Introduction
Rheumatoid arthritis (RA) is a diffuse connective
tissue disease (1,2). RA is a chronic, systemic, autoimmune
disorder that can affect multiple organ systems (2). RA affects 0.5–1% of the population and
is most prevalent in older individuals, with an increased risk of
developing RA in women (2). Due to
structural damage and dysfunction of the joints caused by RA,
patient mobility is limited, daily life is affected and the overall
quality of life for patients and family members is reduced
(3). It is recommended to begin
treating patients with RA as early as possible (1–3).
Traditional RA treatment is relatively affordable, but treatment
efficacy is delayed and is associated with a number of side
effects, which include neurotoxicity following long-term use
(3). Novel bio-drugs, which include
methotrexate (MTX), exhibit improved therapeutic effects, however,
costs are increased and prolonged use can affect cognitive
functions (3). In order to
facilitate the discovery of novel anti-RA treatment is required
without the side effects and costs associated with current
treatment options, the underlying mechanisms of RA will need to be
further examined.
In Traditional Chinese Medicine, various
prescription medicines exhibit pain relieving and bone
strengthening functions, including Strychnosnux vomica
(4–6). Strychnosnux vomica has been used
in Traditional Chinese Medicine for hundreds of years for the
treatment of various diseases, including several types of cancer,
orthopedic diseases and inflammatory disorders (4–6). Brucine
is extracted from the seeds of Strychnosnux vomica (5) and it has been used as clinical
treatment for several types of cancer, including multiple myeloma
(6), diabetes mellitus (7) and inflammatory diseases (8). Brucine activates various signaling
pathways, including the osteoprotegerin/receptor activator of
nuclear factor κ-Β (RANK)/RANK ligand (L), the Jagged1/Notch 1 and
thevascular endothelial growth factor receptor 2 signaling pathways
at cellular levels (5–8). A previous study reported that
immuno-nanoparticles can selectively inhibit tumor cell growth in
hepatocellular carcinoma (9).
Brucine further inhibits fibroblast growth, including in synovial
cells, serves analgesic effects and induces apoptosis in
vitro (10,11). Therefore, the current study explored
effects of brucine on human fibroblast-like synoviocytes (HFLS) of
RA and investigated the associated molecular mechanisms.
Materials and methods
Instruments
FC microplate reader and Class 100 CO2
gas incubator were purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). An HF safe 1200/c biosafety cabinet was
purchased from Shanghai Lishen Scientific Equipment Co., Ltd.
(Shanghai, China). The Mini-Protean Tetra system was purchased from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Reagents preparation
Recombinant tumor necrosis factor-α (TNF-α; cat. no.
300-01A; PeproTech, Inc., Rocky Hill, NJ, USA) dry powder (0.5 g)
was dissolved in PBS and centrifuged at 1,500 × g for 20 min at
room temperature. TNF-α was then dissolved in Dulbecco's modified
Eagle's medium (DMEM)/high glucose (10 µg/l; cat. no. SH30022.01B;
HyClone; GE Healthcare Life Sciences, Logan, UT, USA). Brucine
(cat. no. MUST12122812; Chengdu Must Bio-Technology Co., Ltd.,
Chengdu, China) dry powder (5 g) was dissolved in PBS and
centrifuged at 1,500 × g for 20 min at room temperature. The
supernatant was used to make a 20 mg/ml stock solution. c-Jun
N-terminal kinase (JNK) specific inhibitor SP600125 (Cell Signaling
Technology, Inc., Danvers, MA, USA) was added to the cells (at
final concentration of 25 mol/l) to inhibit JNK
phosphorylation.
Cell Counting Kit-8 (CCK-8) assay
HFLS-RA cells (cat. no. #408RA-05a; Cell
Application, Inc., San Diego, CA, USA) were cultured in DMEM/high
glucose and maintained at 37°Cin a 5% CO2-humidified
incubator. Cells were passaged to the fourth generation prior to
subsequent experimentation. Cell viability was examined using the
CCK-8 kit (QF0025; Shanghai Xiangsheng Biotechnology Co., Ltd.,
Shanghai, China), according to the manufacturer's protocol. In
brief, HFLS-RA cells were seeded into 96-well plates
(5×105/ml) and cultured for 24 h at 37°C. Subsequently,
cells were incubated with 10 µl TNF-α (10 µg/l) for 30 min at 37°C
followed by treatment with various concentrations (0.125, 0.25, 0.5
or 2 mg/ml) brucine or DMEM/high glucose (TNF-α control) for 24 h
at 37°C. For the control group, cells were treated with PBS only.
