Introduction
Various forms of chronic hepatic disease can cause
hepatic stellate cell (HSC) activation and induce liver fibrosis,
an early step in the progression of liver cirrhosis, which can
eventually develop into hepatocellular carcinoma. Notably, these
types of hepatic disease are considered a major global health
concern (1). Liver fibrosis is a
common pathological process, which can eventually lead to liver
failure (2). Liver fibrosis is
defined as the remodeling and excessive deposition of extracellular
matrix (ECM) proteins in the liver (3). The activation of HSC is an important
event in the process of liver fibrosis (4). Activated HSCs are commonly regarded as
major producers of fibrotic ECM proteins (5), which include collagen type I and
fibronectin. Transforming growth factor β1 (TGFβ1) is known as the
strongest effector in liver fibrosis, which acts as a major
pro-fibrotic cytokine, promoting fibroblast recruitment,
proliferation and differentiation into ECM-producing myofibroblasts
(6). There are several known
signaling pathways involved in the progression of liver fibrosis.
TGFβ1 induces liver fibrosis through Smad-dependent and
Smad-independent pathways (7), which
includes the TGFβ1/ERK1/2 signaling pathway (8). The ERK1/2 pathway has been reported to
be important in HSC proliferation and activation via crosstalk with
other signaling pathways (9).
Notably, a previous study indicated that inhibition of the ERK1/2
signaling pathway enhanced liver fibrosis in rats (10). In addition, it has been demonstrated
that the progression of liver fibrosis is reversible (11–14).
There are several therapeutic agents, including Orlistat, Metformin
and Candesartan, typically used for anti-fibrotic therapy (15,16).
However, the major limitation is the lack of effective treatment
strategies for liver fibrosis (17,18).
Therefore, novel and effective therapeutic targets are required for
the treatment of liver fibrosis.
Puerarin is a Chinese herb, which is known to
possess several physiological activities, including anti-oxidative
and anti-inflammatory activity (19,20).
Puerarin has been widely used in the treatment of various diseases,
including ischemic stroke (21),
renal fibrosis (22), myocardial
ischemia and hypertension (23). In
addition, puerarin is also considered to have a therapeutic effect
in liver fibrosis. Previous studies have demonstrated that puerarin
can attenuate liver fibrosis by regulating the expression of TGFβ1
and inhibiting tumor necrosis factor-α (TNF-α)/nuclear factor κB
subunit 1 (NF-κB) (24),
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt (25) and TGFβ1/Smad (26) signaling pathways. However, whether
puerarin reduces liver fibrosis via the ERK1/2 signaling pathway to
inhibit the activation of HSCs and excessive collagen deposition in
liver fibrosis remains unknown.
Thioacetamide (TAA) is a thiono-sulfur containing
compound, which is widely used to induce liver fibrosis in rats to
investigate the underlying mechanisms and therapeutic effects of
potential anti-fibrotic drugs (27).
Therefore, the aim of the current study was to examine the
anti-fibrotic efficacy and underlying mechanism of puerarin in
TAA-induced liver fibrosis in rats. In addition, changes to the
relative protein expression levels of TGFβ1, α-smooth muscle actin
(α-SMA), collagen type I, fibronectin, p-ERK1/2 and ERK1/2 were
analyzed.
Materials and methods
Experimental animals and
treatments
A total of 36 male Sprague-Dawley rats (age, 6–8
weeks; weight, 180~200 g) were obtained from Shanxi Medical
University Laboratory Animal Center (Taiyuan, China) and used to
establish the liver fibrosis in vivo model by
intraperitoneal injection of 200 mg/kg thioacetamide (TAA:
Sigma-Aldrich; Merck KGaA). Rats were housed in an environmentally
controlled breeding room (12-h dark/light cycle; temperature,
25±1°C; humidity, 55±5%) and fed rodent laboratory chow and sterile
water. Rats were randomly divided into three groups: Control, TAA
and puerarin/TAA group. Rats in the control group received the same
dose of PBS once daily (n=12). Rats in the TAA group were injected
with 200 mg/kg TAA three times/week for 8 weeks (n=12). Rats in the
puerarin/TAA group were injected with TAA like those in the TAA
group, but they also received a daily dose of 0.1 ml/10 g puerarin
during week 5 at the same time as TAA administration (n=12). All
rats were anesthetized by intraperitoneal injection of chloral
hydrate (400 mg/kg) and sacrificed at week 8. Liver tissues were
harvested and stored at −80°C for further analysis. All animal
experiment protocols used in the present study followed
internationally accepted principles and were approved by The
Institutional Animal Care and Use Committee of Shanxi Medical
University (Taiyuan, China).
