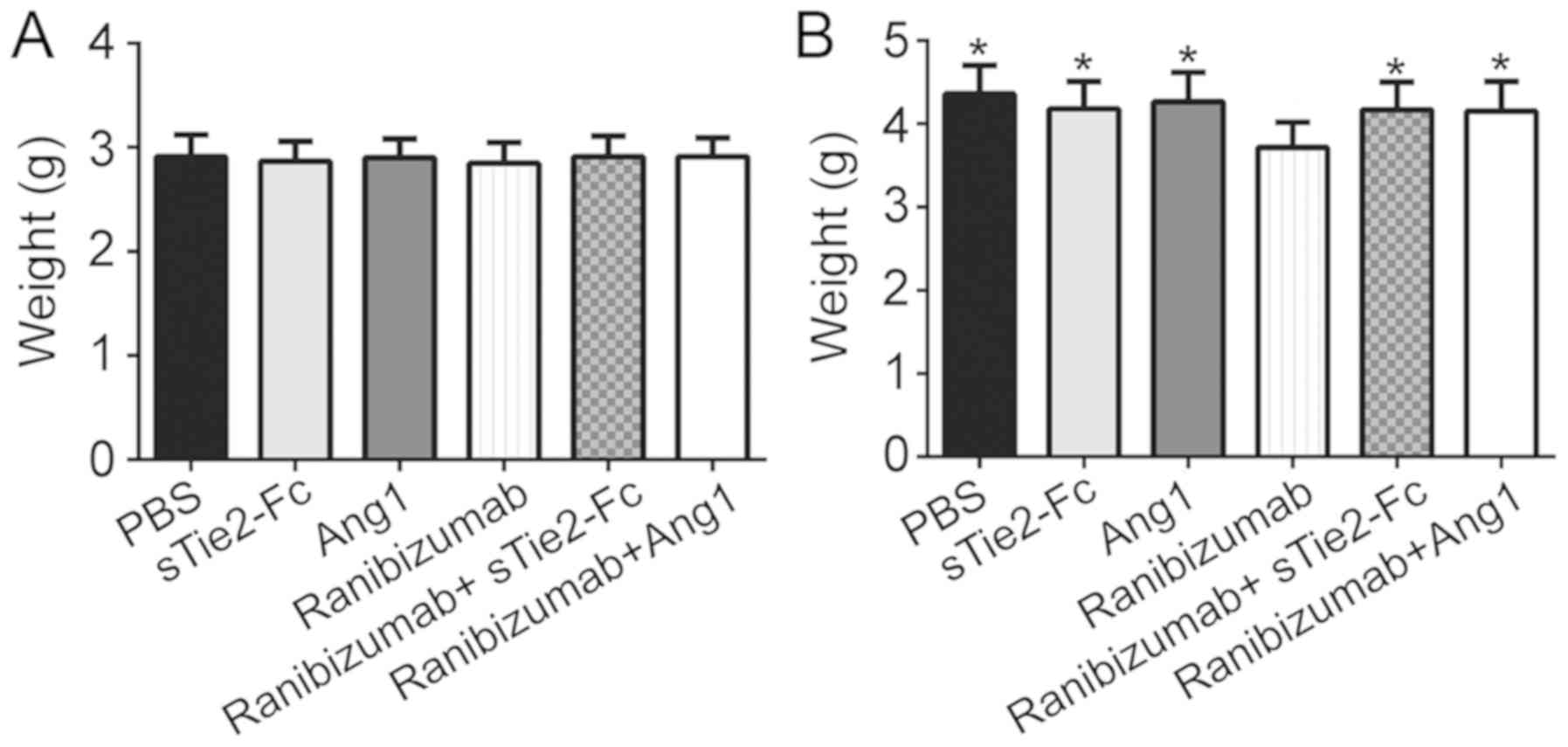
|
1
|
Kahn HA and Hiller R: Blindness caused by
diabetic retinopathy. Am J Ophthalmol. 78:58–67. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gibson DL, Sheps SB, Uh SH, Schechter MT
and McCormick AQ: Retinopathy of prematurity-induced blindness:
Birth weight-specific survival and the new epidemic. Pediatrics.
86:405–412. 1990.PubMed/NCBI
|
|
3
|
Jasani B, Nanavati R and Kabra N:
Mechanisms and management of retinopathy of prematurity. N Engl J
Med. 368:1161–1162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao RC and Dlouhy BJ: Mechanisms and
management of retinopathy of prematurity. N Engl J Med.
368:11612013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartnett ME and Penn JS: Mechanisms and
management of retinopathy of prematurity. N Engl J Med.
