Introduction
Gingival overgrowth (GO), which usually occurs at
the upper and lower anterior teeth, includes gingival enlargement
and hyperplasia. GO is characterized by a change in the color of
the gingiva, bleeding upon probing, compromised appearance of the
teeth, and difficulties regarding mastication, deglutition and
speech (1–5). The pathogenesis of GO is thought to be
multifactorial, involving inflammation, malignancy (e.g., oral
cavity tumor or other solid tumor type), systemic diseases and
undesired effects associated with systemic administration of
certain drugs (6,7). GO that occurs via the latter mechanism
is referred to as drug-induced gingival overgrowth (DIGO) and has a
prevalence of 3–20% (8). To date,
>20 drugs have been reported to be associated with DIGO,
including anti-convulsants (e.g., phenytoin), immunosuppressants
(e.g., cyclosporine A) and calcium channel blockers (CCBs, e.g.,
nifedipine and verapamil) (9–22). CCBs
are administered to treat various cardiac diseases, including
hypertension, angina pectoris and certain arrhythmias (e.g.,
supraventricular tachycardia) and exert their effect on
voltage-dependent Ca2+ channels in smooth muscles
(23), subsequently promoting a
higher concentration of extracellular calcium and decreasing the
level of intracellular calcium. The changes in calcium
concentrations inside and outside of cells affect the activation of
collagenase and processes involved in apoptosis (24–26).
Therefore, CCBs have an important role in the initiation and
progression of tissue overgrowth.
CCBs are primarily divided into three categories:
Phenylalkylamine derivatives (e.g., verapamil), benzothiazepine
derivatives (e.g., diltiazem) and substitute dihydropyridines
(e.g., nifedipine, amlodipine, felodipine, isradipine, nicardipine,
nimodipine, oxodipine, nisodipine and nitrendipine) (23). Nifedipine, as a first-generation
dihydropyridine, has been widely used in the management of cardiac
diseases, including hypertension. In the mid-1980s, Lombardi et
al (27) first reported on a
case of GO caused by nifedipine. Since then, further cases of GO
linked to the use of nifedipine have been reported. The incidence
of nifedipine-induced GO varies among different studies, ranging
from 14–83% (28). Compared to the
first-generation CCBs, the involvement of second- and
third-generation dihydropyridines, including felodipine and
amlodipine, in the pathogenesis of DIGO has been less frequently
reported. One study reported on incidence of amlodipine-induced GO
of 1.4–3.3% (8). Felodipine was
first reported to cause DIGO in 1991 (27), and Fay et al (4) reported on one case of
felodipine-associated GO in a patient with type 2 diabetes, whose
histological characteristics of GO were similar to those in DIGO
and withdrawal of felodipine almost fully resolved the GO. The
present study reports on a case of GO that was attributed to
felodipine treatment. To the best of our knowledge, the present
study is the first to report on felodipine-induced GO in a Chinese
patient with type 2 diabetes.
Case report
A 48-year-old man from Jilin Province in the
northeast region of China presented at the Endocrinology Department
of the First Clinical Hospital of Jilin University (Changchun,
China) with the chief complaint of poor blood glucose control
lasting for nearly 3 days. The patient denied a family history of
hypertension and diabetes mellitus but admitted that his blood
pressure had been high for 4 years, with a peak blood pressure of
180/100 mmHg. Various anti-hypertensive drugs had been used to
control his blood pressure but had a poor efficiency. The patient
had a history of diabetes of ~1 year. Aside from diet control, he
did not take any medications or insulin to control his blood
glucose levels and monitored his fingertip blood glucose regularly.