Immunohistochemistry
Liver tissue was fixed in 10% neutral buffered
formalin for one week at 25–27°C, dehydrated in a 70–100% gradient
of ethyl alcohol, washed in xylene and embedded in paraffin.
Paraffin-embedded liver tissue samples were cut into 4–5 µm thick
sections. For general histology, tissue sections were subsequently
stained with hematoxylin for 5 min at room temperature followed by
eosin staining for 5 min at room temperature. Morphological changes
were observed via Van Gieson's (VG) staining for 2 min at room
temperature. Pathological changes were observed under a light
microscope (magnification, ×200). Collagen content was analyzed
using the Image-Pro Plus analysis software (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA).
Liver hydroxyproline content
analysis
The hydroxyproline content in the liver, as an
indirect index of collagen content, was determined using a
hydroxyproline testing kit (cat. no. 02140R1; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), according to the
manufacturer's protocol. Briefly, liver tissue samples were
hydrolyzed, lyophilized and hydroxyproline content was measured at
a wavelength of 550 nm on a spectrophotometer (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Western blot analysis
Liver tissue samples were homogenized and total
protein was extracted using HEPES extraction buffer (Sangon
Biotech, Co., Ltd.). Total protein was quantified using a
bicinchoninic acid assay (Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and equal amounts of total protein (40 µg protein/lane)
was separated via SDS-PAGE on a 10% gel. The separated proteins
were subsequently transferred onto polyvinyl difluoride membranes
and blocked with 3% skimmed milk for 2 h at room temperature. The
membranes were incubated with primary antibodies against TGFβ1
(1:1,000; cat. no. ab92486), α-SMA (1:1,000; cat. no. ab5694),
collagen type I (1:1,000; cat. no. ab34710), fibronectin (1:1,000;
cat. no. ab2413), phosphorylated (p-) ERK1/2 (1:1000; cat. no.
ab214362), ERK1/2 (1:1,000; cat. no. ab17942) and β-actin (1:1,000;
cat. no. ab8227; all Abcam) overnight at 4°C. Following primary
incubation, membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000;
cat. no. D110066; Sangon Biotech, Co., Ltd.) for 2 h at room
temperature. Protein bands were visualized using the Super ECL
detection kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein expression was quantified using Bio-Rad Quantity One
