Introduction
Thrombelastogram (TEG), first applied in the 1940s,
is used to detect the bleeding risk during surgery and evaluate the
efficacy of blood product infusion, which is widely used in heart
and other operations (1,2). TEG detection of solidification changes
in blood is clinically effective for understanding patients'
platelet function, which is helpful for antiplatelet therapy
(3). Coronary heart disease (CHD) is
caused by myocardial ischemia, hypoxia or necrosis due to vascular
cavity obstruction or stenosis as a result of atherosclerosis in
coronary artery blood vessels. As the first choice for the
treatment of CHD, percutaneous coronary intervention (PCI) is
currently widely used in clinical practice (4,5).
However, the mechanical rotation and expansion of blood vessels
during the operation leads to the rupture of coronary plaques and
the mass release of tissue factors, resulting in platelet
aggregation and thrombosis. Therefore, regular anti-thrombus
therapy should be implemented after operation to prevent thrombosis
(6).
Currently, clopidogrel combined with aspirin for
antiplatelet therapy is a clinically routine treatment regimen for
preventing thrombosis after PCI. However, due to the different drug
metabolism genotypes of clopidogrel and aspirin, as well as
diabetes mellitus, calcification or acute coronary syndrome,
routine treatment is not effective in preventing thrombosis in some
patients. The risk of thrombosis will be increased if the drug
adjustment could not be made in time (7,8).
Therefore, it is of great clinical significance to effectively
prevent thrombosis after PCI. There are studies reporting that
clopidogrel with double maintenance dose (150 mg, 1 time/day) or
double loading dose (600 mg) helps to better improve the
coagulation status of patients after PCI, thereby reducing the
incidence of major adverse cardiovascular events (MACE) (9,10). A
study reported that TEG could be used to guide the clinical
medication by evaluating platelet function in CHD patients after
PCI, thereby promoting the recovery of patients. In that study, TEG
was used to adjust the use of different drugs, but the efficacy of
TEG-guided medication and no TEG-guided medication was not compared
(11).
Therefore, in this study, the antiplatelet effect of
clopidogrel with maintenance dose with the individualized guidance
was studied in order to investigate the application value of TEG in
CHD patients after PCI.
Materials and methods
General information
A retrospective analysis was performed on 203
patients undergoing PCI interventional therapy in the
Cardiovascular Medicine of Weihai Central Hospital (Weihai, China)
from February 2015 to September 2016, including 121 males and 82
females, with an average age of 55.7±2.3 years and an average BMI
of 25.3±3.1 kg/m2. All patients were treated with
clopidogrel and aspirin for anti-thrombus therapy. Among them, 104
patients with the TEG detection of anticoagulant effects for
guiding medication were in experimental group, and 99 patients
without the TEG detection for guiding medication in control group.
There were no significant differences between the two groups in
sex, age, preoperative coagulation function and other aspects
(Table I).
 | Table I.Comparison of general information of
patients between two groups. |
Table I.
Comparison of general information of
patients between two groups.
| Factors | Experimental group
(n=104) | Control group
(n=99) | χ2 | P-value |
|---|
| Sex |
|
| 0.001 | 0.998 |
| Male | 62 (59.62) | 59 (59.60) |
|
|
|
Female | 42 (40.38) | 40 (40.40) |
|
|
| Age (years) |
|
| 0.008 | 0.928 |
| ≤55 | 71 (68.27) | 67 (67.68) |
|
|
|
>55 | 33 (31.73) | 32 (32.32) |
|
|
| BMI
(kg/m2) |
|
| 0.031 | 0.861 |
| ≤25 | 58 (55.77) | 54 (54.55) |
|
|
|
>25 | 46 (44.23) | 45 (45.45) |
|
|
| History of
smoking |
|
| 0.011 | 0.917 |
| Yes | 69 (66.35) | 65 (65.66) |
|
|
| No | 35 (33.65) | 34 (34.34) |
|
|
| History of
drinking |
|
| 0.010 | 0.922 |
| Yes | 75 (72.12) | 72 (72.73) |
|
|
| No | 29 (27.88) | 27 (27.27) |
|
|
| History of
hypertension |
|
| 0.005 | 0.992 |
| Yes | 61 (58.65) | 58 (58.59) |
|
|
| No | 43 (41.35) | 41 (41.41) |
|
|
| History of diabetes
mellitus |
|
| 0.009 | 0.926 |
| Yes | 55 (52.88) | 53 (53.54) |
|
|
| No | 49 (47.12) | 46 (46.46) |
|
|
| Anticoagulant
function |
|
|
|
|
| R value
(min) | 3.76±1.21 | 3.71±1.09 | 0.309 | 0.758 |
| K value
(min) | 1.85±0.69 | 1.79±0.57 | 0.674 | 0.501 |
| MA value
(min) | 66.31±11.08 | 67.19±10.92 | 0.570 | 0.570 |
| Diffuse long
disease |
|
| 0.006 | 0.936 |
| Yes | 31 (29.81) | 29 (29.29) |
|
|
| No | 73 (70.19) | 70 (70.71) |
|
|
| Calcification |
|
| 0.024 | 0.876 |
| Yes | 23 (22.12) | 21 (21.21) |
|
|
| No | 81 (77.88) | 78 (78.79) |
|
|
| Placing stents |
|
| 0.891 | 0.019 |
|
Yes | 43 (41.35) | 40 (40.40) |
|
|
| No | 61 (58.65) | 59 (59.60) |
|
|
This study was approved by the Ethics Committee of
Weihai Central Hospital (Weihai, China). Patients who participated
in this research had complete clinical data. The signed informed
consents were obtained from the patients or the guardians.
