Introduction
Acquired immunodeficiency syndrome (AIDS) is a
serious immunodeficiency disease infected by human immunodeficiency
virus (HIV) (1). AIDS patients are
mostly promiscuous, multi-transfused, homosexual and intravenous
drug addicted. Sexual transmission is the main route of the
transmission of HIV infection, while other routes are
mother-to-child transmission, blood product transfusion, organ
transplantation and drug use with syringes (2). At present, the number of AIDS patients
worldwide has reached 37 million, and the incidence has increased
year by year (3). AIDS is mainly
manifested by fatigue, fever and other clinical symptoms, with the
characteristics of slow onset and high fatality rate. It mainly
invades the immune system of patients, causing serious damage to
their immune function (4). AIDS can
gradually develop into a secondary infection, prone to various
pathogenic bacteria infections. In clinical practice, central
nervous system (CNS) infections are common in AIDS patients
(5).
The CNS of the normal human body can resist the
invasion of various pathogens, but AIDS patients have impaired
immune function and decreased resistance, and the brain and spinal
cord are easily infected by various pathogens, which in turn leads
to CNS infection (6,7). Opportunistic CNS infection is the most
common complication in patients with advanced AIDS (8). CNS infections are usually encephalitis
caused by bacteria invading the CNS and meningitis caused by spinal
pachymeningitis or meninges. The most common CNS infection diseases
in AIDS patients are tuberculous meningitis and cryptococcal
meningitis (9,10). Tuberculous meningitis is a
non-suppurative inflammation of CNS, mostly caused by the invasion
of tubercle bacillus of the ependyma and meninge into subarachnoid
space. Cryptococcal meningitis is a chronic inflammatory disease
with chronic or subacute infection of CNS infected by cryptococcus
neoformans. AIDS complicated with tuberculous meningitis or
cryptococcal meningitis is the main cause of death (11,12).
There is a close relationship between the human immune system and
the nervous system, and when CNS infection occurs, the levels of
various cytokines in the body will be abnormally expressed
(13).
At present, there is no report on the clinical
characteristics and cerebro-spinal fluid (CSF) cytokines changes in
AIDS patients with tuberculous meningitis and cryptococcal
meningitis. The aim of this study was to provide a feasible method
for the early diagnosis and prognosis of AIDS patients with CNS
infectious diseases by observing the clinical symptoms of AIDS
patients with tuberculosis meningitis and cryptococcal meningitis
and the significance of cytokines in CSF.
Materials and methods
General data
The clinical records of 80 AIDS patients with CNS
infection and 40 non-CNS infection patients hospitalized in the
Infection Department of The First Hospital of Changsha (Changsha,
China) from February 2013 to March 2016 were retrospectively
analyzed. Forty-one AIDS patients complicated with tuberculous
meningitis were enrolled as group A, including 29 males and 12
females, with an age range of 23–67 years and an average age of
36.15±10.63 years; 39 AIDS patients complicated with cryptococcal
meningitis were enrolled as group B, including 27 males and 12
females, with an age range of 20–72 years and an average age of
34.14±11.42 years; and 40 patients with non-CNS infection with
lumbar puncture indication were enrolled as group C, including 25
males and 15 females, with an age range of 25–67 years and an
average age of 33.09±9.15 years.
This study was approved by the Ethics Committee of
The First Hospital of Changsha. All the subjects were informed and
agreed to participate in the clinical study, and signed a complete
informed consent form.
Inclusion and exclusion criteria
Inclusion criteria were: in line with the AIDS
diagnostic criteria of the US Centers for Disease Control and
Prevention (CDC) 2015 (14), enzyme
linked immunosorbent assay (ELISA) and western blot confirmed HIV
antibody as positive; the clinical symptoms were headache, fever,
nausea, consciousness disorder and meningeal irritation;
tuberculous meningitis patients with acute and subacute clinical
symptoms, and mycobacterium tuberculosis detected by CSF smear;
patients diagnosed with cryptococcal meningitis by fungal ink
staining, fungal culture, urease test and imaging examination;
patients receiving no anti-tuberculosis, anti-cryptococcus
neoformans and highly active anti-retroviral therapy (HAART) in the
past. Exclusion criteria were: patients complicated with deep
fungal infections such as candidiasis, histoplasmosis and
penicilliosis marneffei; patients with severe liver, kidney and
hematopoietic dysfunction; patients with mental illness or a family
history of mental illness.
Research methods
The general data, clinical symptoms, CSF examination
and prognosis of 3 groups of patients were collected. The CSF
biochemical indexes (including pressure, leucocyte count, glucose,
chloride, protein) were detected within 1 day after admission, and
the death of patients during admission was recorded. The clinical
data, treatment and prognosis of the patients, as well as the
follow-up results were summarized.
Treatment outcome
Patients in group A were given anti-tuberculosis
treatment with 2 HRZE/4HR regimen. Whereas patients in group B were
treated with 1,200 mg/day oral fluconazole for 15 days, followed by
400 mg/day for 45 days and 200 mg/day for life. Patients in the two
groups received HAART at the 3rd week of treatment. If the patients
were able to tolerate anti-infection and anti-retroviral treatment,
HAART was continued. If not, HAART was terminated and other
symptomatic treatment was given. Judgment criteria for improvement:
no meningeal irritation sign, local orientational sign of nervous
system and consciousness disorder. Of the 80 patients, 56 improved
and were discharged, and 24 died after treatment, with a fatality
rate of 30.00%. The 56 patients who improved were considered as the
improvement group and the 24 patients who died as the death
group.
