Introduction
Lazaroids are synthetic 21-aminosteroids; as
derivatives of methylprednisolone, the carbon 21 of the steroid
molecule is replaced by an amino group, thus preserving the
anti-inflammatory properties but lacking the steroidal side
effects. The effects of lazaroids have been extensively examined in
various organs (1–6). The cytoprotective effect of lazaroids
is exerted partly by their anti-oxidant properties but also by
inhibition of arachidonic acid release, membrane stabilization,
suppression of Kuppfer cell activation and downregulation of
cytokine expression and release (7–9).
Structural differences and different mechanisms of action are
responsible for the different efficacy of various lazaroid
compounds (10).
The lazaroid U-74389G has been studied in various
ischemia-reperfusion (I/R) injury models in vitro and in
vivo, including rat liver lyosomes subjected to exogenously
generated oxygen free radicals in vitro (8,11), renal
I/R injury (12), pancreatic I/R
injury in pigs (13,14), intestinal I/R injury in rats
(15), orthotopic heart
transplantation in mongrel dogs (16), as well as in canine liver
preservation (17). The results
indicated various mechanisms of action for the lazaroid U-74389G,
including inhibition of lipid peroxidation, stabilization of the
cellular membrane by incorporation into the lipid bilayer,
suppression of pro-inflammatory gene expression by nuclear factor
(NF)-κB inhibition, prevention of polymorphonuclear cell
infiltration, scavenging of lipid peroxyl radicals and reduction of
tumor necrosis factor-α (TNF-α) release (6,10–12,16–18).
Recently, a novel mechanism of action was assigned to U-74389G:
inhibition of caspase-1, a cysteine-dependent, inflammatory
protease responsible for the production of the pro-inflammatory
cytokine interleukin-1b. This anti-inflammatory action was
indicated to be time-dependent (19).
In the present study, U-74389G was administered to
swine undergoing I/R via the inferior vena cava. A total of four
experimental groups (two sets of experiments, comprising
reperfusion for 60 or 120 min) were examined. Prior to reperfusion,
a 30-min ischemia period was selected, as this appears to best
represent the situation in the clinic, particularly in the
emergency setting. Apart from liver indices, additional attention
was paid to the assessment of intestinal damage, given that
occlusion-reperfusion of the portal vein may respectively imply
venous congestion-reflow in the small bowel, which is often
accompanied by mucosal damage in the small intestine (20). As a whole, the aim of the present
study was to evaluate the efficacy of intracaval administration of
U-74389G in preventing liver I/R injury in a swine model.
Materials and methods
Experimental protocol
The experiments were performed at ELPEN laboratories
(Athens, Greece; license no. EL 09 BIO 03) and were approved by the
veterinary authorities of East Attica Region (ref. no. 3217-June
2007) in accordance with Greek legislation (p.d. 160/91) and
European Community regulations (directive 309 of 1986; license
according to E.U. legislation). This manuscript was written in
accordance to the ARRIVE guidelines (21).
The animals used in the present study were Landrace
Hellenic Domestic pigs (n=28; weight, 28–35 kg; age, 4–5 months)
purchased from the same breeder in Koropi, Greece (E.U. license, EL
090011). Given the study design, the experimental unit was one
animal. The animals were acclimatized to the laboratory conditions
for 3–4 days with free access to food and water and were fasted
during 24 h prior to the experiment, maintaining free access to
water throughout. Pigs were housed in steel cages in a
temperature-controlled environment, (temperature, 19–23°C;
humidity, 50–60%), on a 12-h light/dark cycle, and were fed with
the same diet. All animals received general anesthesia and aseptic
techniques were used for the surgical procedure. All procedures
were performed at fixed time-points in the morning, to minimize any
circadian effects.
A pre-medication injection with midazolam (0.5
mg/kg) and ketamine 15 mg/kg was administered intramuscularly (IM).
Atropine (0.045 mg/kg) was administered IM in the neck at 10 min
prior to intubation (22–26). Two polyethylene intravenous catheters
(18G) were inserted into two peripheral veins in both ears for
infusion of crystalloids and anesthetic drugs. Prior to intubation,
a bolus of propofol (3 mg/kg) and fentanyl (0.012 mg/kg) was
administered. The animals were then intubated and a bolus of
cisatracurium (0.5 mg/kg) was administered. General anesthesia was
maintained with continuous infusion of propofol at 6–8 mg/kg/h, as
well as fentanyl (0.012 mg/kg; Janssen-Cilag International NV;
Beerse, Belgium) and cisatracurium (0.5 mg/kg; GlaxoSmithKline
Manufacturing SpA, Verona, Italy) and the animals were mechanically
ventilated. The femoral vein was also catheterized for blood
collection (23,25,26).
During the experiment, the animals were continuously monitored by
electrocardiography, pulse oximetry and measurement of arterial
blood pressure. At the end of each experiment, the animals were
euthanized using an intravenous overdose of 200 mg/kg pentobarbital
(24).
For the laparotomy, a midline incision and full
asepsis were performed prior to the manipulations. The portal vein
was isolated and prepared for occlusion immediately prior to its
division into the right and left branch at the hepatic hilum.
Occlusion was performed with a bulldog clamp. At the end of the
30-min ischemic period, the test drug U-74389G was administered via
the inferior vena cava, followed by reperfusion by removal of the
clamp. The dose of the lazaroid U-74389G (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was 10 mg/kg.
Two sets of experiments were performed, one adopting
reperfusion of 60 min (REP60 groups) and in another, reperfusion
was performed 120 min (REP120 groups). In each set, the animals
were randomly allocated into either the injection (n=7) or the
control group (n=7). In each set, the injection group was compared
with the control group. Therefore, two comparisons were made:
REP60+U-74389G vs. REP60-controls and REP120+U-74389G vs.
REP120-controls.
In the first set (REP60), liver biopsies were
obtained at 0, 15, 30 and 90 min experimental time (0, 15 and 30
min of ischemia, and 60 min of reperfusion); at 90 min, small bowel
(ileum) biopsies were also obtained. In the second set (REP120),
liver biopsies were obtained at 0, 15, 30 and 150 min experimental
time (0, 15 and 30 min of ischemia and 120 min of reperfusion); at
150 min, ileum biopsies were also taken. All experiments were
acute, meaning that the animals were euthanized by the end of each
experiment. The experimental end-points were as follows: liver I/R
injury (assessed by determination of hepatocyte
vacuolation/degeneration, venous congestion, inflammatory
infiltration, sinus dilatation/congestion), liver malondialdehyde
(MDA) and intestinal damage, expressed as the Chiu score.