Inclusion and exclusion criteria
Inclusion criteria were: Patients diagnosed with
CHD. Exclusion criteria were: Patients who had taken anti-thrombus
drugs recently, with tumors or severe liver and kidney diseases,
who are allergic to clopidogrel and aspirin, with the past history
of peptic hemorrhage and cerebral hemorrhage, with cognitive and
communication disorder and patients who did not cooperate with the
examination were excluded. All subjects signed an informed consent
form and cooperated with medical staff to complete relevant medical
treatment.
Experimental instruments and
drugs
The TEG 5000 coagulation analyzer was purchased from
Haemonetics Management Co., Ltd. (Shanghai, China). Clopidogrel
(SFDA approval number: H31022653) was purchased from Shanghai Fudan
Fuhua Pharmaceutical Co., Ltd. (Shanghai, China). Aspirin (SFDA
approval number: H32024219) was purchased from Nanjing
Pharmaceutical Factory Co., Ltd. (Nanjing, China).
Experimental methods
At 1 day before PCI, all patients were orally
administered with routine 300 mg of load quantity aspirin and 300
mg of clopidogrel. During the operation, patients in both groups
were treated with 3.8% sodium citrate anticoagulation. In the
experimental group, the TEG detection was performed with the
coagulation analyzer. After operation, patients in the control
group were given routine 75 mg/day clopidogrel and 100 mg/day
aspirin. Medication adjustment was performed on patients in the
experimental group based on their TEG detection results. More than
half of the patients in the experimental group had >50% platelet
aggregation rate. When the platelet aggregation rate was >50%,
aspirin was increased to 300 mg and clopidogrel was adjusted to
double loading dose. The TEG detection was performed again on
patients in the two groups on the next day after operation. Their
coagulation function was evaluated, and their platelet inhibition
rates were compared, with the platelet inhibition evaluation
standard as previously described (12). All patients were followed up by
telephone or clinic after discharge, in order to know if they had
MACE (including recurrent angina, myocardial infarction, identified
stent thrombosis and cardiogenic death) and bleeding events
(including eye, nose, gingival, skin, brain, gastrointestinal and
urinary system bleeding) within three months.