Sample collection and detection
CSF (5 ml) from the lower lumbar spine of three
groups of patients was extracted using spinal cord puncture method
and centrifuged (Hunan Hengnuo Instrument Equipment Co., Ltd.,
Changsha, China) at 1,500 × g, 4°C for 10 min, and the separated
supernatant was stored in a refrigerator at −20°C (Shanghai
Coolingway Biotechnology Co., Ltd., Shanghai, China) for later use.
The concentrations of IFN-γ, IL-6, IL-10 and TNF-α in CSF were
detected by ELISA with reference to the instructions of human
IFN-γ, IL-6, IL-10 and TNF-α ELISA kits [Abcam (Shanghai) Trading
Co., Ltd., Shanghai, China]. For the detection method the sample
well, standard well, negative control group and positive control
group were set up. Standard solution (100 µl), test sample,
negative and positive control solution were absorbed into the
reaction wells, and 100 µl of the biological reaction antibody
solution was added quickly, covered with a film, mixed well and
kept for 40 min. Then, 100 µl of streptavidin was added to each
reaction well, covered with a film, mixed evenly and then left to
stand for 40 min. The liquid in the reaction wells was poured out,
and the washing liquid was added to each well, shaken slightly for
1 min, then discarded. The process was repeated five times.
Substrate of reaction solution A (100 µl) and reaction solution B
was added into each reaction well, covered with film, and kept in
the dark for 5 min. Subsequently, 100 µl of elimination agent was
added into wells and then the OD value of each well was immediately
detected at 450 nm using an ELISA Analyzer (Shenzhen Sinothinker
Technology Co., Ltd., Shenzhen, China) to calculate the
concentrations of IFN-γ, IL-6, IL-10 and TNF-α.
Statistical analysis
The SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used
for statistical analysis, and Graph Pad Prism 7 was used to plot
data images. The measurement data are expressed as mean ± standard
deviation (mean ± SD), and independent samples t-test was used to
compare the measurements between groups. The countable data were
expressed as case number/percentage [n (%)], and Chi-square test
was used to compare the countable data between groups. One-way
analysis of variance was used for the comparison between the mean
values of multiple groups, Dunnett-t test was used for pairwise
comparison afterwards. ROC curve was established, the AUC under the
ROC curve of IFN-γ, IL-6, IL-10 and TNF-α concentrations in CSF was
determined, and the sensitivity and specificity under the
diagnostic cut-off were calculated. P<0.05 was considered to
indicate a statistically significant difference.
Results
General data
There was no significant difference in sex, age and
CSF appearance between group A, group B and group C (P>0.05). By
contrast, there were significant differences in clinical
manifestations, CSF pressure, CSF leucocyte count, CSF glucose, CSF
chloride and CSF protein between the three groups (P<0.05). In
group A, 36 cases (87.80%) had CSF pressure ≥180 mmH2O,