software (version 4.6.2; Bio-Rad Laboratories, Inc.) with β-actin
as the loading control.
Statistical analysis
Data were presented as the mean ± standard
deviation. All statistical analyses were performed using SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance followed by Student-Newman-Keuls test was used
to analyze differences among treatment groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Puerarin alleviates inflammation and
fibrosis in TAA-induced liver fibrosis in rats
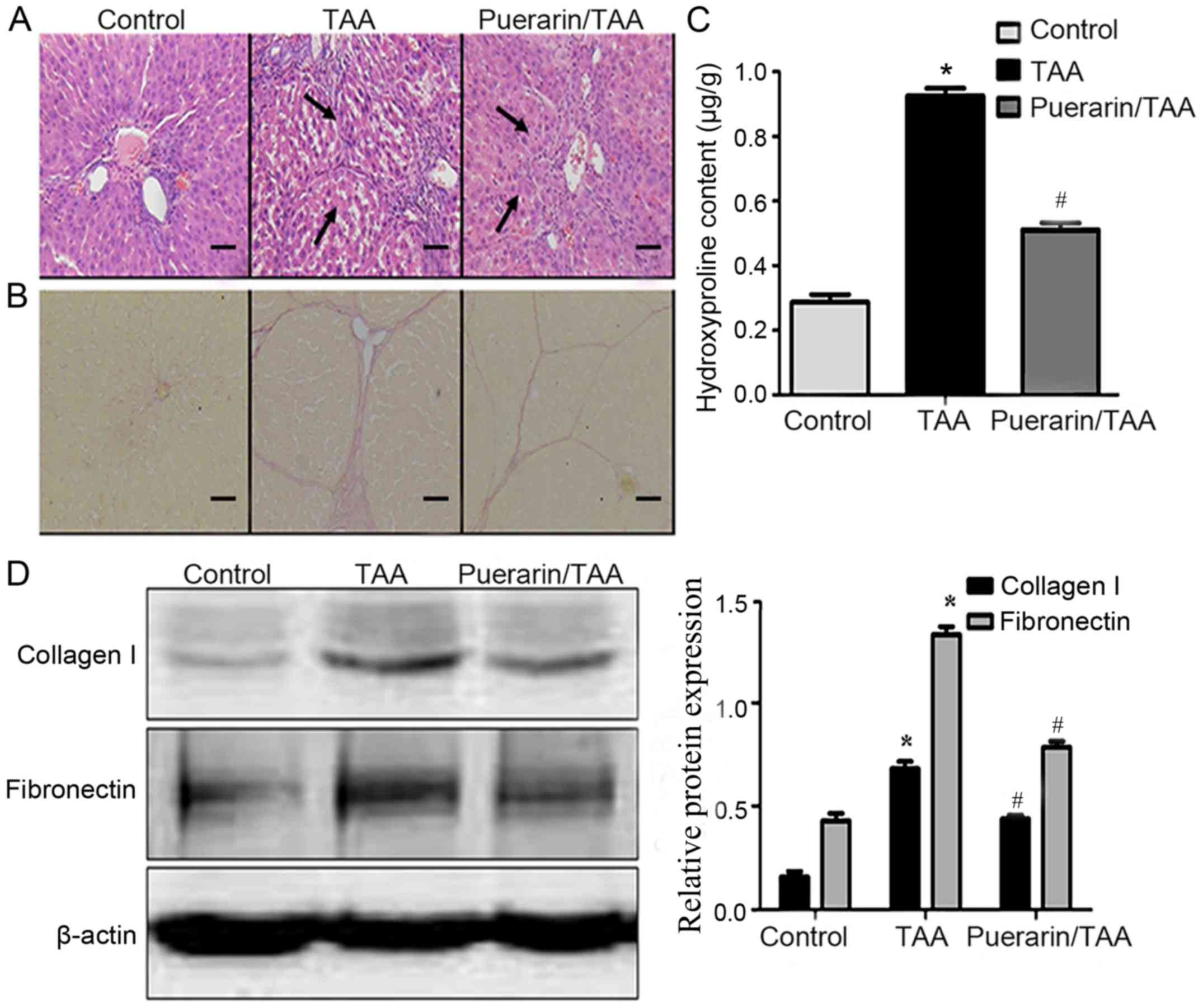
All rats survived the experimental procedure. Liver
tissue samples from rats in each experimental group were stained
using H&E and VG to examine the histopathological changes
associated with liver fibrosis. H&E staining demonstrated that
in the TAA group, there was marked degeneration and necrosis of
liver cells, as well as infiltration of inflammatory cells compared
with the control group. In addition, the normal structure of the
liver was altered, with marked formation of fibrous septum tissue
in the TAA group compared with the control group. However,
pathological changes in the puerarin/TAA group were less severe
compared with the TAA group (Fig.
1A). The presence of collagen type I in the liver tissue was
observed by VG staining. In the TAA group, the level of collagen
fibers were markedly increased with the formation of fibrous septa
surrounding the hepatic lobules compared with the control group
(Fig. 1B). In addition, the collagen
deposition in the puerarin/TAA group was less compared with the TAA
group (Fig. 1B).
Liver hydroxyproline content was significantly
increased in the TAA group compared with the control group
(P<0.05; Fig. 1C). In addition,
the liver hydroxyproline content significantly decreased in the
puerarin/TAA group compared with the TAA group (P<0.05; Fig. 1C). Similarly, western blot analysis
demonstrated that the relative protein expression levels of
collagen type I and fibronectin were significantly upregulated in
the livers of rats in the TAA group compared with the control group
(P<0.05; Fig. 1D). However, the
protein expression levels of collagen type I and fibronectin were
significantly downregulated in the puerarin/TAA group compared with
the TAA group (P<0.05; Fig. 1D).
These results suggest that treatment with puerarin may alleviate
inflammation and fibrosis in liver fibrosis.