The number of 28 animals (7 animals per group) was
based on an a priori power calculation pertaining to the pathology
scores. Specifically, assuming a 10% attrition rate, seven animals
per group were sufficient for the achievement of 80% statistical
power to detect a 0.4-unit change in scores (assuming a standard
deviation per group equal to 0.2 units) at a significance level of
0.05. The power calculation was performed with G*Power 3.1.9.2
statistical software (University of Düsseldorf, Düsseldorf,
Germany).
Histopathologic evaluation
Tissue specimens were fixed in 4% formalin, embedded
in paraffin, sectioned and subjected to hematoxylin-eosin staining.
For the histopathologic evaluation of the liver damage hepatocyte
vacuolation/degeneration, venous congestion, inflammatory
infiltration and sinus dilatation-congestion were rated using
grades from 0 (normal) to 3 (severe). These histological findings
were graded for morphological changes recognized to be secondary to
I/R injury, according to a semi-quantitative scale, depending on
the percentage of positive findings in 30 high-power fields for
each slide with values assigned as 0 (0% positivity, none), 1
(1–25% positivity, mild), 2 (26–50% positivity, moderate) and 3
(51–100% positivity, severe) (27).
For evaluation of intestinal damage in specimens of
ileum, Chiu scoring was used (28).
The samples were assessed by two independent pathologists blinded
to the experimental groups.
Liver MDA
Tissue samples were rinsed with ice-cold isotonic
saline prior to homogenization, which was performed using TBS (20
mmol/; pH 7.4) and an Ultra-Turrax blender (IKA Labortechnik,
Staufen, Germany), with 1 ml buffer used for 0.1 g tissue. A total
of 10 ml 500 mmol/l butylated hydroxytoluene was added to 1 ml
tissue homogenate to prevent sample oxidation. The homogenate was
centrifuged at 704 × g and 4°C for 10 min. and the clear
supernatant was used for protein determination. To quantify the MDA
content, a commercial kit (Bioxytech®-LPO-586™; OxIS
Research™; OXIS Health Products, Inc. BIOXYTECH, Portland, OR, USA)
was used according to the manufacturer's protocol. Measurement was
based on the reaction of a chromogenic reagent,
N-methyl-2-phenylindole, with MDA at 45°C for 60 min. The stable
chromophore product exhibits a maximal absorbance at 586 nm. The
results were expressed as µmol/l solution.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. For each set of experiments repeated-measures analysis of
variance was performed to assess the differences between groups, in
view of the longitudinal nature of data. Given that each set of
experiments comprised measurements at different time-points (0, 15,
30, 90 min for the REP60 set and 0, 15, 30, 150 min for the REP120
set), two separate models were constructed, one for each set.
The Chiu score of intestinal damage was not measured
longitudinally; therefore, the Mann-Whitney U test for independent
samples was used.
Statistical analysis was performed with STATA/SE
version 13 statistical software (StataCorp., College Station, TX,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Animal model
Of the 28 randomized animals, 26 were included in
the final analysis. A total of two animals, one of the
REP120-control group and one of the REP120+U-74389G group, were not
finally included in the study due to death of myocardial infarction
as confirmed by electrocardiography, which is acknowledged as a
common cause of mortality following liver I/R injury (29). The measured parameters in the two
sets of experiments are presented in Table I.
 | Table I.Parameters measured in the two sets
of experiments.a |
Table I.
Parameters measured in the two sets
of experiments.a
|
| First set of
experiments | Second set of
experiments |
|---|
|
|
|
|
|---|
| Parameter/time of
reperfusion (min) | REP60-control
(n=7) | REP60+U-74389G
(n=7) | P-value | REP120-control
(n=6) | REP120+U-74389G
(n=6) | P-value |
|---|
| Liver MDA (pmol/mg
protein) |
|
| 0.056 |
|
| 0.312 |
| 0 | 0.88±0.14 | 0.36±0.05 |
| 0.74±0.09 | 0.52±0.18 |
|
| 15 | 0.71±0.12 | 0.63±0.13 |
| 1.67±0.24 | 1.26±0.33 |
|
| 30 |
1.11±0.25 | 0.62±0.08 |
| 0.84±0.25 | 1.26±0.27 |
|
| 90 | 1.26±0.21 | 0.99±0.09 |
| Not measured | Not measured |
|
|
150 | Not measured | Not measured |
| 1.14±0.19 | 0.52±0.18 |
|
| Hepatocyte
vacuolation-degenerationb |
|
| 0.636 |
|
| 0.207 |
| 0 | 0.00±0.00 | 0.14±0.14 |
| 0.00±0.00 | 0.00±0.00 |
|
| 15 | 0.14±0.14 | 0.00±0.00 |
| 0.00±0.00 | 0.00±0.00 |
|
| 30 | 0.29±0.29 | 0.00±0.00 |
| 0.00±0.00 | 0.17±0.17 |
|
| 90 | 0.43±0.30 | 0.43±0.20 |
| Not measured | Not measured |
|
|
150 | Not measured | Not measured |
| 0.33±0.33 | 0.83±0.31 |
|
| Venous
congestiona |
|
| 0.850 |
|
| 0.223 |
| 0 | 0.14±0.14 | 0.00±0.00 |
| 0.50±0.22 | 0.00±0.00 |
|
| 15 | 0.43±0.20 | 0.29±0.18 |
| 0.83±0.17 | 0.67±0.21 |
|
| 30 | 0.43±0.20 | 0.71±0.18 |
| 1.00±0.26 | 0.83±0.17 |
|
| 90 | 1.00±0.38 | 1.14±0.14 |
| Not measured | Not measured |
|
|
150 | Not measured | Not measured |
| 1.50±0.34 |
1.17±0.31 |
|
| Inflammatory cell
infiltrationa |
|
| 0.017 |
|
| 0.021 |
| 0 | 0.00±0.00 | 0.43±0.20 |
| 0.00±0.00 | 0.67±0.33 |
|
| 15 | 0.14±0.14 | 0.71±0.18 |
| 0.00±0.00 |
1.17±0.31 |
|
| 30 |
0.57±0.20 | 1.14±0.14 |
| 0.33±0.21 |
1.17±0.31 |
|
| 90 |
1.14±0.26 | 1.71±0.29 |
| Not measured | Not measured |
|
|
150 | Not measured | Not measured |
| 1.67±0.42 | 2.50±0.34 |
|
| Sinus
congestion-dilationa |
|
| 0.249 |
|
| 0.797 |
| 0 | 0.29±0.18 | 0.00±0.00 |
| 0.33±0.21 | 0.00±0.00 |
|
| 15 | 0.43±0.20 | 0.71±0.18 |
| 0.50±0.34 | 1.00±0.26 |
|
| 30 | 0.86±0.14 | 0.86±0.26 |
| 1.00±0.45 | 1.17±0.17 |
|
| 90 | 2.00±0.00 | 1.29±0.18 |
| Not measured | Not measured |
|
|
150 | Not measured | Not measured |
| 2.00±0.26 | 2.00±0.45 |
|
| Chiu score of
intestinal damage | 3.43±0.53 | 2.57±0.37 | 0.262 | 4.33±0.21 |
3.17±0.40 | 0.030 |
Chiu score
In the second set of experiments, the Chiu score of
intestinal damage was improved by the administration of U-74389G
(3.17±0.40 in the REP120+U-74389G group vs. 4.33±0.21 in the REP120
controls, P=0.030, Mann-Whitney-U test for independent samples).