29 cases (70.73%) developed headache, 31 cases (75.61%) developed
fever, 24 cases (58.54%) developed nausea and vomiting, 19 cases
(46.34%) developed consciousness disorder, 33 cases (80.49%) had
CSF leucocyte count >8×106/l, 24 cases (58.54%) had
CSF glucose <2.8 mmol/l, 29 cases (70.73%) had CSF chloride
<120 mmol/l, and 33 cases (80.49%) had CSF protein elevation.
The numbers of the above indicators in group B were 33 (84.62%), 25
(64.10%), 28 (71.79%), 26 (66.67%), 18 (46.15%), 25 (64.10%), 26
(66.67%), 27 (69.23%), and 31 (79.49%), respectively (Table I).
 | Table I.Baseline data of patients in the three
groups [n (%)]/(mean ± SD). |
Table I.
Baseline data of patients in the three
groups [n (%)]/(mean ± SD).
| Classification | Group A (n=41) | Group B (n=39) | Group C (n=40) | F/χ2
value | P-value |
|---|
| Sex |
|
|
| 0.704 | 0.706 |
| Male | 29 (70.73) | 27 (69.23) | 25 (62.50) |
|
|
|
Female | 12 (29.27) | 12 (30.77) | 15 (37.50) |
|
|
| Age (years) | 36.15±10.63 | 34.14±11.42 | 33.09±9.15 | 0.9 | 0.409 |
| CSF pressure
(mmH2O) |
|
|
| 68.991 | <0.001 |
| ≥180 | 36 (87.80) | 33 (84.62) | 3 (7.50) |
|
|
|
<180 | 5
(12.20) | 6
(15.38) | 37 (92.50) |
|
|
| Clinical
manifestation |
|
|
| 11.093 | 0.02 |
|
Headache | 29 (70.73) | 25 (64.10) | 0 (0.00) |
|
|
|
Fever | 31 (75.61) | 28 (71.79) | 8
(20.00) |
|
|
| Nausea,
vomiting | 24 (58.54) | 26 (66.67) | 4
(10.00) |
|
|
|
Consciousness disorder | 19 (46.34) | 18 (46.15) | 0 (0.00) |
|
|
| CSF appearance |
|
|
| 4.059 | 0.227 |
|
Colorless | 37 (90.24) | 37 (94.87) | 40
(100.00) |
|
|
| Slightly
red | 2 (4.88) | 1 (2.56) | 0 (0.00) |
|
|
|
Yellow | 2 (4.88) | 1 (2.56) | 0 (0.00) |
|
|
| CSF leukocyte
(×106/l) |
|
|
| 43.862 | <0.001 |
| ≤8 | 8
(19.51) | 14 (35.90) | 36 (90.00) |
|
|
|
>8 | 33 (80.49) | 25 (64.10) | 4
(10.00) |
|
|
| CSF glucose
(mmol/l) |
|
|
| 33.248 | <0.001 |
|
<2.8 | 24 (58.54) | 26 (66.67) | 3 (7.50) |
|
|
|
2.8–4.5 | 17 (41.46) | 13 (33.33) | 37 (92.50) |
|
|
| CSF chloride
(mmol/l) |
|
|
| 48.74 | <0.001 |
|
120–130 | 12 (29.27) | 12 (30.77) | 39 (97.50) |
|
|
|
<120 | 29 (70.73) | 27 (69.23) | 1 (2.50) |
|
|
| CSF protein |
|
|
| 6.733 | 0.014 |
|
Normal | 8
(19.51) | 8
(20.51) | 1 (2.50) |
|
|
|
Elevated | 33 (80.49) | 31 (79.49) | 39 (97.50) |
|
|
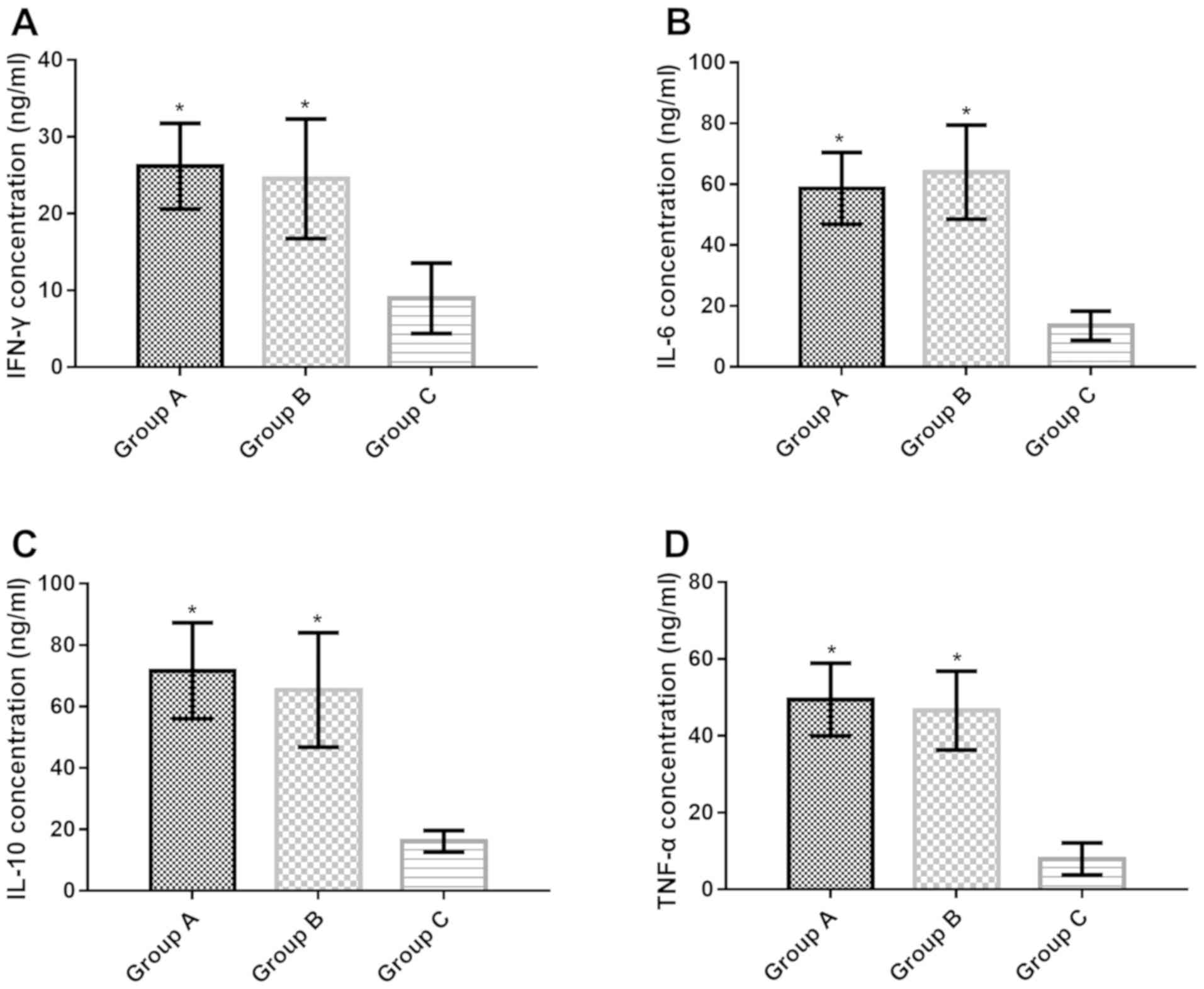
Concentration of IFN-γ, IL-6, IL-10
and TNF-α in CSF of patients in the three groups
Compared with group C, IFN-γ concentration in CSF of
groups A and B increased significantly (t=14.980, P<0.001;
t=10.930, P<0.001), IL-6 concentration increased significantly
(t=22.390, P<0.001; t=19.750, P<0.001), IL-10 concentration
increased significantly (t=17.18, P<0.001; t=16.450,
P<0.001), TNF-α concentration increased significantly (t=25.290,
P<0.001; t=22.070, P<0.001). There was no significant
difference in IFN-γ, IL-6, IL-10 and TNF-α concentrations in CSF of
groups A and B (P>0.05) (Table
II and Fig. 1).
 | Table II.Comparison of IFN-γ, IL-6, IL-10 and
TNF-α concentration in CSF of three groups of patients (mean ±
SD). |
Table II.
