Introduction
Neovascular (NV) ophthalmopathy includes
proliferative diabetic retinopathy, age-related macular
degeneration, and retinopathy of prematurity (ROP), which have
become the main causes of blindness in the elderly (1), infants and young children (2).
The development of NV ophthalmopathy consists of two
stages. First, vascular occlusion and disordered blood vessels
develop from existing conditions. Subsequently, various
hypoxia-related growth factors, including vascular endothelial
growth factor (VEGF), angiopoietin (Ang)-2 and erythropoietin, are
produced due to the insufficient blood and oxygen supply. These
factors further lead to angiogenic lesions (3–6). Animal
models of oxygen-induced retinopathy (OIR) have been frequently
used to study the mechanism of ROP (7–9). Hence,
the present study established an OIR mouse model to explore the
potential mechanism of NV ophthalmopathy.
Retinal neovascularization (RNV) treatments include
retinal laser photocoagulation and vitreous surgery. However, these
treatments were only capable of reducing retinal oxygen
consumption, vitreous proliferation and traction, while the retina
blood supply was not improved or restored to the normal level.
Currently, various anti-VEGF drugs are being utilized in
ophthalmology due to the research into VEGF and its corresponding
receptors. However, anti-VEGF drugs have limitations, for example,
anti-VEGF therapy can transiently reduce systemic VEGF levels
(10). In addition, the formation
and traction of preretinal proliferative membranes was accelerated
following anti-VEGF administration, further worsening the condition
(11,12). Anti-VEGF drugs have a poor
therapeutic effect on macular edema due to a short effective
duration and a high rate of relapse (13–15).
Notably, anti-VEGF therapy for ROP inhibits normal structural
development and functional maturation of retinal nerve cells. It is
also reported that anti-VEGF treatment affects development of
multiple organs, in particular the lungs (16). Therefore, a safer and more effective
therapy for ROP is urgently required.
Ang and its receptor TEK receptor tyrosine kinase 2
(Tie2) have been recently identified to serve a role in the
angiogenic pathway (17–24). Ang1 and Ang2 regulate the stability
of endothelial cells by binding to their coreceptor Tie2. This
indicates that Ang1 can enhance the adhesion of vascular peripheral
tissues and stabilize newly formed blood vessels, thereby limiting
persistent abnormal angiogenesis (23). Ang2 antagonizes the effects of Ang1
and enhances the sensitivity of endothelial cells to mitogenic
signals, leading to instability of endothelial cells and promoting
immature neovascularization (23).
Therefore, it is generally hypothesized that Ang2 inhibition or
Ang1 promotion can inhibit the formation of new blood vessels. The
Ang/Tie2 pathway mainly affects vascular structure and maturation
without significantly overlapping with the VEGF/VEGF receptor (R)
pathway spectrum.
Ranibizumab is a recombinant human monoclonal
antibody that binds to all high-affinity VEGFs and blocks the
corresponding VEGF/VEGFR pathway (25). Soluble Tie2 fusion protein (sTie-Fc)
is the extracellular component of Tie2, which competitively binds
to Ang1 and Ang2, thus regulating the Tie2 pathway in endothelial
cells (26–30).
The present study established a mouse model of OIR
to compare the effects of the VEGF/VEGFR and Ang/Tie2 pathways on
regulating RNV to provide novel directions for RNV treatment.
Materials and methods
Experimental animals
A total of 80 male C57BL/6J mice (7-days-old) were
obtained from Shanghai SLAC Laboratory Animal Co., Ltd. Mice were
housed in a temperature (21±2°C) and humidity (40–70%) controlled
room under a 12 h light/dark cycle (lights on at 06:00). Mice were
maintained in an experimental animal center under
specific-pathogen-free conditions, and were given free access to
water and food. This study was approved by the Animal Ethics
Committee of Liaocheng People's Hospital Animal Center.