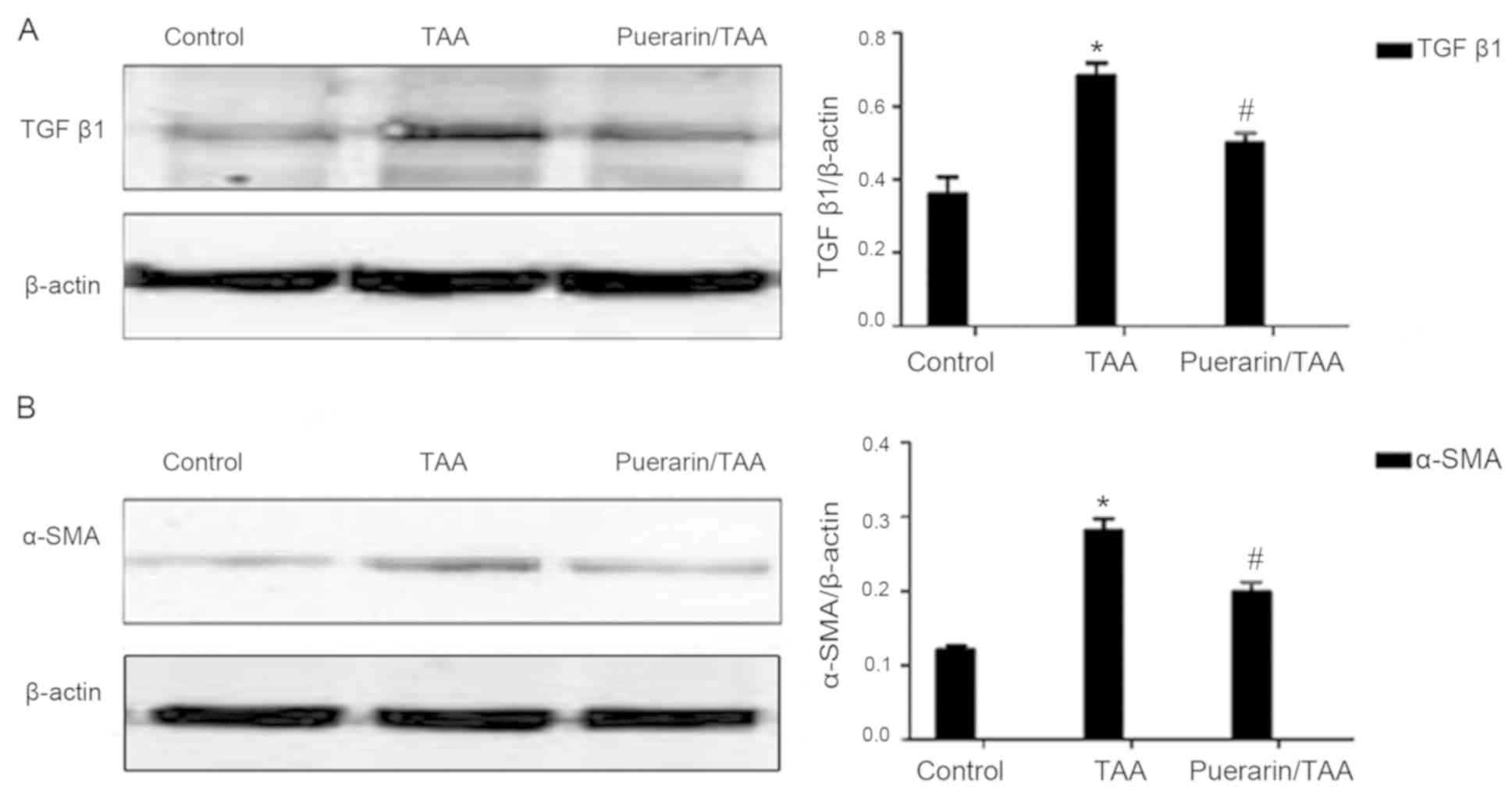
Puerarin alleviates TGFb1 expression
and HSC activation in TAA-induced liver fibrosis in rats
To further investigate the effects of puerarin on
liver fibrosis and HSC activation in vivo following
TAA-induced liver fibrosis, the relative expression levels of TGFβ1
and α-SMA were examined. TGFβ1 is known as the strongest effector
for liver fibrosis, while α-SMA is a marker of HSC activation
(28,29). The relative protein expression levels
of TGFβ1 and α-SMA were significantly increased in the TAA group
compared with the control group, whereas TGFβ1 and α-SMA expression
levels were significantly decreased in the puerarin/TAA group
compared with the TAA group (P<0.05; Fig. 2A and B). These results indicated that
puerarin may reduce TGFβ1 production and HSC activation.