Following 24-h incubation, CCK-8 solution (10 µl) was added to each
well and further incubated at 37°C for 3 h. Absorbance measurements
at 450 nm were performed to evaluate the viability of HFLS-RA.
Viability was reported as the percentage of optical density (OD) of
treatment groups/OD of blank control (PBS only).
Western blotting
HFLS-RA were seeded into 96-well plates at a density
of 5×104 cells/well for 24 h at 37°C. Subsequently,
cells were incubated with 10 µl TNF-α (10 µg/l) for 30 min at 37°C
followed by treatment with various concentrations (0.125, 0.25, 0.5
and 2 mg/ml) of brucine or PBS (blank control group) for 24 h at
37°C. Total protein was extracted from cells using the
ProteoPrep® Total Extraction Sample kit (cat. no.
PROTTOT-1KT; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
according to the manufacturer's protocol. Total protein was
quantified using the bicinchoninic acid assay kit (cat. no. BCA1;
Sigma-Aldrich; Merck KGaA) and 0.2 µg protein/lane was separated
via SDS-PAGE on a 15% gel. The separated proteins were transferred
onto polyvinylidene difluoride membranes (Amersham; GE Healthcare,
Chicago, IL, USA) and blocked for 2 h at 37°C with 5% skimmed milk.
The membranes were rinsed withPBSTween-20 (PBST) prior to
incubation with mouse anti-human JNK monoclonal antibody (1:3,000;
cat. no. sc136205), mouse anti-human phosphorylated (p)-JNK
monoclonal antibody (1:2,000; cat. no. sc6254) or mouse anti-human
β-actin monoclonal antibody (1:3,000; cat. no. sc376421; all Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room
temperature. Membranes were washed three times with PBST. The
membranes were incubated with horseradish peroxidase-conjugated
rabbit anti-mouse polyclonal antibody (1:2,000; cat. no. sc358914;
Santa Cruz Biotechnology, Inc.) for 30 min at 37°C. Membranes were
washed six times with PBST. Protein bands were visualized using
enhanced chemiluminescence (Amersham; GE Healthcare) and images
were captured and analyzed using Quantity One (version 4.52;
Bio-Rad Laboratories, Inc.).
Statistical analysis
Data presented as the mean ± standard deviation were
and analyzed with SPSS (version 17.0; SPSS, Inc., Chicago, IL,
USA). Data are representative of ≥6 independent tests. One-way
analysis of variance followed by Tukey's post-hoc test was used in
the comparison of multiple groups. The association between brucine
dosage and JNK phosphorylation was analyzed using a linear
regression analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Brucine treatment reverses increased
TNF-α-induced cell viability
Effect of brucine on cell growth was observed in
TNF-α-treated HFLS-RA. TNF-α significantly improved cell viability
compared with the untreated Control as determined by CCK-8 assay
(P<0.05; Table I). Brucine
treatment of TNF-α-treated HFLS-RA significantly reversed the
observed viability increases induced by TNF-α (P<0.05; Table I). Low doses of brucine (0.125 and
0.25 mg/ml) reduced the cell viability of TNF-α-treated HFLS-RA to
levels just above those determined for the untreated Control
(P>0.05 and P<0.05, respectively; Table I). Higher doses of brucine (≥0.5
mg/ml) decreased the cell viability of TNF-α-treated HFLS-RA to
levels significantly lower compared with the untreated control
(P<0.05; Table I).
 | Table I.HFLS-RA viability with
TNF-αpretreatment followed by treatment with various concentrations
of brucine. |
Table I.
