Introduction
The number of malformed fetuses further increases
with the ever-changing living conditions, which significantly
affects the family well-being and national population. Fetal
abnormality is referred to as developmental defects in fetal
intelligence development, morphology and physiology attributable to
various adverse factors in the course of fetal growth and
development (1). Among all
abnormalities, the congenital microtia, a common facial defect with
an incidence of 5/10,000, is early detected during the prenatal
examination, which is crucial for ensuring fetal quality. The
chromosome 10q26 of fibroblast growth factor receptor 2 (FGFR2)
gene, which primarily encodes tyrosine kinases, exerts a great
effect on the cell growth and development as well as angiogenesis,
and is involved in embryogenesis, especially in the process of
cartilage and bone formation. Previous studies have demonstrated
that the polymorphism of FGFR2 gene is bound up with the long bone
mineral density of Chinese people, but there are no reports on the
role of FGFR2 in bone formation and remodeling in adults (2,3).
Therefore, this study places emphasis on exploring the association
of the single-nucleotide polymorphism (SNP) of rs3135718 site in
FGFR2 gene with congenital microtia.
Patients and methods
General data
A total of 193 patients treated in Maternity and
Child Health Care of Zaozhuang (Zaozhuang, China) and meeting the
diagnostic criteria for congenital microtia and diagnosed with the
congenital microtia from January 2010 to October 2017 were randomly
selected as observation group. Moreover, 150 normal healthy
children with the same age from January 2010 to October 2017 were
selected as the control group. There were 105 males and 88 females
with an average age of 14.33±6.58 years in observation group, and
87 males and 63 females with an average age of 13.75±7.51 years in
control group. The statistical difference between the two groups
was in sex (P<0.05), but not in age (P>0.05).
The study was approved by the Ethics Committee of
Maternity and Child Health Care of Zaozhuang. Patients who
participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Sample collection
After 2 ml venous blood was collected from all
subjects enrolled, it was anti-coagulated with ethylenediamine
tetracetic acid, sub-packaged and stored at −80°C. The genomic
deoxyribonucleic acid (DNA) was extracted using the TIANamp Genomic
DNA kit (Tiangen Biotech Co., Ltd., Beijing, China) in accordance
with the instructions of the kit, and stored in a refrigerator at
−20°C for standby application.
Quantitative polymerase chain reaction (qPCR). The
primers (synthesized by Shanghai Invitrogen Biotechnology Co.,
Ltd., Shanghai, China) were designed using the Primer Premier5.0
software according to the DNA sequences of human FGFR2 gene
reported in the NCBI database, and the rs3135718 site in FGFR2 gene
was typed via Taq-man PCR. The primer sequences are shown in
Table I. The PCR system was 20 µl in
total: A total of 50 ng genomic DNA, 1X PCR buffer, 3 mmol/l
MgCl2, 4 mmol/l dNTPs, 5 pmol/l primers and 1 unit
TaqDNA polymerase (Qiagen, Inc., Valencia, CA, USA), and it was
loaded on a CFX96 instrument for amplification (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Parameters of qPCR
amplification: 95°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec,
and then extension at 72°C for 7 min after 30 cycles. The
experimental results were generated by the built-in software. Three
repeated wells were set for each sample. The experimental results
were analyzed using the 2−ΔΔCq method (4). The DEPC-treated water was used as the
negative control, and the positive plasmid containing this sequence
as the positive control (synthesized by Shanghai Sangon Biotech
Co., Ltd., Shanghai, China).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| SNP | Fragment
amplification primers | Probe primers |
|---|
| Rs3135718 | F:
5′-CCTCCAACAGTTTAGCTTTC-3′ |
FAM-CAACCCAAGCATTTTTAA |
|
| R:
5′-TGAGCAGAAGCAACGTGACC-3′ |
VIC-CAACCCGAGCATTTTTAA |
Statistical analysis
The Hardy-Weinberg equilibrium test was performed
for the genotype frequency of rs3135718 site in FGFR2 gene, and
SPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for processing. The
difference in allele frequency between the observation and control
group was analyzed using χ2 test. P<0.05 was
considered to indicate a statistically significant difference. The
associations of rs3135718 polymorphism with the risk and
clinicopathological parameters of congenital microtia were assessed
via the unconditional logistic regression odds ratio (OR) and
confidence interval (CI).