At 3 days prior to presentation, his fingertip blood glucose were
14 mmol/l and glycated hemoglobin (HbA1c) levels were 8.4%, which
prompted him to visit our department. On physical examination, the
patient had a body temperature of 36.5°C, heart rate of 88
beats/min, respiratory rate of 20 breaths/min and blood pressure of
160/100 mmHg. He was well developed and moderately nourished with a
body mass index of 26.2 kg/m2. His skin and sclera had
no yellow staining. The trachea was in the midline and the thyroid
was not enlarged. No abnormal breath or heart sound was noted on
auscultation. No abdominal positive signs were identified. Routine
examination indicated the following: Normal liver and kidney
function, fasting blood glucose of 7.9 mmol/l, blood potassium of
3.1 mmol/l (normal range, 3.5–5.3 mmol/l), carbon dioxide binding
capacity of 35.6 mmol/l (normal range, 22–30 mmol/l) and no
abnormalities in electrocardiogram and chest X-ray. Abdominal
computed tomography indicated slight hyperplasia of the bilateral
adrenal grands and thick insides and thin outsides of limbs. No
retinopathy or diabetic peripheral neuropathy was observed.
On physical examination, GO was identified. The
patient had mild gingival soreness when chewing hard foods, but did
not have any difficulties with mastication. Review of the patient's
medical history revealed the use of oral medicines to control
hypertension. He had started taking a combination of irbesartan, an
angiotensin II receptor antagonist, and felodipine ~4 years
previously, but after ~6 months on this medication, he discontinued
irbesartan due to the increasing incidence of hypopiesia. Since
then, he had taken only felodipine for ~3.5 years. He mentioned
that GO first occurred 8 months previously while he was using
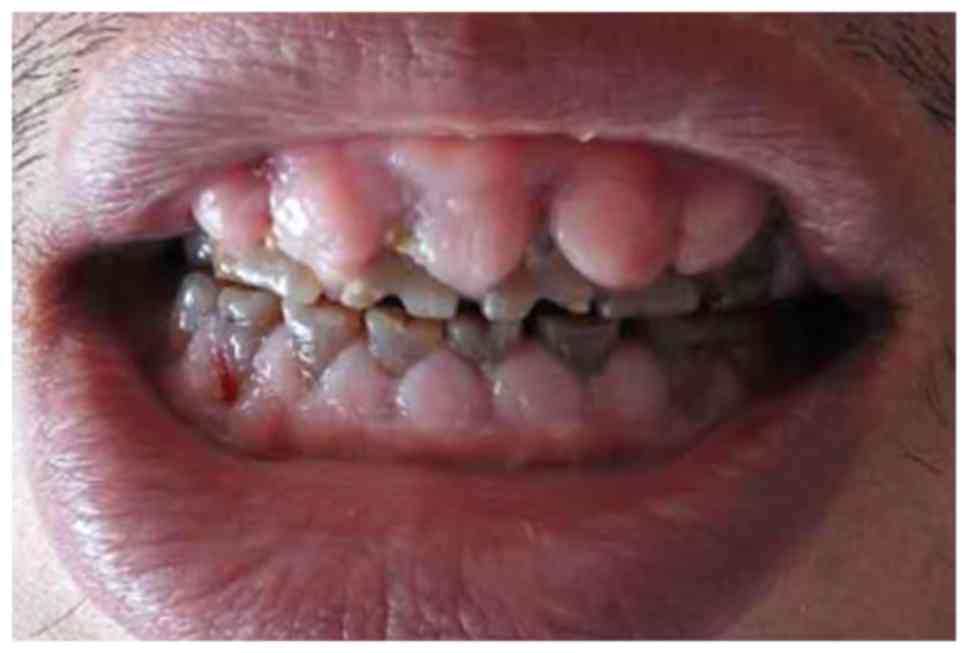
felodipine. Oral examination revealed a mouth opening index of III
and GO throughout all quadrants, which was particularly pronounced
under the dental papilla (Fig. 1).
In addition, the gums bled when probed. All teeth were dark brown
due to poor oral hygiene. Based on the patient's medical history
and consultation with a dental professional, it is likely that GO
in this patient was caused by felodipine. Therefore, felodipine was
first replaced with a combination of spironolactone (a diuretic)
and terazosin (an α-receptor blocker) to control the hypertension.