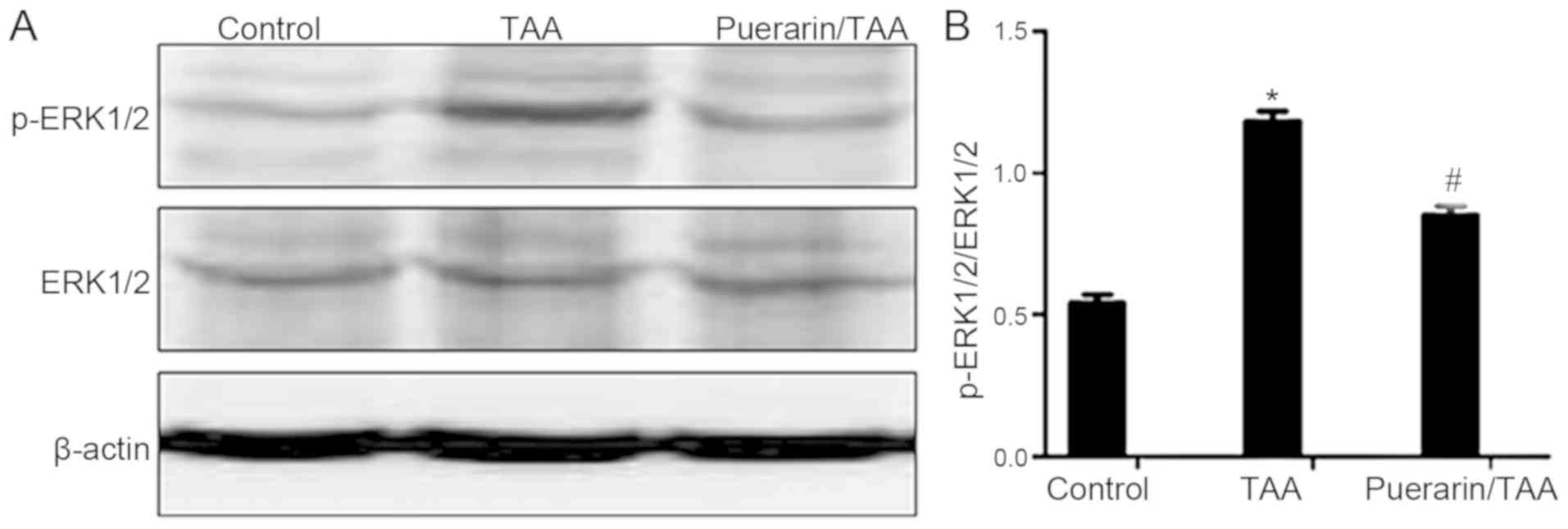
Puerarin inhibits the ERK1/2 signaling
pathway
The ERK1/2 signaling pathway serves an important
role in liver fibrosis (10,30). Therefore, to examine whether puerarin
is involved in regulating ERK1/2 in the reduction of HSC activation
and ECM deposition, the relative expression levels of ERK1/2 and
the activated form, p-ERK1/2, were examined. The relative protein
expression level of p-ERK1/2 was markedly increased in the TAA
group compared with the control group (Fig. 3A). By contrast, the relative protein
expression level of p-ERK1/2 was markedly decreased in the
puerarin/TAA group compared with the TAA group (Fig. 3A). The ratio of p-ERK1/2 to ERK1/2
was also significantly decreased in the puerarin/TAA group compared
with the TAA group (P<0.05; Fig.
3B). There was no marked difference in the protein expression
level of ERK1/2 between the experimental groups (Fig. 3A). These results demonstrated that
ERK1/2 phosphorylation may be suppressed by puerarin in liver
fibrosis.
Discussion
Liver fibrosis is a major global public health
problem (31) and is caused by
several forms of chronic liver disease (32). The pathogenesis of liver fibrosis is
complex and is characterized by enhanced ECM production and altered
deposition of ECM proteins. Notably, uncontrolled liver fibrosis
can develop into cirrhosis (33).
Treatment options for liver fibrosis currently available are not
highly effective (34). Therefore,
more effective treatments for liver fibrosis are required.
TAA can interfere with protein synthesis and enzyme
metabolism in the liver, which can lead to chronic liver injury
(35,36). Previous studies have demonstrated
that TAA could induce hepatic fibrosis in rats (37,38). In
the current study, H&E and VG staining revealed a marked
increase in the number of necrotic hepatocytes and infiltrating
inflammatory cells, with a markedly increased deposition of
collagen fibers in the TAA group, as well as liver fibrosis were
directly observed. Furthermore, liver hydroxyproline content in the
TAA group was significantly increased compared with the control
group. These results suggest that the liver fibrosis in vivo
model was successfully established in rats.
Chinese herbs, including puerarin, can induce
favorable effects in liver fibrosis, whereby ECM components can
change from normal basement matrix, which includes collagen type
IV, to a fibrotic basement matrix, which includes collagen type I
(39). In previous studies, puerarin
significantly reduced levels of hydroxyproline and collagen type I
in liver fibrosis (25,26). In the current study, hepatic
inflammation and the protein expression levels of collagen type I
and fibronectin were significantly alleviated following treatment
with puerarin, which was consistent with previous reports (40,41). The
results from the current study suggest that puerarin may have an
anti-fibrotic effect in liver fibrosis. However, the underlying
mechanism of puerarin in liver fibrosis remains unknown.
