Introduction
Capsaicin and its related vanilloids have a complex
action on primary sensory neurons with major role in physiology of
pain by detection of high threshold to physical and noxious
chemical stimuli, as the first step in producing the pain session
(1). Initially, capsaicin induces
their activation, characterized by a local burning and stinging
sensation (2), possibly associated
with hyperalgesia and allodynia after exposure to heat and
mechanical stimuli (3). These
nociceptive effects are accompanied by a localized transient
inflammatory response denominated as neurogenic inflammation,
activated by the neuropeptides released from the peripheral sensory
nerve fibers (2,4). In case of subsequent or prolonged
applications of capsaicin, initial excitation is followed by loss
of responsiveness, known as desensitization of nociceptive neurons
(5), which stands at the base of
analgesic/anti-nociceptive effect of topical application of
capsaicin. Low-concentration topical creams, gels, lotions (0.025,
0.075 and 0.1%) and high concentration patches (8%) with capsaicin
were developed to ‘defunctionalize’ cutaneous nociceptors and treat
painful conditions (6). Moreover,
capsaicin further depletes the neuropeptides from the sensory nerve
endings and reduces the initial inflammatory response (7). Capsaicin can also induce a progressive
neurotoxic degeneration of cutaneous nerves when used in high
concentrations or for a long period of time (8,9).
Given its analgesic and anti-nociceptive effect,
capsaicin has been used in the management of neuropathic discomfort
(10–17), post-herpetic neuralgia (18,19),
neuropathy of patients with diabetes and/or HIV (20–25),
burning mouth syndrome (26),
temporomandibular joint disorder (27), chemotherapy-induced peripheral
neuropathy (28) and fibromyalgia
(29). In trials enrolling patients
with osteoarthritis and rheumatoid arthritis, topical application
of capsaicin proved its efficacy and safety as an alternative to
systemic analgesics, which frequently may trigger serious adverse
effects (30,31).
Furthermore, capsaicin-induced local inflammation
can be observed and quantified using laser-Doppler flowmetry
(32) and more recently through
means of in vivo reflectance confocal microscopy (33), suggesting its potential diagnostic
value in various functional alterations of cutaneous sensory nerve
fibers (34,35).
Physicochemical properties of capsaicin
Capsaicin is a natural protoalkaloid and the major
pungent component of hot peppers (Capsicum annuum L.). Also
known as trans−8-methyl-N-vanillyl-6-nonenamide, this
chemical compound is crystalline, off-white solid, lipophilic,
colorless and odorless. It has a melting point of 62–65°C and
though not water soluble, it is soluble in ethanol, acetone, and
fatty oils.
Capsaicin is a member of the vanilloid family of
compounds such as vanillin (derived from vanilla), eugenol
(extracted from bay leaves and cloves), and zingerone (encountered
in ginger) (36,37). Capsaicin shares structural
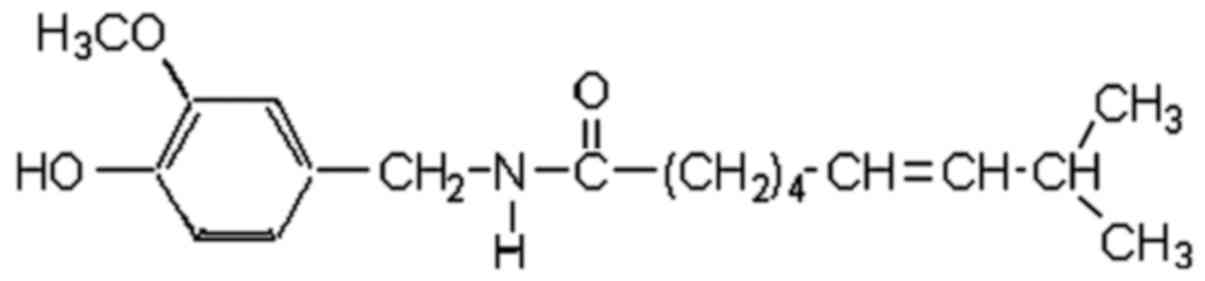
similitudes with other vanilloids, namely an aromatic ring and a
long hydrophobic chain with a polar amide group (Fig. 1).
Capsaicin may also be found in fruits of other
plants belonging to the genus Capsicum (38). In 1816, Bucholtz was the first to
succeed extraction in solution of the pungent hot pepper compound.
In 1846, the name Capsaicine was assigned to this pungent
ingredient by Thresh, who also isolated it in crystalline form.
After identification by Nelson in 1919, Darling and Späth
established a chemical process for its synthesis as a crystalline
compound with hydrophobic/lipophilic, colourless and odourless
properties, in 1930 (39).
Several investigative methods are available for
capsaicinoid analysis, varying from colorimetric photometry, liquid
and gas chromatography, mass spectrometry, nuclear magnetic
resonance, spectroscopy, amperometry, modified capillary
electrophoresis, as well as olfactory electronic sensing (40–48).
High-performance liquid chromatography (HPLC) is
currently employed on the largest scale, as it can provide
satisfactory reliability and accuracy, being preferred by the
American Spice Trade Association (ASTA; Washington, DC, USA) as
well (49).
Recently, several HPLC methodologies for capsaicin
purification were published. The main capsaicinoids from Naga
jolokia peppers were separated using an HPLC method with a C18
reverse-phase fused-core column. The separation was obtained
rapidly with a gradient method with very good repeatability and
precision. This method is suggested also for the separation of
major capsaicinoids from commercial products that have chilli
peppers (50). Using a methodology
with aqueous two-phase system (ATPS) comprising an ethylene
oxide-propylene oxide (EOPO) copolymer, salt and ethanol, capsaicin
was extracted from capsicum oleoresin with a 95.5% yield (51).
For specimens whose concentration of capsaicin
and/or related resins exceeds 700 ppm, identification and analysis
by UV absorption is preferred, whilst for specimens with lower
concentration fluorescence assessment is used.
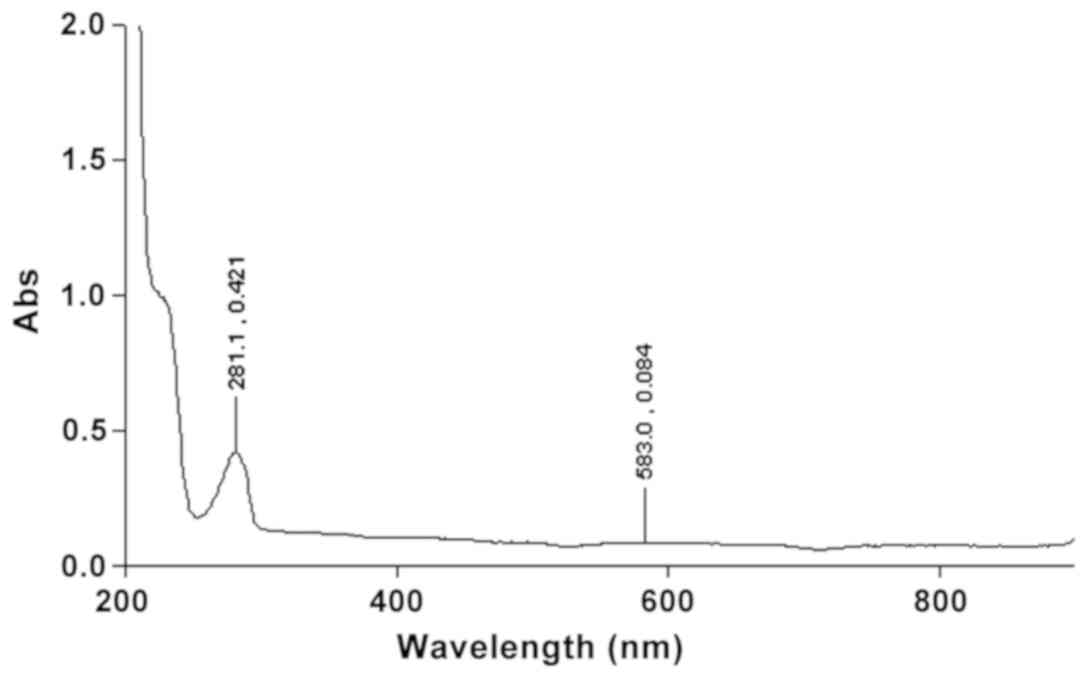
UV-visible spectrophotometry is often highly
sensitive, particularly for analytes with high selectivity for
molar absorptivities. Fig. 2
presents the UV absorption spectrum of Capsicum chinense
Jacq. extract; the wide absorption peaks at 230 and 280 nm are
highly suggestive for capsaicinoids and derived resins.