Model and treatment
For the OIR model group, 14 C57BL/6J mice (age, 7
days) were housed in a 75% oxygen concentration hyperoxia container
for 5 consecutive days, followed by 5 days in a normal air
environment. For the control group, 10 C57BL/6J mice (age, 7 days)
were housed in a normal air environment for 10 days. In addition,
120 C57BL/6J mice (age, 7 days) were selected and divided into 6
groups (n=20). The mice were kept in a container with 75% oxygen
concentration for 5 consecutive days. At 12-days old, the weight of
the mice was measured and recorded. PBS (1 µl), 1 µl sTie2-Fc (3
mol/l; cat. no. SFC-014; Ankang Biotechnology Co., Ltd.), 1 µl Angl
(4 mol/l; cat. no. An-002; Ankang Biotechnology Co., Ltd.), 1 µl
ranibizumab (10 mol/l; Shanghai TheraMabs Bio-technology Co.,
Ltd.), 1 µl sTie2-Fc + 1 µl ranibizumab (sTie2-Fc 3 mol/l;
ranibizumab 10 mol/l) or 1 µl Angl + 1 µl ranibizumab (Angl 4
mol/l; ranibizumab 10 mol/l) were injected into the vitreous cavity
of mice. Mice were reared for further 5 days then the weight of the
mice was again measured and recorded at 10 days. Half of the mice
were subsequently sacrificed at 5 days and the remainder were
sacrificed at 10 days.
Fluorescein isothiocyanate
(FITC)-dextran cardiac perfusion and stretched retina
preparation
A total of 10 mice were anesthetized by
intraperitoneal injection of ketamine (120 mg/kg) and xylazine (12
mg/kg), as previously descried (31–33). The
heart was exposed and 1 ml of FITC-dextran (2%; cat. no. 141270;
Shanghai Huicheng Biotechnology Co., Ltd.) was administrated into
the apex of left ventricle. After 3–5 min, pupils were harvested
then the mucous membranes stained and fixed in 4% paraformaldehyde
at room temperature for 20–30 min. The retinas were stripped from
the lens and cut into 6 µm sections. The total retina area, area of
instillation, microvascular network density, area of new blood
vessels, width of vein and the tortuosity of the arteries were
observed.
Quantification of endotheliocyte
nuclei of new vessels beyond the inner limiting membrane
A total of 5 mouse pupils from each group harvested
as aforementioned were collected and fixed in 4% paraformaldehyde
at room temperature for 24 h. The lens was removed under a
microscope, then subjected to a gradient dehydration in alcohol
(100, 95, 85 and 75%) and xylene. Subsequently, tissue samples were
paraffin embedded and cut into 5 µm sections for hematoxylin (5
min) and eosin (1–3 min; HE) staining (Boster Biological
Technology; 5 min). The total number of endotheliocyte nuclei of
new vessels beyond the inner limiting membrane were counted in each
sample via light microscopy. Three retinal tissue sections were
analyzed for each mouse and three fields of view were analyzed for
each sample. The total number of endotheliocyte nuclei was taken as
an average from three technicians who were blinded to the study
groups.
Intravitreal injection
Following anesthesia by intraperitoneal injection of
ketamine (120 mg/kg) and xylazine (12 mg/kg), 30 mice were
sacrificed at day 10 and their eyelids were separated with
ophthalmic smooth forceps. Pupils were immediately dilated with
tropicamide (0.01%; Sigma-Aldrich; Merck KGaA; cat. no. T9778) 2–4
times. The eyeball was protruded by slight pressure on the orbital
margin then 1 µl sTie2-Fc (3 mol/l), Angl (4 mol/l), ranibizumab
(10 mol/l), sTie2-Fc + ranibizumab (sTie2-Fc 3 mol/l; ranibizumab
10 mol/l) or Angl + ranibizumab (Angl 4 mol/l; ranibizumab 10
mol/l) were administrated from the superior corneal limbus to the
vitreous chamber. The needle of microinjector was maintained in the
chamber for 15 sec and immediately pulled out. After intravitreal
injection, erythromycin ophthalmic ointment was applied.
