Introduction
Gallstone disease (GD) is the most common disease of
the biliary system and the majority of cases are asymptomatic
(1). The prevalence of GD has grown
with the increasing occurrence of unhealthy lifestyles and the
obesity epidemic (2). Patients with
gallstones are characterized by distorted bile acid secretion,
which is associated with an increased risk of cardiovascular
disease (CVD). Several genetic, metabolic and environmental factors
for GD and CVD development have been identified. Environmental CVD
risk factors include a sedentary lifestyle, a high-fat diet and
cigarette smoking, which in turn have all been associated with GD
(3,4). Certain lipid metabolizing genes have
been identified as susceptibility genes for GD and CVD in
genome-wide association studies or meta-analyses (5,6).
Recently, numerous cohort studies have reported an association
between GD and CVD (7–9), while others have revealed no
association (7). Therefore, a
meta-analysis was performed in the present study to better explore
the possible associations between CVD and GD.
Materials and methods
Data search
Published studies indexed in EMBASE (http://www.embase.com/), PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and the
Cochrane Library (http://www.thecochranelibrary.com/) were
systematically searched from their dates of inception to March 3rd,
2018. Search terms included ‘carotid’ OR ‘artery disease’ OR
‘carotid atherosclerosis’ OR ‘carotid plaques’ OR ‘carotid intimal
thickness’ OR ‘carotid artery’ OR ‘atherosclerosis’ OR
‘cardiovascular disease’ OR ‘cerebrovascular disease’ AND
‘gallstone diseases’ OR ‘cholelithiasis’ OR ‘cholecystolithiasis’
OR ‘stone’ OR ‘cholecystectomy’. References in the included studies
were examined to identify additional studies that were not captured
by the initial literature search strategy.
Study selection
Studies were selected for the meta-analysis based on
the following criteria: i) Only cohort studies were included for
observational studies; ii) CVD, including coronary heart disease,
stroke, coronary artery calcification, aortic atherosclerosis,
peripheral artery disease, arterial stiffness and cerebral vascular
accident; iii) hazard ratios (HRs), odds ratios (ORs) and 95%
confidence intervals (CIs) were either extracted directly from the
selected articles or calculated according to the provided data in
the studies; and iv) letters, review articles, comments, case
reports and systematic reviews were excluded. No language
exclusions were applied.
Data extraction and quality
assessment
Two authors (SZ and GS) independently extracted data
from the selected studies. Disagreements were resolved by
discussion; if this process did not solve a disagreement, a third
author (AW) was consulted. The extracted information included
authors, publication year, study design, age, sex, with or without
CVD in GD group and control, years of follow-up, outcomes, ORs,
HRs, confounder adjustment and CIs. The methodological quality of
the selected studies was evaluated with the Newcastle-Ottawa Scale
(10). The scale contained nine