HFLS-RA viability with
TNF-αpretreatment followed by treatment with various concentrations
of brucine.
| Group | Repeats (n) | Cell viability
(%) |
|---|
| 0.125 mg/ml brucine +
TNF-α | 6 |
78.59±1.70b,c |
| 0.25 mg/ml brucine +
TNF-α | 6 |
86.95±1.60a–c |
| 0.5 mg/ml brucine +
TNF-α | 6 |
74.37±0.91a–c |
| 2 mg/ml brucine +
TNF-α | 6 |
69.22±0.90a–c |
| TNF-α | 6 |
109.1±1.10a |
| Control | 6 | 77.25±2.01 |
| Blank cell | 6 | 100±0.00 |
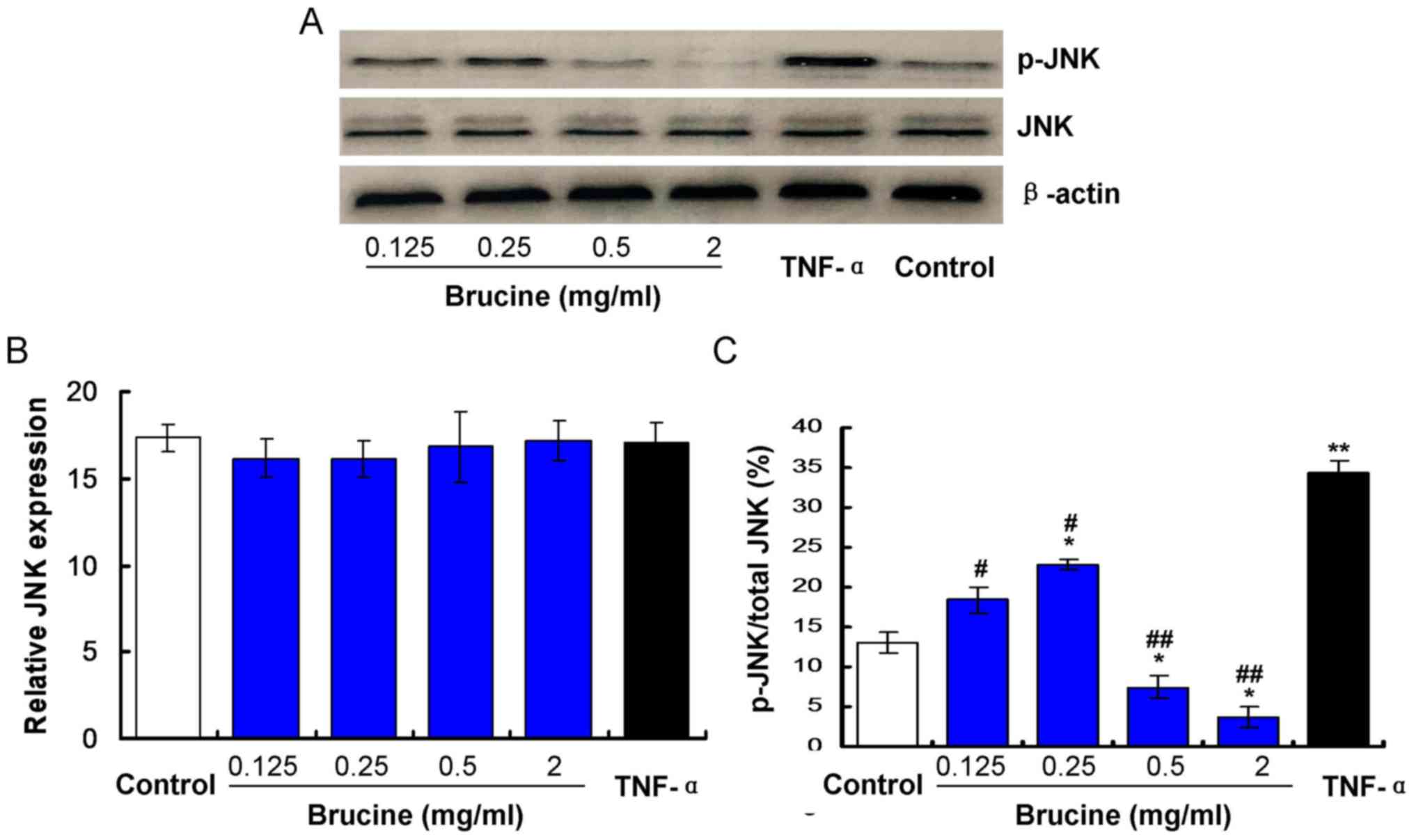
Brucine reduces JNK phosphorylation in
TNF-α-treated HFLS-RA
Expression of JNK in TNF-α-induced HFLS-RA cells
exposed to varying concentrations of brucine was evaluated. The
results indicated no significant differences in JNK expression
between the TNF-α and the Control groups, the brucine and the TNF-α
groups, the brucine and the Control groups or among the brucine
groups (P>0.05; Fig. 1). To
investigate effects of brucine on JNK phosphorylation, p-JNK/JNK
levels were examined by western blotting. Results indicated that
the p-JNK/JNK value was significantly increased in TNF-α-treated
HFLS-RA compared with the untreated Control (P<0.05; Fig. 1C). Significant decreases were
observed in the brucine-treated groups compared with the
TNF-α-treated HFLS-RA (P<0.05; Fig.