Statistical methods
SPSS19.0 (IBM Corp., Armonk, NY, USA) was used to
analyze the data. The Chi-square test was used for enumeration
data. Measurement data were expressed as mean ± SD, and tested by
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of postoperative TEG
detection of coagulation function of patients between two
groups
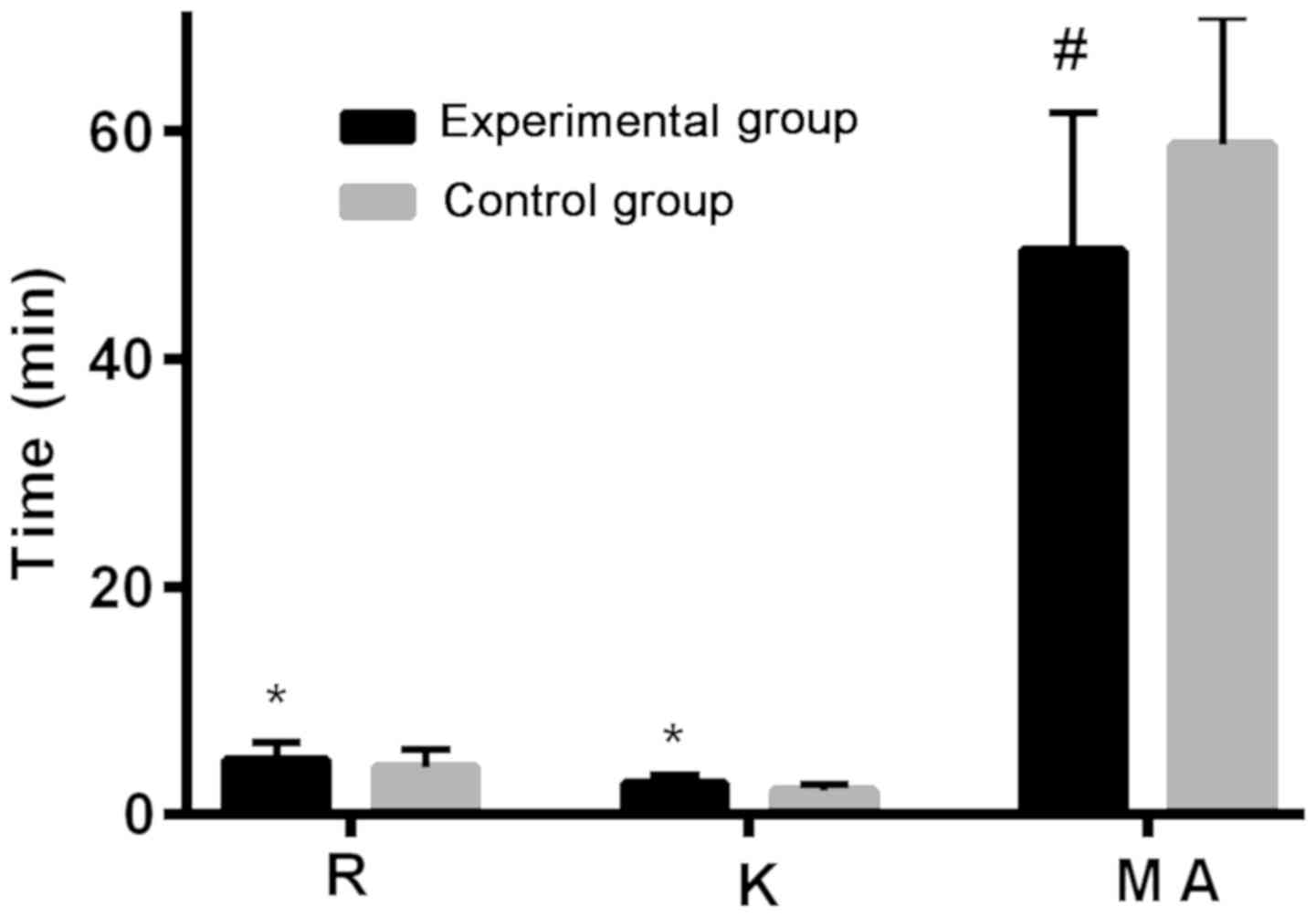
Patients in the experimental group had higher R
(coagulation reaction time) value and K (blood clot formation time)
value than those in the control group after treatment (P<0.05),
and lower MA (maximum amplitude) value than those in the control
group (P<0.05) (Table II and
Fig. 1).
 | Table II.Comparison of postoperative TEG
detection of coagulation function between two groups of
patients. |
Table II.
Comparison of postoperative TEG
detection of coagulation function between two groups of
patients.