points: Comparability (two stars), estimation of outcomes or
exposures (three stars) and selection (four stars). Studies that
received more than six stars were judged as high quality.
Statistical analysis and subgroup
analysis
STATA version 12.0 (STATA Corp LP, College Station,
TX, USA) was used in the present meta-analysis. The combined
unadjusted OR and HR were calculated using the random-effects model
(11). When information was reported
for more than one subpopulation (for example, subjects with HRs and
unadjusted ORs, subjects from different geographical areas or
subjects with different sexes) in one study, each subpopulation was
treated as a separate comparison in the meta-analysis. To assess
across-study heterogeneity, the inconsistency index (I2)
was calculated with the significance level set at P<0.01. A
high, moderate and low degree of heterogeneity was assessed using
I2≥75, I2≥50 and I2≥25%,
respectively. A fixed-effect model for the various studies without
heterogeneity was performed. By contrast, the random-effects model
was used in the meta-analysis and subgroups to analyze the source
of heterogeneity and potential factors. Subgroup analysis was
stratified by area, sex, age, years of follow-up, study design and
sample size. Between trial heterogeneity was assessed using the
χ2 test. The effect of each study on the overall
estimates was assessed with sensitivity analysis. Direct
observation of funnel plots was performed to assess the presence of
publication bias and confirmed with an Egger's test (12).
Results
Study characteristics
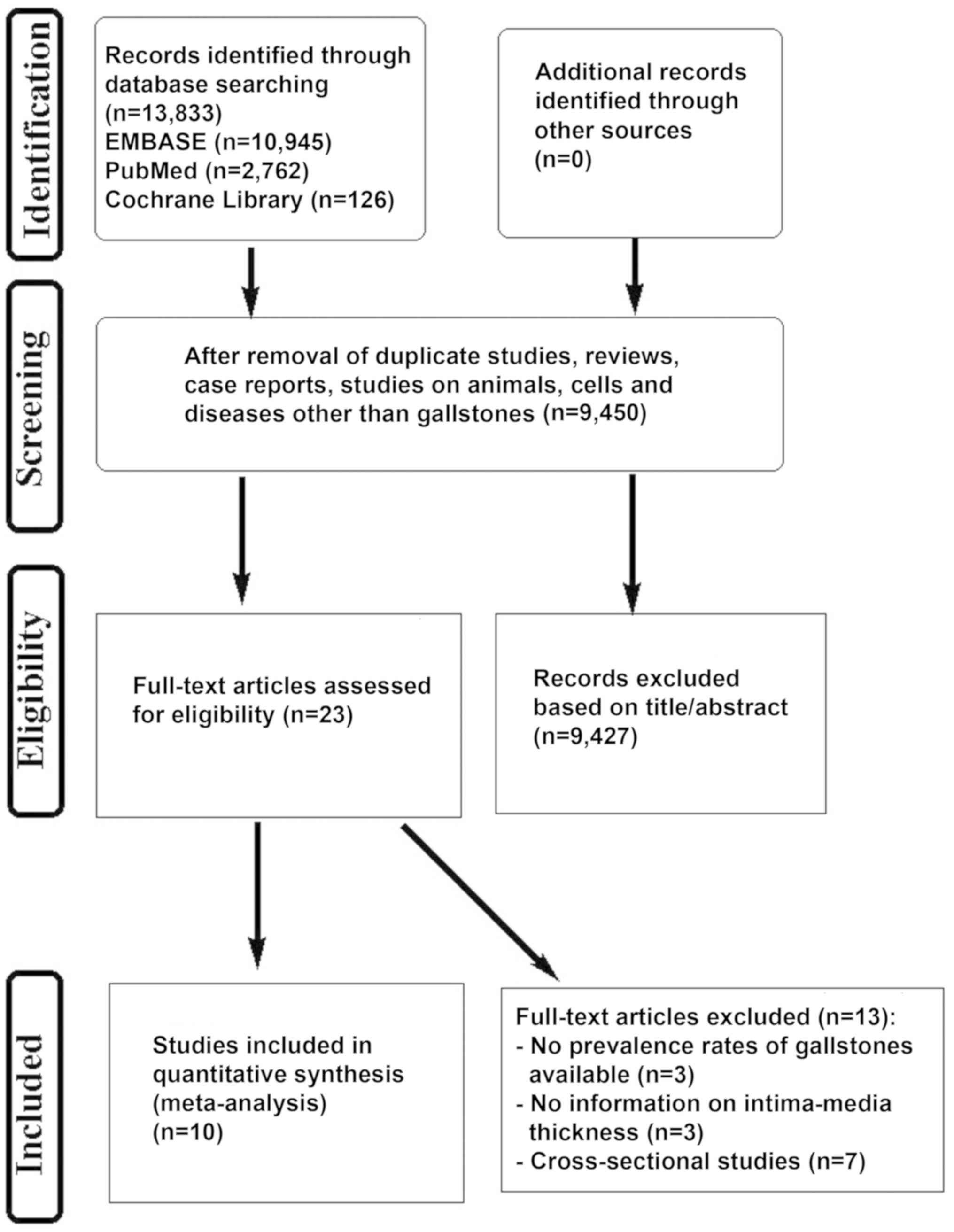
The flowchart of the included studies was presented
in Fig. 1. Overall, 13,833 records
were identified through the EMBASE, PubMed and Cochrane Library
search. According to the predefined inclusion and exclusion
criteria, 10 studies, including retrospective cohort studies (n=7)
and prospective cohort studies (n=3) published between 1985 and
2017 were included in the meta-analysis.
In terms of the location, five studies were from
Asia (9,13–16) and
five studies were from Western countries (2,8,17–19). Of
the 10 studies included in the meta-analysis, seven reported HRs
(2,8,9,13,14,16,17) and eight reported original
data, from which unadjusted ORs were calculated (2,8,9,13–17).
Among these studies, there were five prospective cohort studies
(2,9,15,17,18)
and five retrospective cohort studies (8,13,14,16,19).
Cardiovascular diseases and cerebrovascular diseases were studied
in nine and two included articles, respectively. For end-point
incidence of these studies, two included mortality as an end-point
(2,18) and eight studies used morbidity
(8,9,13–17,19).
The characteristics of the selected studies were summarized in
Table I.
 | Table I.Characteristics of the included
studies summarized in the meta-analysis. |
Table I.
Characteristics of the included
studies summarized in the meta-analysis.
| Author, year | Group | Sex/age
(years) | Gallstone patients
and control with/without cardio-cerebrovascular diseases | OR/HR (95% CI) | Followed-up
(years) | Study design | Region | Outcome | Confounder