The capsaicin receptor - structure and
functioning
Capsaicin is able to link to transient receptor
potential vanilloid 1 (TRPV1), mostly present in afferent neural
cells (52–54). The TRPV1 receptor is a protein
consisting of 838 aminoacids, with a molecular weight of 95 kDa
(containing 6 transmembrane areas and belonging to the transient
receptor potential (TRP) family (55). TRP family has three classes:
Canonical, melastatin, and vanilloid, where TRPV1 belongs to the
vanilloid class group (56). TRPV1
has a pore domain created by the fifth and sixth transmembrane
regions, and intracellular N and C termini (57,58).
TRPV1 is a non-selective cation channel with high calcium
permeability (permeability sequence
Ca2+>Mg2+>Na+≈K+≈Cs+)
(59,60).
At intracellular level, TRPV1 is expressed in
several compartments such as in the cytoplasmic membrane,
endoplasmic reticulum (ER), and cytoplasmic vesicles (61). At the membrane level, TRPV1 functions
as a classical receptor that generates the intracellular signaling
cascade when it is activated. TRPV1 is probably stored in the
cytoplasmic vesicles and upon stimulation (e.g., activation of
protein kinase C), can be translocated to the membrane (61). TRPV1 function at the ER level is
still under thorough research. The first findings suggested that
activation of TRPV1 at the ER level increases Ca2+
mobilization from intracellular compartments and they regulate
Ca2+ intracellular homeostasis (62,63).
Recent studies have shown that TRPV1 in ER could be involved in the
ER stress-related apoptotic intracellular signaling pathway in
neurodegenerative disorders (64).
TRPV1 is able to integrate various signals and has
several regulators, activators, inhibitors or even compounds with
dual action on TRPV1 functioning.
This receptor is activated by chemical stimuli, but
also by physical triggers like temperature. It is activated by
vanilloids (65,66) like capsaicin and by several
endogenous ligands [e.g., anandamide (67), reactive metabolites of acetaminophen,
N-arachidonoyl-dopamine (68), lipoxygenase products such as
12-hydroperoxyeicosatetraenoic acid (69)]. Activation can additionally be
induced by capsaicin analogues such as resiniferatoxin, and
agonists like olvanil and camphor (69–75).
The capsaicin receptor is stimulated by temperatures
over 43°C and protons (pH <5.2). Moreover, heat and low pH
sensitize its responses to other activators (76). Inflammatory mediators such as
bradykinin (77) and prostaglandins
prostaglandin E2 (PGE2) and PGI2
(78) have a facilitating effect on
TRPV1. Nerve growth factor (NGF), which is released during
inflammatory processes, can be associated with increased expression
of TRPV1 on nociceptive neurons, and may also act directly on this
receptor, increasing its response to capsaicin (79). There are various other compounds,
such as histamine, serotonin, mannitol, catecholamines, botulinum
neurotoxin type A and ethanol, able to potentiate TRPV1 activity
(80–85). In addition, ATP reduces the
temperature threshold for TRPV1 and increases responses induced by
capsaicin and protons (86). At the
same time, protease-activated receptor 2 (PAR2) agonists, such as
trypsin and mast cell tryptase, sensitize TRPV1, increasing its
response to capsaicin (87).
Inhibitors of these receptors comprise also chemical
and physical factors. Hence, low temperatures strongly inhibit the
activity of the TRPV1 receptor, whereas effects of TRPV1 activation
may be deterred or diminished through action of capsazepine,
receptor's competitive antagonist (88).
Other compounds may have dual action on TRPV1.
Omega-3 fatty acids activate TRPV1 and enhance responses to low pH
on one hand, while they may competitively inhibit vanilloid
agonists' responses on the other hand. Of omega-3 fatty acids,
docosahexaenoic acid mainly acts as a TRPV1 activator, whereas
eicosapentaenoic acid and linolenic acid are primarily inhibitory
(88).
Studies of the effect of phosphatidylinositol
4,5-bisphosphate (PIP2) on TRPV1 have also provided contradictory
results: certain studies have shown PIP2 effect of reducing TRPV1
sensitivity to protons, capsaicin and heat (77), whereas other research suggests the
opposite (89).
One of the intracellular communication pathways may
be induced by TRPV1 sensitization mediated by PKC phosphorylation
of the receptor (90) or PKA
(91). The phosphorylation status of
the channel plays also an important role in receptor
desensitization. Channel dephosphorylation occurs through action of
protein phosphatase 2A (92), and
protein phosphatase 2B, known as calcineurin (93), induces an inhibitory action on TRPV1
receptor activity. In addition, other research suggests that the
calmodulin/Ca2+ complex may be involved in the channel
inactivation process (94,95).
However, many unknowns persist regarding the
function and modulation of TRPV1 activity and further investigation
is of great interest for both scientific research and clinical
practice e.g., in pain control and/or neurodegenerative
disorders.
Expression and roles of the capsaicin
receptor
The capsaicin receptor is highly expressed in the
unmyelinated type C nerve fibers originating from small diameter
sensory neurons in dorsal root ganglia and cranial nerve ganglia
correspondents (96). It can also be
found in the thin myelinated A-delta fibers (97). In adult rats, the majority of neurons
from the dorsal root ganglion are immunoreactive for TRPV1. The
positive marking for TRPV1 has been identified at the level of both
the cell membrane, inducing a cyclic pattern, and intracytoplasmic
structures (98). The peripheral
endings of primary sensory neurons that are positive for TRPV1 also
contain proinflammatory neuropeptides, such as substance P (SP) and
the calcitonin gene-related peptide (CGRP) (4,99),
released as result of activation. Thus, TRPV1 is involved in both
nociception, by integration of various noxious stimuli, and
neurogenic inflammation and inflammatory pain (74,100–102).
TRPV1 may also be found within the spinal cord and
the brain, where it is involved in mediation of the sensation of
pain as well as in thermoregulation (103).
Evidence from murine and human research has shown
that, in addition to the nervous structures, TRPV1 is also present
in other tissues as well, such as skin, adipose tissue,
gastrointestinal tract, pancreatic islets, respiratory mucosa,
urinary bladder, cornea, synoviocytes, myocardium, vascular smooth
muscle, blood mononuclear cells. However, further studies are
needed in order to clarify TRPV1 expression patterns and role in
various tissues (104).
In the skin, the capsaicin receptor may work as an
extraneuronal receptor (105) as it
is also expressed by non-neural structures, such as keratinocytes,
mast cells and dermal blood vessels (7).
In epidermal keratinocytes, activation by capsaicin
induces a calcium influx (106), a
similar effect being observed in human skin fibroblasts (107). Further effects of capsaicin
receptor activation in keratinocytes are the result of intensified
expression of cyclooxygenase-2 (COX-2) and of increased synthesis
of IL-8 and PGE2 (106).
Capsazepine, the antagonist of TRPV1 receptor, reduces the
elevation of intracellular calcium concentration and inhibits the
capsaicin-induced release of these pro-inflammatory mediators.
Thus, keratinocytes, via TRPV1 receptor, appear to be actively
involved in inducing inflammation determined by noxious skin
stimulation. It makes an interesting hypothesis that
pro-inflammatory mediators synthesized and released by
keratinocytes following TRPV1 activation may act on sensory skin
nerve endings (108), enhancing
neurogenic inflammation and nociceptive signaling. Indeed,
PGE2 stimulates capsaicin-induced SP release from
sensory neuron terminals (109) and
prostaglandin synthesis inhibitors are able to reduce the wheal
response produced by capsaicin (110). Moreover, recent research suggests
that increased TRPV1 expression in human skin is involved in the
photo-ageing process (111) and
TRPV1 activation in keratinocytes induces an upregulation of matrix
metalloproteinases leading to an increased breakdown of protein
components of the extracellular matrix (112,113).
Furthermore, capsaicin and TRPV1 have been proposed
to be involved in mast cell activation (114,115)
and histamine-induced pruritus, suggesting an even more complex
role of capsaicin receptor in inflammatory processes (116).
TRPV1 can also be found in skin annex structures
such as hair follicles, sebaceous and sweat glands. In the hair
follicles it participates in modulation of outer root sheath
keratinocytes proliferation, differentiation and apoptosis
(117).