| Indicator | Experimental group
(n=104) | Control group
(n=99) | t value | P-value |
|---|
| R value (min) | 4.78±1.50 | 4.12±1.53 | 3.103 | <0.050 |
| K value (min) | 2.63±0.76 | 2.07±0.60 | 5.808 | <0.001 |
| MA value (min) | 49.52±12.04 | 58.76±11.21 | 5.652 | <0.001 |
Comparison of postoperative platelet
inhibition and platelet count of patients between two groups
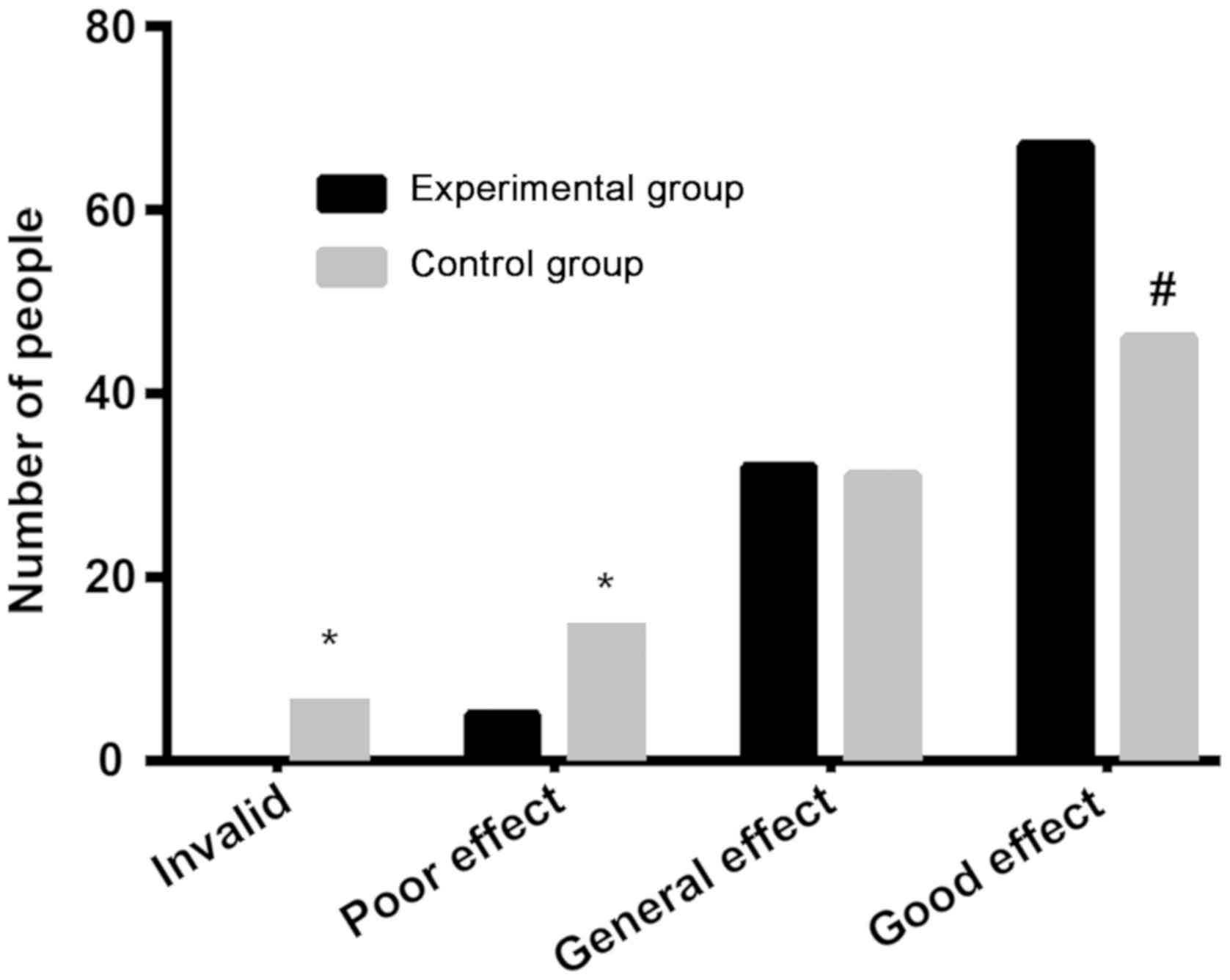
The ineffective rate and poor effect rate of the
platelet inhibition of patients in the experimental group were 0
and 4.81%, respectively, significantly lower than 7.07 and 15.15%
in the control group (P<0.05). There was no significant
difference in the general effect rate (P>0.05). The good effect
rate of patients in the experimental group was 64.42%,
significantly higher than 46.46% in the control group (P<0.05).
There was no significant difference in preoperative platelet count
between the two groups (P>0.05), but the platelet count in the
experimental group was significantly lower than that in the control
group, with a statistically significant difference (P<0.05)
(Tables III and IV, and Fig.
2).
 | Table III.Comparison of postoperative platelet
inhibition between two groups of patients [n (%)]. |
Table III.
Comparison of postoperative platelet
inhibition between two groups of patients [n (%)].
| Inhibitory
effect | Experimental group
(n=104) | Control group
(n=99) | χ2 | P-value |
|---|
| Ineffective | 0 (0.00) | 7 (7.07) | 7.616 | <0.050 |
| Poor effect | 5 (4.81) | 15 (15.15) | 6.110 | <0.050 |
| General effect | 32 (30.77) | 31 (31.31) | 0.007 |
0.933 |
| Good effect | 67 (64.42) | 46 (46.46) | 6.628 | <0.050 |
 | Table IV.Platelet counts before and after
surgery in two groups (×109/l). |
Table IV.