It was then proposed that the patient retains his original dietary
habits combined with the use of metformin (a hypoglycemic drug) to
reduce blood glucose levels. Finally, the patient was instructed on
methods to improve his oral hygiene, including rinsing of the teeth
after each meal and increasing the frequency of tooth brushing per
day. At the 3-month follow-up, the patient had a blood pressure
ranging from 130/85 to 140/90 mmHg, fasting blood glucose of 7–8
mmol/l and postprandial blood glucose of 9–10 mmol/l. The level of
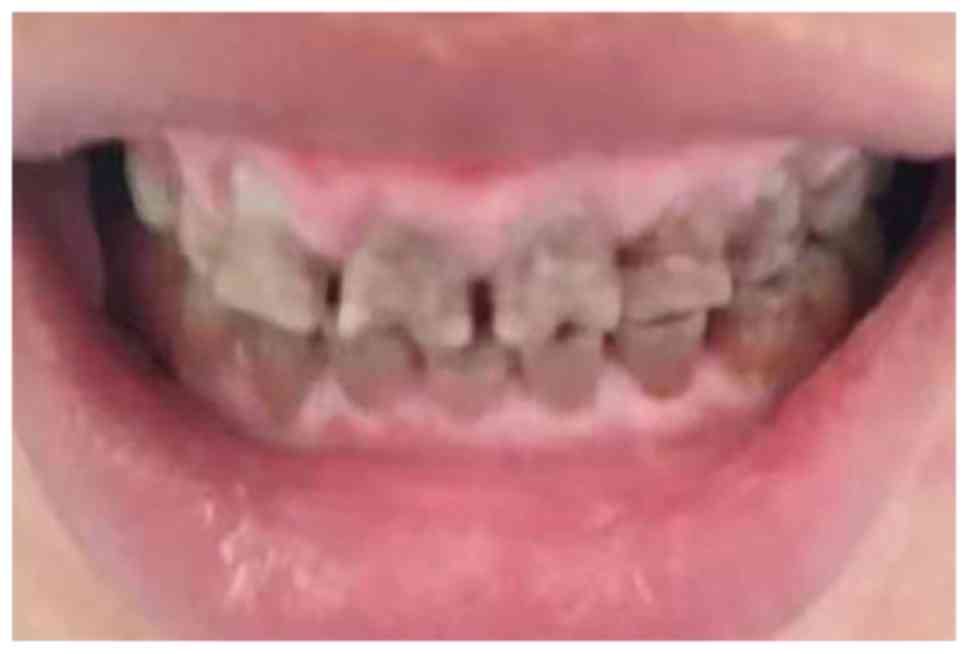
HbA1c had decreased to 7.5%. Although the GO had not been
completely eliminated, the symptoms of tooth soreness disappeared
after the triggers of GO were eliminated (Fig. 2).
Discussion
Felodipine was first reported to cause DIGO in 1991
(27). However to the best of our
knowledge, felodipine-induced GO in a Chinese patient with diabetes
mellitus has not been previously reported. The present study
reports on one case of felodipine-associated GO in a type 2
diabetic patient.
Although it is well established that CCBs are
associated with the development of GO, the exact underlying
mechanisms remain to be fully elucidated. Inflammatory as well as
non-inflammatory pathways have been indicated to be involved in
CCB-induced GO (28). The
non-inflammatory pathway includes the upregulation of keratinocyte
growth factor, which promotes epithelial cell growth in the oral
cavity and matrix synthesis around gingival connected tissues. Such
increased matrix synthesis leads to defective collagenase activity
attributed to the reduced uptake of folic acid, thereby resulting
in the accumulation of connective tissue and development of GO
(28).
On the other hand, the inflammatory pathway also has
an important role in the interaction between drugs and fibroblasts
(28). First, the higher drug
concentration in crevicular gingival fluid, which was identified to
be up to 292-fold of that detected in serum, may have direct toxic
effects. This drug-induced inflammation causes an upregulation of
the levels of several cytokine factors, including fibroblast growth
factor-2, transforming growth factor-β1, interleukin-6 (IL-6),
IL-1B and platelet derived growth factor-β to generate excessive
fibroblasts, which contributes to the development of fibrotic
gingival hyperplasia (28,29). In addition, the pro-inflammatory
cytokines released from inflammatory corpuscles participate in
inflammation-associated local cellular events, including mast cell
migration, fibroblast proliferation, as well as an increase in
extracellular matrix synthesis and decrease in degradation. These
processes may create a vicious cycle to promote the pathogenesis of
DIGO. Mechanistically, the CCB-induced fluctuation in intra- and
extra-cellular calcium levels is mediated via inflammatory
signaling (30). It has been
reported that a high intracellular free Ca2+
concentration potentiates the activity of growth factors and cell
cycle regulators, and thus, promotes cell proliferation and
collagen synthesis (30).