Comparison of IFN-γ, IL-6, IL-10 and
TNF-α concentration in CSF of three groups of patients (mean ±
SD).
| Group | n | IFN-γ (ng/ml) | IL-6 (ng/ml) | IL-10 (ng/ml) | TNF-α (ng/ml) |
|---|
| Group A | 41 | 26.17±5.58 | 58.63±11.79 | 71.63±20.13 | 49.43±9.48 |
| Group B | 39 | 24.53±7.78 | 63.97±15.45 | 65.41±18.63 | 46.58±10.28 |
| Group C | 40 | 8.97±4.59 | 13.52±4.78 | 16.13±3.47 | 7.93±4.17 |
| F value | – | 96.800 | 230.900 | 185.200 | 303.900 |
| P-value | – | <0.001 | <0.001 | <0.001 | <0.001 |
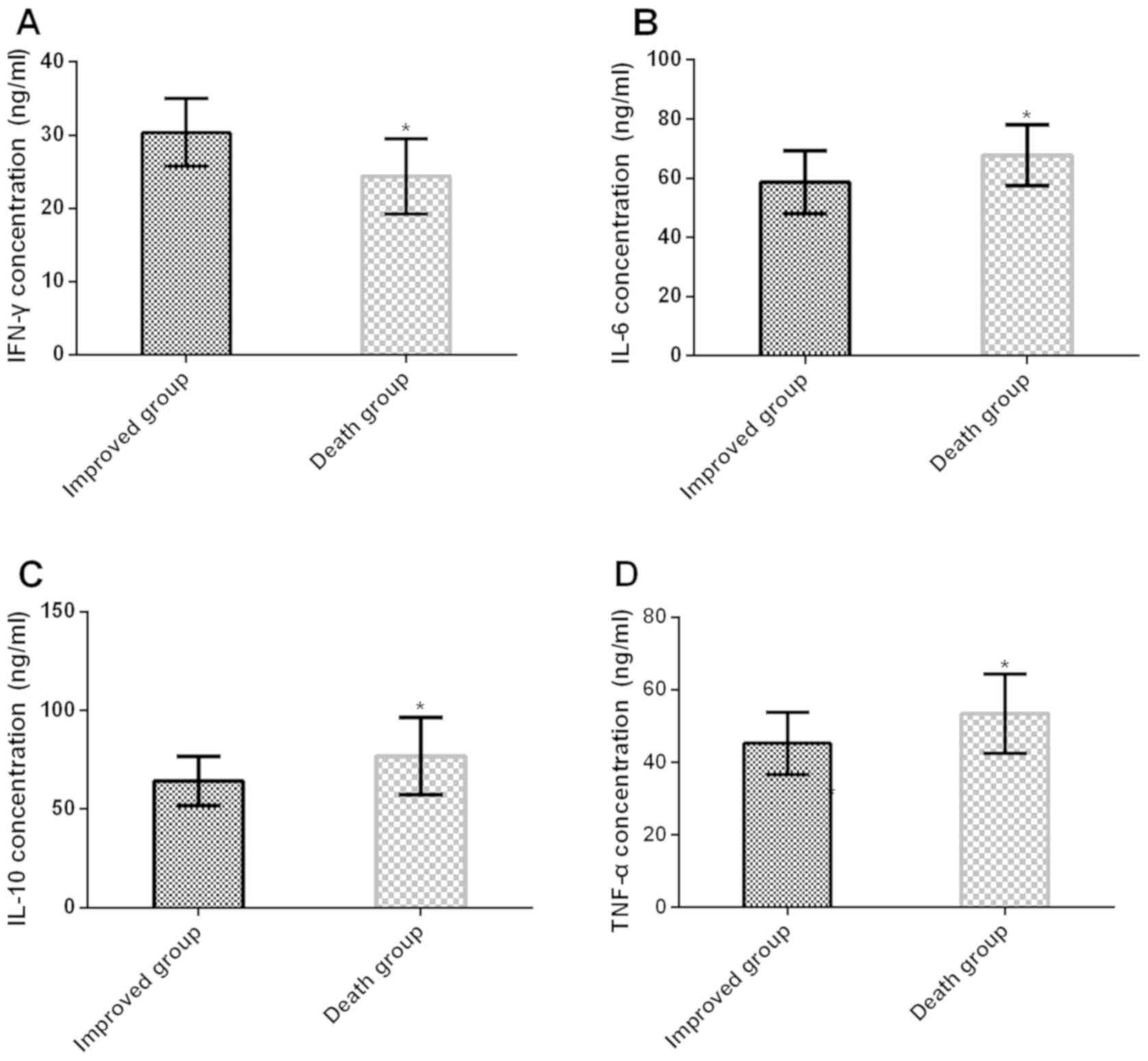
Concentration of IFN-γ, IL-6, IL-10
and TNF-α in CSF of patients in the improvement group and the death
group
The concentration of IL-6, IL-10 and TNF-α in CSF of
patients in the improvement group was significantly lower than
those in the death group (t=3.534, P<0.001; t=3.460, P<0.001;
t=3.557, P<0.001), while the IFN-γ concentration was increased
significantly (t=4.904, P<0.001) (Table III and Fig. 2).
 | Table III.Comparison of IFN-γ, IL-6, IL-10 and
TNF-α concentrations in CSF of patients between the improvement
group and the death group (mean ± SD). |
Table III.