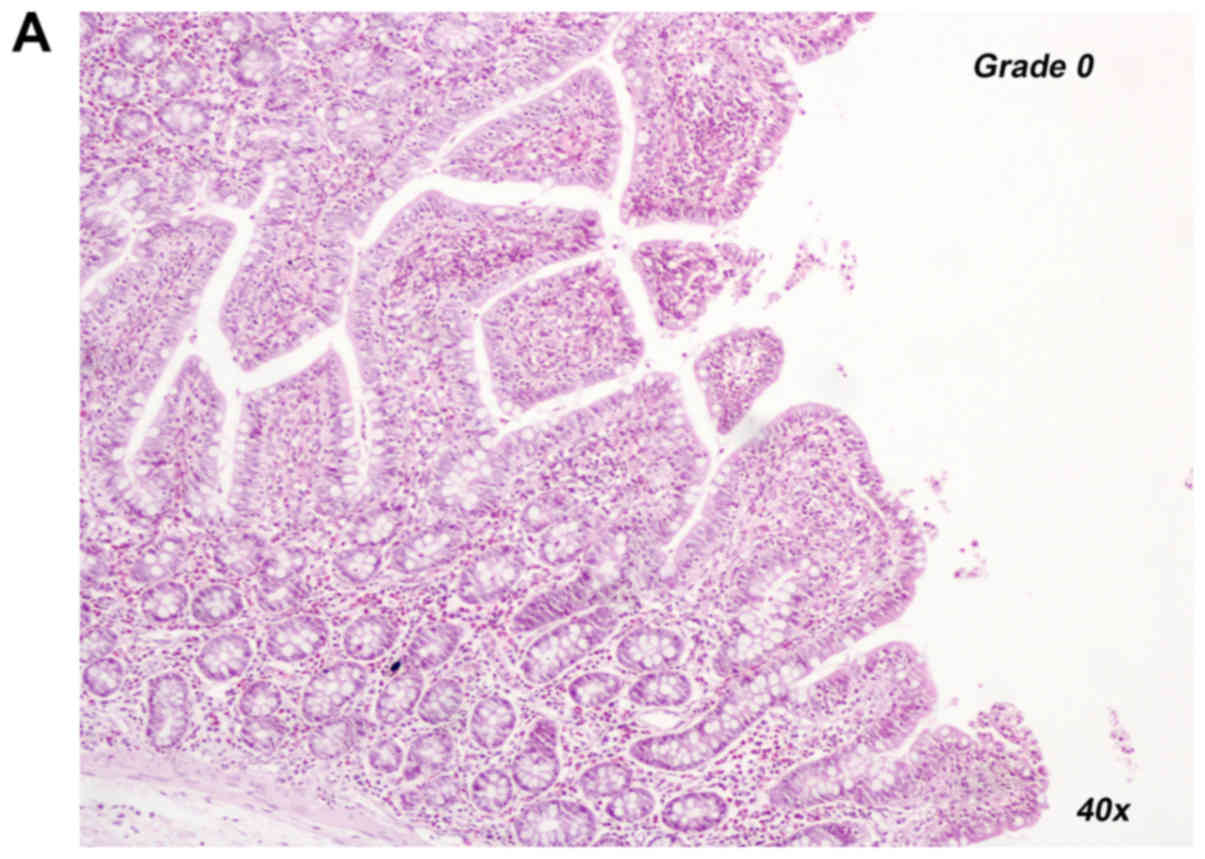
Representative histological images for a Chiu score rating of 0
(REP120+U-74389G group) and 2 (REP120 control group) are provided
in Fig. 1A and B, respectively.