HSC is widely regarded as the key fibrogenic cell
that coordinates hepatic ECM formation (5). Activation of HSCs serve an important
role in the process of liver fibrosis (4). TGFβ1 is a potent activator of HSC
(42) and a potent inducer of
collagen type I production (43).
α-SMA is a widely accepted marker of activated HSC (44). Previous studies demonstrated that
treatment with puerarin reduced the production of TGF-βl and α-SMA
(24,45). In the current study, protein
expression levels of TGFβ1 and α-SMA were significantly decreased
following treatment with puerarin, which is similar to previous
reports (26,40,45).
Taken together, these results suggest that puerarin may reduce
TGFβ1 production and HSC activation to alleviate ECM protein
expression in liver fibrosis.
Previous studies revealed that puerarin can regulate
serum enzymes, reduce the production of TGF-β1 and inhibit
excessive collagen deposition to attenuate liver fibrosis via the
inhibition of TNF-α/NF-κB (24),
PI3K/Akt (25) and TGFβ1/Smad
(46) signaling pathways. However,
whether puerarin alleviates liver fibrosis via the ERK1/2 signaling
pathway remains unknown. Therefore, the current study hypothesized
that puerarin may reduce liver fibrosis via the ERK1/2 signaling
pathway. In the current study, the ratio of p-ERK1/2 to ERK1/2
protein expression levels were significantly downregulated in the
puerarin/TAA group compared with the TAA group, which suggests that
the anti-fibrotic effect of puerarin in liver fibrosis may occur
via the ERK1/2 signaling pathway. These results suggest that
puerarin may ameliorate liver fibrosis by inhibiting the ERK1/2
pathway to reduce HSC activation and ECM deposition. Liu et
al (47) reported that puerarin
inhibited ERK phosphorylation in breast cancer cells. Therefore,
puerarin may be involved in regulating ERK1/2 phosphorylation,
thereby inhibiting ERK1/2 signaling in liver fibrosis. However,
whether puerarin binds to other functional domains of ERK1/2
requires further investigation.
In conclusion, the current study demonstrated that
puerarin may inhibit the TGF-β/ERK1/2 signaling pathway to reduce
HSC activation, thereby alleviating ECM protein expression in liver
fibrosis. Therefore, puerarin may be a potential therapeutic
candidate in the treatment of liver fibrosis. However, further
investigations are required to validate the potential therapeutic
effects of puerarin in liver fibrosis.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
Foundation of Lianyungang Oriental Hospital (grant no.
2016-12).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW designed the experiments. HaZ, LP, HuZ, XM and JC
performed the experiments. XL analyzed the data and prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shanxi Medical University (SCXK2009-0001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Higashi T, Friedman SL and Hoshida Y:
Hepatic stellate cells as key target in liver fibrosis. Adv Drug
Deliv Rev. 121:27–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qian H, Deng X, Huang ZW, Wei J, Ding CH,
Feng RX, Zeng X, Chen YX, Ding J, Qiu L, et al: An HNF1α-regulated
feedback circuit modulates hepatic fibrogenesis via the crosstalk
between hepatocytes and hepatic stellate cells. Cell Res.
25:930–945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palmer DH, Hussain SA and Johnson PJ:
Gene- and immunotherapy for hepatocellular carcinoma. Expert Opin
Biol Ther. 5:507–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tao LL, Zhai YZ, Ding D, Yin WH, Liu XP
and Yu GY: The role of C/EBP-α expression in human liver and liver
fibrosis and its relationship with autophagy. Int J Clin Exp
Pathol. 8:13102–13107. 2015.PubMed/NCBI
|
|
5
|
Lewindon PJ, Pereira TN, Hoskins AC,
Bridle KR, Williamson RM, Shepherd RW and Ramm GA: The role of
hepatic stellate cells and transforming growth factor-beta(1) in
cystic fibrosis liver disease. Am J Pathol. 160:1705–1715. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sheppard D: Transforming growth factor
beta: A central modulator of pulmonary and airway inflammation and
fibrosis. Proc Am Thorac Soc. 3:413–417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabregat I, Moreno-Càceres J, Sánchez A,
Dooley S, Dewidar B, Giannelli G and Ten Dijke P; IT-LIVER
Consortium, : TGF-β signalling and liver disease. FEBS J.
283:2219–2232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai FF, Wu R, Song YN, Xiong AZ, Chen XL,
Yang MD, Yang L, Hu Y, Sun MY and Su SB: Yinchenhao decoction
alleviates liver fibrosis by regulating bile acid metabolism and
TGF-β/Smad/ERK signalling pathway. Sci Rep. 8:153672018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lestari N, Louisa M, Soetikno V, Suwana
AG, Ramadhan PA, Akmal T and Arozal W: Alpha mangostin inhibits the
proliferation and activation of acetaldehyde induced hepatic
stellate cells through TGF-β and ERK 1/2 pathways. J Toxicol.