Platelet counts before and after
surgery in two groups (×109/l).
| Time | Experimental group
(n=104) | Control group
(n=99) | t value | P-value |
|---|
| Before surgery |
213.62±12.37 |
214.25±13.01 | 0.354 | 0.754 |
| After surgery | 126.58±7.59 | 176.65±6.31 | 50.98 | <0.001 |
Comparison of MACE occurrence of
patients within three months between two groups
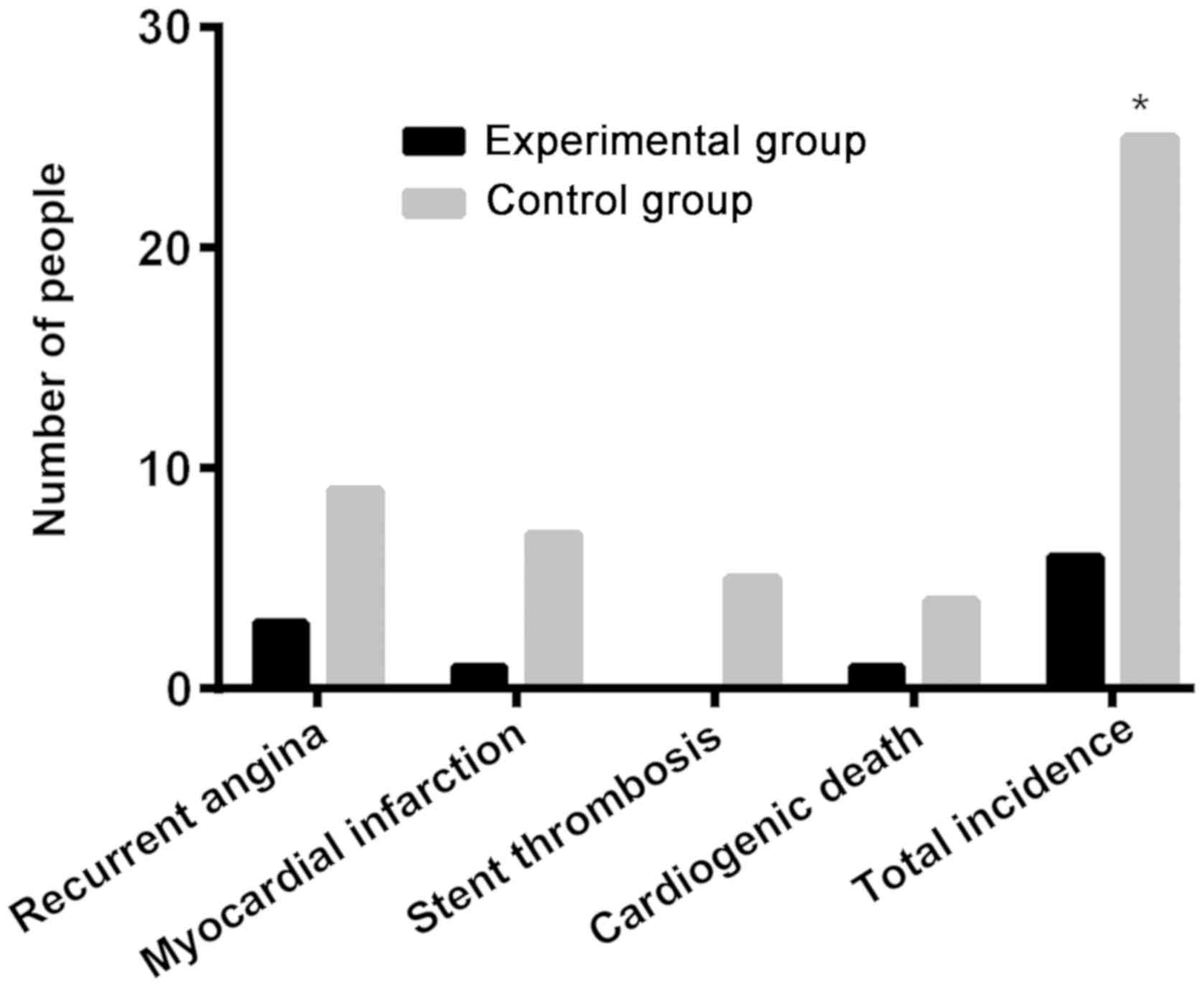
The numbers of patients with recurrent angina,
myocardial infarction, stent thrombosis and cardiogenic death in
the experimental group were 3, 2, 0, and 1, respectively. Whereas
in the control group they were 9, 7, 5 and 4, respectively. The
total incidence of MACE in the experimental group was 5.77%,
significantly lower than 25.25% in the control group, with a
statistically significant difference (P<0.05) (Table V and Fig.