Capsaicin-induced neurogenic
inflammation
The main role in capsaicin-induced neurogenic
inflammation is played by the peripheral endings of small diameter
primary sensory neurons, which are able to release bioactive
substances, thus playing an ‘efferent’ or ‘local effector function’
(118,119). Upon activation, the nociceptive
nerve endings can release SP and CGRP, neurokinin A, neurokinin B,
somatostatin, galanin, corticotropin-releasing hormone, vasoactive
intestinal peptide, and pituitary adenylate cyclase-activating
polypeptide (120). Additionally,
there are other substances such as cytokines and prostaglandins
(106) that may be involved in this
process as well.
Substances released from the nerve endings under the
influence of capsaicin interact with endothelial cells, mast cells,
immune cells and arteriolar smooth muscle cells, causing neurogenic
inflammation characterized by redness, warmth (secondary
vasodilation), swelling (induced by plasma extravasation), and
hyperesthesia (secondary influence of certain sensory neurons
excitability) (121). Mast cells
appear to play an important role in production and expansion of
capsaicin-induced inflammatory reaction. This hypothesis is
supported by the close contacts between mast cells and small
diameter fibers of sensory neurons sensitive to capsaicin, that
were highlighted in a variety of tissues (122), and by the fact that neuropeptides
released by the sensory neurons activated by capsaicin can induce
mast cell degranulation (release of serotonin, proteoglycans and
histamine), as well as synthesis of pro-inflammatory cytokines such
as interleukins, and tumor necrosis factor-α (TNF-α) (123). These mast cell mediators can
further stimulate the release of SP and other peptides from sensory
nerve endings, which can induce a supplementary activation of mast
cells (124). Another piece of
evidence supporting mast cell involvement comes from the important
decrease of capsaicin-induced inflammatory response produced by
inhibitors of mast cell degranulation and by histamine or serotonin
antagonists (110). This
bidirectional autocatalytic loop can amplify the mast cell -
sensory nerve fiber activation, eventually leading to the
well-known wheal and flare reaction.
Other research suggests that, in addition to its
indirect effects via substance P and other neuropeptides, capsaicin
may also exert direct effects on mast cells (114). Capsaicin receptor was identified on
the surface of mast cells, and its activation induces a calcium
influx and subsequent release of pro-inflammatory cytokine IL-4.
Moreover, it can induce mast cell desensitization in case of
further stimulation.
Another contribution to capsaicin-induced
inflammation can be related to its vascular effects. For example,
an in vitro study on human umbilical vein endothelial cells
has shown that capsaicin increases both expression and secretion of
CGRP, a potent vasodilator and this process is mediated by TRPV1
(125).
Capsaicin-induced hyperalgesia
In addition to local inflammation accompanied by the
sensation of pain, hyperalgesia is another possible effect of
capsaicin administration to the skin. Primary hyperalgesia has been
described, occurring on administration area (126–129),
as well as secondary hyperalgesia, arising in adjacent regions
(130). Primary hyperalgesia is
manifested by an exaggerated response to different stimuli, such as
thermal or mechanic (131,132). The mechanisms of primary
hyperalgesia induced by capsaicin are complex and not completely
understood. One of the theories that have tried to explain this
phenomenon argues that the main mechanism directly involved is
sensitization of nociceptive nerve endings that undergo capsaicin
action. There is experimental evidence supporting this hypothesis.
Use of capsaicin on a single type C nerve fiber induces
sensitization of this specific nerve fiber and not of the other
adjacent nerve endings that have not been exposed to capsaicin
(133). These results highlight the
ability of capsaicin to cause direct sensitization of nociceptive
nerve endings.
Secondary hyperalgesia has been described primarily
to mechanical stimuli (129) and is
probably determined by sensitization of dorsal horn neurons in the
spinal cord (134). Another
hypothesis regarding the mechanism of secondary hyperalgesia
production supports the involvement of ‘silent’ nociceptor-free
nerve endings of unmyelinated type C fibers that can respond to
noxious stimuli only after their recruitment through
pro-inflammatory mediators (135).
In addition to activation and sensitization of multimodal
nociceptors, other peripheral nerve mechanisms that may modulate
the sensation of pain and hyperalgesia induced by capsaicin also
include activation of α-adrenergic receptors, local application of
norepinephrine enhancing the painful effects of capsaicin (136).
Involvement of TRPV1/other vanilloid receptors in
inflammation-associated pain is increasingly acknowledged (102,137).
Experimental data show that TRPV1 is required for the thermal
hyperalgesia associated with acute inflammation (102,138).
Hyperalgesia develops in certain pathological conditions
characterized by an increased expression of TRPV1 but the
mechanisms involved are yet revealed only in part (77,139).
For example, in post-inflammatory states or some clinical pain
conditions, the increase in TRPV1 is associated with higher levels
of NGF and glial cell-derived neurotropic factor (GDNF). Moreover,
peripheral production of NGF with an enhanced retrograde transport
of NGF to the neuronal cell body activates p38 MAPK in the primary
neurons, inducing an increased expression of TRPV1 in the
nociceptive nerve endings and further thermal hypersensitivity
(140–144). The occurrence of thermal
hyperalgesia also involves sensitization of existing peripheral
TRPV1 channels by a number of mediators such as protons (during
states of tissue injury or ischemia), prostaglandins, including
PGE2 and PGI2 bradykinin (BK), ATP, and endothelin
(ET)-1, possibly also responsible for the persistent burning pain
often encountered in clinical practice (78,145,146).
Capsaicin-induced desensitization
Mechanisms underlying desensitization and numbness
resulting from treatment with capsaicin are still not well
understood. One possibility is depletion of neuropeptides SP and
CGRP from type C nerve fibers, leading to desensitization of
nociceptors, as capsaicin is known to trigger the release of these
peptides from primary afferent terminals (118,121).
However, electrophysiological studies suggest that initial rapid
desensitization would be the effect of capsaicin on sensory neurons
ion channels.
Capsaicin excites nociceptors by interacting with
its receptor TRPV1 (147), inducing
depolarization of sensory neurons. The membrane permeability to
ions increases, a process involving mainly calcium ion channels
(148,149). This is followed by inactivation of
voltage-gated ion channels, which affects the formation of action
potentials that is possibly responsible for the initial rapid
desensitization and subsequent hypoesthesia. Moreover, capsaicin
may also interfere with formation of action potentials by inducing
mitochondrial ultrastructural alterations in the nociceptive
endings as a consequence of prolonged activation of ion channels
(9).
There are two types of desensitization resulting
from capsaicin application: i) pharmacological desensitization
under repeated or prolonged application of capsaicin, leading to
gradual reduction of subsequent responses to capsaicin; and ii)
functional desensitization, in which capsaicin decreases neuronal
sensitivity to a variety of noxious stimuli (heat, pressure,
chemical irritants, endogenous or exogenous agents). Though often
occurring together, the two phenomena can be separated on very low
capsaicin concentrations. In such circumstances, the ability to
elicit a response to capsaicin is selectively diminished or
abolished, while the response to other stimuli remains unaltered
(150). Functional desensitization
arising on increased concentrations of capsaicin is considered the
foundation for the analgesic and anti-inflammatory effects of
capsaicin.
Although initially stimulating the release of
neuropeptides, capsaicin has a long-term inhibitory effect on the
efferent function of sensory neurons, which may underlie its
analgesic and anti-inflammatory actions. Following capsaicin
application, injurious stimuli no longer trigger the release of
neurotransmitters and neuropeptides, in spite of the nearly normal
levels of neuropeptides in the sensory nerve endings (151–153).
Inflammation arising from the injection of histamine and vasoactive
agents SP, VIP and somatostatin were also reduced in skin
previously treated with capsaicin (151–153).
This long-term inhibitory effect of capsaicin has
also been connected to inhibition of voltage-gated calcium channels
(148,149) at the level of central and
peripheral nerve endings. From animal studies, it appears that the
efferent function of sensory neurons is preferentially inhibited by
capsaicin, suggesting a higher sensitivity to capsaicin for the
mechanism of peptide release as compared to the process of sensory
transmission. Another possible explanation is the involvement of
different nerve fiber subpopulations in this process (154).
Capsaicin neurotoxicity
Capsaicin elicits a wide variety of effects on the
sensory neurons ranging from excitation to conduction blockage
accompanied by reversible ultrastructural changes and going up to
apoptosis and irreversible changes, such as mitochondrial damage
and intracellular release of reactive oxygen species triggering DNA
fragmentation and activation of the caspase cascade (155–157).