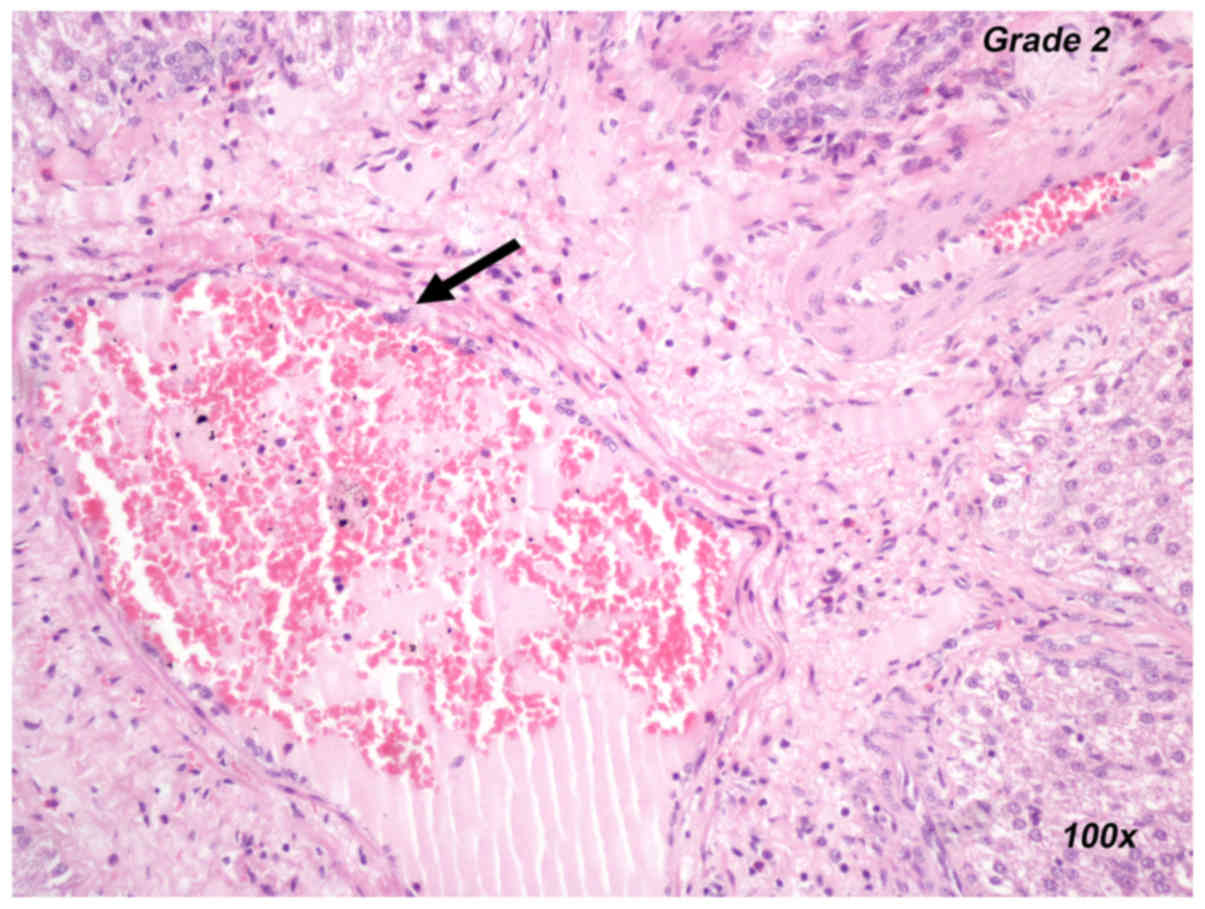
Inflammatory reaction
However, in the two sets of experiments, the liver
inflammatory reaction was more pronounced in the U-74389G groups
(P=0.017 for the REP60+U-74389G vs. REP60 comparison, P=0.021 for
the REP120+U-74389G vs. REP120 comparison; repeated-measures
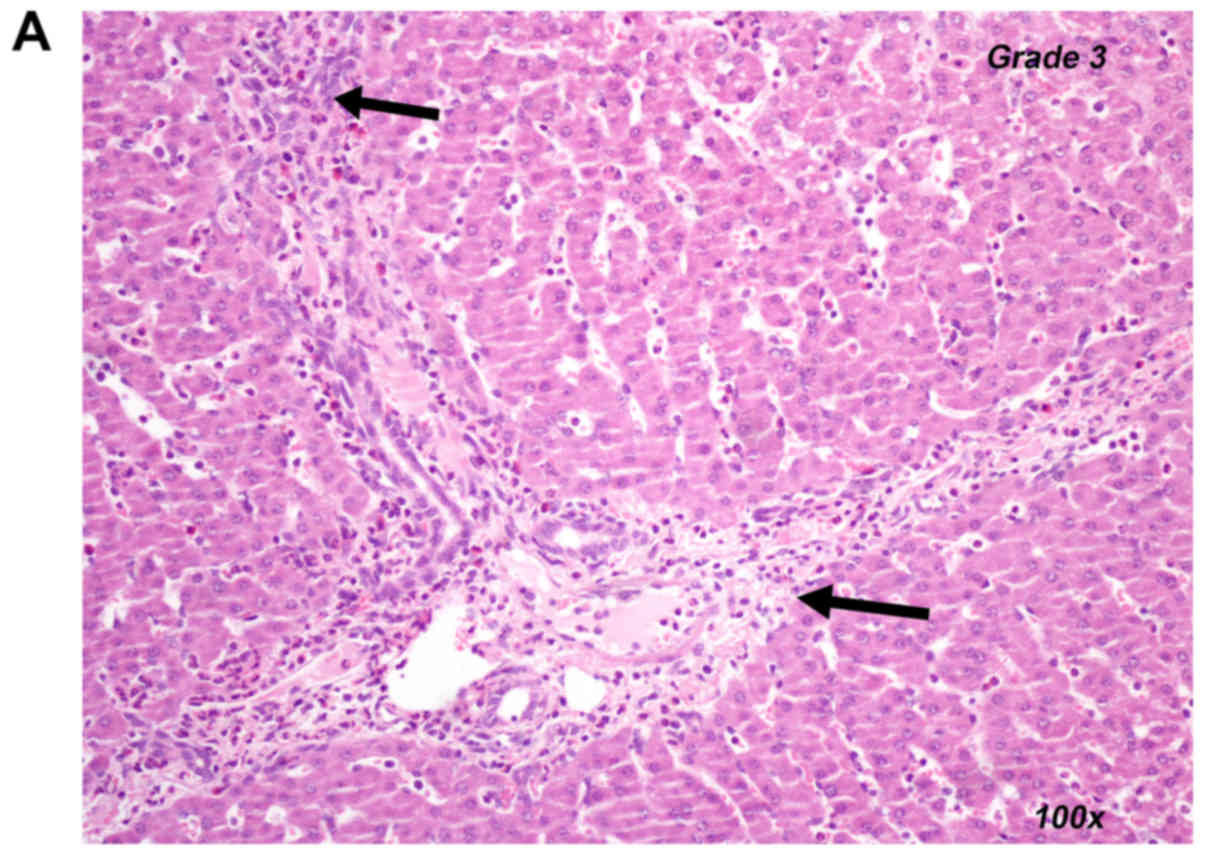
ANOVA). Representative histological images for an inflammatory
reaction grade of 0 (control group) and 3 (U-74389G group) are
provided in Fig. 2A and B,
respectively.