3).
 | Table V.Comparison of MACE occurrence of
patients within three months between two groups [n (%)]. |
Table V.
Comparison of MACE occurrence of
patients within three months between two groups [n (%)].
| Factors | Experimental group
(n=104) | Control group
(n=99) | χ2 | P-value |
|---|
| Recurrent
angina | 3 (2.88) | 9 (9.09) | – | – |
| Myocardial
infarction | 2 (1.92) | 7 (7.07) | – | – |
| Stent
thrombosis | 0 (0.00) | 5 (5.05) | – | – |
| Cardiogenic
death | 1 (0.96) | 4 (4.04) | – | – |
| Total
incidence | 6 (5.77) | 25 (25.25) | 14.88 | <0.001 |
Comparison of occurrence of bleeding
events of patients between two groups
There was 1 patient with gingival bleeding and 1
patient with skin bleeding in the experimental group; 1 patient
with eye and nose bleeding, 4 patients with gingival bleeding, 4
patients with skin bleeding and 2 patients with gastrointestinal
bleeding in the control group. The total incidence of bleeding was
1.92% in the experimental group, and 11.11% in the control group.
There was a significant difference in the incidence of bleeding
events between the two groups (P<0.05) (Table VI).
 | Table VI.Comparison of occurrence of bleeding
events of patients between two groups [n (%)]. |
Table VI.
Comparison of occurrence of bleeding
events of patients between two groups [n (%)].
| Bleeding site | Experimental group
(n=104) | Control group
(n=99) | χ2 | P-value |
|---|
| Eye and nose | 0 | 1 | – | – |
| Gingival | 1 | 4 | – | – |
| Skin | 1 | 4 | – | – |
| Brain | 0 | 0 | – | – |
|
Gastrointestinal | 0 | 2 | – | – |
| Urinary system | 0 | 0 | – | – |
| Total
incidence | 2 (1.92) | 11 (11.11) | 7.144 | <0.050 |
Discussion
CHD, a common disease in heart medicine, has a
serious impact on patients' health and quality of life (13). There is a study (14) showing that the pathogenic factors of
CHD are complicated, for which age, hypertension and diabetes
mellitus can be independent risk factors. It has been reported
(15) that CHD patients usually have
a high coagulation tendency, especially after PCI. Their
coagulation status and platelet activity are affected. At present,
clopidogrel combined with aspirin is a routine treatment after PCI.
However, even if it is a conventional treatment, due to different
degrees of tolerance to drugs and different efficacy in different
patients, some patients may have MACE (16). TEG is an instrument that obtains
blood coagulation patterns and related parameters. Through TEG
analysis, the coagulation function and platelet changes of patients
are reflected in a more comprehensive way (17). Currently, TEG is widely used in the
detection of coagulation function in patients after PCI to improve
the efficacy and reduce the risk of thrombosis and other
complications by guiding the selection and dose adjustment of
anticoagulant drugs (18). Previous
studies (19,20) reported that TEG can evaluate the
platelet function of patients after PCI, but there are few studies
on whether to use TEG to guide patients in medication and the
comparison of the efficacy between TEG-guided medication and no
TEG-guided medication. Therefore, in this study, the antiplatelet
effect of individual-guided clopidogrel with maintenance dose was
studied to investigate the application value of TEG in CHD patients
after PCI.
In this study, TEG was used to analyze and compare
the coagulation function of patients between the two groups. The
results showed that patients in the experimental group with
TEG-guided medication adjustment had higher R value and K value
than those in the control group, and lower MA value than those in
the control group. The function of coagulation factor can be
reflected by R value, K value and MA value. When R value and K
value are increased and MA value is decreased, coagulation factor
and platelet coagulation acti vity are both decreased (21). It is indicated that the overall
coagulation function of patients was better in the experimental
group than that in the control group. Then, the platelet inhibition
and platelet count of patients were compared between the two
groups. The results showed that the ineffective rate and poor
effect rate of patients in the experimental group were 0% and
4.81%, respectively, significantly lower than the 7.07% and 15.15%
in the control group. There was no significant difference in the
general effect rate (P>0.05). The good effect rate of patients
in the experimental group was 64.42%, significantly higher than the
46.46% in the control group. There was no significant difference in
preoperative platelet count between the two groups, but the
platelet count in the experimental group was significantly lower
than that in the control group. It is suggested that the platelet
inhibitory effect on patients is better in the experimental group
than that in the control group, indicating that the TEG-guided
adjustment of anticoagulant drugs is beneficial to improve
patients' coagulation function and platelet inhibition.