Capsaicin-induced neurotoxicity may arise at high
doses, administered systemically or topically (injected
intradermally) (9). The first
studies on capsaicin neurotoxic effects have demonstrated that
systemic administration of high-dose capsaicin in either adult or
new-born rats causes degeneration of a subset of primary afferent
small diameter fibers and their cell bodies (118,158).
In humans, systemic administration of capsaicin was proven to
induce a certain degree of degeneration of the sub-epidermal nerve
plexus (159), indicating the
susceptibility of skin nerve fibers to the neurotoxic effects of
capsaicin. Intradermal injection of capsaicin produces a rapid,
dose-dependent degeneration of epidermal and sub-epidermal nerve
fibers; such degeneration is limited to the injection site and only
to the nerve fibers in direct contact with capsaicin (9). This progressive denervation occurs
during the first two weeks after the injection and can be
highlighted by the loss of protein gene product 9.5 (PGP 9.5)
immunoreactive nerve fibers. For capsaicin in low doses,
denervation is mainly limited to the epidermis, whereas
administration of higher doses determines a complete loss of
epidermal PGP 9.5 immunoreactive nerve fibers and injury of various
degrees of sub-epidermal nerve fibers (9). Moreover, 72 h after capsaicin
injection, a loss of immunoreactivity for CGRP and a decrease of
nerve fibers immunoreactive for SP can be observed. Reduction of
epidermal nerve fibers is associated with a reduced pain sensation
from heat and mechanical stimulation, capsaicin exerting a greater
effect on pain from heat stimulation. The touch threshold is not
significantly modified following injection of capsaicin (9).
Reinnervation of the epidermis begins during the
first 3–4 weeks after capsaicin injection and is characterized by
re-emergence of an intact sub-epidermal nerve plexus, of CGRP
immunoreactive nerve fibers and scarce intraepidermal fibers. At
the same time, one can observe the gradual restoration of pain
sensation induced by heat and mechanical stimuli. Immunoreactivity
for PGP 9.5 is gradually restored and is associated with
progressive regeneration of the sub-epidermal nervous plexus and
with reinnervation of the epidermis, although the number of
regenerated fibers immunoreactive for PGP 9.5 is lower than that of
normal skin, even 4 to 6 weeks following capsaicin injection. The
loss and further reoccurrence of immunoreactivity for PGP 9.5 is
correlated with loss and recovery of somatic sensations (9).
Epidermal nerve fiber degeneration occurs also after
topical administration of capsaicin, although slower and less
intense than that produced by intradermal injection (8). Because of capsaicin capacity to
diffuse, degeneration only develops at the application site and in
the fibers directly exposed to the neurotoxin. Moreover,
degeneration develops progressively, epidermal nerve fibers being
affected 24 h after capsaicin administration, while sub-epidermal
nerve plexus and nerve fibers immunoreactive for CGRP and SP after
1 week. This phenomenon has been well illustrated in a study in
which multiple topical applications of capsaicin caused progressive
degeneration of the nerve fibers in the epidermis (8).
Mechanisms underlying neurotoxicity evoked by
capsaicin were investigated in vitro using cell cultures of
DRG neurons as well as in vivo using murine experimental
models (160). Studies revealed
that capsaicin-induced alterations are caused by both osmotic
changes and alterations of calcium influx levels, inducing
activation of calcium-sensitive proteases (160). Moreover, unlike adult sensory
neurons, the presence of NGF is necessary for the survival of
immature neurons in the dorsal root ganglia and administration of
capsaicin in neonate rats leads to destruction of most primary
nociceptive neurons, probably by disrupting intra-axonal transport
of NGF (155).
Conclusion
Capsaicin, the major pungent ingredient of hot
peppers activates TRPV1 receptor that is widely expressed in the
cutaneous peripheral sensory nerve fibers. At first topical
application, capsaicin induces a local burning sensation,
associated with allodynia and hyperesthesia and a transient
inflammatory response secondary to the release of neuromodulators
from the sensory nerve fibers. The extent of the local inflammatory
reaction can be quantified noninvasively and seems a promising
diagnostic tool in functional alterations of cutaneous sensory
nerve fibers. Repeated applications of capsaicin lead to
desensitization of nociceptive neurons, gradual reduction of the
inflammatory response and further to neurotoxic degeneration of
cutaneous nerve fibers when used in high concentrations. These
effects explain the analgesic/anti-nociceptive and
anti-inflammatory effects of topical capsaicin and its potential
use in the management of painful and inflammatory conditions.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant of the Romanian
National Authority for Scientific Research and Innovation
(CNCS/CCCDI - UEFISCDI, project no. PN-III-P2-2.1-BG-2016-0443,
120BG/01.10.2016, PN-III-P1-1.2-PCCDI-2017-0341) financed by the
Executive Agency for Higher Education, Research, Development and
Innovation, and PN 18.21.02.02/2018 financed by the Ministry of
Research and Innovation (Bucharest Romania).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MAI, CC, MN were responsible for the research
creation and design, data acquisition, analysis and interpretation
of data, statistical analysis and contributed to drafting the
manuscript, and revising it critically for important intellectual
content. MT and SRG contributed to the data acquisition, analysis
and interpretation of data, drafting the manuscript, and revising
it critically for important intellectual content. CM, CC, CN and
RMI were responsible for the analysis and interpretation of data,
statistical analysis, drafting the manuscript, and revising it
critically for important intellectual content. DB contributed to
the research creation and design, analysis and interpretation of
data, drafting the manuscript, and revising it critically for
important intellectual content. All authors agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dubin AE and Patapoutian A: Nociceptors:
The sensors of the pain pathway. J Clin Invest. 120:3760–3772.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Căruntu C, Negrei C, Ghiţă MA, Căruntu A,
Bădărău AI, Buraga I, Boda D, Albu A and Brănişteanu D: Capsaicin,
a hot topic in skin pharmacology and physiology. Farmacia.
63:487–491. 2015.
|
|
3
|
du Jardin KG, Gregersen LS, Røsland T,
Uggerhøj KH, Petersen LJ, Arendt-Nielsen L and Gazerani P:
Assessment of pain response in capsaicin-induced dynamic mechanical
allodynia using a novel and fully automated brushing device. Pain
Res Manag. 18:6–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caruntu C, Boda D, Musat S, Caruntu A,
Poenaru E, Calenic B, Savulescu-Fiedler I, Draghia A, Rotaru M and
Badarau AI: Stress effects on cutaneous nociceptive nerve fibers
and their neurons of origin in rats. Rom Biotechnol Lett.
19:9517–9530. 2014.
|
|
5
|
Szallasi A and Blumberg PM: Vanilloid
(Capsaicin) receptors and mechanisms. Pharmacol Rev. 51:159–212.
1999.PubMed/NCBI
|
|
6
|
Derry S, Rice AS, Cole P, Tan T and Moore
RA: Topical capsaicin (high concentration) for chronic neuropathic
pain in adults. Cochrane Database Syst Rev.
1:CD0073932017.PubMed/NCBI
|
|
7
|
Ständer S, Moormann C, Schumacher M,
Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz
BM, Luger TA, et al: Expression of vanilloid receptor subtype 1 in
cutaneous sensory nerve fibers, mast cells, and epithelial cells of
appendage structures. Exp Dermatol. 13:129–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nolano M, Simone DA, Wendelschafer-Crabb G
and Kennedy WR: Decreased sensation and loss of epidermal nerve
fibers following repeated topical application of capsaicin in
humans. Soc Neurosci Abstr. 22:18021996.
|
|
9
|
Simone DA, Nolano M, Johnson T,
Wendelschafer-Crabb G and Kennedy WR: Intradermal injection of
capsaicin in humans produces degeneration and subsequent
reinnervation of epidermal nerve fibers: Correlation with sensory
function. J Neurosci. 18:8947–8959. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mankowski C, Poole CD, Ernault E, Thomas
R, Berni E, Currie CJ, Treadwell C, Calvo JI, Plastira C,
Zafeiropoulou E, et al: Effectiveness of the capsaicin 8% patch in
the management of peripheral neuropathic pain in European clinical
practice: The ASCEND study. BMC Neurol. 17:802017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burness CB and McCormack PL: Capsaicin 8%
patch: A review in peripheral neuropathic pain. Drugs. 76:123–134.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haanpää M, Cruccu G, Nurmikko TJ, McBride
WT, Docu Axelarad A, Bosilkov A, Chambers C, Ernault E and
Abdulahad AK: Capsaicin 8% patch versus oral pregabalin in patients
with peripheral neuropathic pain. Eur J Pain. 20:316–328. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giménez-Milà M, Videla S, Navarro MA,
Faulí A, Ojeda A, Bogdanovich A, Moreno LA, Hernández-Cera C and
Busquets C: Assessment of the feasibility of high-concentration
capsaicin patches in the pain unit of a tertiary hospital for a
population of mixed refractory peripheral neuropathic pain
syndromes in non-diabetic patients. BMC Anesthesiol. 14:1202014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zis P, Apsokardos A, Isaia C, Sykioti P
and Vadalouca A: Posttraumatic and postsurgical neuropathic pain
responsive to treatment with capsaicin 8% topical patch. Pain
Physician. 17:E213–E218. 2014.PubMed/NCBI
|
|
15
|
Serrano A, Torres D, Veciana M, Caro C,
Montero J and Mayoral V: Quantitative thermal testing profiles as a
predictor of treatment response to topical capsaicin in patients
with localized neuropathic pain. Pain Res Treat.