Comparison of IFN-γ, IL-6, IL-10 and
TNF-α concentrations in CSF of patients between the improvement
group and the death group (mean ± SD).
| Group | n | IFN-γ (ng/ml) | IL-6 (ng/ml) | IL-10 (ng/ml) | TNF-α (ng/ml) |
|---|
| Improvement
group | 56 | 30.36±4.62 | 58.64±10.67 | 64.23±12.43 | 45.23±8.64 |
| Death group | 24 | 24.37±5.16 | 67.73±10.23 | 76.84±19.68 |
53.37±10.95 |
| t value | – | 4.904 | 3.534 | 3.460 | 3.557 |
| P-value | – | <0.001 | <0.001 | <0.001 | <0.001 |
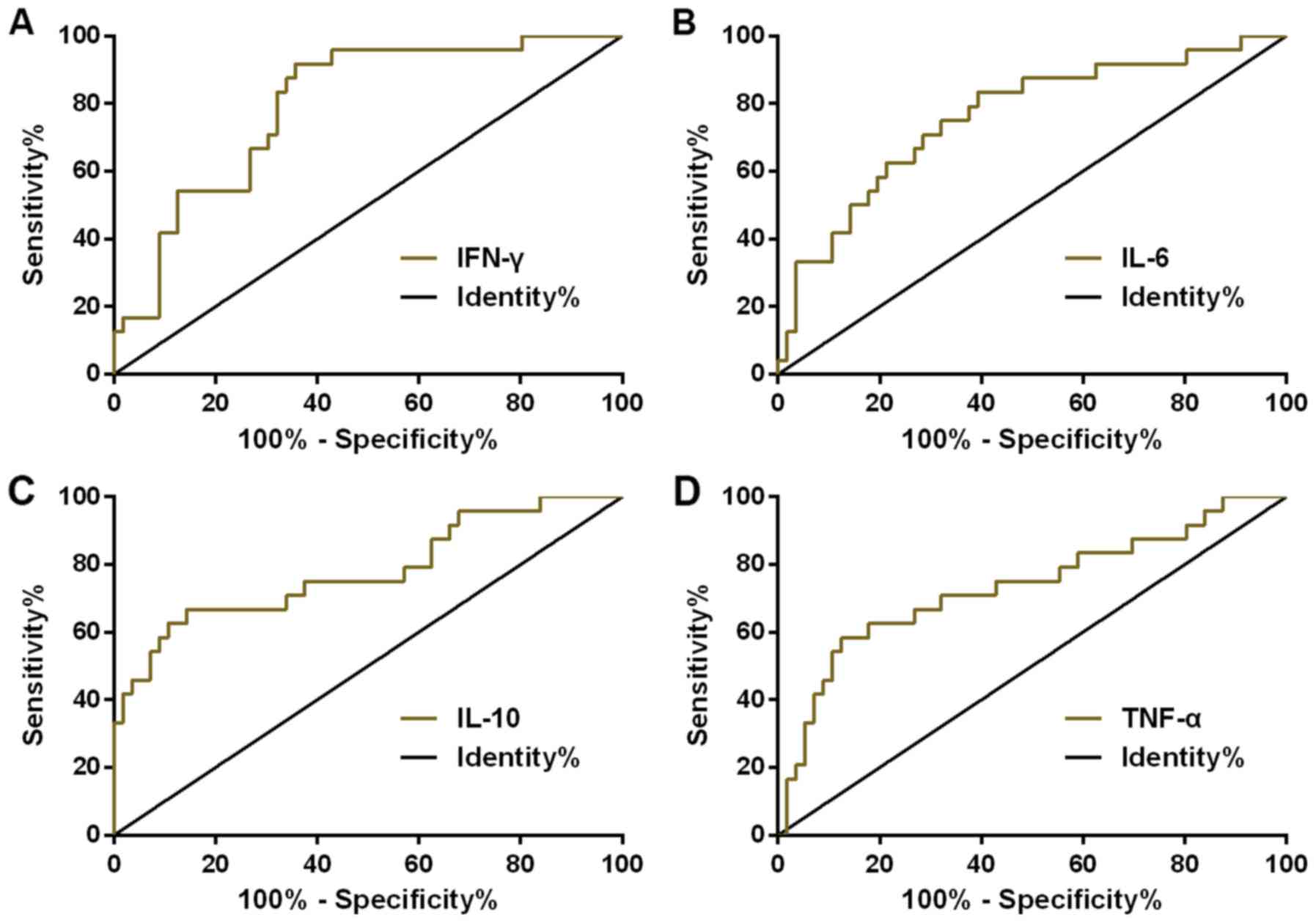
ROC curve of IFN-γ, IL-6, IL-10 and
TNF-α concentration in CSF for diagnosing the prognosis of AIDS
patients with CNS infection
ROC curve of IFN-γ, IL-6, IL-10 and TNF-α
concentration in CSF for diagnosing prognosis of AIDS patients with
CNS infection was plotted. The AUC, diagnostic sensitivity,
specificity, and optimal cut-off value of IFN-γ in diagnosing
prognosis of AIDS patients with CNS infection were 0.795 (95% CI:
0.694–0.896), 89.29, 66.67, and 25.41%, respectively. While those
of IL-6 were 0.760 (95% CI: 0.643–0.876), 83.93, 62.50 and 59.80%,
respectively. Those of IL-10 were 0.780 (95% CI: 0.660–0.901),
67.86, 87.50, and 76.38%, respectively. Those of TNF-α were 0.734
(95% CI: 0.604–0.863), 58.93, 83.33, and 55.82%, respectively
(Table IV and Fig. 3).
 | Table IV.Diagnostic value of IFN-γ, IL-6,
IL-10 and TNF-α concentration in CSF in patients with AIDS
complicated with CNS infection. |
Table IV.