2018:53604962018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Zhang Y, Zhang Q, Guo X, Zhang H,
Zheng G and Liu L: Insulin-like growth factor binding
protein-related protein 1 (IGFBPrP1) contributes to liver
inflammation and fibrosis via activation of the ERK1/2 pathway.
Hepatol Int. 9:130–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benyon RC and Iredale JP: Is liver
fibrosis reversible? Gut. 46:443–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Issa R, Zhou X, Constandinou CM,
Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim
N, Knorr A, et al: Spontaneous recovery from micronodular
cirrhosis: Evidence for incomplete resolution associated with
matrix cross-linking. Gastroenterology. 126:1795–1808. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dienstag JL, Goldin RD, Heathcote EJ, Hann
HW, Woessner M, Stephenson SL, Gardner S, Gray DF and Schiff ER:
Histological outcome during long-term lamivudine therapy.
Gastroenterology. 124:105–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bataller R and Brenner DA: Hepatic
stellate cells as a target for the treatment of liver fibrosis.
Semin Liver Dis. 21:437–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iredale JP, Pellicoro A and Fallowfield
JA: Liver fibrosis: Understanding the dynamics of bidirectional
wound repair to inform the design of markers and therapies. Dig
Dis. 35:310–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schuppan D: Liver fibrosis: Common
mechanisms and antifibrotic therapies. Clin Res Hepatol
Gastroenterol. 39 (Suppl 1):S51–S59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JH, Jeon YD, Lee YM and Kim DK: The
suppressive effect of puerarin on atopic dermatitis-like skin
lesions through regulation of inflammatory mediators in vitro and
in vivo. Biochem Biophys Res Commun. 498:707–714. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Yu J and Shi J: Management of
diabetes mellitus with puerarin, a natural isoflavone from pueraria
lobata. Am J Chin Med. 46:1771–1789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan M, Liu G, Zheng X, Li P, Liu J, Wang
S and Cao Y: Effects of puerarin combined with conventional therapy
on ischemic stroke. Exp Ther Med. 14:2943–2946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X, Bai C, Sun X, Gong X, Yang Y, Chen
C, Shan G and Yao Q: Puerarin attenuates renal fibrosis by reducing
oxidative stress induced-epithelial cell apoptosis via MAPK signal
pathways in vivo and in vitro. Ren Fail. 39:423–431. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo BQ, Xu JB, Xiao M, Ding M and Duan LJ:
Puerarin reduces ischemia/reperfusion-induced myocardial injury in
diabetic rats via upregulation of vascular endothelial growth
factor A/angiotensin-1 and suppression of apoptosis. Mol Med Rep.
17:7421–7427. 2018.PubMed/NCBI
|
|
24
|
Li R, Xu L, Liang T, Li Y, Zhang S and
Duan X: Puerarin mediates hepatoprotection against CCl4-induced
hepatic fibrosis rats through attenuation of inflammation response
and amelioration of metabolic function. Food Chem Toxicol.
52:69–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo C, Xu L, He Q, Liang T, Duan X and Li
R: Anti-fibrotic effects of puerarin on CCl4-induced hepatic
fibrosis in rats possibly through the regulation of PPAR-γ
expression and inhibition of PI3K/Akt pathway. Food Chem Toxicol.
56:436–442. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu L, Zheng N, He Q, Li R, Zhang K and
Liang T: Puerarin, isolated from Pueraria lobata (Willd.), protects
against hepatotoxicity via specific inhibition of the TGF-β1/Smad
signaling pathway, thereby leading to anti-fibrotic effect.
Phytomedicine. 20:1172–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Attar AM, Alrobai AA and Almalki DA:
Effect of Olea oleaster and Juniperus procera leaves extracts on
thioacetamide induced hepatic cirrhosis in male albino mice. Saudi
J Biol Sci. 23:363–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wickert L, Steinkrüger S, Abiaka M,
Bolkenius U, Purps O, Schnabel C and Gressner AM: Quantitative
monitoring of the mRNA expression pattern of the TGF-beta-isoforms
(beta 1, beta 2, beta 3) during transdifferentiation of hepatic
stellate cells using a newly developed real-time SYBR Green PCR.