2017:74259072017.PubMed/NCBI
|
|
16
|
Bauchy F, Mouraux A, Deumens R, Leerink M,
Ulpiano Trillig A, le Polain de Waroux B, Steyaert A, Joëlle QL and
Forget P: Feasibility of topical applications of natural
high-concentration capsaicinoid solutions in patients with
peripheral neuropathic pain: A retrospective analysis. Pain Res
Manag. 2016:97030362016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baranidharan G, Das S and Bhaskar A: A
review of the high-concentration capsaicin patch and experience in
its use in the management of neuropathic pain. Ther Adv Neurol
Disorder. 6:287–297. 2013. View Article : Google Scholar
|
|
18
|
Yong YL, Tan LT, Ming LC, Chan KG, Lee LH,
Goh BH and Khan TM: The effectiveness and safety of topical
capsaicin in postherpetic neuralgia: A systematic review and
meta-analysis. Front Pharmacol. 7:5382017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyd K, Shea SM and Patterson JW: The role
of capsaicin in dermatology. In: Capsaicin as a Therapeutic
Molecule. Springer; Basel: pp. 293–306. 2014, PubMed/NCBI
|
|
20
|
Ostrovsky DA: Single treatment with
capsaicin 8% patch may reduce pain and sleep interference up to 12
weeks in patients with painful diabetic peripheral neuropathy.
Explore (NY). 13:351–353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gálvez R, Navez ML, Moyle G, Maihöfner C,
Stoker M, Ernault E, Nurmikko TJ and Attal N: Capsaicin 8% patch
repeat treatment in nondiabetic peripheral neuropathic pain: A
52-week, open-label, single-arm, safety study. Clin J Pain.
33:921–931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiani J, Ahmad Nasrollahi S, Esna-Ashari
F, Fallah P and Sajedi F: Amitriptyline 2% cream vs. capsaicin
0.75% cream in the treatment of painful diabetic neuropathy (Double
blind, randomized clinical trial of efficacy and safety). Iran J
Pharm Res. 14:1263–1268. 2015.PubMed/NCBI
|
|
23
|
Kulkantrakorn K, Lorsuwansiri C and
Meesawatsom P: 0.025% capsaicin gel for the treatment of painful
diabetic neuropathy: A randomized, double-blind, crossover,
placebo-controlled trial. Pain Pract. 13:497–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown S, Simpson DM, Moyle G, Brew BJ,
Schifitto G, Larbalestier N, Orkin C, Fisher M, Vanhove GF and
Tobias JK: NGX-4010, a capsaicin 8% patch, for the treatment of
painful HIV-associated distal sensory polyneuropathy: Integrated
analysis of two phase III, randomized, controlled trials. AIDS Res
Ther. 10:52013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simpson DM, Brown S, Tobias JK and Vanhove
GF; NGX-4010 C107 Study Group, : NGX-4010, a capsaicin 8% dermal
patch, for the treatment of painful HIV-associated distal sensory
polyneuropathy: Results of a 52-week open-label study. Clin J Pain.
30:134–142. 2014.PubMed/NCBI
|
|
26
|
Feller L, Fourie J, Bouckaert M, Khammissa
RAG, Ballyram R and Lemmer J: Burning mouth syndrome:
Aetiopathogenesis and principles of management. Pain Res Manag.
2017:19262692017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campbell BK, Fillingim RB, Lee S, Brao R,
Price DD and Neubert JK: Effects of high-dose capsaicin on TMD
subjects: A randomized clinical study. JDR Clin Trans Res. 2:58–65.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Filipczak-Bryniarska I, Krzyzewski RM,
Kucharz J, Michalowska-Kaczmarczyk A, Kleja J, Woron J, Strzepek K,
Kazior L, Wordliczek J, Grodzicki T, et al: High-dose 8% capsaicin
patch in treatment of chemotherapy-induced peripheral neuropathy:
Single-center experience. Med Oncol. 34:1622017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casanueva B, Rodero B, Quintial C, Llorca
J and González-Gay MA: Short-term efficacy of topical capsaicin
therapy in severely affected fibromyalgia patients. Rheumatol Int.
33:2665–2670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deal CL, Schnitzer TJ, Lipstein E, Seibold
JR, Stevens RM, Levy MD, Albert D and Renold F: Treatment of
arthritis with topical capsaicin: A double-blind trial. Clin Ther.
13:383–395. 1991.PubMed/NCBI
|
|
31
|
Laslett LL and Jones G: Capsaicin for
osteoarthritis pain. In: Capsaicin as a Therapeutic Molecule.
Springer; Basel: pp. 277–291. 2014, PubMed/NCBI
|
|
32
|
Caselli A, Spallone V, Marfia GA, Battista
C, Pachatz C, Veves A and Uccioli L: Validation of the nerve axon
reflex for the assessment of small nerve fibre dysfunction. J
Neurol Neurosurg Psychiatry. 77:927–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous neurogenic
inflammatory reaction induced by capsaicin in human subjects. J
Biomed Opt. 17:0850032012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Căruntu C, Negrei C, Boda D, Constantin C,
Căruntu A and Neagu M: Biotechnological advances for diagnosis of
peripheral diabetic neuropathy. Rom Biotechnol Lett. 19:9846–9858.
2014.
|
|
35
|
Adriana Ghita M, Caruntu C, Lixandru D,
Pitea A, Batani A and Boda D: The quest for novel biomarkers in
early diagnosis of diabetic neuropathy. Curr Proteomics. 14:86–99.
2017. View Article : Google Scholar
|
|
36
|
Fattori V, Hohmann MS, Rossaneis AC,
Pinho-Ribeiro FA and Verri WA: Capsaicin: Current understanding of
its mechanisms and therapy of pain and other pre-clinical and
clinical uses. Molecules. 21:8442016. View Article : Google Scholar
|
|
37
|
Rollyson WD, Stover CA, Brown KC, Perry
HE, Stevenson CD, McNees CA, Ball JG, Valentovic MA and Dasgupta P:
Bioavailability of capsaicin and its implications for drug
delivery. J Control Release. 196:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reyes-Escogido ML, Gonzalez-Mondragon EG
and Vazquez-Tzompantzi E: Chemical and pharmacological aspects of
capsaicin. Molecules. 16:1253–1270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bode AM and Dong Z: The two faces of
capsaicin. Cancer Res. 71:2809–2814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
North H: Colorimetric determination of
capsaicin in oleoresin of capsicum. Anal Chem. 21:934–936. 1949.
View Article : Google Scholar
|
|
41
|
Hartman KT: A rapid gas-liquid
chromatographic determination for capsaicin in capsicum spices. J
Food Sci. 35:543–547. 1970. View Article : Google Scholar
|
|
42
|
Cooper TH, Guzinski JA and Fisher C:
Improved high-performance liquid chromatography method for the
determination of major capsaicinoids in capsicum oleoresins. J
Agric Food Chem. 39:2253–2256. 1991. View Article : Google Scholar
|
|
43
|
Iwai K, Suzuki T, Fujiwake H and Oka S:
Simultaneous microdetermination of capsaicin and its four analogues
by using high-performance liquid chromatography and gas
chromatography - mass spectrometry. J Chromatogr A. 172:303–311.
1979. View Article : Google Scholar
|
|
44
|
Nyberg NT, Baumann H and Kenne L:
Application of solid-phase extraction coupled to an NMR flow-probe
in the analysis of HPLC fractions. Magn Reson Chem. 39:236–240.