In the study of Gurbel et al (22), it is reported that the TEG
determination of the coagulation function and platelet inhibition
after PCI is used to adjust the dosage of anticoagulant drugs in
patients with low response to aspirin and clopidogrel. There is no
stent thrombosis during the 2-year follow-up, but some patients in
the control group have it. This confirms our conclusion. After
that, the incidence of MACE and bleeding events of patients was
compared between the two groups. The results showed that the total
incidence of MACE in the experimental group was 5.77%,
significantly lower than the 25.25% in the control group, with a
statistically significant difference (P<0.05). The total
incidence of bleeding was 1.92% in the experimental group, and
11.11% in the control group. There was a significant difference in
the incidence of bleeding events between the two groups
(P<0.05). It is indicated that the individual-guided
anticoagulant drugs after PCI through TEG detection is beneficial
to reduce the incidence of MACE and bleeding events. There is a
study confirming (23) that
optimizing anticoagulant therapy after TEG detection can reduce the
postoperative incidence and fatality rate of MACE as well as
bleeding events in patients undergoing PCI.
In summary, CHD patients after PCI with the
TEG-guided dose adjustment of clopidogrel have better treatment
effects than patients without the TEG guidance. TEG makes the
treatment of patients more targeted and is worthy of promotion.
However, due to the small sample size and limited time in this
study, the side effects of long-term high doses of antithrombotic
drugs were not investigated. The safety of TEG detection after PCI
remains to be studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL wrote the manuscript. YL and HoC recorded and
analyzed coagulation function index. LN and PX analyzed the general
data of patients. CL, LY and HaC were responsible for treatment of
patients. CY helped with statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Weihai Central Hospital (Weihai, China). Patients who participated
in this research had complete clinical data. The signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reikvam H, Steien E, Hauge B, Liseth K,
Hagen KG, Størkson R and Hervig T: Thrombelastography. Transfus
Apheresis Sci. 40:119–123. 2009. View Article : Google Scholar
|
|
2
|
Craft RM, Chavez JJ, Bresee SJ, Wortham
DC, Cohen E and Carroll RC: A novel modification of the
thrombelastograph assay, isolating platelet function, correlates
with optical platelet aggregation. J Lab Clin Med. 143:301–309.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patti G, Grieco D, Dicuonzo G, Pasceri V,
Nusca A and Di Sciascio G: High versus standard clopidogrel
maintenance dose after percutaneous coronary intervention and
effects on platelet inhibition, endothelial function, and
inflammation results of the ARMYDA-150 mg (antiplatelet therapy for
reduction of myocardial damage during angioplasty) randomized
study. J Am Coll Cardiol. 57:771–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart RAH, Colquhoun DM, Marschner SL,
Kirby AC, Simes J, Nestel PJ, Glozier N, O'Neil A, Oldenburg B,
White HD, et al LIPID Study Investigators, : Persistent
psychological distress and mortality in patients with stable
coronary artery disease. Heart. 103:1860–1866. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen KJ and Liu Y: Stable coronary heart
disease: A choice for PCI or drug therapy - inspiration from
results of a new meta-analysis. Zhongguo Zhong Xi Yi Jie He Za Zhi.
32:583–584. 2012.(In Chinese). PubMed/NCBI
|
|
6
|
Khan AR, Golwala H, Tripathi A, Riaz H,
Kumar A, Flaherty MP and Bhatt DL: Meta-analysis of percutaneous
coronary intervention versus coronary artery bypass grafting in
left main coronary artery disease. Am J Cardiol. 119:1949–1956.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ford NF and Taubert D: Clopidogrel,
CYP2C19, and a black box. J Clin Pharmacol. 53:241–248. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duzenli MA, Ozdemir K, Aygul N, Soylu A
and Tokac M: Comparison of increased aspirin dose versus combined
aspirin plus clopidogrel therapy in patients with diabetes mellitus
and coronary heart disease and impaired antiplatelet response to
low-dose aspirin. Am J Cardiol. 102:396–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Yang J, Zhu X, Wang X, Peng L, Li
X, Cheng P and Yin T: Effect of high-dose clopidogrel according to
CYP2C19*2 genotype in patients undergoing percutaneous coronary
intervention- a systematic review and meta-analysis. Thromb Res.