Liver MD, hepatocyte vacuolization,
venous congestion and sinus dilation
Liver MDA (P=0.056 for the first set and P=0.312 for
the second set), hepatocyte vacuolation-degeneration (P=0.636 for
the first set and P=0.207 for the second set), venous congestion
(P=0.850 for the first set and P=0.223 for the second set) and
sinus congestion-dilation (P=0.249 for the first set and P=0.797
for the second set) did not differ between the U-74389 injection
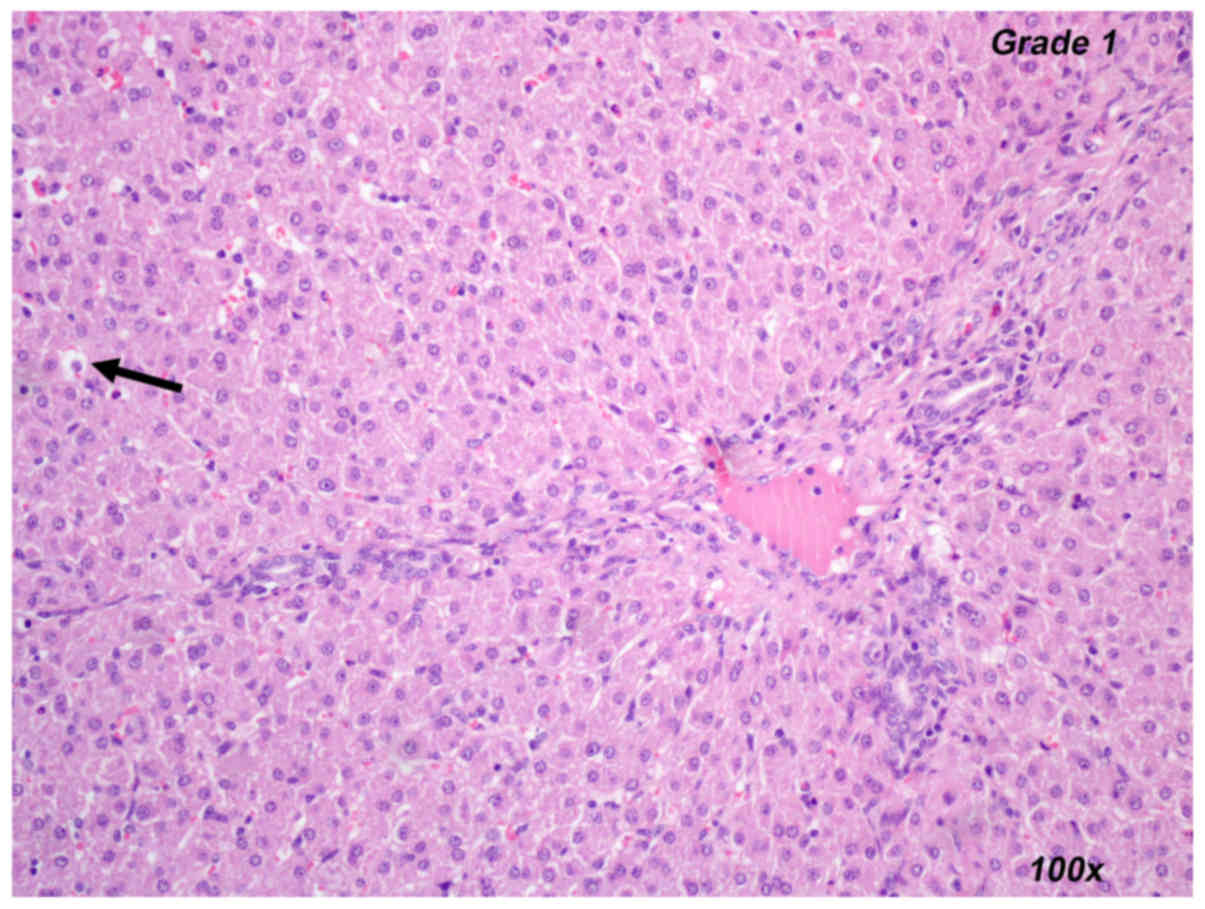
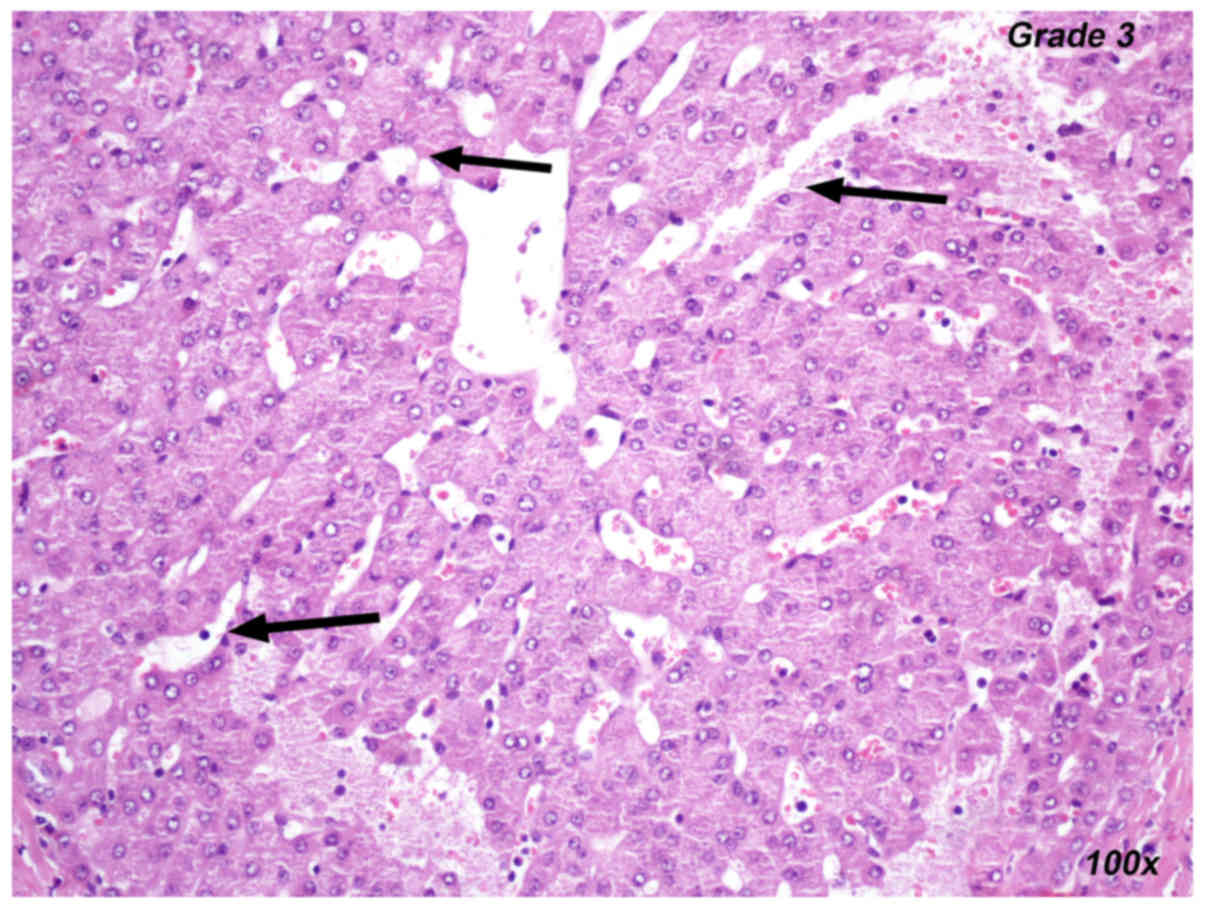
and control groups in either set of experiments. Representative
images for the histopathological evaluation of liver ischemia are
provided in Figs. 3–5, including the presentation of hepatocyte
vacuolization-degeneration grade 1 (Fig.