2001. View Article : Google Scholar
|
|
45
|
Nikolaeva DA: Spectrophotometric
determination of capsaicin in peppers (Capsicum annuum L.).
Biokhim. Metody Analiza Plodov; Kishinev: pp. 99–102. 1984
|
|
46
|
Pryakhin OR, Tkach VI, Golovkin VA,
Gladyshev VV and Kuleshova ND: Method for determination of the
total amount of capsaicinoids in thick red pepper extract by
amperometric titration. U.S.S.R. 90:48803301992.
|
|
47
|
Laskaridou-Monnerville A: Determination of
capsaicin and dihydrocapsaicin by micellar electrokinetic capillary
chromatography and its application to various species of
Capsicum, Solanaceae. J Chromatogr A. 838:293–302. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Korel F, Baǧdatlioǧlu N, Balaban MÖ and
Hişil Y: Ground red peppers: Capsaicinoids content, Scoville
scores, and discrimination by an electronic nose. J Agric Food
Chem. 50:3257–3261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Way RM: Official Analytical Methods of the
American SpiceTrade Association. 3. American Spice Trade
Association; Washington, DC: pp. 51–52. 1985
|
|
50
|
Stipcovich T, Barbero GF,
Ferreiro-González M, Palma M and Barroso CG: Fast analysis of
capsaicinoids in Naga Jolokia extracts (Capsicum
chinense) by high-performance liquid chromatography using fused
core columns. Food Chem. 239:217–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fan Y, Lu YM, Yu B, Tan CP and Cui B:
Extraction and purification of capsaicin from capsicum oleoresin
using an aqueous two-phase system combined with chromatography. J
Chromatogr B Analyt Technol Biomed Life Sci. 1063:11–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Darré L and Domene C: Binding of capsaicin
to the TRPV1 ion channel. Mol Pharm. 12:4454–4465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Srinivasan K: Biological activities of red
pepper (Capsicum annuum) and its pungent principle
capsaicin: A review. Crit Rev Food Sci Nutr. 56:1488–1500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Clapham DE: TRP channels as cellular
sensors. Nature. 426:517–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Szolcsányi J and Jancsó-Gábor A: Sensory
effects of capsaicin congeners I. Relationship between chemical
structure and pain-producing potency of pungent agents.
Arzneimittelforschung. 25:1877–1881. 1975.PubMed/NCBI
|
|
56
|
Montell C, Birnbaumer L and Flockerzi V:
The TRP channels, a remarkably functional family. Cell.
108:595–598. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ferrer-Montiel A, García-Martínez C,
Morenilla-Palao C, García-Sanz N, Fernández-Carvajal A,
Fernández-Ballester G and Planells-Cases R: Molecular architecture
of the vanilloid receptor. Insights for drug design. Eur J Biochem.
271:1820–1826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
García-Sanz N, Fernández-Carvajal A,
Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E,
Fernández-Ballester G and Ferrer-Montiel A: Identification of a
tetramerization domain in the C terminus of the vanilloid receptor.
J Neurosci. 24:5307–5314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Song S, Ayon RJ, Yamamura A, Yamamura H,
Dash S, Babicheva A, Tang H, Sun X, Cordery AG, Khalpey Z, et al:
Capsaicin-induced Ca2+ signaling is enhanced via
upregulated TRPV1 channels in pulmonary artery smooth muscle cells
from patients with idiopathic PAH. Am J Physiol Lung Cell Mol
Physiol. 312:L309–L325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Caterina MJ and Julius D: The vanilloid
receptor: A molecular gateway to the pain pathway. Annu Rev
Neurosci. 24:487–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Morenilla-Palao C, Planells-Cases R,
García-Sanz N and Ferrer-Montiel A: Regulated exocytosis
contributes to protein kinase C potentiation of vanilloid receptor
activity. J Biol Chem. 279:25665–25672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kárai LJ, Russell JT, Iadarola MJ and Oláh
Z: Vanilloid receptor 1 regulates multiple calcium compartments and
contributes to Ca2+-induced Ca2+ release in
sensory neurons. J Biol Chem. 279:16377–16387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Marshall IC, Owen DE, Cripps TV, Davis JB,
McNulty S and Smart D: Activation of vanilloid receptor 1 by
resiniferatoxin mobilizes calcium from inositol
1,4,5-trisphosphate-sensitive stores. Br J Pharmacol. 138:172–176.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vrechi TA, Crunfli F, Costa AP and Torrão
AS: Cannabinoid receptor type 1 agonist ACEA protects neurons from
death and attenuates endoplasmic reticulum stress-related apoptotic
pathway signaling. Neurotox Res. 33:846–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Van Der Stelt M and Di Marzo V:
Endovanilloids. Putative endogenous ligands of transient receptor
potential vanilloid 1 channels. Eur J Biochem. 271:1827–1834. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim SR, Lee DY, Chung ES, Oh UT, Kim SU
and Jin BK: Transient receptor potential vanilloid subtype 1
mediates cell death of mesencephalic dopaminergic neurons in vivo
and in vitro. J Neurosci. 25:662–671. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Smart D, Gunthorpe MJ, Jerman JC, Nasir S,
Gray J, Muir AI, Chambers JK, Randall AD and Davis JB: The
endogenous lipid anandamide is a full agonist at the human
vanilloid receptor (hVR1). Br J Pharmacol. 129:227–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Marinelli S, Di Marzo V, Florenzano F,
Fezza F, Viscomi MT, van der Stelt M, Bernardi G, Molinari M,
Maccarrone M and Mercuri NB: N-arachidonoyl-dopamine tunes synaptic
transmission onto dopaminergic neurons by activating both
cannabinoid and vanilloid receptors. Neuropsychopharmacology.
32:298–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ,
Jung J, Cho S, Min KH, Suh YG, Kim D, et al: Direct activation of
capsaicin receptors by products of lipoxygenases: Endogenous
capsaicin-like substances. Proc Natl Acad Sci USA. 97:6155–6160.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Eberhardt MJ, Schillers F, Eberhardt EM,
Risser L, de la Roche J, Herzog C, Echtermeyer F and Leffler A:
Reactive metabolites of acetaminophen activate and sensitize the
capsaicin receptor TRPV1. Sci Rep. 7:127752017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Smutzer G and Devassy RK: Integrating
TRPV1 receptor function with capsaicin psychophysics. Adv Pharmacol
Sci. 2016:15124572016.PubMed/NCBI
|
|
72
|
Elokely K, Velisetty P, Delemotte L,
Palovcak E, Klein ML, Rohacs T and Carnevale V: Understanding TRPV1
activation by ligands: Insights from the binding modes of capsaicin
and resiniferatoxin. Proc Natl Acad Sci USA. 113:E137–E145. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nagy I, Friston D, Valente JS, Torres
Perez JV and Andreou AP: Pharmacology of the capsaicin receptor,
transient receptor potential vanilloid type-1 ion channel. Prog
Drug Res. 68:39–76. 2014.PubMed/NCBI
|
|
74
|
Tominaga M, Caterina MJ, Malmberg AB,
Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI and Julius
D: The cloned capsaicin receptor integrates multiple pain-producing
stimuli. Neuron. 21:531–543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Moreira FA, Aguiar DC, Terzian AL,
Guimarães FS and Wotjak CT: Cannabinoid type 1 receptors and
transient receptor potential vanilloid type 1 channels in fear and
anxiety-two sides of one coin? Neuroscience. 204:186–192. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ryu S, Liu B and Qin F: Low pH potentiates
both capsaicin binding and channel gating of VR1 receptors. J Gen
Physiol. 122:45–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chuang HH, Prescott ED, Kong H, Shields S,
Jordt SE, Basbaum AI, Chao MV and Julius D: Bradykinin and nerve
growth factor release the capsaicin receptor from
PtdIns(4,5)P2-mediated inhibition. Nature. 411:957–962. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Moriyama T, Higashi T, Togashi K, Iida T,
Segi E, Sugimoto Y, Tominaga T, Narumiya S and Tominaga M:
Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive
mechanism of prostaglandins. Mol Pain. 1:32005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang X, Huang J and McNaughton PA: NGF
rapidly increases membrane expression of TRPV1 heat-gated ion
channels. EMBO J. 24:4211–4223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nakagawa H and Hiura A: Four possible
itching pathways related to the TRPV1 channel, histamine, PAR-2 and
serotonin. Malays J Med Sci. 20:5–12. 2013.PubMed/NCBI
|
|
81
|
Bertrand H, Kyriazis M, Reeves KD, Lyftogt
J and Rabago D: Topical mannitol reduces capsaicin-induced pain:
Results of a pilot-level, double-blind, randomized controlled
trial. PM R. 7:1111–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Luvisetto S, Vacca V and Cianchetti C:
Analgesic effects of botulinum neurotoxin type A in a model of
allyl isothiocyanate- and capsaicin-induced pain in mice. Toxicon.