adjusted | Quality assessment
(newcastle-ottawa scale) | (Refs.) |
|---|
| Wang et al,
2017 | Whole cohort | 51.7 | A:192, B:215,
T1:407, C:1851, D:3953, T2:5804 | OR:1.9
(1.55,2.33) | 3 | Prospective
cohort | Taiwan | AS | 33 | 7 | (15) |
| Zheng et al,
2016 | Independent cohort
1 | Female/48.6 | T1:8796,
T2:103724 | HR:1.15
(1.1,1.21) | 30 | Retrospective
cohort | USA | CHD |
5,9,11,12,13,19,20,22,24,25,27,28 | 8 | (8) |
|
| Independent cohort
2 | Female/36.4 | T1:5227,
T2:107692 | HR:1.33
(1.17,1.51) | 22 |
| USA | CHD |
5,9,11,12,13,19,20,22,24,25,27,28 | 8 | (8) |
|
| Independent cohort
3 | Male/60.1 | T1:1449,
T2:42254 | HR:1.11
(1.04,1.2) | 24 |
| USA | CHD |
5,9,11,12,13,19,20,22,24,25,27,28 | 8 | (8) |
| Lv et al,
2015 | Whole cohort |
30–79 | A:1942, B:26403,
T1:28345, C:23017, D:436011, T2:459028 | HR:1.23
(1.17,1.28) | 7.2 | Prospective
cohort | China | IHD |
1,2,7,12,14,15,16,17,19,21,22,24,29,31,32 | 8 | (9) |
|
| Sex subgroup 1 | Male/30–79 | T1:7374,
T2:191918 | HR:1.11
(1.02,1.22) |
|
| China | IHD |
1,2,7,12,14,15,16,17,19,21,22,24,29,31,32 | 8 | (9) |
|
| Sex subgroup 2 | Female/30–79 | T1:21029,
T2:267052 | HR:1.27
(1.2,1.34) |
|
| China | IHD |
1,2,7,12,14,15,16,17,19,21,22,24,29,31,32 | 8 | (9) |
| Wei et al,
2014 | Whole cohort | NA | A:12234, B:123278,
T1:135512, C:20680, D:250344, T2:271024 | HR:1.29
(1.26,1.32) | 8 | Retrospective
cohort Retrospective cohort | Taiwan | Stroke | 1,11,17,19 | 7 | (16) |
|
| Disease subgroup
1 | NA | A:10500, B:125012,
T1:135512, C:17748, D:253276, T2:271024 | HR:1.33
(1.25,1.41) |
|
| Taiwan | HS | 1,11,17,19 | 7 | (16) |
|
| Disease subgroup
2 | NA | A:1734, B:133778,
T1:135512, C:2932, D:268092, T2:271024 | HR:1.28
(1.25,1.31) |
|
| Taiwan | IS | 1,11,17,19 | 7 | (16) |
|
| Sex subgroup 1 | Male | T1:66792,
T2:133584 | HR:1.39
(1.28,1.51) |
|
| Taiwan | Stroke | 1,17,18,19 | 7 | (16) |
|
| Sex subgroup 2 | Female | T1:68720,
T2:137440 | HR:1.25
(1.14,1.37) |
|
| Taiwan | Stroke | 1,17,18,19 | 7 | (16) |
|
| Age subgroup 1 | <45 | T1:30655,
T2:61310 | HR:2.41
(1.93,3.01) |
|
| Taiwan | Stroke | 1,17,18,19 | 7 | (16) |
|
| Age subgroup 2 |
45–64 | T1:50510,
T2:101020 | HR:1.46
(1.31,1.62) |
|
| Taiwan | Stroke | 1,17,18,19 | 7 | (16) |
|
| Age subgroup 3 |
≥65 | T1:54347,
T2:108694 | HR:1.12
(1.04,1.21) |
|
| Taiwan | Stroke | 1,17,18,19 | 7 | (16) |
| Lee et al,
2013 | Whole cohort | 60.9 | A:126, B:252,
T1:378 | HR:2.11
(1.14,3.9) | 3.9 | Retrospective
cohort | Korea | CAD | 33 | 7 | (13) |
| Wirth et al,
2013 | Whole cohort | 54.1 | A:134, B:4696,
T1:4828, C:785, D:40873, T2:41658 | HR:1.09
(0.8,1.5) | 8.2 | Prospective
cohort | German | CVD |
1,2,7,14,19,24,26, | 8 29,30,32 | (17) |
| Olaiya et
al, 2013 | Whole cohort | NA | A:935, B:6046,
T1:6981, C:2758, D:25166, T2:27924 | HR:1.32
(1.22,1.43) | 6 | Retrospective
cohort Retrospective cohort | Taiwan | CVD | 1,4,17 | 7 | (14) |
|
| Disease subgroup
1 | NA | NA | HR:1.15
(1.01,1.32) |
|
| Taiwan | Stroke | 1,4,17 | 7 | (14) |
|
| Disease subgroup
2 | NA | NA | HR:1.42
(1.28,1.58) |
|
| Taiwan | CHD | 1,4,17 | 7 | (14) |