367:2515–2526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connor KM, Krah NM, Dennison RJ, Aderman
CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL and Smith LE:
Quantification of oxygen-induced retinopathy in the mouse: A model
of vessel loss, vessel regrowth and pathological angiogenesis. Nat
Protoc. 4:1565–1573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Liang X, Xu C, Xie S, Kuang W and
Liu Z: Quantification of oxygen-induced retinopathy in the mouse.
Yan Ke Xue Bao. 22:103–106. 2006.(In Chinese). PubMed/NCBI
|
|
8
|
Ding X, Liang X, Xie S, Zhu X and Tang S:
A modified mouse model of oxygen-induced retinopathy. Yan Ke Xue
Bao. 22:98–102. 2006.(In Chinese). PubMed/NCBI
|
|
9
|
Smith LE, Wesolowski E, McLellan A, Kostyk
SK, D'Amato R, Sullivan R and D'Amore PA: Oxygen-induced
retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–111.
1994.PubMed/NCBI
|
|
10
|
Sato T, Wada K, Arahori H, Kuno N, Imoto
K, Iwahashi-Shima C and Kusaka S: Serum concentrations of
bevacizumab (avastin) and vascular endothelial growth factor in
infants with retinopathy of prematurity. Am J Ophthalmol.
153:327–333.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Käll A: Is Avastin the right choice of
treatment for retinopathy of prematurity? Acta Paediatr.
101:796–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Avery RL: Bevacizumab (Avastin) for
retinopathy of prematurity: Wrong dose, wrong drug, or both? J
AAPOS. 16:2–4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nuti E, Traversi C, Marigliani D,
Balestrazzi A, Alegente M, Martone G, Malandrini A, Romeo N,
Mazzotta C and Tosi GM: Treatment of macular edema because of
occlusive vasculitis with bevacizumab (avastin): Efficacy of three
consecutive monthly injections. Retina. 31:1863–1870. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu L, Arevalo JF, Roca JA, Maia M,
Berrocal MH, Rodriguez FJ, Evans T, Costa RA and Cardillo J;
Pan-American Collaborative Retina Study Group (PACORES), :
Comparison of two doses of intravitreal bevacizumab (Avastin) for
treatment of macular edema secondary to branch retinal vein
occlusion: Results from the Pan-American Collaborative Retina Study
Group at 6 months of follow-up. Retina. 28:212–219. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kook D, Wolf A, Kreutzer T, Neubauer A,
Strauss R, Ulbig M, Kampik A and Haritoglou C: Long-term effect of
intravitreal bevacizumab (avastin) in patients with chronic diffuse
diabetic macular edema. Retina. 28:1053–1060. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC and
Chen SN: An updated study of the use of bevacizumab in the
treatment of patients with prethreshold retinopathy of prematurity
in taiwan. Am J Ophthalmol. 155:150–158.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Bullock AJ, Zhang L, Wei L, Yu D,
Mahagaokar K, Alsop DC, Mier JW, Atkins MB, Coxon A, et al: The
role of angiopoietins as potential therapeutic targets in renal
cell carcinoma. Transl Oncol. 7:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saulle E, Guerriero R, Petronelli A,
Coppotelli E, Gabbianelli M, Morsilli O, Spinello I, Pelosi E,
Castelli G, Testa U and Coppola S: Autocrine role of angiopoietins
during megakaryocytic differentiation. PLoS One. 7:e397962012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas M and Augustin HG: The role of the
Angiopoietins in vascular morphogenesis. Angiogenesis. 12:125–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuo QH, Zeng H, Stinnett A, Yu H, Aschner
JL, Liao DF and Chen JX: Critical role of angiopoietins/Tie-2 in
hyperglycemic exacerbation of myocardial infarction and impaired
angiogenesis. Am J Physiol Heart Circ Physiol. 294:H2547–H2557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hildbrand P, Cirulli V, Prinsen RC, Smith
KA, Torbett BE, Salomon DR and Crisa L: The role of angiopoietins
in the development of endothelial cells from cord blood CD34+
progenitors. Blood. 104:2010–2019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plank MJ, Sleeman BD and Jones PF: The
role of the angiopoietins in tumour angiogenesis. Growth Factors.
22:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thurston G: Role of Angiopoietins and Tie
receptor tyrosine kinases in angiogenesis and lymphangiogenesis.
Cell Tissue Res. 314:61–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ellis LM, Ahmad S, Fan F, Liu W, Jung YD,
Stoeltzing O, Reinmuth N and Parikh AA: Angiopoietins and their
role in colon cancer angiogenesis. Oncology (Williston Park).