135:449–458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen S, Zhang Y, Wang L, Geng Y, Gu J, Hao
Q, Wang H and Qi P: Effects of dual-dose clopidogrel, clopidogrel
combined with tongxinluo capsule, and ticagrelor on patients with
coronary heart disease and CYP2C19*2 gene mutation after
percutaneous coronary interventions (PCI). Med Sci Monit.
23:3824–3830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Wang L, Yang X, Li K, Sun H, Zhang
D, Wang H, Li W, Ni Z, Xia K, et al: Platelet function monitoring
guided antiplatelet therapy in patients receiving high-risk
coronary interventions. Chin Med J (Engl). 127:3364–3370.
2014.PubMed/NCBI
|
|
12
|
Louis SG, Van PY, Riha GM, Barton JS,
Kunio NR, Underwood SJ, Differding JA, Rick E, Ginzburg E and
Schreiber MA: Thromboelastogram-guided enoxaparin dosing does not
confer protection from deep venous thrombosis: A randomized
controlled pilot trial. J Trauma Acute Care Surg. 76:937–942;
discussion 942–943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jolliffe JA, Rees K, Taylor RS, Thompson
D, Oldridge N and Ebrahim S: Exercise-based rehabilitation for
coronary heart disease. Cochrane Database Syst Rev.
1:CD0018002001.
|
|
14
|
Anderson JL, Adams CD, Antman EM, Bridges
CR, Califf RM, Casey DE Jr, Chavey WE II, Fesmire FM, Hochman JS,
Levin TN, et al 2011 Writing Group Members; ACCF/AHA Task Force
Members, : 2011 ACCF/AHA focused update incorporated into the
ACC/AHA 2007 guidelines for the management of patients with
unstable angina/non-ST-elevation myocardial infarction: A report of
the American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines. Circulation.
123:e426–e579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishan PV, Uday KC, Shobha JC, Usharani P
and Chandrasekhar E: Effect of oral anti-platelet regimens on
platelet aggregation using chronolog light transmittance
aggregometry in coronary heart disease patients: An observational
study. J Clin Diagn Res. 7:2478–2482. 2013.PubMed/NCBI
|
|
16
|
Steg PG, James S, Harrington RA, Ardissino
D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S,
Katus H, et al PLATO Study Group, : Ticagrelor versus clopidogrel
in patients with ST-elevation acute coronary syndromes intended for
reperfusion with primary percutaneous coronary intervention: A
Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup
analysis. Circulation. 122:2131–2141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hiller KN: Clinically relevant exaggerated
pharmacodynamic response to dual antiplatelet therapy detected by
Thromboelastogram® Platelet Mapping™. J Anaesthesiol
Clin Pharmacol. 32:112–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jain R and Sood J: Antiplatelet therapy in
patients with coronary artery stents for noncardiac surgery: Role
of thromboelastography. J Anaesthesiol Clin Pharmacol. 27:537–540.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao SW, Wang YP, Xu LD and Gang W: The
application of thromboelastogram in detection of indexes of
antiplatelet therapy for coronary heart disease. J Thorac Dis.
8:3515–3520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yildirim F, Tuncer B, Ozbakkaloglu A,
Kurdal AT, Ozturk T and Iskesen I: Thromboelastogram reduces blood
use by inspecting coagulation in heart surgery. Asian Cardiovasc
Thorac Ann. 24:441–444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berezovskaya G, Smirnova O, Malev E,
Khromov-Borisov N, Klokova E, Karpenko M, Papayan L and Petrishchev
N: Thrombin generation test for evaluation of antiplatelet
treatment in patients with coronary artery disease after
percutaneous coronary intervention. Platelets. 29:185–191. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gurbel PA, Bliden KP, Kreutz RP, Dichiara
J, Antonino MJ and Tantry US: The link between heightened
thrombogenicity and inflammation: Pre-procedure characterization of
the patient at high risk for recurrent events after stenting.
Platelets. 20:97–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gurbel PA, Bliden KP, Navickas IA, Mahla
E, Dichiara J, Suarez TA, Antonino MJ, Tantry US and Cohen E:
Adenosine diphosphate-induced platelet-fibrin clot strength: A new
thrombelastographic indicator of long-term poststenting ischemic
events. Am Heart J. 160:346–354. 2010. View Article : Google Scholar : PubMed/NCBI
|