|
| Sex subgroup 1 | Male | A:425, B:2632,
T1:3057, C:1265, D:10963, T2:12228 | HR:1.29
(1.15,1.44) |
|
| Taiwan | CVD |
1,2,6,8,9,12,19,23 | 7 | (14) |
|
| Sex subgroup 2 | Female | A:510, B:3414,
T1:3924, C:1493, D:14203, T2:15696 | HR:1.35
(1.22,1.5) |
|
| Taiwan | CVD |
1,2,6,8,9,12,19,23 | 7 | (14) |
|
| Age subgroup 1 | 18–40 | A:88, B:2057,
T1:2145, C:228, D:8352, T2:8580 | HR:1.42
(1.09,1.84) |
|
| Taiwan | CVD |
1,2,6,8,9,11,17,19,23 | 7 | (14) |
|
| Age subgroup 2 | 41–60 | A:464, B:2923,
T1:3387, C:1273, D:12278, T2:13551 | HR:1.35
(1.21,1.51) |
|
| Taiwan | CVD |
1,2,6,8,9,11,17,19,23 | 7 | (14) |
|
| Age subgroup 3 | >60 | A:383, B:1066,
T1:1449, C:1257, D:4536, T2:5793 | HR:1.24
(1.1,1.39) |
|
| Taiwan | CVD |
1,2,6,8,9,11,17,19,23 | 7 | (14) |
| Ruhl and Everhart,
2011 | Whole cohort | 69.5 | A:247, B:1771,
T1:2018, C:639, D:11571, T2:12210 | HR:1.5
(1.3,1.8) | 18 | Prospective
cohort | USA | CVD | 1 | 9 | (2) |
| Khan et al,
2009 | Whole cohort | 78 | A:889, B:1321,
T1:2210, C:874, D:1336, T2:2210 | OR:0.97
(0.86,1.90) | 10 | Prospective
cohort | South East
England | CHD | 1,17 | 7 | (18) |
| Bortnichak et
al, 1985 | Whole cohort |
28–62 | A:111, B:391,
T1:502, C:649, D:3557, T2:4206 | OR:1.56
(1.24,1.95) | 26 | Retrospective
cohort | USA | CHD | 33 | 7 | (19) |
|
| Sex subgroup 1 | Female/28–62 | A:44, B:278,
T1:322, C:189, D:1989, T2:2178 | OR:1.67
(1.17,2.37) |
|
| USA | CHD | 33 | 7 | (19) |
|
| Sex subgroup 2 | Male/28–62 | A:67, B:113,
T1:180, C:460, D:1568,T2:2028 | OR:2.02
(1.47,2.78) |
|
| USA | CHD | 33 | 7 | (19) |
Main analysis and publication
bias
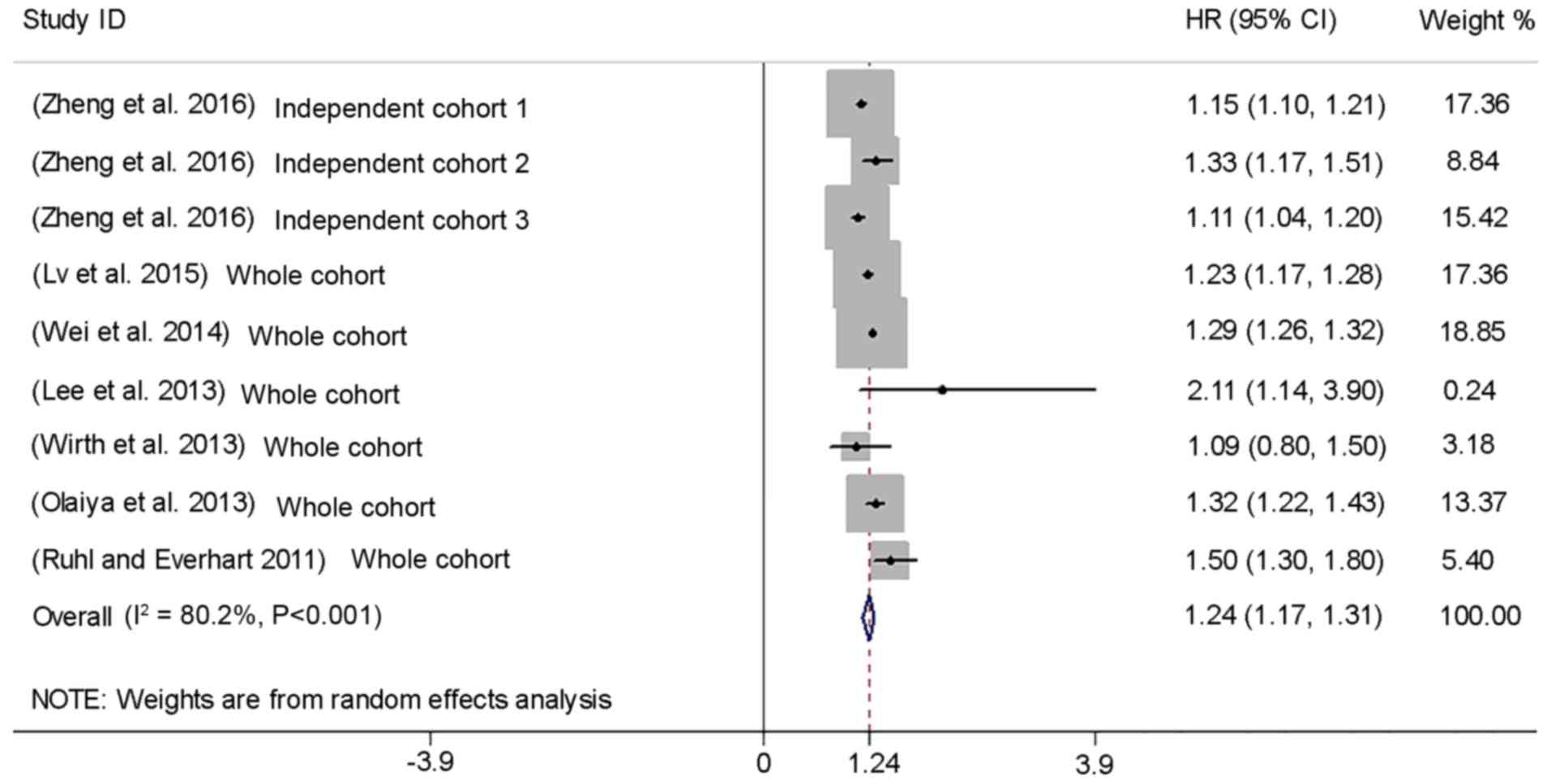
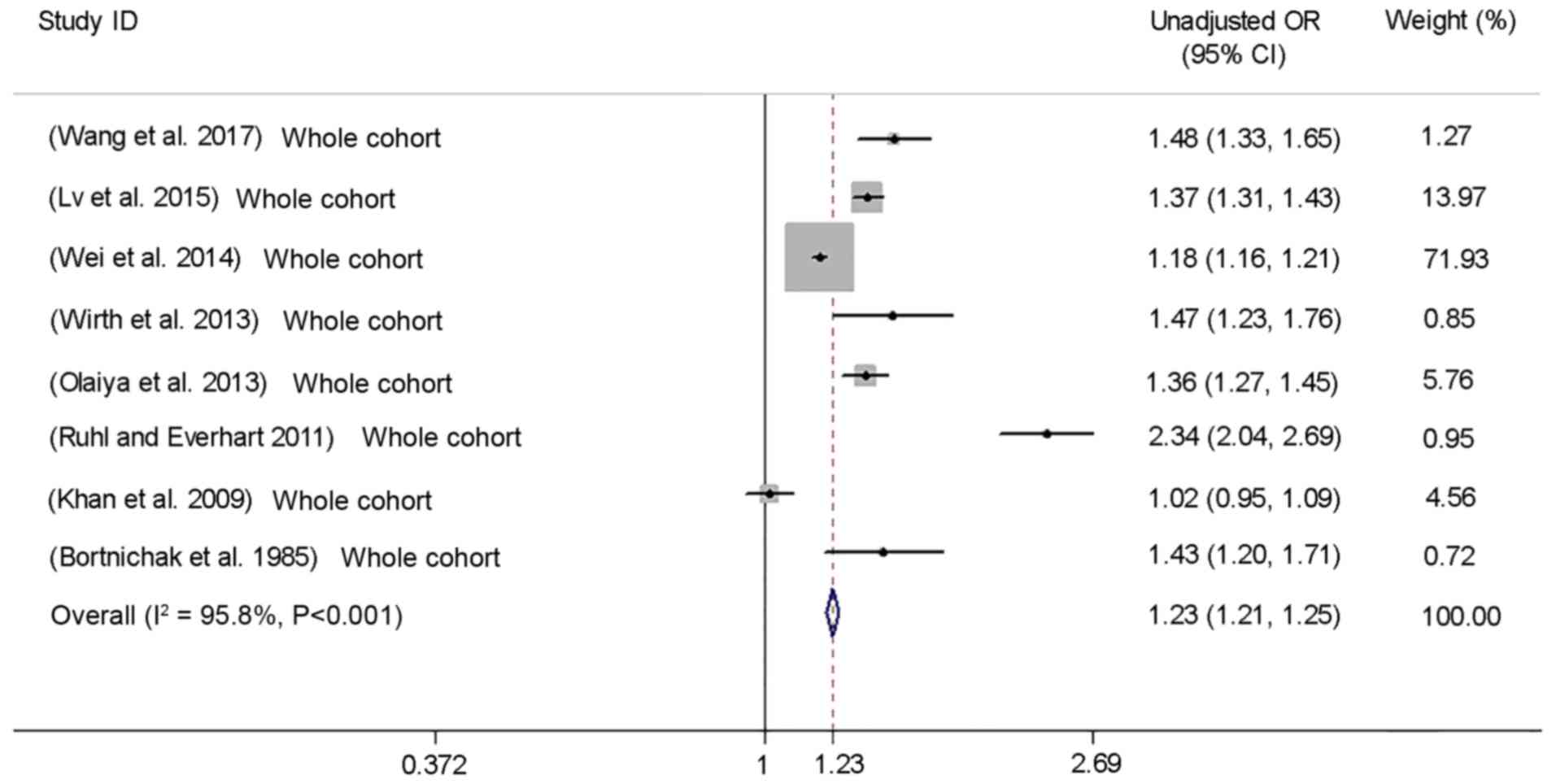
The meta-analysis suggested a significantly
increased risk of incidence (HR=1.24, 95% CI: 1.17–1.31; Fig. 2) and prevalence (unadjusted OR=1.23,
95% CI: 1.21–1.25; Fig. 3) of CVD
with GD, but with substantial heterogeneity [P<0.001,
I2=80.2% (HR); P<0.001, I2=95.8%
(unadjusted OR); Figs. 2 and
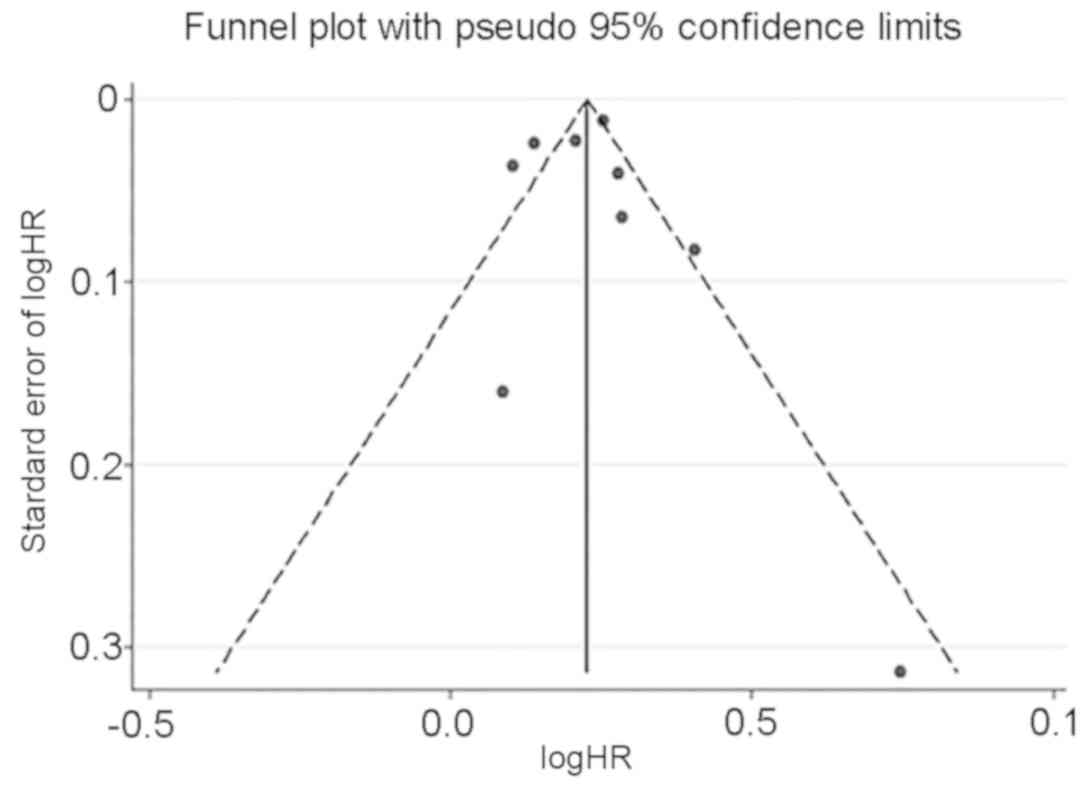
3]. Publication bias of the HR was
analyzed using the funnel plot, and the results revealed no
symmetric distribution of the included studies on both sides of the
funnel plot, suggesting a possibility of publication bias (Fig. 4). No publication bias of HR was
identified by Egger's test (Pr>|z|=0.679). However, because the
number of included studies for unadjusted OR was <10, the
publication bias for it was not assessed. Collectively, it was not
certain that the included studies had no publication bias, but
there was no evidence to doubt the validity of the results.
Subgroup analysis
To investigate the influencing factors of
heterogeneity, the data were initially stratified by HR and
unadjusted OR, then group 1 was further stratified by mean age
(HR≥45, unadjusted OR≤60), sex (female), geographical region,
follow-up (<10 years), prospective cohort study and sample size
(patients with gallstones>10,000). Group 2 was stratified by
mean age (HR>45, unadjusted OR>60), sex (male), geographical
region, years of follow-up (≥10 years), retrospective cohort study