Results
Results of SNP genotyping
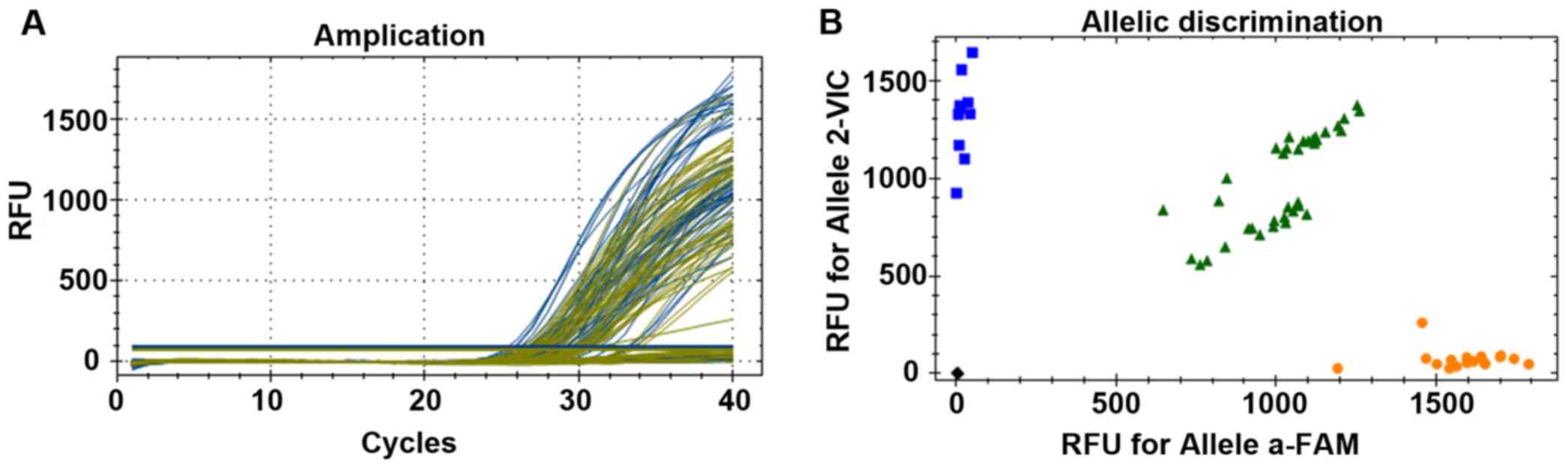
As shown in Fig. 1, a
wild homozygous type (AA type) was near the FAM abscissa, a mutant
homozygous type (GG type) was near the VIC ordinate, and a
heterogeneous type (AG type) was near the 45° line.
Hardy-Weinberg equilibrium analysis of distribution
of rs3135718 genotype in FGFR2 gene. As shown in Table II, the distribution of gene and
genotype frequency of rs3135718 site in FGFR2 gene in control group
were consistent with the Hardy-Weinberg equilibrium, indicating
that the subjects in this study were representative of the
population (P>0.05).
 | Table II.Hardy-Weinberg equilibrium analysis of
distribution of rs3135718 genotype in FGFR2 gene. |
Table II.
Hardy-Weinberg equilibrium analysis of
distribution of rs3135718 genotype in FGFR2 gene.
| Site | Genotype | Control group | Composition ratio
(%) | χ2
value | P-value |
|---|
| FGFR2 rs3135718 | AA | 78 | 52.0 |
|
|
|
| AG | 61 | 40.7 | 0.278 | 0.654 |
|
| GG | 11 | 7.33 |
|
|
Distribution of genotypes and their
influences on congenital microtia
As shown in Table
III, the influence of rs3135718 genotype (AG) on the prevalence
of congenital microtia had no statistically significant difference
between the observation group and control group (P>0.05).
 | Table III.Distribution of genotype and allele
frequency of rs3135718 site [n (%)]. |
Table III.