94:23–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matak I, Rossetto O and Lacković Z:
Botulinum toxin type A selectivity for certain types of pain is
associated with capsaicin-sensitive neurons. Pain. 155:1516–1526.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Arout CA, Perrino AC Jr, Ralevski E,
Acampora G, Koretski J, Limoncelli D, Newcomb J and Petrakis IL:
Effect of intravenous ethanol on capsaicin-induced hyperalgesia in
human subjects. Alcohol Clin Exp Res. 40:1425–1429. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Filippi A, Caruntu C, Gheorghe RO, Deftu
A, Amuzescu B and Ristoiu V: Catecholamines reduce transient
receptor potential vanilloid type 1 desensitization in cultured
dorsal root ganglia neurons. J Physiol Pharmacol. 67:843–850.
2016.PubMed/NCBI
|
|
86
|
Tominaga M, Wada M and Masu M:
Potentiation of capsaicin receptor activity by metabotropic ATP
receptors as a possible mechanism for ATP-evoked pain and
hyperalgesia. Proc Natl Acad Sci USA. 98:6951–6956. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Amadesi S, Nie J, Vergnolle N, Cottrell
GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes
H, et al: Protease-activated receptor 2 sensitizes the capsaicin
receptor transient receptor potential vanilloid receptor 1 to
induce hyperalgesia. J Neurosci. 24:4300–4312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Matta JA, Miyares RL and Ahern GP: TRPV1
is a novel target for omega-3 polyunsaturated fatty acids. J
Physiol. 578:397–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sowa NA, Street SE, Vihko P and Zylka MJ:
Prostatic acid phosphatase reduces thermal sensitivity and chronic
pain sensitization by depleting phosphatidylinositol
4,5-bisphosphate. J Neurosci. 30:10282–10293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Premkumar LS and Ahern GP: Induction of
vanilloid receptor channel activity by protein kinase C. Nature.
408:985–990. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bhave G, Zhu W, Wang H, Brasier DJ, Oxford
GS and Gereau RW IV: cAMP-dependent protein kinase regulates
desensitization of the capsaicin receptor (VR1) by direct
phosphorylation. Neuron. 35:721–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang X, Wu J, Fang L and Willis WD: The
effects of protein phosphatase inhibitors on the duration of
central sensitization of rat dorsal horn neurons following
injection of capsaicin. Mol Pain. 2:232006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Por ED, Samelson BK, Belugin S, Akopian
AN, Scott JD and Jeske NA: PP2B/calcineurin-mediated
desensitization of TRPV1 does not require AKAP150. Biochem J.
432:549–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Numazaki M, Tominaga T, Takeuchi K,
Murayama N, Toyooka H and Tominaga M: Structural determinant of
TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci
USA. 100:8002–8006. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pecze L, Blum W and Schwaller B: Mechanism
of capsaicin receptor TRPV1-mediated toxicity in pain-sensing
neurons focusing on the effects of Na(+)/Ca(2+) fluxes and the
Ca(2+)-binding protein calretinin. Biochim Biophys Acta.
1833:1680–1691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kobayashi K, Fukuoka T, Obata K, Yamanaka
H, Dai Y, Tokunaga A and Noguchi K: Distinct expression of TRPM8,
TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with
adelta/c-fibers and colocalization with trk receptors. J Comp
Neurol. 493:596–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lumpkin EA and Caterina MJ: Mechanisms of
sensory transduction in the skin. Nature. 445:858–865. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hong S, Morrow TJ, Paulson PE, Isom LL and
Wiley JW: Early painful diabetic neuropathy is associated with
differential changes in tetrodotoxin-sensitive and -resistant
sodium channels in dorsal root ganglion neurons in the rat. J Biol
Chem. 279:29341–29350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Michael GJ and Priestley JV: Differential
expression of the mRNA for the vanilloid receptor subtype 1 in
cells of the adult rat dorsal root and nodose ganglia and its
downregulation by axotomy. J Neurosci. 19:1844–1854. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chung MK and Campbell JN: Use of capsaicin
to treat pain: Mechanistic and therapeutic considerations.
Pharmaceuticals (Basel). 9:662016. View Article : Google Scholar
|
|
101
|
Davis JB, Gray J, Gunthorpe MJ, Hatcher
JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson
K, et al: Vanilloid receptor-1 is essential for inflammatory
thermal hyperalgesia. Nature. 405:183–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Julius D and Basbaum AI: Molecular
mechanisms of nociception. Nature. 413:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mezey E, Tóth ZE, Cortright DN, Arzubi MK,
Krause JE, Elde R, Guo A, Blumberg PM and Szallasi A: Distribution
of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like
immunoreactivity, in the central nervous system of the rat and
human. Proc Natl Acad Sci USA. 97:3655–3660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fernandes ES, Fernandes MA and Keeble JE:
The functions of TRPA1 and TRPV1: Moving away from sensory nerves.
Br J Pharmacol. 166:510–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Roosterman D, Goerge T, Schneider SW,
Bunnett NW and Steinhoff M: Neuronal control of skin function: The
skin as a neuroimmunoendocrine organ. Physiol Rev. 86:1309–1379.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Southall MD, Li T, Gharibova LS, Pei Y,
Nicol GD and Travers JB: Activation of epidermal vanilloid
receptor-1 induces release of proinflammatory mediators in human
keratinocytes. J Pharmacol Exp Ther. 304:217–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kim SJ, Lee SA, Yun SJ, Kim JK, Park JS,
Jeong HS, Lee JH, Moon SJ and Won YH: Expression of vanilloid
receptor 1 in cultured fibroblast. Exp Dermatol. 15:362–367. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Treede RD, Meyer RA, Raja SN and Campbell
JN: Peripheral and central mechanisms of cutaneous hyperalgesia.
Prog Neurobiol. 38:397–421. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Southall MD and Vasko MR: Prostaglandin
receptor subtypes, EP3C and EP4, mediate the prostaglandin
E2-induced cAMP production and sensitization of sensory neurons. J
Biol Chem. 276:16083–16091. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gábor M and Rázga Z: Development and
inhibition of mouse ear oedema induced with capsaicin. Agents
Actions. 36:83–86. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lee YM, Kim YK and Chung JH: Increased
expression of TRPV1 channel in intrinsically aged and photoaged
human skin in vivo. Exp Dermatol. 18:431–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lee YM, Kang SM and Chung JH: The role of
TRPV1 channel in aged human skin. J Dermatol Sci. 65:81–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lee YM, Kim YK, Kim KH, Park SJ, Kim SJ
and Chung JH: A novel role for the TRPV1 channel in UV-induced
matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J Cell
Physiol. 219:766–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bíró T, Maurer M, Modarres S, Lewin NE,
Brodie C, Acs G, Acs P, Paus R and Blumberg PM: Characterization of
functional vanilloid receptors expressed by mast cells. Blood.
91:1332–1340. 1998.PubMed/NCBI
|
|
115
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1059502014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo
JY, Lee CH, Kim M and Oh U: TRPV1 mediates histamine-induced
itching via the activation of phospholipase A2 and 12-lipoxygenase.
J Neurosci. 27:2331–2337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bodó E, Bíró T, Telek A, Czifra G, Griger
Z, Tóth BI, Mescalchin A, Ito T, Bettermann A, Kovács L, et al: A
hot new twist to hair biology: Involvement of vanilloid receptor-1
(VR1/TRPV1) signaling in human hair growth control. Am J Pathol.
166:985–998. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Holzer P: Local effector functions of
capsaicin-sensitive sensory nerve endings: Involvement of
tachykinins, calcitonin gene-related peptide and other
neuropeptides. Neuroscience. 24:739–768. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Richardson JD and Vasko MR: Cellular
mechanisms of neurogenic inflammation. J Pharmacol Exp Ther.
302:839–845. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Birklein F and Schmelz M: Neuropeptides,
neurogenic inflammation and complex regional pain syndrome (CRPS).
Neurosci Lett. 437:199–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Maggi CA and Meli A: The sensory-efferent
function of capsaicin-sensitive sensory neurons. Gen Pharmacol.