1C). At low brucine treatment doses (0.25 mg/ml) the p-JNK/JNK
values were significantly increased compared with the untreated
control (P<0.05) and at higher treatment doses (≥0.5 mg/ml)
p-JNK/JNK values were decreased compared with the untreated Control
(P<0.05; Fig. 1C).
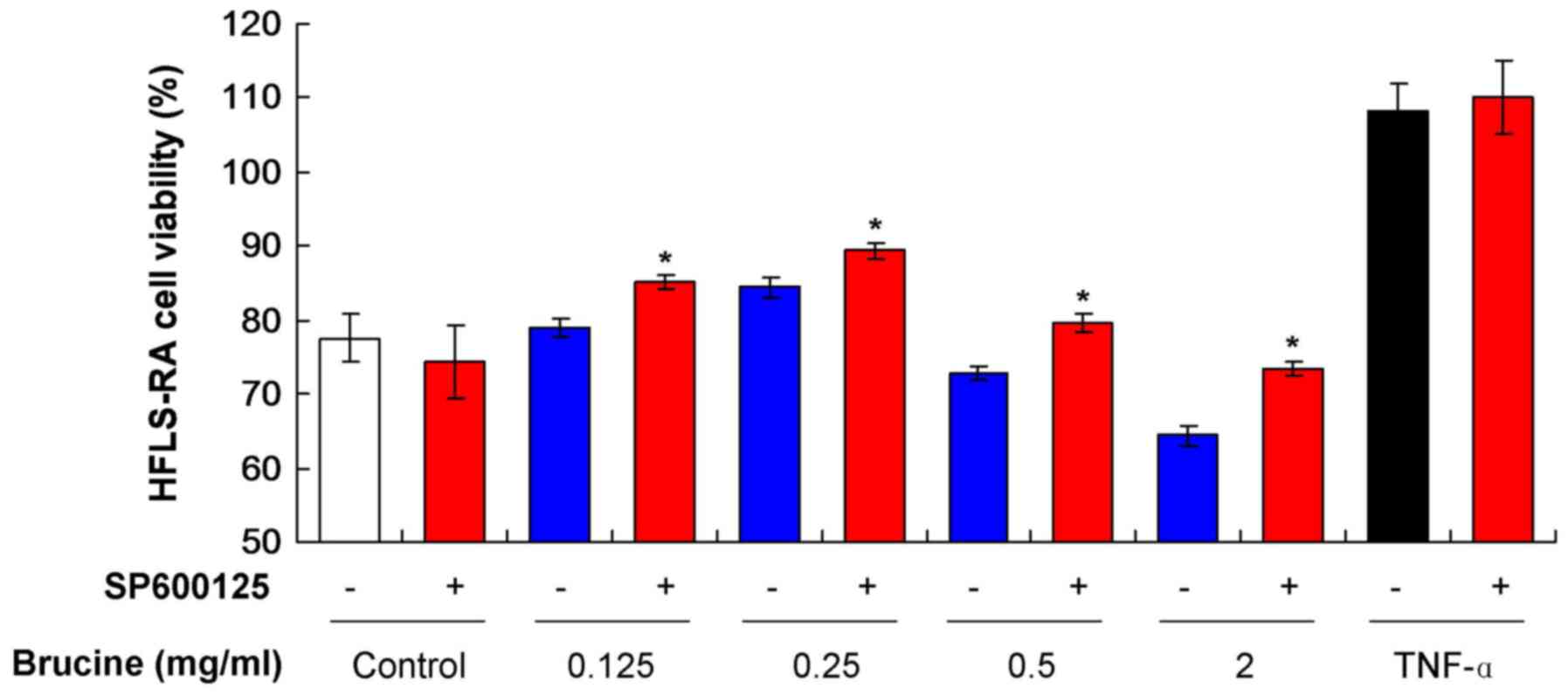
Brucine reduces HFLS-RA viability by
activating the JNK signaling pathway
To assess whether HFLS-RA viability was affected by
the activation of the JNK signaling pathway, SP600125, a JNK
specific inhibitor, was added to the various TNF-α-induced HFLS-RA
treated with brucine. The result indicated that SP600125 treatment
increased cell viability in all TNF-α-induced HFLS-RA groups
(Fig. 2). SP600125 induced a
decrease in viability in the untreated Control group. Significant
increases were observed in all groups treated with SP600125 and
brucine compared with the groups only treated with brucine
(P<0.05; Fig. 2). These results
suggest that brucine reduced HFLS-RA viability by activating the
JNK signaling pathway, however, as HFLS-RA viability did not fully
recover, other pathways may also be involved.
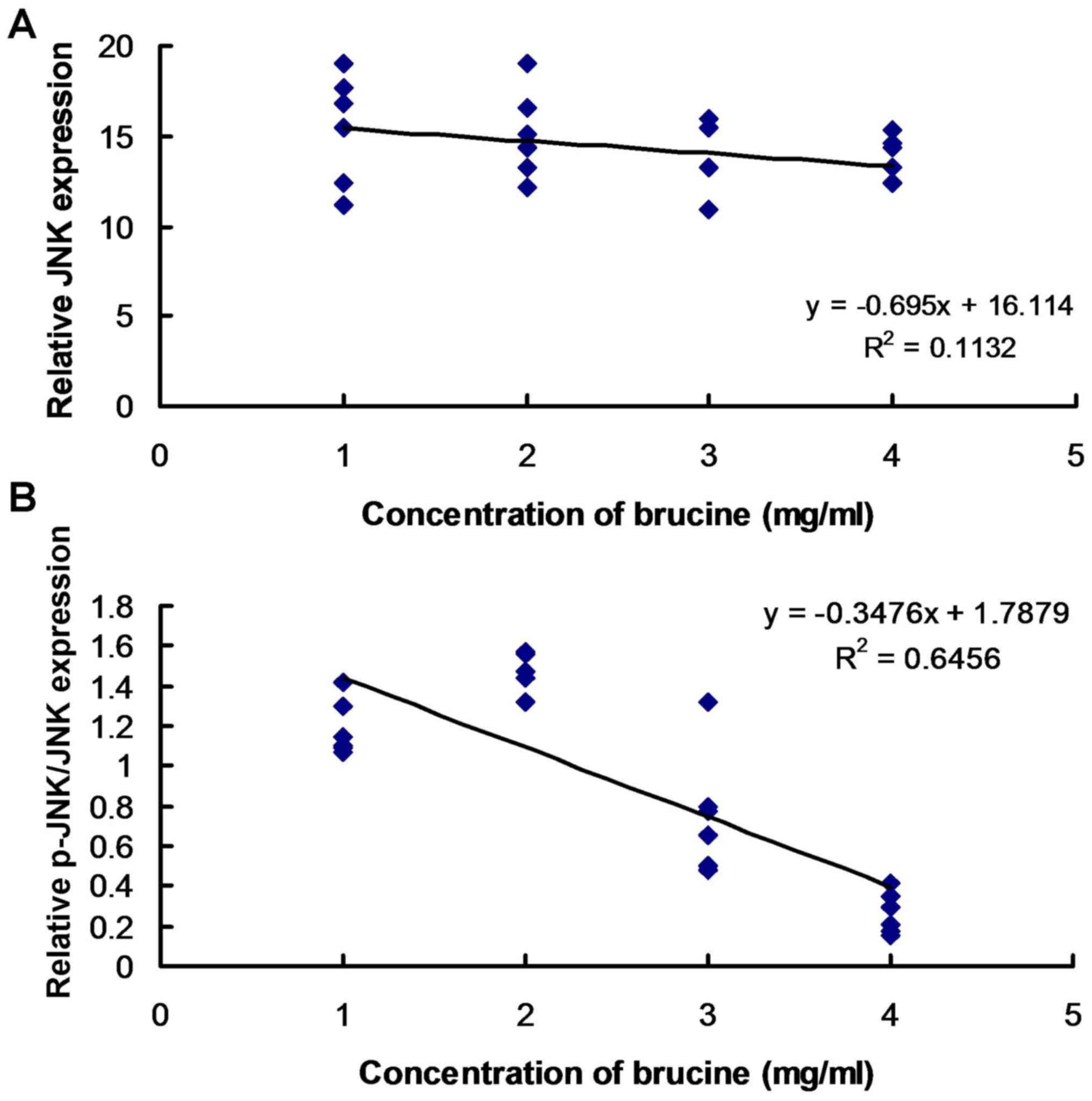
Brucine is associated with JNK
phosphorylation in TNF-α-treated HFLS-RA
Linear regression analysis was performed to assess
the association between JNK expression and phosphorylation and
brucine concentration. The results suggest that there was no
association between JNK expression and brucine concentration
(Fig. 3A). JNK phosphorylation
exhibited an association with varying concentrations of brucine in
TNF-α-induced HFLS-RA (Fig. 3B).
Discussion
In recent years, excessive proliferation and