Diagnostic value of IFN-γ, IL-6,
IL-10 and TNF-α concentration in CSF in patients with AIDS
complicated with CNS infection.
| Diagnostic
index | AUC | 95% CI | Standard error | Cut-off | Sensitivity
(%) | Specificity
(%) |
|---|
| IFN-γ | 0.795 | 0.694–0.896 | 0.051 | 25.41 | 89.29 | 66.67 |
| IL-6 | 0.760 | 0.643–0.876 | 0.060 | 59.80 | 83.93 | 62.50 |
| IL-10 | 0.780 | 0.660–0.901 | 0.062 | 76.38 | 67.86 | 87.50 |
| TNF-α | 0.734 | 0.604–0.863 | 0.066 | 55.82 | 58.93 | 83.33 |
Discussion
HIV belongs to retroviruses and is the pathogen of
AIDS, both neurotic and lymphotropic. HIV invades T lymphocytes and
multiplies in a large number of helper CD4+ lymphocytes,
resulting in a large number of their progressive reductions,
further leading to serious damage to the immune function of the
organism, which may cause opportunistic infections (15,16). HIV
can infect B lymphocytes, bone marrow stem cells and mononuclear
phagocytes simultaneously. CNS is the vacuum area of body immunity,
and tuberculosis and cryptococcosis are common opportunistic
infections of AIDS (17).
Tuberculous meningitis caused by mycobacterium tuberculosis
infection and cryptococcal meningitis caused by cryptococcal
neoformans infection are the most common types of CNS infection in
AIDS patients. Delays in treatment caused by untimely diagnosis of
CNS infection are the main causes of AIDS deaths (18,19).
Once the body has immune deficiency, the integrity
of the blood-brain barrier is destroyed by HIV, which facilitates
the intracranial spread of mycobacterium tuberculosis and
cryptococcus neoformans, resulting in CNS infection in the body.
CSF undergoes corresponding pathological changes when CNS infection
occurs (20). In the study of Price
et al (21), it was pointed
out that there were difficulties in the clinical diagnosis and
management of HIV-related CNS infection, while changes in CSF
biomarkers could provide an objective and valuable evaluation
method. The results of this study showed that both group A and
group B patients presented with clinical manifestations of
meningitis such as headache, fever, nausea, vomiting and
consciousness disorder. In group A, 87.80% of patients had
intracranial pressure ≥180 mmH2O, 80.49% had leucocyte
count >8×106/l, 58.54% had glucose <2.8 mmol/l,
70.73% had chloride <120 mmol/l, and 80.49% had protein
elevation; while the rate of those 5 biochemical components of CSF
in the group B were 84.62, 64.10, 66.67, 69.23, and 79.49%,
respectively. The significant changes of CSF biochemical indexes
after CNS infection may be caused by the infection of mycobacterium
tuberculosis and cryptococcus neoformans, causing an increase of
permeability of choroid plexus capillaries and meninges, leading to
the increase of protein and intracranial pressure and the decrease
of glucose and chloride. Graybill et al (22) pointed out that the increased
intracranial pressure and decreased glucose content were the main
reasons for the poor prognosis of patients. Therefore, by observing
the clinical symptoms of AIDS patients with CNS infection and the
changes of CSF biochemical indexes, timely drug symptomatic
treatment can be given.
Mycobacterium tuberculosis, viruses and fungi
infections can cause T-helper 1 (Th1) mediated cellular immune
response in humans, while cellular immunity plays an important role
in resisting pathogen infection (23). HIV infection is a disorder of immune
function characterized by reduction of CD4+ T cells,
imbalance of cytokines and activation of polyclonal cells, and
cytokines play an important role in balancing and maintaining
immune response (24). IFN-γ, IL-6,
IL-10, TNF-α and other cytokines are secreted by activated Th1
cells, Th2 cells, B cells and other cells, which mediate the immune
responses of body fluids (25). In a
study by Chakrabarti et al (26), the levels of inflammatory cytokines
and chemokines IL-6, IL-8/CXCL 8, IP-10/CXCL 10, TNF-α in patients
infected with AIDS and Mycobacterium tuberculosis increased, and
soluble IL-2 receptors were released after activation of
CD4+ T cells in the patients. These inflammatory
cytokines and chemokines had very important effects on the
development of the disease. The results of this study showed that
the concentrations of IFN-γ, IL-6, IL-10 and TNF-α in CSF of
patients in group A and B were significantly higher than those in
group C, suggesting their involvement in the inflammatory reaction
and immune response of AIDS complicated with tuberculous meningitis
and cryptococcal meningitis, which is similar to previous studies.
Clinically, selectively blocking HIV infected patients can
up-regulate the secretion of HIV-1 expressing cytokines, implement
cytokines to rebuild the immune function of the body's defect,
stimulate the recovery and improve the immune imbalance, which is
an important strategy for the treatment of AIDS (27). A study by Worsley et al
(28) showed that, the severity of
HIV was manifested through the reduction of CD4+ T cells
and the occurrence of opportunistic infections; the levels of IL-10
mRNA and TNF-α mRNA increased with the aggravation of the disease,
and the decrease of IFN-γ mRNA was one of the reasons leading to
the deterioration of HIV disease; with the increase of virus
replication, the levels of TNF-α, IL-4 and IL-10 increased and
IFN-γ decreased, making children vulnerable to HIV-related
opportunistic infections. In our study, the levels of IL-6, IL-10
and TNF-α in CSF of the patients in the improvement group were
significantly lower than those in the death group, while the levels
of IFN-γ increased significantly, indicating that they may be
involved in the development of AIDS patients with CNS infection and
related to the patients' poor prognosis. The ROC curve of IFN-γ,
IL-6, IL-10 and TNF-α in the diagnosis of AIDS patients with CNS
infection was further evaluated, and the results indicated their
certain values in the diagnosis of AIDS patients with CNS
infection. Therefore, detecting the concentrations of IL-6, IL-10
and TNF-α in CSF of AIDS patients with CNS infection has certain
predictive value for the poor prognosis of the patients.