Apart from certain drugs, GO has been reported to be
associated with other factors, including diabetes. For instance,
Van Dis et al (31) reported
on four cases of hyperplastic gingival enlargement in diabetes
mellitus patients and Fay et al (4) reported one case of
felodipine-influenced GO in a patient with uncontrolled type 2
diabetes mellitus. Hence, diabetes mellitus may be regarded as one
of the contributing factors to the development of GO, particularly
CCB-induced GO. The exact mechanisms by which hyperglycemia
contributes to the development of GO remain elusive. It may be
speculated that hyperglycemia is associated with an elevated local
inflammatory response, which may have a role in the pathogenesis of
GO. Furthermore, the clinical manifestations in previously reported
cases of GO included erythematous and edematous hyperplastic
gingival tissues, numerous food sediments and severe periodontitis
(32,33), all of which were accompanied by poor
oral hygiene (34). Therefore, it
may be reasoned that the poor gingival hygienic conditions of the
present case served as another contributing factor to DIGO
(35). However, how these
contributing factors act synergistically remains elusive.
The case of the present study had been diagnosed
with type 2 diabetes 1 year previously and had experienced poor
blood glucose control for 3 days. GO occurred at 8 months prior to
the patient's presentation at the department of Endocrinology and
Metabolism of the First Hospital of Jilin University, and after the
replacement of the angiotensin II receptor antagonist with the CCB
felodipine. In addition, his gingival hygienic condition was poor.
Of note, the degree of severity of GO, drug dosage and the
occurrence time of DIGO were similar to those of a previous case
reported by Fay et al (4),
except for the controlled diabetes mellitus. Hence, it may be
concluded that the gingival hygienic status may be regarded as a
major promoting factor for felodipine-induced DIGO. As mentioned
above, hyperglycemia is likely to be another contributing
factor.
Previously, Fay et al (4) and Khzam et al (35) suggested that surgery and application
of antibiotics should be considered as the second choice for
treating DIGO. Indeed, upon revision of the medication strategy for
the present case, i.e., replacement of the CCB with a diuretic, the
symptoms of gingival soreness were relieved and GO had been
partially eliminated within 3 months. Therefore, removal of the
causative factors, including the improvement of oral hygiene,
control of blood glucose levels, and most importantly, replacement
of the CCB with a different appropriate anti-hypertensive drug,
reduced the clinical symptoms and prevented the recurrence of
lesions. Indeed, the clinical significance of the present case is
that a simple change of the dihydropyridine drug in combination
with improvements in the patient's health care (i.e., controlling
diabetes and oral hygiene) permitted the avoidance of surgical
intervention.
A literature search did not provide any studies that
linked angiotensin II receptor antagonists to the pathogenesis of
GO and this possibility was therefore excluded. The present case
had recurrent hypokalemia and adrenal hyperplasia. After a series
of endocrine system examinations, the patient was determined to
have adrenal hyperplasia. In combination with the presence of
gingival hyperplasia in the present case, the treatment was
switched to aldosterone antagonist spironolactone combined with
terazosin anti-hypertensive drug in order to control blood pressure
and hypokalemia. From the start of the new treatment, the patient's
blood pressure was under control. Trazosin and spironolactone are
not known to be associated with drug-induced gingival hyperplasia.
The patient was advised to return periodically for follow-up with
regard to adrenal hyperplasia and associated endocrine hormone
levels.