activity of FLS have been reported to be the major reason for joint
injury in RA (12,13). Stimuli from inflammatory factors
induce tumor-type unlimited proliferation in FLS (14). TNF-α is a proinflammatory factor
produced by lymphomonocytes and macrophages with multiple roles,
including the magnification of inflammatory signals and the
induction of excessive FLS proliferation (15). Amplified inflammatory signals can
trigger secretion of inflammatory factors, including interleukin
(IL)-1 and IL-6, which aggravate synovitis and further damage the
joints (16).
Brucine is a herbal compound extensively used in
China, Thailand, East India and Northern Australia (17). Previous studies reported various
functions of brucine, including anti-inflammation, antitumor,
antianemia, antianalgesia, antidiabetes and antigonorrhea (18,19). Wu
et al (8) recently reported
that brucine inhibits arthritis symptoms and synoviocytes growth in
an arthritis rat model. The current study investigated whether
brucine acts as a TNF-α inhibitor and exerts antiproliferation
effects in TNF-α-induced HFLS-RA. The present study illustrated
that lower brucine doses (≤0.25 mg/ml) significantly decreased
TNF-α-induced viability changes to levels increased compared with
the untreated Control, thus, not fully reversing the effects
exerted by TNF-α. High brucine doses (≥0.5 mg/ml) fully reversed
TNF-α-induced effects and further decreased cell viability compared
with the untreated Control. The results of the present study are
consistent with findings by Xin et al (20) regarding the induction of apoptosis
inhuman monocytic leukemia cells by brucine.
A previous study reported that the mitogen-activated