In this study, the subjects were screened strictly
according to the inclusion and exclusion criteria. The collection
of samples and the detection of cytokines were the same in
methodology, eliminating the differences caused by experimental
methods and ensuring the rigor and reliability of this study. Among
CNS infections, the expression levels of cytokines in AIDS patients
infected with different severity levels may be different, and the
network formed by cytokines is extremely complex, with mutual
regulation and interaction (29).
The regulatory mechanism of cytokines in AIDS complicated with CNS
infection was not included in this study. Future study should
expand the sample size, and group the course and treatment of
patients with different severity of infection.
Collectively, AIDS patients with tuberculous
meningitis and cryptococcal meningitis in CNS infection diseases
are mainly manifested by headache, fever, nausea, vomiting, and
consciousness disorder. CSF biochemistry is characterized by
increased pressure, leucocyte count and protein, and decreased
chloride and glucose. IFN-γ, IL-6, IL-10 and TNF-α in CSF have
certain predictive value for poor prognosis of AIDS patients with
CNS infection.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC wrote the manuscript. ZC and NW were responsible
for ELISA. YH analyzed and interpreted the patients' data. MW
assisted with statistical analysis. All the authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First Hospital of Changsha (Changsha, China). Patients who
participated in this research had complete clinical data. Signed
written informed consents were obtained from the patients and/or
the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Priya D, Sudharshan S and Biswas J:
Management of a rare presentation of Vogt-Koyanagi-Harada disease
in human immunodeficiency virus/acquired immunodeficiency disease
syndrome patient. Indian J Ophthalmol. 65:413–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moon S, Van Leemput L, Durier N, Jambert
E, Dahmane A, Jie Y, Wu G, Philips M, Hu Y and Saranchuk P:
Out-of-pocket costs of AIDS care in China: Are free antiretroviral
drugs enough? AIDS Care. 20:984–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zafer M, Horvath H, Mmeje O, van der Poel
S, Semprini AE, Rutherford G and Brown J: Effectiveness of semen
washing to prevent human immunodeficiency virus (HIV) transmission
and assist pregnancy in HIV-discordant couples: A systematic review
and meta-analysis. Fertil Steril. 105:645–655.e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iroezindu MO, Agbaji OO, Daniyam CA,
Isiguzo GC, Isichei C and Akanbi MO: Liver function test
abnormalities in Nigerian patients with human immunodeficiency
virus and hepatitis B virus co-infection. Int J STD AIDS.
24:461–467. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Swanson PA II and McGavern DB: Viral
diseases of the central nervous system. Curr Opin Virol. 11:44–54.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cordova E, Boschi A, Ambrosioni J, Cudos C
and Corti M: Reactivation of Chagas disease with central nervous
system involvement in HIV-infected patients in Argentina,
1992–2007. Int J Infect Dis. 12:587–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aoki M, Hayashi H, Rao KV, Das D,
Higashi-Kuwata N, Bulut H, Aoki-Ogata H, Takamatsu Y, Yedidi RS,
Davis DA, et al: A novel central nervous system-penetrating
protease inhibitor overcomes human immunodeficiency virus 1
resistance with unprecedented aM to pM potency. eLife. 6:62017.
View Article : Google Scholar
|
|
8
|
Kugathasan R, Collier DA, Haddow LJ, El
Bouzidi K, Edwards SG, Cartledge JD, Miller RF and Gupta RK:
Diffuse white matter signal abnormalities on magnetic resonance
imaging are associated with human immunodeficiency virus type 1
viral escape in the central nervous system among patients with
neurological symptoms. Clin Infect Dis. 64:1059–1065. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walker NF, Wilkinson KA, Meintjes G,
Tezera LB, Goliath R, Peyper JM, Tadokera R, Opondo C, Coussens AK,
Wilkinson RJ, et al: Matrix degradation in human immunodeficiency
virus type 1-associated tuberculosis and tuberculosis immune
reconstitution inflammatory syndrome: A prospective observational
study. Clin Infect Dis. 65:121–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Torres RG, Etchebehere RM, Adad SJ,
Micheletti AR, Ribeiro BM, Silva LE, Mora DJ, Paim KF and
Silva-Vergara ML: Cryptococcosis in acquired immunodeficiency
syndrome patients clinically confirmed and/or diagnosed at necropsy
in a teaching hospital in Brazil. Am J Trop Med Hyg. 95:781–785.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uldrick TS, Pipkin S, Scheer S and Hessol
NA: Factors associated with survival among patients with
AIDS-related primary central nervous system lymphoma. AIDS.