3), venous congestion grade 2 (Fig.
4) and sinus congestion-dilation grade 3 (Fig. 5).
Discussion
In the present study, intracaval administration of
the lazaroid U-74389G was not able to favorably modify any of the
examined histopathologic variables of hepatic I/R injury; namely
hepatocyte vacuolization-degeneration, venous congestion, sinus
congestion-dilation, as well as the biochemical markers of liver
MDA. However, a protective effect at the level of the ileum was
noted, as measured by the improved values of Chiu intestinal damage
score. It therefore appears that U-74389G mitigates the damage
associated with portal venous congestion-reflow (20).
The protective role of U-74389G at the level of the
ileum was in accordance with that previously reported in the
literature. Lazaroid U-74389G has been indicated to exert
protective effects in rat models of experimental colitis and also
on intestinal grafts after heterotopic small bowel transplantation,
when administered to donor as well as recipient animals (30,31).
Similarly, U-74389G treatment, in addition to cold storage in
University of Wisconsin solution, has been indicated to improve the
recovery of graft function and to minimize morphological damage to
the small intestinal mucosa in a rat model of cold preservation
(4). Beneficial effects of U-74389G
were also demonstrated in the context of warm ischemia of the small
bowel in a rat model (31). In
addition, a study evaluating the effect of U-74389G on intestinal
recovery after acute mesenteric ischemia and reperfusion in rats
indicated that U-74389G was capable of protecting the small
intestine from oxidative damage by inhibiting lipid peroxidation
(32). Similar results were reported
in pig models of bowel and liver ischemia, with U-74389G achieving
an attenuation of the tissue levels of MDA and TNF-α, and
improvement of the intestinal architecture (33,18).
The result that the inflammatory cell infiltration
was more pronounced in the lazaroid group may not be surprising.
Lazaroids at high concentrations may be associated with cell
injury, although they are still effective radical scavengers
(34). Indeed, this potentially
detrimental effect was previously noted in a swine pancreatic I/R
model, where edema was more pronounced in the U-74389G group, in
contrary to the protective action that may have been expected
(13).
Although the present study did not point to the
beneficial effect of U-74389G in liver ischemia, other studies have
supported favourable effects of this agent. Todo et al
(17) used a canine liver
transplantation protocol with preservation using lazaroid U-74389G,
revealing that MDA increased in all experimental groups during
preservation and decreased after reperfusion. Fukuma et al
(6) assessed the protective effect
of U-74389G in an experimental rat model of endotoxin-induced liver
injury. They revealed that U-74389G reduced lipid peroxidation as
indicated by the reduction in MDA (the end product of lipid
peroxidation), suppressed pro-inflammatory gene expression by NF-κB
inhibition and prevented polymorphonuclear cell infiltration in the
liver (6).
In an experimental study on intestinal I/R in
swines, Flessas et al (5)
obtained a statistically significant reduction in MDA, TNF-α and
histopathological scores in tissue treated with U-74389G. In their
study, four groups of swine were examined: i) control
group-ischemia for 30 min and reperfusion for 60 min; ii) control
group-ischemia for 30 min and reperfusion for 120 min; iii)
ischemia for 30 min, immediately followed by intravenous injection
of lazaroid U-74389G and reperfusion for 60 min; and iv) ischemia
for 30 min, immediately followed by intravenous injection of
lazaroid U-74389G and reperfusion for 120 min. The present protocol
followed the same setting of experimental conditions as the study
by Flessas et al (5).
Conversely, a recent study using an experimental
protocol to induce hemorrhagic shock in swine revealed no
protective effect of U-74389G in the small intestine according to
the tissue MDA and serum alkaline phosphatase levels. Three groups
underwent resuscitation with fluid alone, and in another 3 groups,
the lazaroid U-74389G was administered in addition to fluid, while
the control group underwent all the surgical procedures without
hemorrhagic shock (23).
To the best of our knowledge, only one previous
study, namely that by Tsaroucha et al (18), has assessed the use of the lazaroid
U-74389G to prevent liver I/R injury in a swine model. The study
comprised three experimental groups and lazaroid was administered
at the end of a 30-min ischemia period, and immediately prior to
reperfusion. U-74389G was administered intraportally at a dose of
10 mg/kg. Portal infiltration, MDA and TNF-α levels were
significantly lower in the U-74389G-treated groups compared with
those in the control (untreated) animals (18).
In the present study, U-74389G was administered by
injection into the inferior vena cava; whereas in the study by
Tsaroucha et al (18), the
compound was administered intraportally. This difference may be
accountable for a reduced effectiveness, as intraportal
administration may maximize the effects of the agent on the liver
while systemic distribution is obviated; on the contrary, by
intracaval administration, the lazaroid is distributed to the
systemic circulation prior to being metabolized in the liver.
Furthermore, in most experimental protocols used in the past, the
administration of the lazaroid was performed prior to the onset of
ischemia (35,36), a situation that cannot be encountered
in real clinical practice. In the present study, the compound was
administered at the end of ischemia and prior to reperfusion.
The appropriate dose of lazaroids is a topic under
investigation. The dose of 10 mg/kg appears to be the most
effective in various studies with liver I/R injury (13–15). The
mechanism of action of U-74389G in liver or intestinal ischemia in
swine is based on the inhibition of lipid peroxidation (as
evidenced by the attenuation of MDA), the reduction of TNF-α tissue
levels and the improvement of intestinal and liver
histopathological parameters The reduction in the serum levels of
TNF-α may also contribute to the reduction of leukocyte
infiltration (36).