16:31–35. 2002.PubMed/NCBI
|
|
25
|
Campochiaro PA, Sophie R, Pearlman J,
Brown DM, Boyer DS, Heier JS, Marcus DM, Feiner L and Patel A:
Long-term outcomes in patients with retinal vein occlusion treated
with ranibizumab: The RETAIN study. Ophthalmology. 121:209–219.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung NA, Makin AJ and Lip GY: Measurement
of the soluble angiopoietin receptor tie-2 in patients with
coronary artery disease: Development and application of an
immunoassay. Eur J Clin Invest. 33:529–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Fu P, Li H, Gao R and Xiu R:
Soluble angiopoietin receptor Tie-2 in patients with acute
myocardial infarction and its effects on angiogenesis. Clin
Hemorheol Microcirc. 33:1–10. 2005.PubMed/NCBI
|
|
28
|
Singh N, Macnamara E, Rashid S, Ambati J,
Kontos CD, Higgins E and Ambati BK: Systemic soluble Tie2
expression inhibits and regresses corneal neovascularization.
Biochem Biophys Res Commun. 332:194–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takagi H, Koyama S, Seike H, Oh H, Otani
A, Matsumura M and Honda Y: Potential role of the angiopoietin/tie2
system in ischemia-induced retinal neovascularization. Invest
Ophthalmol Vis Sci. 44:393–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hangai M, Moon YS, Kitaya N, Chan CK, Wu
DY, Peters KG, Ryan SJ and Hinton DR: Systemically expressed
soluble Tie2 inhibits intraocular neovascularization. Hum Gene
Ther. 12:1311–1321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hohlbaum K, Bert B, Dietze S, Palme R,
Fink H and Thone-Reineke C: Impact of repeated anesthesia with
ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS
One. 13:e2035592018. View Article : Google Scholar
|
|
32
|
Shim HJ, Jung WB, Schlegel F, Lee J, Kim
S, Lee J and Kim SG: Mouse fMRI under ketamine and xylazine
anesthesia: Robust contralateral somatosensory cortex activation in
response to forepaw stimulation. Neuroimage. 177:30–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stewart KA, Wilcox KS, Fujinami RS and
White HS: Development of postinfection epilepsy after Theiler's
virus infection of C57BL/6 mice. J Neuropathol Exp Neurol.
69:1210–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
You JY, Chung H and Kim HC: Evaluation of
changes in choroidal neovascularization secondary to age-related
macular degeneration after anti-VEGF therapy using spectral domain
optical coherence tomography. Curr Eye Res. 37:438–445. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oishi A, Yamashiro K, Tsujikawa A, Ooto S,
Tamura H, Nakata I, Miyake M and Yoshimura N: Long-term effect of
intravitreal injection of anti-VEGF agent for visual acuity and
chorioretinal atrophy progression in myopic choroidal
neovascularization. Graefes Arch Clin Exp Ophthalmol. 251:1–7.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Introini U, Casalino G, Querques G, Gimeno
AT, Scotti F and Bandello F: Spectral-domain OCT in anti-VEGF
treatment of myopic choroidal neovascularization. Eye (Lond).
26:976–982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee J, Park DY, Park DY, Park I, Chang W,
Nakaoka Y, Komuro I, Yoo OJ and Koh GY: Angiopoietin-1 suppresses
choroidal neovascularization and vascular leakage. Invest
Ophthalmol Vis Sci. 55:2191–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Bi H, Teng D, Zou Y, Pan X, Guo D
and Cui Y: Potential protective effect of angiopoietin-1 on the
leakage of rat choroidal neovascularization. Saudi Med J.
34:584–590. 2013.PubMed/NCBI
|
|
39
|
Feng Y, Wang Y, Pfister F, Hillebrands JL,
Deutsch U and Hammes HP: Decreased hypoxia-induced
neovascularization in angiopoietin-2 heterozygous knockout mouse
through reduced MMP activity. Cell Physiol Biochem. 23:277–284.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Natarajan G, Johnson YR, Brozanski B,
Farrow KN, Zaniletti I, Padula MA, Asselin JM, Durand DJ, Short BL,
Pallotto EK, et al: Postnatal weight gain in preterm infants with
severe bronchopulmonary dysplasia. Am J Perinatol. 31:223–230.
2014.PubMed/NCBI
|
|
41
|
Turner S, Zhang G, Young S, Cox M,
Goldblatt J, Landau L and Le Souëf P: Associations between
postnatal weight gain, change in postnatal pulmonary function,
formula feeding and early asthma. Thorax. 63:234–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stahl A, Krohne TU, Eter N,
Oberacher-Velten I, Guthoff R, Meltendorf S, Ehrt O, Aisenbrey S,
Roider J, Gerding H, et al: Comparing alternative ranibizumab
dosages for safety and efficacy in retinopathy of prematurity: A
randomized clinical trial. Jama Pediatr. 172:278–286. 2018.
View Article : Google Scholar : PubMed/NCBI
|