protein kinase (MAPK) signaling pathway induces expression of TNF-α
in FLS (21). There are various
enzymes associated with the MAPK signaling pathway, including JNK,
p38 and extracellular signal-regulated kinase (21). Phosphorylation of these kinases
triggers participation in pathogenic processes of synovitis
(22). Therefore, the present study
examined JNK expression and phosphorylation in TNF-α-induced
HFLS-RA with brucine treatment. It was observed that JNK expression
was not affected by brucine treatment, but phosphorylation levels
were decreased in the brucine treatment groups compared with the
TNF-α group. Only doses ≥0.5 mg/ml brucine fully reversed
TNF-α-induced effects and further decreased phosphorylation levels
compared with the untreated Control. A linear regression analysis
suggested that brucine dose-dependently activated JNK
phosphorylationin TNF-α-induced HFLS-RA. To further confirm the
effects of brucine were mediated by the JNK signaling pathway,
SP600125, a specific inhibitor, was employed in TNF-α-induced
HFLS-RA. It was observed that the presence of the inhibitor
alleviated the inhibitory effects exerted by brucine. However,
viability levels did not fully recover to match the TNF-α-induced
levels. The results indicated that brucine partially modulated cell
viability of HFLS-RA by activating the JNK signaling pathway. The
results indicated that TNF-α significantly activated JNK
phosphorylation in HFLS-RA and triggered proliferation. The results
are consistent with previous findings reporting that TNF-α promoted
HFLS-RA proliferation through JNK activation of the MAPK signaling
pathway (23).