Another limitation of the present study is the
application of two separate repeated-measures ANOVA models instead
of one; this was deemed necessary, given that each set of
experiments comprised measurements at different time-points (0, 15,
30 and 90 min for the REP60 set and 0, 15, 30 and 150 min for the
REP120 set). Indeed, the inherently missing values at the last two
time-points (for 50% of the whole dataset) would have introduced
bias in the calculations. It is possible that a future
meta-analysis of the data may answer if the difference in the last
two time points is responsible for bias in the present results.
There are also certain technical limitations to the
present study, given the lack of more elabοrate biochemical
end-points, e.g., the levels of NF-κB or TNF-α, which may be
mediators of the mechanism of action of U-74389G. Although the
focus of the present study was on whether U-74389G has a beneficial
or no effect on liver I/R injury in swine, further studies are
required to determine the exact biochemical pathways involved in
this effect.
In conclusion, intracaval administration of U-74389G
does not appear to exert any protective effects on liver
I/R-associated injury. However, the intestinal architecture appears
to be protected by the lazaroid U-74389G. Further studies that also
evaluate intrahepatic tissue concentrations of the agent appear to
be required for the optimal protection of the liver in the context
of I/R injury.
Acknowledgements
The authors are pleased to acknowledge the
contribution of the personnel of the Experimental Research Center
of ELPEN Pharmaceuticals (Athens, Greece), namely Mrs Eleftheria
Karampela, Mrs Maria Karamperi, Mrs Kalliopi Tsarea, Mrs Stergios
Gerakis and Mrs Evripidis Gerakis, in the performance of the
experiments.
Funding
This study was funded by ELPEN Pharmaceuticals.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MK, AP and GZ conceived the study, TS and GA
analyzed and interpreted the data and TS performed the statistical
analysis. AG, GA and EP performed the histological examination, the
laboratory tests, and were major contributors in writing the
manuscript. AG, AP and EP provided biological materials, tools and
intruments vital for the experimental results. MK, IF and DC
reviewed the literature, performed the experiments and took
responsibility for data management and reporting. MK and GT took
responsibility for the logical interpretation and presentation of
the results. GT, GZ and AP reviewed the article prior to submission
and gave the final aproval of the version to be published. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments were performed at ELPEN laboratories
(license no. EL 09 BIO 03) and were approved by the veterinary
authorities of East Attica Region (ref. no. 3217-June 2007) in
accordance with Greek legislation (p.d. 160/91) and European
Community regulations (directive 309 of 1986; license according to
E.U. legislation).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
I/R injury
|
ischemia-reperfusion injury
|
|
TNF-α
|
tumor necrosis factor-α
|
|
REP
|
reperfusion
|
References
|
1
|
Hillinger S, Schmid RA, Stammberger U,
Boehler A, Schöb OM, Zollinger A and Weder W: Donor and recipient
treatment with the Lazaroid U-74006F do not improve post-transplant
lung function in swine. Eur J Cardiothorac Surg. 15:475–480. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shackleton CR, Ettinger SL, Scudamore CH,
Toleikis PF and Keown PA: Effect of a 21-aminosteroid, U74006F, on
lipid peroxidation and glomerulotubular function following
experimental renal ischemia. J Surg Res. 57:433–437. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haynes J Jr, Seibert A, Bass JB and Taylor
AE: U74500A inhibition of oxidant-mediated lung injury. Am J
Physiol. 259:H144–H148. 1990.PubMed/NCBI
|
|
4
|
Katz SM, Sun S, Schechner RS, Tellis VA,
Alt ER and Greenstein SM: Improved small intestinal preservation
after lazaroid U74389G treatment and cold storage in University of
Wisconsin solution. Transplantation. 59:694–698. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flessas II, Papalois AE, Toutouzas K,
Zagouri F and Zografos GC: Effects of lazaroids on intestinal
ischemia and reperfusion injury in experimental models. J Surg Res.
166:265–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukuma K, Marubayashi S, Okada K, Yamada
K, Kimura A and Dohi K: Effect of lazaroid U-74389G and
methylprednisolone on endotoxin-induced shock in mice. Surgery.
125:421–430. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braughler JM, Chase RL, Neff GL, Yonkers
PA, Day JS, Hall ED, Sethy VH and Lahti RA: A new 21-aminosteroid
antioxidant lacking glucocorticoid activity stimulates
adrenocorticotropin secretion and blocks arachidonic acid release
from mouse pituitary tumor (AtT-20) cells. J Pharmacol Exp Ther.
244:423–427. 1988.PubMed/NCBI
|
|
8
|
Currin RT, Reinstein LJ, Lichtman SN,
Thurman RG and Lemasters JJ: Inhibition of tumor necrosis factor
release from cultured rat Kupffer cells by agents that reduce graft
failure from storage injury. Transplant Proc. 25:1631–1632.
1993.PubMed/NCBI
|
|
9
|
Shenkar R and Abraham E: Effects of
treatment with the 21-aminosteroid, U7438F, on pulmonary cytokine
expression following hemorrhage and resuscitation. Crit Care Med.
23:132–139. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishizaki N, Zhu Y, Zhang S, Nemoto A,
Kobayashi Y, Subbotin VM, Lee RG, Starzl TE and Todo S: Comparison
of various lazaroid compounds for protection against ischemic liver
injury. Transplant Proc. 29:1333–1334. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalra J, Mantha SV, Kumar P and Prasad K:
Protective effects of lazaroids against oxygen-free radicals
induced lysosomal damage. Mol Cell Biochem. 136:125–129. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garvin PJ, Niehoff ML, Robinson SM, Mistry
B, Esterl R, Heisler T, Combs C, Berson A, Solomon H and
Salinas-Madrigal L: Renoprotective effects of the 21-aminosteroid
U74389G in ischemia-reperfusion injury and cold storage
preservation. Transplantation. 63:194–201. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chrysikos DT, Sergentanis TN, Zagouri F,
Psaltopoulou T, Theodoropoulos G, Flessas I, Agrogiannis G,
Alexakis N, Lymperi M, Katsarou AI, et al: Lazaroid U-74389G
administration in pancreatic ischemia-reperfusion injury: a swine
model encompassing ischemic preconditioning. JOP. 16:176–184.
2015.PubMed/NCBI
|
|
14
|
Chrysikos DT, Sergentanis TN, Zagouri F,
Psaltopoulou T, Flessas I, Agrogiannis G, Alexakis N, Bramis I,
Patsouri EE, Patsouris ES, et al: The effect of U-74389G on
pancreas ischemia-reperfusion injury in a swine model. J Surg Res.