and sample size (patients with gallstone>10,000; Table II). The two groups with GD had a
risk of CVD; although the risk in group 1 was greater than that of
group 2, indicating that the association between GD and CVD was
stronger in the younger population or female individuals. The
heterogeneity of the HR was significantly reduced in the stratified
analysis by years of follow-up and prospective cohorts, indicating
that years of follow-up and study design factors may be potential
sources of heterogeneity.
 | Table II.Stratified analyses of the risk of
cardiovascular disease among patients with gallstones. |
Table II.
Stratified analyses of the risk of
cardiovascular disease among patients with gallstones.
| A, Group 1 |
|---|
|
|---|
| Subgroup | HR/unadjusted
OR | RR (95% CI) | Reports | I2
(%) |
P(heterogeneity) | Weight % |
|---|
| Mean age |
|
<45 | HR: 1.65 | (1.14, 2.40) | 3 | 90.5 | <0.001 | 29.85 |
|
≤60 | Unadjusted OR:
1.47 | (1.38, 1.58) | 4 | 0 | 0.979 | 39.23 |
| Sex |
|
Female | HR: 1.25 | (1.18, 1.34) | 5 | 70.5 | 0.009 | 56.13 |
| Geographical
region |
|
Asia | HR: 1.27 | (1.21, 1.32) | 5 | 55.5 | 0.061 | 53.89 |
|
Western | Unadjusted OR:
1.29 | (1.22, 1.37) | 4 | 97.4 | <0.001 | 7.08 |
| Follow-up
(year) |
|
<10 | HR: 1.26 | (1.21, 1.32) | 6 | 49.8 | 0.076 | 56.30 |
|
<10 | Unadjusted OR:
1.27 | (1.19, 1.34) | 3 | 98.2 | <0.001 | 6.23 |
| Study design |
|
Prospective cohort study | HR: 1.29 | (1.10, 1.50) | 3 | 66.7 | 0.050 | 24.48 |
|
Prospective cohort study | Unadjusted OR:
1.35 | (1.30, 1.39) | 5 | 96.7 | <0.001 | 21.59 |
| Sample size
(gallstone) |
|
>10,000 | Unadjusted OR:
1.24 | (1.21, 1.26) | 5 | 96.8 | <0.001 | 93.45 |
|
| B, Group
2 |
|
|
Subgroup | HR/unadjusted
OR | RR (95%
CI) | Reports | I2
(%) |
P(heterogeneity) | Weight
% |
|
| Mean age |
|
≥45 | HR: 1.20 | (1.11, 1.30) | 5 | 81.4 | <0.001 | 70.15 |
|
>60 | Unadjusted OR:
1.24 | (1.17, 1.30) | 3 | 98.2 | <0.001 | 60.77 |
| Sex |
|
Male | HR: 1.22 | (1.08, 1.37) | 4 | 85.8 | <0.001 | 43.87 |
| Geographical
region |
|
Western | HR: 1.22 | (1.11, 1.34) | 5 | 74.4 | 0.004 | 46.11 |
|
Asia | Unadjusted OR:
1.23 | (1.20, 1.25) | 4 | 94.3 | <0.001 | 92.92 |
| Follow-up
(year) |
|
≥10 | HR: 1.23 | (1.11, 1.36) | 4 | 80.5 | 0.001 | 43.70 |
|
≥10 | Unadjusted OR:
1.23 | (1.21, 1.25) | 5 | 92.9 | <0.001 | 93.77 |
| Study design |
|
Retrospective cohort
study | HR: 1.23 | (1.15, 1.31) | 7 | 83.5 | <0.001 | 75.52 |
|
Retrospective cohort
study | Unadjusted OR:
1.20 | (1.17, 1.22) | 3 | 88.5 | <0.001 | 78.41 |
| Sample size
(gallstone) |
|
≤10,000 | Unadjusted OR:
1.15 | (1.09, 1.22) | 3 | 94.6 | <0.001 | 6.55 |
Discussion
In the present meta-analysis, it was demonstrated
that compared with controls, GD was associated with a 1.24-fold
increase in prevalence and a 1.23-fold increase in incidence for
CVD, including cardiovascular disease, coronary artery disease and
stroke. Previous studies have reached conflicting conclusions about
the association between GD and CVD. Several reports have indicated
that GD is associated with CVD (8,9), but
others have concluded otherwise (7).