Distribution of genotype and allele
frequency of rs3135718 site [n (%)].
| Genotype | Observation group
(n=193) | Control group
(n=150) | χ2
value | OR (95% CI) | 95% CI | P-value |
|---|
| AA | 62 (32.1) | 78 (52) | 0.145 | 1.562 | 0.512–3.016 | 0.012 |
| AG | 68 (35.2) | 61 (40.7) | 0.040 | 0.328 | 0.411–2.568 | 0.850 |
| GG | 63 (32.6) | 11 (7.33) | 0.230 | 1.957 | 0.604–1.516 | 0.050 |
Logistic regression analysis of
association between genotypes and risk of fetal microtia
The GG and G genotypes in rs3135718 were closely
related to fetal microtia (P<0.05) (Table IV).
 | Table IV.Correlation between genotypes and risk
of fetal microtia, |
Table IV.
Correlation between genotypes and risk
of fetal microtia,
| SNP | Genotype | Crude OR (95%
CI) | P-value | Corrected OR (95%
CI) | P-value |
|---|
| rs3135718 | AA | 0.943
(0.749–1.186) | 0.614 | 0.987
(0.783–1.243) | 0.910 |
|
| GG | 0.630
(0.434–0.914) | 0.015 | 0.725
(0.497–1.057) | 0.095 |
|
| AG | 0.652
(0.461–0.923) | 0.016 | 0.731
(0.514–1.039) | 0.180 |
|
| A | 0.838
(0.713–0.985) | 0.032 | 0.893
(0.758–1.052) | 0.177 |
|
| G | 0.723
(0.686–0.875) | 0.052 | 0.783
(0.652–1.112) | 0.065 |
Analysis of association between
distribution of rs3135718 genotype frequency and risk of congenital
microtia in the sexes
The results revealed that the mutation of
rs3135718-GG site was more correlated with the risk of microtia in
male (P<0.05), but not correlated with the risk of microtia in
female (P>0.05), which could explain why the risk of microtia
was higher in male to a certain extent (Table V).
 | Table V.Analysis of correlation between
distribution of rs3135718 genotype frequency and risk of congenital
microtia in the sexes. |
Table V.
Analysis of correlation between
distribution of rs3135718 genotype frequency and risk of congenital
microtia in the sexes.
| Site | Sex | Genotype | Observation group
composition ratio (%) | Control group
composition ratio (%) | χ2
value | P-value | OR | 95% CI |
|---|
| rs3135718 | Male | AA | 54 | 44 | 0.218 | 0.756 | 1.000 |
|
|
|
|
| 51.43 | 50.57 |
|
|
|
|
|
|
| GG | 43 | 28 | 0.020 | 0.025 | 0.056 | 1.421–3.582 |
|
|
|
| 40.95 | 26.7 |
|
|
|
|
|
|
| AG | 6 | 4 | 0 | 0.956 | 1.312 | 0.451–4.238 |
|
|
|
| 4.8 | 4.41 |
|
|
|
|
|
| Female | AA | 50 | 34 | 0.059 | 0.952 | 1.000 |
|
|
|
|
| 56.8 | 53.97 |
|
|
|
|
|
|
| GG | 31 | 23 | 0.040 | 0.85 | 0.924 | 0.401–2.128 |
|
|
|
| 35.22 | 36.51 |
|
|
|
|
|
|
| AG | 6 | 5 | 0 | 1.00 | 0.85 | 0.185–3.901 |
|
|
|
| 6.82 | 7.94 |
|
|
|
|
Association between distribution of
allele frequency and risk of microtia
The distribution of allele frequency in rs3135718
gene in observation group and control group was analyzed using
χ2 test. The results confirmed that there was no
statistically significant difference in the distribution of allele
frequency in total population (P>0.05), while the difference in
distribution of allele frequency of rs3135718-G in male between the
two groups was statistically significant (P<0.05) (Table VI).
 | Table VI.Correlation between distribution of
allele frequency and risk of microtia. |
Table VI.