Biochem Biophys Res Commun. 295:330–335. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carpino G, Morini S, Ginanni Corradini S,
Franchitto A, Merli M, Siciliano M, Gentili F, Onetti Muda A,
Berloco P, Rossi M, et al: Alpha-SMA expression in hepatic stellate
cells and quantitative analysis of hepatic fibrosis in cirrhosis
and in recurrent chronic hepatitis after liver transplantation. Dig
Liver Dis. 37:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Tanbouly DM, Wadie W and Sayed RH:
Modulation of TGF-β/Smad and ERK signaling pathways mediates the
anti-fibrotic effect of mirtazapine in mice. Toxicol Appl
Pharmacol. 329:224–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sánchez-Valle V, Chávez-Tapia NC, Uribe M
and Méndez-Sánchez N: Role of oxidative stress and molecular
changes in liver fibrosis: A review. Curr Med Chem. 19:4850–4860.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mormone E, George J and Nieto N: Molecular
pathogenesis of hepatic fibrosis and current therapeutic
approaches. Chem Biol Interact. 193:225–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elpek GÖ: Cellular and molecular
mechanisms in the pathogenesis of liver fibrosis: An update. World
J Gastroenterol. 20:7260–7276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Toosi AE: Liver fibrosis: Causes and
methods of assessment, a review. Rom J Intern Med. 53:304–314.
2015.PubMed/NCBI
|
|
35
|
Sharma L, Gupta D and Abdullah ST:
Thioacetamide potentiates high cholesterol and high fat diet
induced steato-hepatitic changes in livers of C57BL/6J mice: A
novel eight weeks model of fibrosing NASH. Toxicol Lett. 304:21–29.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schyman P, Printz RL, Estes SK, Boyd KL,
Shiota M and Wallqvist A: Identification of the toxicity pathways
associated with thioacetamide-induced injuries in rat liver and
kidney. Front Pharmacol. 9:12722018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amirtharaj GJ, Natarajan SK, Pulimood A,
Balasubramanian KA, Venkatraman A and Ramachandran A: Role of
oxygen free radicals, nitric oxide and mitochondria in mediating
cardiac alterations during liver cirrhosis induced by
thioacetamide. Cardiovasc Toxicol. 17:175–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Golbar HM, Izawa T, Wijesundera KK, Bondoc
A, Tennakoon AH, Kuwamura M and Yamate J: Depletion of hepatic
macrophages aggravates liver lesions induced in rats by
thioacetamide (TAA). Toxicol Pathol. 44:246–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lakner AM, Moore CC, Gulledge AA and
Schrum LW: Daily genetic profiling indicates JAK/STAT signaling
promotes early hepatic stellate cell transdifferentiation. World J
Gastroenterol. 16:5047–5056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang S, Shi XL, Feng M, Wang X, Zhang ZH,
Zhao X, Han B, Ma HC, Dai B and Ding YT: Puerarin protects against
CCl4-induced liver fibrosis in mice: Possible role of PARP-1
inhibition. Int Immunopharmacol. 38:238–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hou B, Zhao Y, Qiang G, Yang X, Xu C, Chen
X, Liu C, Wang X, Zhang L and Du G: Puerarin mitigates diabetic
hepatic steatosis and fibrosis by inhibiting TGF-β signaling
pathway activation in type 2 diabetic rats. Oxid Med Cell Longev.
2018:45453212018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen A: Acetaldehyde stimulates the
activation of latent transforming growth factor-beta1 and induces
expression of the type II receptor of the cytokine in rat cultured
hepatic stellate cells. Biochem J. 368:683–693. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jin L, Gao H, Wang J, Yang S, Wang J, Liu
J, Yang Y, Yan T, Chen T, Zhao Y and He Y: Role and regulation of
autophagy and apoptosis by nitric oxide in hepatic stellate cells
during acute liver failure. Liver Int. 37:1651–1659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu GL, Chen J, Yu GY, Li JP and Lu WW:
Effect of puerarin on levels of TGF-beta1 and alpha-SMA in rats
with alcoholic injury liver. Zhongguo Zhong Yao Za Zhi.
33:2245–2249. 2008.(In Chinese). PubMed/NCBI
|
|
46
|
Wu T, Liu T, Xing L and Ji G: Baicalin and
puerarin reverse epithelial-mesenchymal transition via the
TGF-β1/Smad3 pathway in vitro. Exp Ther Med. 16:1968–1974.
2018.PubMed/NCBI
|
|
47
|
Liu X, Zhao W, Wang W, Lin S and Yang L:
Puerarin suppresses LPS-induced breast cancer cell migration,
invasion and adhesion by blockage NF-κB and Erk pathway. Biomed
Pharmacother. 92:429–436. 2017. View Article : Google Scholar : PubMed/NCBI
|