The conflicting findings may be due to differences in the mean age,
sex, geographical region, sample size, study design, follow-up and
outcome. In the present study, subgroup analysis by mean
participant age revealed that young participants had higher CVD
risks, suggesting that more attention should be paid to the
prevention of CVD in younger patients with GD. It is generally
acknowledged that the prevalence of GD in younger individuals is
lower than that in older individuals (20). Therefore, GD may have a greater
impact on CVD risk in populations with a lower prevalence. The
effect of GD on CVD was stronger in females in the current
meta-analysis, which was consistent with the conclusion of a
previous meta-analysis (21). Female
patients with GD may have more CVD risks, such a pregnancy or oral
contraceptive use (22).
Furthermore, in postmenopausal women, a decrease in high-density
lipoprotein (HDL) levels contributes to the development of GD
(23). Recently, Fan et al
(21) also conducted a meta-analysis
on GD and CVD. A notable difference between their study and the
present study was that they pooled OR to calculate the risk of CVD
(21), whereas the current analysis
included HR and unadjusted OR, which were pooled separately to
calculate the risk of CVD. A previous meta-analysis also separated
pooled HR and OR, but did not perform subgroup analysis (24).
Various pathogenic mechanisms have been suggested as
possible explanations for GD and CVD. Cholesterol accumulation is a
major component in atherosclerotic CVD and GD (15,25).
These disorders share common risk factors, including age, sex and
obesity, as well as lipid and glucose metabolism disorders, which
are also key components of metabolic syndrome (MS) (26,27). MS
is strongly associated with coronary artery disease, and gallstones
may be considered a biliary feature of this syndrome (28,29). It
was assumed that patients with GD had a higher risk of having the
comorbidities of hypertension, diabetes, coronary arterial disease,
atrial fibrillation and hyperlipidemia, all of which have been
proved to be conventional risk factors for stroke (16). However, Olaiya et al (14) revealed that GD and diabetes or GD and
hyperlipidemia also interacted to increase the stroke risk.
Diabetes and hyperlipidemia present the same risks in GD and
stroke. Therefore, diabetes or hyperlipidemia with GD may have
synergistic effects that can promote strokes. Considering this,
checking for GD in patients with diabetes or hyperlipidemia maybe a
new strategy for stroke prevention (16).
Aberrant inflammation is involved in the development
of GD and CVD (30,31). Several inflammatory factors,
including von Willebrand factor, lectin-like oxidized
low-density-lipoprotein receptor-1, soluble urokinase plasminogen
activator receptor, regulated upon activation, normal T-cell
expressed and secreted, as well as microparticles, have been
proposed as reducing the expression levels of these factors may
improve CVD (32). The inflammatory
process in GD may promote atherosclerosis or vasculopathy in the
cerebral vasculature, thereby increasing the risk of CVD.
Associated liver diseases, including non-alcoholic fatty liver
disease and pyogenic liver abscesses, have been determined to
increase the risk of subsequent CVD via a similar mechanism.
Inflammation may also be a vascular risk factor in GD (31).
Dysbiosis is an alteration in the composition of the
gut microbiota, and has been associated with several diseases,
including CVD and GD (33,34). Specifically, dysbiosis has been
implicated in CVD, with various aspects of cardiometabolic
syndrome: Obesity, hypertension, chronic kidney disease and
diabetes (35). A mechanistic link
between gut microbiota formation of trimethylamine-N-oxide (TMAO)
and CVD has been repeatedly demonstrated. TMAO has been implicated
in atherosclerosis, platelet aggregation, diabetes and hypertension
(36). Gut and biliary tract
dysbiosis disequilibrates the enterohepatic circulation, leading to
gallstone formation (37,38). Various mechanisms have been
consequently speculated, including Helicobacter spp. in the
gallstone nuclei, systemic immune response alteration and
modulation of enterohepatic cycling of conjugated bile acids
(39). Infection with
Helicobacter pylori is positively associated with high
low-density lipoprotein cholesterol (LDL-C), low HDL-C and CVD
(40). Patients that are
Helicobacter seropositive with the TT genotype of the
polymorphic gene adiponectin receptor 2 rs1044471 constitute a risk
group of cardiovascular event formation (41). In light of these findings, therapies
targeting the gut microbiota present novel opportunities for CVD
and GD treatment.