The limitations of the present case report should be
acknowledged. For instance, the local concentration of CCB and
glucose around the gingival tissue was not determined and no
histological examination was performed. Furthermore, the glucose
levels in blood vs. gingival interstitial fluid were not compared,
and changes in the gingival tissues by histological examination
prior to and following treatment were not determined. These changes
may be investigated in further intensive studies.
Acknowledgements
Not applicable.
Funding
The present study was sponsored by Jilin Province
Science and Technology Development Plan Project (grant nos.
20160623092TC-03 and 20180623083TC-03).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW and XG contributed a lot to the design of the
study and revision of the manuscript. LS and CW wrote the first
draft of the manuscript. TZ and SX edited this manuscript and
interpreted the data. LS, CW and XG contributed a lot to the
acquisition of the data as well as the revision of the final draft.
All authors have approved the submitted version and agreed to be
accountable for all aspects of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Hospital of Jilin University (Changchun,
China).
Patient consent for publication
The patient provided written informed consent for
the publication of his data and images in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nyska A, Shemesh M, Tal H and Dayan D:
Gingival hyperplasia induced by calcium channel blockers: Mode of
action. Med Hypotheses. 43:115–118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lafzi A, Farahani RM and Shoja MA:
Amlodipine-induced gingival hyperplasia. Med Oral Patol Oral Cir
Bucal. 11:E480–E482. 2006.PubMed/NCBI
|
|
3
|
Joshi S and Bansal S: A rare case report
of amlodipine-induced gingival enlargement and review of its
pathogenesis. Case Rep Dent. 2013:1382482013.PubMed/NCBI
|
|
4
|
Fay AA, Satheesh K and Gapski R:
Felodipine-influenced gingival enlargement in an uncontrolled type
2 diabetic patient. J Periodontol. 76:12172005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sucu M, Yuce M and Davutoglu V:
Amlodipine-induced massive gingival hypertrophy. Can Fam Physician.
57:436–437. 2011.PubMed/NCBI
|
|
6
|
Hallmon WW and Rossmann JA: The role of
drugs in the pathogenesis of gingival overgrowth. A collective
review of current concepts. Periodontol 2000. 21:176–196. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seymour RA, Thomason JM and Ellis JS: The
pathogenesis of drug-induced gingival overgrowth. J Clin
Periodontol. 23:165–175. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ono M, Tanaka S, Takeuchi R, Matsumoto H,
Okada H, Yamamoto H, Makiyama Y, Hirayama T, Sakamaki T, Fujii A
and Akimoto Y: Prevalence of Amlodipine-induced Gingival
Overgrowth. Int J Oral-Med Sci. 9:96–100. 2010. View Article : Google Scholar
|
|
9
|
Hassell TM and Gilbert GH: Phenytoin
sensitivity of fibroblasts as the basis for susceptibility to
gingival enlargement. Am J Pathol. 112:218–223. 1983.PubMed/NCBI
|
|
10
|
Casetta I, Granieri E, Desiderá M, Monetti
VC, Tola MR, Paolino E, Govoni V and Calura G: Phenytoin-induced
gingival overgrowth: A community-based cross-sectional study in
Ferrara, Italy. Neuroepidemiology. 16:296–303. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunsvold M, Tomasovic J and Ruemping D:
The measured effect of phenytoin withdrawal on gingival hyperplasia
in children. ASDC J Dent Child. 52:417–421. 1985.PubMed/NCBI
|
|
12
|
Rateitschak-Plüss EM, Hefti A, Lörtscher R
and Thiel G: Initial observation that cyclosporin-A induces
gingival enlargement in man. J Clin Periodontol. 10:237–246. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pisanty S, Rahamim E, Ben-Ezra D and
Shoshan S: Prolonged systemic administration of cyclosporin A
affects gingival epithelium. J Periodontol. 61:138–141. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daly CG: Resolution of cyclosporin A
(CsA)-induced gingival enlargement following reduction in CsA
dosage. J Clin Periodontol. 19:143–145. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barak S, Engelberg IS and Hiss J: Gingival
hyperplasia caused by nifedipine. Histopathologic findings. J
Periodontol. 58:639–642. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romanos GE, Schröter-Kermani C, Hinz N,
Herrmann D, Strub JR and Bernimoulin JP: Extracellular matrix
analysis of nifedipine-induced gingival overgrowth:
Immunohistochemical distribution of different collagen types as
well as the glycoprotein fibronectin. J Periodontal Res. 28:10–16.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miranda J, Brunet L, Roset P, Berini L,
Farré M and Mendieta C: Prevalence and risk of gingival enlargement
in patients treated with nifedipine. J Periodontol. 72:605–611.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pernu HE, Oikarinen K, Hietanen J and
Knuuttila M: Verapamil-induced gingival overgrowth: A clinical,
histologic, and biochemic approach. J Oral Pathol Med. 18:422–425.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miller CS and Damm DD: Incidence of
verapamil-induced gingival hyperplasia in a dental population. J
Periodontol. 63:453–456. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mehta AV, Chidambaram B and O'Riordan AC:
Verapamil-induced gingival hyperplasia in children. Am Heart J.