Correlation between distribution of
allele frequency and risk of microtia.
| Site | Sex | Genotype | Observation group
composition ratio (%) | Control group
composition ratio (%) | χ2 | P-value | OR | 95% CI |
|---|
| rs3135718 | General | A | 130 | 139 | 0.006 | 0.936 | 1.000 | – |
|
|
|
| 67.36 | 92.67 |
|
|
|
|
|
|
| G | 131 | 72 |
|
| 0.0486 | 1.682–2.451 |
|
|
|
| 67.88 | 61.07 |
|
|
|
|
|
| Male | A | 60 | 48 | 0.000 | 0.993 | 1.000 | – |
|
|
|
| 31.09 | 32 |
|
|
|
|
|
|
| G | 49 | 32 |
|
| 0.038 | 1.653–2.649 |
|
|
|
| 25.39 | 21.3 |
|
|
|
|
|
| Female | A | 56 | 39 | 0.068 | 0.795 | 1.000 | – |
|
|
|
| 29.02 | 26 |
|
|
|
|
|
|
| G | 37 | 28 |
|
| 0.858 | 0.487–1.734 |
|
|
|
| 19.17 | 18.67 |
|
|
|
|
Discussion
Microtia is one of the most common manifestations of
facial deformity with a high incidence (4,5). It was
reported that the prevalence of neonatal microtia is
0.84–17.5/10,000 in the world and 3.79/10,000 in Australia, and it
is higher in South America (3.2/10, 000) than that in other regions
(6,7). However, its etiology is still unclear.
It is currently believed that scuh a congenital defect is largely
attributable to both genetic and environmental factors (8,9). The
congenital microtia is primarily characterized by the abnormalities
in external and middle ears, conductive or mixed deafness, mostly
accompanied by preauricular pit, preauricular tag or accessory
auricles, and some patients may also suffer from cleft palate,
facial nerve dysplasia and facial asymmetry (10,11). The
SNP may be one of the original causes of congenital deformity.
Guizar-Vázquez et al first discovered patients with familial
aggregation microtia in the 1870s (12). Additionally, numerous studies have
proposed that microtia is an autosomal dominant genetic disorder
(13,14). Along with the advancement of
technology and the development of the times, the DNA detection has
been one of the essential means to diagnose autosomal dominant
genetic disorder. SNP is the most abundant form of DNA variation in
human genome (15,16). FGFRs, which belong to the family of
receptor tyrosine protein kinases and contain at least 22 exons,
act primarily by binding between their receptors and fibroblast
growth factors (17,18). Genetic studies have found that FGFR
plays a vital role in the growth and development of bone and
cartilage (19,20). This study was designed to investigate
the association of rs3135718 site in FGFR2 gene SNP with congenital
microtia.
In this study, the association between the
polymorphism of rs3135718 gene in FGFRs and congenital microtia was
investigated for the first time, and the genotype frequency
distribution in the sexes and the association of the distribution
of allele frequency with the risk of microtia were analyzed. The
data analysis indicated that the distribution of genotypes was in
line with the H-W equilibrium law, and the distribution of genotype
frequency of rs3135718 polymorphism site in FGFRs in observation
group and control group was statistically significant. Further
stratification analysis in the sexes revealed that the polymorphism
of rs3135718-G gene in FGFRs was related to the occurrence of
congenital microtia. Due to a quite complicated etiology of
congenital microtia and limited sample size in this experiment, the
study results need further confirmation.
The influence of rs3135718 genotype (AG) on the
prevalence of congenital microtia had no statistically significant
difference between the observation group and control group
(P>0.05). The GG and G genotypes in rs3135718 were closely
related to fetal microtia (P<0.05). The results revealed that
the rs3135718-GG mutation was more correlated with the risk of
microtia in male (P<0.05), but not correlated with the risk of
microtia in female (P>0.05). Moreover, there was a statistically
significant difference in the distribution of rs3135718-G allele
frequency in male between the groups (P<0.05), which accounted
for the high incidence of congenital microtia in male patients.
In conclusion, the rs3135718-G gene in FGFR2 has a
certain association with the incidence of fetal microtia with high
prevalence and risk.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RZ wrote the manuscript. PD helped with sample
collection. RZ and HS performed qPCR. LY and PL contributed to
statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Maternity and Child Health Care of Zaozhuang (Zaozhuang, China).