Genetic studies have highlighted several single
nucleotide polymorphisms that may characterize patients with a high
risk for GD and CVD. Several genes in the cholesterol metabolism
pathway serve an important role in lipid biosynthesis, metabolism
and transport. Variants of these genes may significantly influence
plasma total cholesterol and LDL-C levels, and in turn affect the
pathogenesis of GD and CVD. For example, the ATP-binding cassette
G8 (ABCG8) gene is expressed exclusively in the liver and
intestine, and forms heterodimers to regulate the efflux of sterols
into the intestinal lumen and controls the hepatic secretion of
sterols into the bile. The ABCG8 D19H variant is associated with
plasma total cholesterol and LDL-C levels (42,43), and
confers risk for CVD and GD. Previous research has revealed that
the presence of the e4 allele of apolipoprotein E gene is a risk
factor for CVD and GD, and is associated with significantly higher
levels of LDL-C, TC and non-HDL-C (44,45).
Notably, this meta-analysis has several limitations.
First, heterogeneity was revealed through the pooled analysis among
studies. The present meta-analysis is unlikely to fully account for
heterogeneity, even with the explanation of certain clues from the
subgroup analysis. Therefore, the results of the meta-analysis must
be interpreted with caution. Because of the potential heterogeneity
in age, sex, follow-up, geographical region and study design, it
was assumed that the estimated true effect would vary between study
designs, in addition to the years of follow-up. Heterogeneity was
accounted for by using combined results of the eligible studies
with the random-effect model. Although the random-effect approach
provides some heterogeneity allowance beyond sampling error or a
limited influence, it does not necessarily rule out the
heterogeneity effect. Second, although Egger's test suggested no
publication bias for the HR; the inverted funnel plots of the HR
were not symmetrical. The source of publication bias may originate
from the nonrandomized studies, including prospective cohort and
retrospective cohorts. Finally, unpublished data/studies that may
have met the inclusion criteria were not included. In addition,
potential biases may have been produced in the present
meta-analysis.
In conclusion, the present study demonstrated that
the presence of GD was associated with an increased risk of CVD
incidence and prevalence. Further studies are required with more
randomized controlled trials to elucidate the molecular mechanisms
underlying the association between these two diseases, and whether
preventive therapies would prevent the progression of CVD in
patients with GD.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
SFZ performed the literature review, designed the
study, was a major contributor in writing the manuscript and
performed statistical analysis. GJS performed the literature review
and designed the study. SFZ and GJS independently extracted the
data from the selected studies. If there was a disagreement in the
interpretation of the results, AMW was consulted. XJY was involved
in writing the manuscript. LLW designed the workflow for the
current meta-analysis. XNX was involved in writing the manuscript
and revised the tables. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors have declared that no competing
interests.
References
|
1
|
Sakorafas GH, Milingos D and Peros G:
Asymptomatic cholelithiasis: Is cholecystectomy really needed? A
critical reappraisal 15 years after the introduction of
laparoscopic cholecystectomy. Dig Dis Sci. 52:1313–1325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ruhl CE and Everhart JE: Gallstone disease
is associated with increased mortality in the United States.
Gastroenterology. 140:508–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matveeva SA: The role of lipid-protein
interplay in the development of atherogenesis during coronary heart
disease and metabolic syndrome. Klin Med (Mosk). 90:30–34. 2012.(In
Russian). PubMed/NCBI
|
|
4
|
Ahmed MH, Barakat S and Almobarak AO: The
association between renal stone disease and cholesterol gallstones:
The easy to believe and not hard to retrieve theory of the
metabolic syndrome. Ren Fail. 36:957–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chuang SC, Hsi E and Lee KT: Genetics of
gallstone disease. Adv Clin Chem. 60:143–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allen NB, Lloyd-Jones D, Hwang SJ,
Rasmussen-Torvik L, Fornage M, Morrison AC, Baldridge AS,
Boerwinkle E, Levy D, Cupples LA, et al: Genetic loci associated
with ideal cardiovascular health: A meta-analysis of genome-wide
association studies. Am Heart J. 175:112–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kwon CH, Kang JG, Lee HJ, Kim NH, Sung JW,
Cheong E and Sung KC: Absence of association between gallstone and
coronary artery calcification. Atherosclerosis. 258:51–55. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng Y, Xu M, Li Y, Hruby A, Rimm EB, Hu
FB, Wirth J, Albert CM, Rexrode KM, Manson JE and Qi L: Gallstones
and risk of coronary heart disease: Prospective analysis of 270000
men and women from 3 US cohorts and meta-analysis. Arterioscler
Thromb Vasc Biol. 36:1997–2003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv J, Qi L, Yu C, Guo Y, Bian Z, Chen Y,
Yang L, Shen J, Wang S, Li M, et al: Gallstone disease and the risk
of ischemic heart disease. Arterioscler Thromb Vasc Biol.
35:2232–2237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Athanasiou T, Alruzzeh S, Kumar P,
Crossman MC, Amrani M, Pepper JR, Del Stanbridge R, Casula R and
Glenville B: Off-pump myocardial revascularization is associated
with less incidence of stroke in elderly patients. Ann Thorac Surg.
77:745–753. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–788. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee YS, Jang SE, Lee BS, Lee SJ, Lee MG,
Park JK, Lee SH, Ryu JK, Kim YT, Yoon YB and Hwang JH: Presence of
coronary artery disease increases the risk of biliary events in
patients with asymptomatic gallstones. J Gastroenterol Hepatol.
28:1578–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olaiya MT, Chiou HY, Jeng JS, Lien LM and
Hsieh FI: Significantly increased risk of cardiovascular disease
among patients with gallstone disease: A population-based cohort
study. PLoS One. 8:e764482013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang JY, Lu FH, Sun ZJ, Wu JS, Yang YC,
Lee CT and Chang CJ: Gallstone disease associated with increased
risk of arterial stiffness in a Taiwanese population. J Hum
Hypertens. 31:616–619. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei CY, Chung TC, Chen CH, Lin CC, Sung
FC, Chung WT, Kung WM, Hsu CY and Yeh YH: Gallstone disease and the
risk of stroke: A nationwide population-based study. J Stroke
Cerebrovasc Dis. 23:1813–1820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wirth J, di Giuseppe R, Wientzek A, Katzke
VA, Kloss M, Kaaks R, Boeing H and Weikert C: Presence of
gallstones and the risk of cardiovascular diseases: The
EPIC-Germany cohort study. Eur J Prev Cardiol. 22:326–334. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khan HN, Harrison M, Bassett EE and Bates
T: A 10-year follow-up of a longitudinal study of gallstone
prevalence at necropsy in South East England. Dig Dis Sci.