124:535–536. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giustiniani S, Robustelli della Cuna F and
Marieni M: Hyperplastic gingivitis during diltiazem therapy. Int J
Cardiol. 15:247–249. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fattore L, Stablein M, Bredfeldt G, Semla
T, Moran M and Doherty-Greenberg JM: Gingival hyperplasia: A side
effect of nifedipine and diltiazem. Spec Care Dentist. 11:107–109.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Godfraind T: Calcium channel blockers in
cardiovascular pharmacotherapy. J Cardiovasc Pharmacol Ther.
19:501–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godfraind T: Mechanisms of action of
calcium entry blockers. Fed Proc. 40:2866–2871. 1981.PubMed/NCBI
|
|
25
|
Soward AL, Vanhaleweyk GL and Serruys PW:
The haemodynamic effects of nifedipine, verapamil and diltiazem in
patients with coronary artery disease. A review. Drugs. 32:66–101.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bose T, Cieślar-Pobuda A and Wiechec E:
Role of ion channels in regulating Ca2+ homeostasis
during the interplay between immune and cancer cells. Cell Death
Dis. 6:e16482015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lombardi T, Fiore-Donno G, Belser U and Di
Felice R: Felodipine-induced gingival hyperplasia: A clinical and
histologic study. J Oral Pathol Med. 20:89–92. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thomas P, Tolstoy R and Duraisingh L:
Amlodipine induced gingival hyperplasia: A case report. Int J Basic
Clin Pharmacol. 805–807. 2015.(In Chinese). View Article : Google Scholar
|
|
29
|
Gong Y, Lu J, Ding X and Yu Y: Effect of
adjunctive roxithromycin therapy on interleukin-1β, transforming
growth factor-β1 and vascular endothelial growth factor in gingival
crevicular fluid of cyclosporine A-treated patients with gingival
overgrowth. J Periodontal Res. 49:448–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsumoto H, Takeuchi R, Ono M, Akimoto Y,
Kobayashi N and Fujii A: Drug-induced gingival overgrowth and its
tentative pharmacotherapy. Jpn Dent Sci Rev. 46:11–16. 2010.
View Article : Google Scholar
|
|
31
|
Van Dis ML, Allen CM and Neville BW:
Erythematous gingival enlargement in diabetic patients: A report of
four cases. J Oral Maxillofac Surg. 46:794–798. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Padmanabhan S and Dwarakanath CD: Severe
gingival enlargement associated with aggressive periodontitis. J
Indian Soc Periodontol. 17:115–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agrawal AA: Gingival enlargements:
Differential diagnosis and review of literature. World J Clin
Cases. 3:779–788. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reali L, Zuliani E, Gabutti L, Schönholzer
C and Marone C: Poor oral hygiene enhances gingival overgrowth
caused by calcineurin inhibitors. J Clin Pharm Ther. 34:255–260.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khzam N, Bailey D, Yie HS and Bakr MM:
Gingival enlargement induced by felodipine resolves with a
conventional periodontal treatment and drug modification. Case Rep
Dent. 2016:10959272016.PubMed/NCBI
|