19:1–43. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Botchkarev VA, Eichmüller S, Peters EM,
Pietsch P, Johansson O, Maurer M and Paus R: A simple
immunofluorescence technique for simultaneous visualization of mast
cells and nerve fibers reveals selectivity and hair cycle-dependent
changes in mast cell - nerve fiber contacts in murine skin. Arch
Dermatol Res. 289:292–302. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ansel JC, Brown JR, Payan DG and Brown MA:
Substance P selectively activates TNF-alpha gene expression in
murine mast cells. J Immunol. 150:4478–4485. 1993.PubMed/NCBI
|
|
124
|
Kowalski ML and Kaliner MA: Neurogenic
inflammation, vascular permeability, and mast cells. J Immunol.
140:3905–3911. 1988.PubMed/NCBI
|
|
125
|
Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ,
Li D, Deng HW and Li YJ: Transient receptor potential vanilloid
1-mediated expression and secretion of endothelial cell-derived
calcitonin gene-related peptide. Regul Pept. 150:66–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Price RC, Gandhi W, Nadeau C, Tarnavskiy
R, Qu A, Fahey E, Stone L and Schweinhardt P: Characterization of a
novel capsaicin/heat ongoing pain model. Eur J Pain. 22:370–384.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Szolcsányi J: Capsaicin and sensory
neurones: A historical perspective. In: Capsaicin as a Therapeutic
Molecule. Springer; Basel: pp. 1–37. 2014
|
|
128
|
Simone DA, Ngeow JY, Putterman GJ and
LaMotte RH: Hyperalgesia to heat after intradermal injection of
capsaicin. Brain Res. 418:201–203. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
LaMotte RH, Shain CN, Simone DA and Tsai
EF: Neurogenic hyperalgesia: Psychophysical studies of underlying
mechanisms. J Neurophysiol. 66:190–211. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Torebjörk HE, Lundberg LE and LaMotte RH:
Central changes in processing of mechanoreceptive input in
capsaicin-induced secondary hyperalgesia in humans. J Physiol.
448:765–780. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Simone DA and Ochoa J: Early and late
effects of prolonged topical capsaicin on cutaneous sensibility and
neurogenic vasodilatation in humans. Pain. 47:285–294. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Carpenter SE and Lynn B: Vascular and
sensory responses of human skin to mild injury after topical
treatment with capsaicin. Br J Pharmacol. 73:755–758. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Schmelz M, Schmid R, Handwerker HO and
Torebjörk HE: Encoding of burning pain from capsaicin-treated human
skin in two categories of unmyelinated nerve fibres. Brain.
123:560–571. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Simone DA, Baumann TK and LaMotte RH:
Dose-dependent pain and mechanical hyperalgesia in humans after
intradermal injection of capsaicin. Pain. 38:99–107. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Serra J, Campero M and Ochoa J: Flare and
hyperalgesia after intradermal capsaicin injection in human skin. J
Neurophysiol. 80:2801–2810. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kinnman E, Nygårds EB and Hansson P:
Peripheral α-adrenoreceptors are involved in the development of
capsaicin induced ongoing and stimulus evoked pain in humans. Pain.
69:79–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ma XL, Zhang FX, Dong F, Bao L and Zhang
X: Experimental evidence for alleviating nociceptive
hypersensitivity by single application of capsaicin. Mol Pain.
11:222015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
White JPM, Urban L and Nagy I: TRPV1
function in health and disease. Curr Pharm Biotechnol. 12:130–144.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Amaya F, Shimosato G, Nagano M, Ueda M,
Hashimoto S, Tanaka Y, Suzuki H and Tanaka M: NGF and GDNF
differentially regulate TRPV1 expression that contributes to
development of inflammatory thermal hyperalgesia. Eur J Neurosci.
20:2303–2310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Urban L, White JPM and Nagy I: Molecular
structure of transient receptor potential vanilloid type 1 ion
channel (TRPV1). Curr Pharm Biotechnol. 12:115–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tympanidis P, Casula MA, Yiangou Y,
Terenghi G, Dowd P and Anand P: Increased vanilloid receptor VR1
innervation in vulvodynia. Eur J Pain. 8:129–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yilmaz Z, Renton T, Yiangou Y, Zakrzewska
J, Chessell IP, Bountra C and Anand P: Burning mouth syndrome as a
trigeminal small fibre neuropathy: Increased heat and capsaicin
receptor TRPV1 in nerve fibres correlates with pain score. J Clin
Neurosci. 14:864–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Haanpää M and Treede RD: Capsaicin for
neuropathic pain: Linking traditional medicine and molecular
biology. Eur Neurol. 68:264–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ji RR, Samad TA, Jin SX, Schmoll R and
Woolf CJ: p38 MAPK activation by NGF in primary sensory neurons
after inflammation increases TRPV1 levels and maintains heat
hyperalgesia. Neuron. 36:57–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Jordt SE, Tominaga M and Julius D: Acid
potentiation of the capsaicin receptor determined by a key
extracellular site. Proc Natl Acad Sci USA. 97:8134–8139. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Huang J, Zhang X and McNaughton PA:
Inflammatory pain: The cellular basis of heat hyperalgesia. Curr
Neuropharmacol. 4:197–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Szallasi A and Blumberg PM: Specific
binding of resiniferatoxin, an ultrapotent capsaicin analog, by
dorsal root ganglion membranes. Brain Res. 524:106–111. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bleakman D, Brorson JR and Miller RJ: The
effect of capsaicin on voltage-gated calcium currents and calcium
signals in cultured dorsal root ganglion cells. Br J Pharmacol.
101:423–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Docherty RJ, Robertson B and Bevan S:
Capsaicin causes prolonged inhibition of voltage-activated calcium
currents in adult rat dorsal root ganglion neurons in culture.
Neuroscience. 40:513–521. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dray A, Bettaney J and Forster P: Actions
of capsaicin on peripheral nociceptors of the neonatal rat spinal
cord-tail in vitro: Dependence of extracellular ions and
independence of second messengers. Br J Pharmacol. 101:727–733.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Anand P, Bloom SR and McGregor GP: Topical
capsaicin pretreatment inhibits axon reflex vasodilatation caused
by somatostatin and vasoactive intestinal polypeptide in human
skin. Br J Pharmacol. 78:665–669. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Bjerring P and Arendt-Nielsen L:
Inhibition of histamine skin flare reaction following repeated
topical applications of capsaicin. Allergy. 45:121–125. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Tóth-Kása I, Jancsó G, Bognár A, Husz S
and Obál F Jr: Capsaicin prevents histamine-induced itching. Int J
Clin Pharmacol Res. 6:163–169. 1986.PubMed/NCBI
|
|
154
|
Winter J, Bevan S and Campbell EA:
Capsaicin and pain mechanisms. Br J Anaesth. 75:157–168. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hartel M, di Mola FF, Selvaggi F, Mascetta
G, Wente MN, Felix K, Giese NA, Hinz U, Di Sebastiano P, Büchler
MW, et al: Vanilloids in pancreatic cancer: Potential for
chemotherapy and pain management. Gut. 55:519–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Shin CY, Shin J, Kim BM, Wang MH, Jang JH,
Surh YJ and Oh U: Essential role of mitochondrial permeability
transition in vanilloid receptor 1-dependent cell death of sensory
neurons. Mol Cell Neurosci. 24:57–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Athanasiou A, Smith PA, Vakilpour S,
Kumaran NM, Turner AE, Bagiokou D, Layfield R, Ray DE, Westwell AD,
Alexander SP, et al: Vanilloid receptor agonists and antagonists
are mitochondrial inhibitors: How vanilloids cause non-vanilloid
receptor mediated cell death. Biochem Biophys Res Commun.
354:50–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Buck SH and Burks TF: The
neuropharmacology of capsaicin: Review of some recent observations.
Pharmacol Rev. 38:179–226. 1986.PubMed/NCBI
|
|
159
|
Chung K, Klein CM and Coggeshall RE: The
receptive part of the primary afferent axon is most vulnerable to
systemic capsaicin in adult rats. Brain Res. 511:222–226. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wood JN, Coote PR, Minhas A, Mullaney I,
McNeill M and Burgess GM: Capsaicin-induced ion fluxes increase
cyclic GMP but not cyclic AMP levels in rat sensory neurones in
culture. J Neurochem. 53:1203–1211. 1989. View Article : Google Scholar : PubMed/NCBI
|