari N, Vahabzadeh V, Jebran AF, et al:
Transplantation of bone marrow stem cells during cardiac surgery.
Asian Cardiovasc Thorac Ann. 23:363–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donndorf P, Kundt G, Kaminski A, Yerebakan
C, Liebold A, Steinhoff G and Glass A: Intramyocardial bone marrow
stem cell transplantation during coronary artery bypass surgery: A
meta-analysis. J Thorac Cardiovasc Surg. 142:911–920. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions version 5.1.0 [updated
March 2011]. The Cochrane Collaboration. 2011, http://handbook.cochrane.org
|
|
24
|
Hozo SP, Djulbegovic B and Hozo I:
Estimating the mean and variance from the median, range, and the
size of a sample. BMC Med Res Methodol. 5:132005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duran JM, Makarewich CA, Sharp TE,
Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo
H, et al: Bone-derived stem cells repair the heart after myocardial
infarction through transdifferentiation and paracrine signaling
mechanisms. Circulation research. 113:539–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li TS, Cheng K, Malliaras K, Smith RR,
Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H,
et al: Direct comparison of different stem cell types and
subpopulations reveals superior paracrine potency and myocardial
repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol.
59:942–953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoon CH, Koyanagi M, Iekushi K, Seeger F,
Urbich C, Zeiher AM and Dimmeler S: Mechanism of improved cardiac
function after bone marrow mononuclear cell therapy: Role of
cardiovascular lineage commitment. Circulation. 121:2001–2011.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li SH, Sun Z, Brunt KR, Shi X, Chen MS,
Weisel RD and Li RK: Reconstitution of aged bone marrow with young
cells repopulates cardiac-resident bone marrow-derived progenitor
cells and prevents cardiac dysfunction after a myocardial
infarction. Eur Heart J. 34:1157–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rota M, Kajstura J, Hosoda T, Bearzi C,
Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A,
Rizzi R, et al: Bone marrow cells adopt the cardiomyogenic fate in
vivo. Proc Natl Acad Sci USA. 104:17783–17788. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beohar N, Rapp J, Pandya S and Losordo DW:
Rebuilding the damaged heart: The potential of cytokines and growth
factors in the treatment of ischemic heart disease. J Am Coll
Cardiol. 56:1287–1297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alvarez-Dolado M, Pardal R, Garcia-Verdugo
JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ and
Alvarez-Buylla A: Fusion of bone-marrow-derived cells with Purkinje
neurons, cardiomyocytes and hepatocytes. Nature. 425:968–973. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeevanantham V, Butler M, Saad A,
Abdel-Latif A, Zuba-Surma EK and Dawn B: Adult bone marrow cell
therapy improves survival and induces long-term improvement in
cardiac parameters: A systematic review and meta-analysis.
Circulation. 126:551–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nesteruk J, Voronina N, Kundt G, Donndorf
P, Klopsch C, Kaminski A, Duckers HJ and Steinhoff G: Stem cell
registry programme for patients with ischemic cardiomyopathy
undergoing coronary artery bypass grafting: What benefits does it
derive? ESC Heart Fail. 4:105–111. 2017. View Article : Google Scholar : PubMed/NCBI
|