187:450–457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andreadou I, Poussios D, Papalois A,
Gavalakis N, Aroni K, Gazouli M, Gorgoulis VG and Fotiadis C:
Effect of U-74389G (21-lazaroid) on intestinal recovery after acute
mesenteric ischemia and reperfusion in rats. In Vivo. 17:463–468.
2003.PubMed/NCBI
|
|
16
|
Takahashi T, Takeyoshi I, Hasegawa Y,
Koyano T, Yamagishi T, Oshima K, Matsumoto K and Morishita Y:
Cardioprotective effects of Lazaroid U-74389G on
ischemia-reperfusion injury in canine hearts. J Heart Lung
Transplant. 18:285–291. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Todo S, Hamada N, Zhu Y, Zhang S, Subbotin
V, Nemoto A, Takeyoshi I and Starzl TE: Lazaroid U-74389G for
48-hour canine liver preservation. Transplantation. 61:189–194.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsaroucha AK, Papalois A, Vernadakis S,
Adamopoulos S, Papadopoulos K, Lambropoulou M, Constadinidis T,
Kyriazi A, Papadopoulos N and Simopoulos C: The effect of U-74389G
on liver recovery after acute liver ischemia-reperfusion injury in
a swine model. J Surg Res. 151:10–14. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawarski M, Hagerman TK and Karver CE:
Lazaroids U83836E and U74389G are potent, time-dependent inhibitors
of caspase-1. Chem Biol Drug Des. 86:1049–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu B, Fujise T, Iwakiri R, Ootani A,
Amemori S, Tsunada S, Toda S and Fujimoto K: Venous congestion
induces mucosal apoptosis via tumor necrosis factor-alpha-mediated
cell death in the rat small intestine. J Gastroenterol.
39:1056–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kilkenny C, Browne WJ, Cuthill IC, Emerson
M and Altman DG: Improving bioscience research reporting: The
ARRIVE guidelines for reporting animal research. PLoS Biol.
8:e10004122010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karamtsoukis SL, Trigka EA, Stasinopoulou
M, Stavridou A, Zacharioudaki A, Tsarea K, Karamperi M, Pittaras T,
Papadopoulos O, Patsouris E, et al: Beneficial effect of U-74389G
and Sildenafil in an experimental model of flap Iscemia/Reperfusion
Injury is swine. Histological and Biochemical evaluation of the
model. J Invest Surg. Nov 30–2018.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Bouboulis G, Bonatsos VG, Katsarou AI,
Karameris A, Galanos A, Zacharioudaki A, Theodoropoulos G, Zografos
G, Papalois AE and Toutouzas K: Experimental hemorrhagic shock
protocol in swine models: The effects of 21-Aminosteroid on the
small intestine. Curr Ther Res Clin Exp. 88:18–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swindle MM and Smith AC: Swine in the
laboratory: surgery, anesthesia, imaging and experimental
techniques, Third Edition. CRC Press. (Boca Raton, FL). pp58–59,
81. 2015.
|
|
25
|
Orfanos NF, Mylonas AI, Karmaniolou II,
Stergiou IP, Lolis ED, Dimas C, Papalois AE, Kondi-Pafiti AI,
Smyrniotis VE and Arkadopoulos NF: The effects of antioxidants on a
porcine model of liver hemorrhage. J Trauma Acute Care Surg.
80:964–971. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mylonas AI, Orfanos NF, Karmaniolou II,
Lolis ED, Stergiou EP, Papalois AE, Nomikos TN, Kondi-Pafiti AI,
Smyrniotis VE and Arkadopoulos NF: The effects of hemorrhagic shock
secondary to hepatectomy in a swine model. J Surg Res. 195:228–234.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hafez T, Moussa M, Nesim I, Baligh N,
Davidson B and Abdul-Hadi A: The effect of intraportal
prostaglandin E1 on adhesion molecule expression, inflammatory
modulator function, and histology in canine hepatic
ischemia/reperfusion injury. J Surg Res. 138:88–99. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chiu CJ, McArdle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesion in low-flow states. I. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nastos C, Kalimeris K, Papoutsidakis N,
Tasoulis MK, Lykoudis P, Theodoraki K, Nastou D, Smyrniotis V and
Arkadopoulos N: Global consequences of liver Ischemia/reperfusion
injury. Oxid Med Cell Longev. 2014:9069652014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Margonis N, Christoloukas N, Antoniou E,
Arkadopoulos N, Theodoropoulos G, Agrogiannis G, Pikoulis E,
Patsouris ES, Zografos GC and Papalois AE: Effectiveness of
sildenafil and U-74389G in a rat model of colitis. J Surg Res.
193:667–674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Oca J, Cuadrado S, Vallet J, Benasco C,
Martín E, Ardanuy C, Closa D, Hotter G and Jaurrieta E: Protective
effects of lazaroid U74389G on intestinal graft after heterotopic
small bowel transplantation in rats. J Surg Res. 75:18–23. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen H, Xu D, Qi S, Aboujaoude M, Sénéchal
J and Daloze P: 21-Aminosteroid lipid peroxidation inhibitor
U74389G protects the small bowel in the rat against warm and cold
ischemia damage. Transplant Proc. 26:1483–1484. 1994.PubMed/NCBI
|
|
33
|
Flessas I, Bramis I, Menenakos E,
Toutouzas K, Agrogiannis G, Patsouris E, Nonni A, Chrysikos D,
Korontzi M, Gioxari A, et al: Effects of lazaroid U-74389G on
intestinal ischemia and reperfusion injury in porcine experimental
model. Int J Surg. 13:42–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niederau C, Schulz HU and Klonowski H:
Lazaroids protect isolated rat pancreatic acinar cells against
damage induced by free radicals. Pancreas. 11:107–121. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Collard CD and Gelman S: Pathophysiology,
clinical manifestations, and prevention of ischemia-reperfusion
injury. Anesthesiology. 94:1133–1138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Squadrito F, Altavilla D, Ammendolia L,
Squadrito G, Campo GM, Sperandeo A, Canale P, Ioculano M, Saitta A
and Caputi AP: Improved survival and reversal of endothelial
dysfunction by the 21-aminosteroid, U-74389G in splanchnic
ischaemia-reperfusion injury in the rat. Br J Pharmacol.
115:395–400. 1995. View Article : Google Scholar : PubMed/NCBI
|