A previous study demonstrated that brucine modulates
physiological functions by regulating cell immune responses
(9). Furthermore, brucine serves a
role in several cellular processes, which include inhibiting tumor
cell growth, conducting analgesic effects and inducing cell
apoptosis (9–11). Based on a preliminary data (not
shown), brucine has low bioavailability, however the current study
used various concentrations of brucine ranging from 0.125–2 mg/ml,
including optimal and higher brucine concentrations. However, a
limitation associated with the current study is the lack of IC50
assay to measure the potency of brucine.
In conclusion, TNF-α promoted HFLS-RA proliferation
and brucine significantly inhibited TNF-α-induced effects in
HFLS-RA partially by activating the JNK signaling pathway. Brucine
was further associated with JNK phosphorylation in TNF-α-treated
HFLS-RA. Therefore, brucine may be physiologically relevant and
have potential applications in the therapy of RA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated and/or analyzed during the
current study is available from the corresponding author on
reasonable request.
Authors' contributions
MT, WJZ and ZCY performed the experiments. ZCY
performed the statistical analysis. MT and CSH contributed to the
design of the study and prepared the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Muraki Y, Mizuno S, Nakatani K,
Wakabayashi H, Ishikawa E, Araki T, Taniguchi A, Isaji S and Okuda
M: Monitoring of peripheral blood cluster of differentiation
4+ adenosine triphosphate activity and CYP3A5 genotype
to determine the pharmacokinetics, clinical effects and
complications of tacrolimus in patients with autoimmune diseases.
Exp Ther Med. 15:532–538. 2018.PubMed/NCBI
|
|
2
|
Shiraishi T, Ishimoto H, Akata K, Kawanami
T, Yatera K and Mukae H: An autopsy case report of adult T-cell
leukemia accompanied by rheumatoid arthritis mimicking diffuse
panbronchiolitis. J UOEH. 39:55–61. 2017.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van den Bemt BJ, Zwikker HE and van den
Ende CH: Medication adherence in patients with rheumatoid
arthritis: A critical appraisal of the existing literature. Expert
Rev Clin Immunol. 8:337–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Qu Y, Wang D, Peng P, Cai H, Gao
Y, Chen Z and Cai B: Pharmacological evaluation of total alkaloids
from nux vomica: Effect of reducing strychnine contents.
Molecules. 19:4395–4408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shu G, Mi X, Cai J, Zhang X, Yin W, Yang
X, Li Y, Chen L and Deng X: Brucine, an alkaloid from seeds of
Strychnos nux-vomica Linn., represses hepatocellular
carcinoma cell migration and metastasis: The role of hypoxia
inducible factor 1 pathway. Toxicol Lett. 222:91–101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rao PS and Prasad MN: Strychnos
nux-vomica root extract induces apoptosis in the human multiple
myeloma cell line-U266B1. Cell Biochem Biophys. 66:443–450. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhati R, Singh A, Saharan VA, Ram V and
Bhandari A: Strychnos nux-vomica seeds: Pharmacognostical
standardization, extraction, and antidiabetic activity. J Ayurveda
Integr Med. 3:80–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu P, Liang Q, Feng P, Li C, Yang C, Liang
H, Tang H and Shuai C: A novel brucine gel transdermal delivery
system designed for anti-inflammatory and analgesic activities. Int
J Mol Sci. 18:E7572017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin JM, Yin PH, Li Q, Sa ZQ, Sheng X, Yang
L, Huang T, Zhang M, Gao KP, Chen QH, et al: Anti-tumor effects of
brucine immuno-nanoparticles on hepatocellular carcinoma. Int J
Nanomedicine. 7:369–379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jakubík J, Krejcí A and Dolezal V:
Asparagine, valine, and threonine in the third extracellular loop
of muscarinic receptor have essential roles in the positive
cooperativity of strychnine-like allosteric modulators. J Pharmacol
Exp Ther. 313:688–696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin W, Wang TS, Yin FZ and Cai BC:
Analgesic and anti-inflammatory properties of brucine and brucine
N-oxide extracted from seeds of Strychnos nux-vomica. J
Ethnopharmacol. 88:205–214. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Zhao F and Nie J: Anti-rheumatic
effects of Aconitum leucostomum Worosch. on human fibroblast-like
synoviocyte rheumatoid arthritis cells. Exp Ther Med. 14:453–460.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan F, Zhu L, Lv H and Pei C: Quercetin
promotes the apoptosis of fibroblast-like synoviocytes in
rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med.