54:2736–2741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bortnichak EA, Freeman DH Jr, Ostfeld AM,
Castelli WP, Kannel WB, Feinleib M and McNamara PM: The association
between cholesterol cholelithiasis and coronary heart disease in
Framingham, Massachusetts. Am J Epidemiol. 121:19–30. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dua A, Aziz A, Desai SS, McMaster J and
Kuy S: National trends in the adoption of laparoscopic
cholecystectomy over 7 Years in the United States and impact of
laparoscopic approaches stratified by age. Minim Invasive Surg.
2014:6354612014.PubMed/NCBI
|
|
21
|
Fan LL, Chen BH and Dai ZJ: The relation
between gallstone disease and cardiovascular disease. Sci Rep.
7:151042017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Lin H, Zhang C, Wang L, Wu S, Zhang
D, Tang F, Xue F and Liu Y: Non-alcoholic fatty liver disease
associated with gallstones in females rather than males: A
longitudinal cohort study in Chinese urban population. BMC
Gastroenterol. 14:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sieron D, Czerny B, Sieron-Stoltny K,
Karasiewicz M, Bogacz A, Seremak-Mrozikiewicz A, Kotrych D, Boron D
and Mrozikiewicz P: The effect of chronic estrogen application on
bile and gallstone composition in women with cholelithiasis.
Minerva Endocrinol. 41:19–27. 2016.PubMed/NCBI
|
|
24
|
Upala S, Sanguankeo A and Jaruvongvanich
V: Gallstone disease and the risk of cardiovascular disease: A
systematic review and meta-analysis of observational studies. Scand
J Surg. 106:21–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahmed MH, Hamad MA, Routh C and Connolly
V: Statins as potential treatment for cholesterol gallstones: An
attempt to understand the underlying mechanism of actions. Expert
Opin Pharmacother. 12:2673–2681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada T, Hara K and Kadowaki T: Chewing
betel quid and the risk of metabolic disease, cardiovascular
disease, and all-cause mortality: A meta-analysis. PLoS One.
8:e706792013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen HC, Hu YC, Chen YF and Tung TH:
Prevalence and associated metabolic factors of gallstone disease in
the elderly agricultural and fishing population of Taiwan.
Gastroenterol Res Pract. 2014:8769182014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zidi W, Allal-Elasmi M, Zayani Y, Zaroui
A, Guizani I, Feki M, Mourali MS, Mechmeche R and Kaabachi N:
Metabolic syndrome, independent predictor for coronary artery
disease. Clin Lab. 61:1545–1552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen LY, Qiao QH, Zhang SC, Chen YH, Chao
GQ and Fang LZ: Metabolic syndrome and gallstone disease. World J
Gastroenterol. 18:4215–4220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fiorito G, Vlaanderen J, Polidoro S,
Gulliver J, Galassi C, Ranzi A, Krogh V, Grioni S, Agnoli C,
Sacerdote C, et al: Oxidative stress and inflammation mediate the
effect of air pollution on cardio- and cerebrovascular disease: A
prospective study in nonsmokers. Environ Mol Mutagen. 59:234–246.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maurer KJ, Carey MC and Fox JG: Roles of
infection, inflammation, and the immune system in cholesterol
gallstone formation. Gastroenterology. 136:425–440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv JX, Kong Q and Ma X: Current advances
in circulating inflammatory biomarkers in atherosclerosis and
related cardio-cerebrovascular diseases. Chronic Dis Transl Med.
3:207–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu T, Zhang Z, Liu B, Hou D, Liang Y,
Zhang J and Shi P: Gut microbiota dysbiosis and bacterial community
assembly associated with cholesterol gallstones in large-scale
study. BMC Genomics. 14:6692013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang WH, Kitai T and Hazen SL: Gut
microbiota in cardiovascular health and disease. Circ Res.
120:1183–1196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Z and Zhao Y: Gut microbiota derived
metabolites in cardiovascular health and disease. Protein Cell. May
3–2018.(Epub ahead of print). View Article : Google Scholar
|
|
36
|
Ahmadmehrabi S and Tang WHW: Gut
microbiome and its role in cardiovascular diseases. Curr Opin
Cardiol. 32:761–766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai JS and Chen JH: The mechanism of
enterohepatic circulation in the formation of gallstone disease. J
Membr Biol. 247:1067–1082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Q, Jiao L, He C, Sun H, Cai Q, Han T
and Hu H: Alteration of gut microbiota in association with
cholesterol gallstone formation in mice. BMC Gastroenterol.
17:742017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Neri V, Margiotta M, de Francesco V,
Ambrosi A, Valle ND, Fersini A, Tartaglia N, Minenna MF,
Ricciardelli C, Giorgio F, et al: DNA sequences and proteic
antigens of H. pylori in cholecystic bile and tissue of patients
with gallstones. Aliment Pharmacol Ther. 22:715–720. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nam SY, Ryu KH, Park BJ and Park S:
Effects of Helicobacter pylori infection and its eradication on
lipid profiles and cardiovascular diseases. Helicobacter.
20:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kozyrieva T, Kolesnikova E and Shut I:
Correlation of helicobacter pylori infection with development of
cardiovascular risk in patients with coronary heart disease in
association with type 2 diabetes mellitus. Georgian Med News.
24–29. 2016.PubMed/NCBI
|
|
42
|
Xu HL, Cheng JR, Andreotti G, Gao YT,
Rashid A, Wang BS, Shen MC, Chu LW, Yu K and Hsing AW: Cholesterol
metabolism gene polymorphisms and the risk of biliary tract cancers
and stones: A population-based case-control study in Shanghai,
China. Carcinogenesis. 32:58–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Srivastava A, Garg N, Srivastava A,
Srivastava K and Mittal B: Effect of genetic variant (rs11887534)
in ABCG8 gene in coronary artery disease and response to
atorvastatin therapy. Dis Markers. 28:307–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xue P, Niu WQ, Jiang ZY, Zheng MH and Fei
J: A meta-analysis of apolipoprotein E gene ε2/ε3/ε4 polymorphism
for gallbladder stone disease disease. PLoS One. 7:e458492012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
El-Lebedy D, Raslan HM and Mohammed AM:
Apolipoprotein E gene polymorphism and risk of type 2 diabetes and
cardiovascular disease. Cardiovasc Diabetol. 15:122016. View Article : Google Scholar : PubMed/NCBI
|