28:397–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoffmann C, Tabrizian S, Wolf E, Eggers C,
Stoehr A, Plettenberg A, Buhk T, Stellbrink HJ, Horst HA, Jäger H,
et al: Survival of AIDS patients with primary central nervous
system lymphoma is dramatically improved by HAART-induced immune
recovery. AIDS. 15:2119–2127. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hofer MJ and Campbell IL:
Immunoinflammatory diseases of the central nervous system - the
tale of two cytokines. Br J Pharmacol. 173:716–728. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao L, Xing H, Su B, Wang Z, Ruan Y, Wang
X, Liu Z, Lu Y, Yang S, Zhao Q, et al: Impact of HIV drug
resistance on virologic and immunologic failure and mortality in a
cohort of patients on antiretroviral therapy in China. AIDS.
27:1815–1824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pollpeter D, Parsons M, Sobala AE, Coxhead
S, Lang RD, Bruns AM, Papaioannou S, McDonnell JM, Apolonia L, et
al: Deep sequencing of HIV-1 reverse transcripts reveals the
multifaceted antiviral functions of APOBEC3G. Nat Microbiol.
3:220–233. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coiras M, Bermejo M, Descours B, Mateos E,
García-Pérez J, López-Huertas MR, Lederman MM, Benkirane M and
Alcamí J: IL-7 induces SAMHD1 phosphorylation in CD4+ T
lymphocytes, improving early steps of HIV-1 life cycle. Cell Rep.
14:2100–2107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sawai T, Nakao T, Koga S, Ide S, Yoshioka
S, Matsuo N and Mukae H: Miliary tuberculosis with co-existing
pulmonary cryptococcosis in non-HIV patient without underlying
diseases: A case report. BMC Pulm Med. 18:62018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Day CL, Abrahams DA, Harris LD, van Rooyen
M, Stone L, de Kock M and Hanekom WA: HIV-1 infection is associated
with depletion and functional impairment of mycobacterium
tuberculosis-specific CD4 T cells in individuals with latent
tuberculosis infection. J Immunol. 199:2069–2080. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He X, Shi X, Puthiyakunnon S, Zhang L,
Zeng Q, Li Y, Boddu S, Qiu J, Lai Z, Ma C, et al: CD44-mediated
monocyte transmigration across Cryptococcus neoformans-infected
brain microvascular endothelial cells is enhanced by HIV-1 gp41-I90
ectodomain. J Biomed Sci. 23:282016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahimy E, Li FY, Hagberg L, Fuchs D,
Robertson K, Meyerhoff DJ, Zetterberg H, Price RW, Gisslén M and
Spudich S: Blood-brain barrier disruption is initiated during
primary HIV infection and not rapidly altered by antiretroviral
therapy. J Infect Dis. 215:1132–1140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Price RW, Peterson J, Fuchs D, Angel TE,
Zetterberg H, Hagberg L, Spudich S, Smith RD, Jacobs JM, Brown JN,
et al: Approach to cerebrospinal fluid (CSF) biomarker discovery
and evaluation in HIV infection. J Neuroimmune Pharmacol.
8:1147–1158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Graybill JR, Sobel J, Saag M, van Der
Horst C, Powderly W, Cloud G, Riser L, Hamill R and Dismukes W; The
NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups, :
Diagnosis and management of increased intracranial pressure in
patients with AIDS and cryptococcal meningitis. Clin Infect Dis.
30:47–54. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Umemura M, Okamoto-Yoshida Y, Yahagi A,
Touyama S, Nakae S, Iwakura Y and Matsuzaki G: Involvement of
IL-17A-producing TCR γδ T cells in late protective immunity against
pulmonary Mycobacterium tuberculosis infection. Immun Inflamm Dis.
4:401–412. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hogan LE, Vasquez J, Hobbs KS, Hanhauser
E, Aguilar-Rodriguez B, Hussien R, Thanh C, Gibson EA, Carvidi AB,
Smith LCB, et al: Increased HIV-1 transcriptional activity and
infectious burden in peripheral blood and gut-associated
CD4+ T cells expressing CD30. PLoS Pathog.
14:e10068562018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akdis M, Aab A, Altunbulakli C, Azkur K,
Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R,
et al: Interleukins (from IL-1 to IL-38), interferons, transforming
growth factor β, and TNF-α: Receptors, functions, and roles in
diseases. J Allergy Clin Immunol. 138:984–1010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chakrabarti LA, Boucherie C, Bugault F,
Cumont MC, Roussillon C, Breton G, Patey O, Chêne G, Richert L and
Lortholary O; ANRS 129 BKVIR-CYTOK STUDY GROUP, : Biomarkers of
CD4+ T-cell activation as risk factors for
tuberculosis-associated immune reconstitution inflammatory
syndrome. AIDS. 28:1593–1602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kassa D, de Jager W, Gebremichael G,
Alemayehu Y, Ran L, Fransen J, Wolday D, Messele T, Tegbaru B,
Ottenhoff TH, et al: The effect of HIV coinfection, HAART and TB
treatment on cytokine/chemokine responses to Mycobacterium
tuberculosis (Mtb) antigens in active TB patients and latently Mtb
infected individuals. Tuberculosis (Edinb). 96:131–140. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Worsley CM, Suchard MS, Stevens WS, Van
Rie A and Murdoch DM: Multi-analyte profiling of ten cytokines in
South African HIV-infected patients with Immune Reconstitution
Inflammatory Syndrome (IRIS). AIDS Res Ther. 7:362010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bulli L, Apolonia L, Kutzner J, Pollpeter
D, Goujon C, Herold N, Schwarz SM, Giernat Y, Keppler OT, Malim MH,
et al: Complex interplay between HIV-1 capsid and MX2-independent
alpha interferon-induced antiviral factors. J Virol. 90:7469–7480.
2016. View Article : Google Scholar : PubMed/NCBI
|