38:1507–1514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Lei M, Yu C, Lv Y, Song Y and Yang
L: Mechano growth factor-E regulates apoptosis and inflammatory
responses in fibroblast-like synoviocytes of knee osteoarthritis.
Int Orthop. 39:2503–2509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kadkhoda Z, Amirzargar A, Esmaili Z,
Vojdanian M and Akbari S: Effect of TNF-α blockade in gingival
crevicular fluid on periodontal condition of patients with
rheumatoid arthritis. Iran J Immunol. 13:197–203. 2016.PubMed/NCBI
|
|
16
|
Harigai M, Hara M, Kawamoto M, Kawaguchi
Y, Sugiura T, Tanaka M, Nakagawa M, Ichida H, Takagi K,
Higami-Ohsako S, et al: Amplification of the synovial inflammatory
response through activation of mitogen-activated protein kinases
and nuclear factor kappaB using ligation of CD40 on CD14+ synovial
cells from patients with rheumatoid arthritis. Arthritis Rheum.
50:2167–2177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo W, Wang X, Zheng L, Zhan Y, Zhang D,
Zhang J and Zhang Y: Brucine suppresses colon cancer cells growth
via mediating KDR signaling pathway. J Cell Mol Med. 17:1316–3124.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deng XK, Yin W, Li WD, Yin FZ, Lu XY,
Zhang XC, Hua ZC and Cai BC: The anti-tumor effects of alkaloids
from the seeds of Strychnos nux-vomica on HepG2 cells and
its possible mechanism. J Ethnopharmacol. 106:179–186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agrawal SS, Saraswati S, Mathur R and
Pandey M: Cytotoxic and antitumor effects of brucine on Ehrlich
ascites tumor and human cancer cell line. Life Sci. 89:147–158.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin F, Wei W, Ji AF, Shen XL, Zhang GX,
Zhang MX, Li XX and Zhang HY: Inducing-apoptosis effect of brucine
on human monocytic leukemia cell line THP-1 and its mechanism.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 22:681–686. 2014.(In Chinese).
PubMed/NCBI
|
|
21
|
Zuo J, Xia Y, Li X, Ou-Yang Z and Chen JW:
Selective modulation of MAPKs contribute to the anti-proliferative
and anti-inflammatory activities of
1,7-dihydroxy-3,4-dimethoxyxanthone in rheumatoid arthritis-derived
fibroblast-like synoviocyte MH7A cells. J Ethnopharmacol.
168:248–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan JX: LncRNA H19 promotes
atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur
Rev Med Pharmacol Sci. 21:322–328. 2017.PubMed/NCBI
|
|
23
|
Knies N, Alankus B, Weilemann A, Tzankov
A, Brunner K, Ruff T, Kremer M, Keller UB, Lenz G and Ruland J:
Lymphomagenic CARD11/BCL10/MALT1 signaling drives malignant B-cell
proliferation via cooperative NF-κB and JNK activation. Proc Natl
Acad Sci USA. 112:E7230–E7238. 2015. View Article : Google Scholar : PubMed/NCBI
|