Patients who participated in this research had complete clinical
data. The signed informed consents were obtained from the patients
or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fischer S, Hirsch T, Hirche C, Kiefer J,
Kueckelhaus M, Germann G and Reichenberger MA: Surgical treatment
of primary gynecomastia in children and adolescents. Pediatr Surg
Int. 30:641–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yin L, Du X, Li C, Xu X, Chen Z, Su N,
Zhao L, Qi H, Li F, Xue J, et al: A Pro253Arg mutation in
fibroblast growth factor receptor 2 (Fgfr2) causes skeleton
malformation mimicking human Apert syndrome by affecting both
chondrogenesis and osteogenesis. Bone. 42:631–643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Xiao R, Yang F, Karim BO,
Iacovelli AJ, Cai J, Lerner CP, Richtsmeier JT, Leszl JM, Hill CA,
et al: Abnormalities in cartilage and bone development in the Apert
syndrome FGFR2(+/S252W) mouse. Development. 132:3537–3548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Canfield MA, Langlois PH, Nguyen LM and
Scheuerle AE: Epidemiologic features and clinical subgroups of
anotia/microtia in Texas. Birth Defects Res A Clin Mol Teratol.
85:905–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suutarla S, Rautio J, Ritvanen A,
Ala-Mello S, Jero J and Klockars T: Microtia in Finland: Comparison
of characteristics in different populations. Int J Pediatr
Otorhinolaryngol. 71:1211–1217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forrester MB and Merz RD: Descriptive
epidemiology of anotia and microtia, Hawaii, 1986–2002. Congenit
Anom (Kyoto). 45:119–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harris J, Källén B and Robert E: The
epidemiology of anotia and microtia. J Med Genet. 33:809–813. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel-Friedrich S: Congenital auricular
malformations: Description of anomalies and syndromes. Facial Plast
Surg. 31:567–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Artunduaga MA, Quintanilla-Dieck ML,
Greenway S, Betensky R, Nicolau Y, Hamdan U, Jarrin P, Osorno G,
Brent B, Eavey R, et al: A classic twin study of external ear
malformations, including microtia. N Engl J Med. 361:1216–1218.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luquetti DV, Heike CL, Hing AV, Cunningham
ML and Cox TC: Microtia: Epidemiology and genetics. Am J Med Genet
A 158A. 124–139. 2012. View Article : Google Scholar
|
|
12
|
Guizar-Vázquez J, Arredondo-Vega F,
Rostenberg I, Manzano C and Armendares S: Microtia and meatal
atresia in mother and son. Clin Genet. 14:80–82. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zankl M and Zang KD: Inheritance of
microtia and aural atresia in a family with five affected members.
Clin Genet. 16:331–334. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orstavik KH, Medbø S and Mair IW:
Right-sided microtia and conductive hearing loss with variable
expressivity in three generations. Clin Genet. 38:117–120. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chafai Elalaoui S, Cherkaoui Jaouad I,
Rifai L and Sefiani A: Autosomal dominant microtia. Eur J Med
Genet. 53:100–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Konigsmark BW, Nager GT and Haskins HL:
Recessive microtia, meatal atresia, and hearing loss. Report of a
sibship. Arch Otolaryngol. 96:105–109. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmid M, Schröder M, Langenbeck U, Opitz
JM and Reynolds JF: Familial microtia, meatal atresia, and
conductive deafness in three siblings. Am J Med Genet. 22:327–332.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strisciuglio P, Ballabio A and Parenti G:
Microtia with meatal atresia and conductive deafness: Mild and
severe manifestations within the same sibship. J Med Genet.
23:459–460. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ornitz DM and Marie PJ: FGF signaling
pathways in endochondral and intramembranous bone development and
human genetic disease. Genes Dev. 16:1446–1465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su N, Sun Q, Li C, Lu X, Qi H, Chen S,
Yang J, Du X, Zhao L, He Q, et al: Gain-of-function mutation in
FGFR3 in mice leads to decreased bone mass by affecting both
osteoblastogenesis and osteoclastogenesis. Hum Mol Genet.
19:1199–1210. 2010. View Article : Google Scholar : PubMed/NCBI
|