Statistical analysis
Stata statistical software (version 7.0; StataCorp
LP) was used for data analysis. Data are presented as the mean ±
standard deviation. The differences amongst groups were compared
using one-way analysis of variance, followed by Fisher's least
significant difference post hoc test with Bonferroni adjustment for
comparisons between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Evaluation of retinal vascular
development
The OIR model was constructed for evaluating the
total amount of endotheliocyte nuclei of new vessels beyond the
inner limiting membrane. Mice were randomly assigned into the OIR
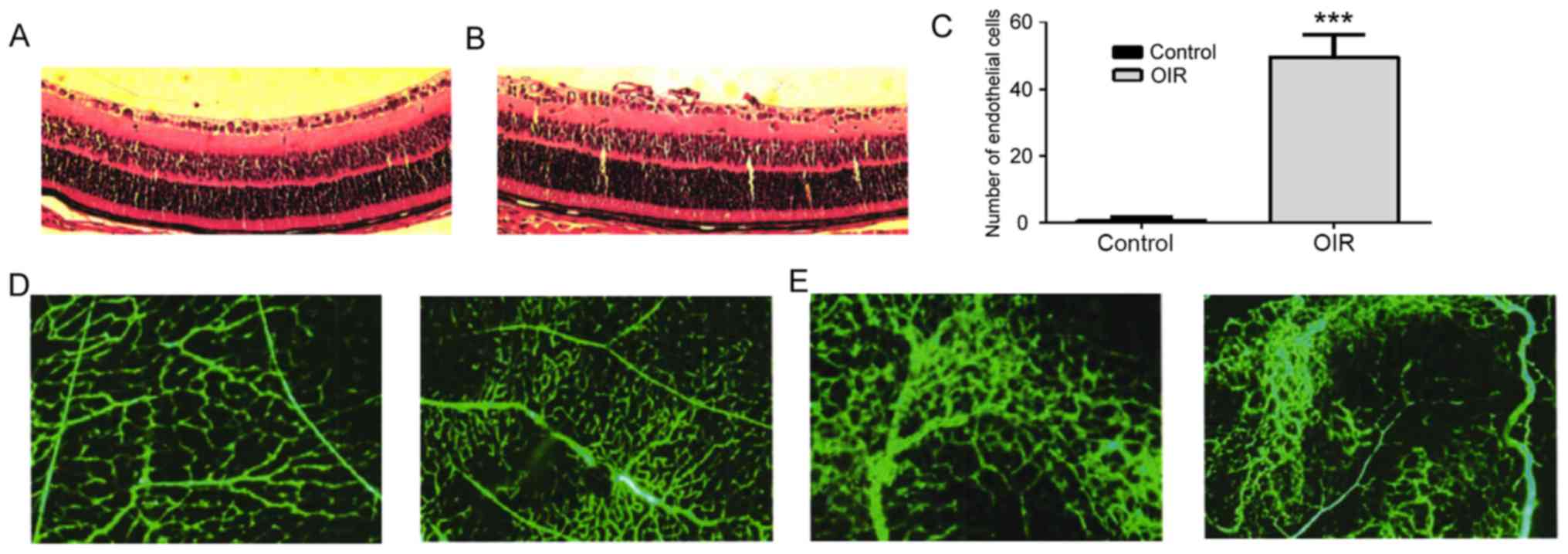
group (n=10) or control group (n=10). Results demonstrated that
mice in the OIR group presented multiple neovascular clusters
(Fig. 1A and B) and a significantly
higher amount of endotheliocyte nuclei of new vessels compared with
the control group (Fig. 1C). Retinal
vein dilatation and arterial circuitous degree was used to assess
the disease condition. Both parameters were more pronounced in the
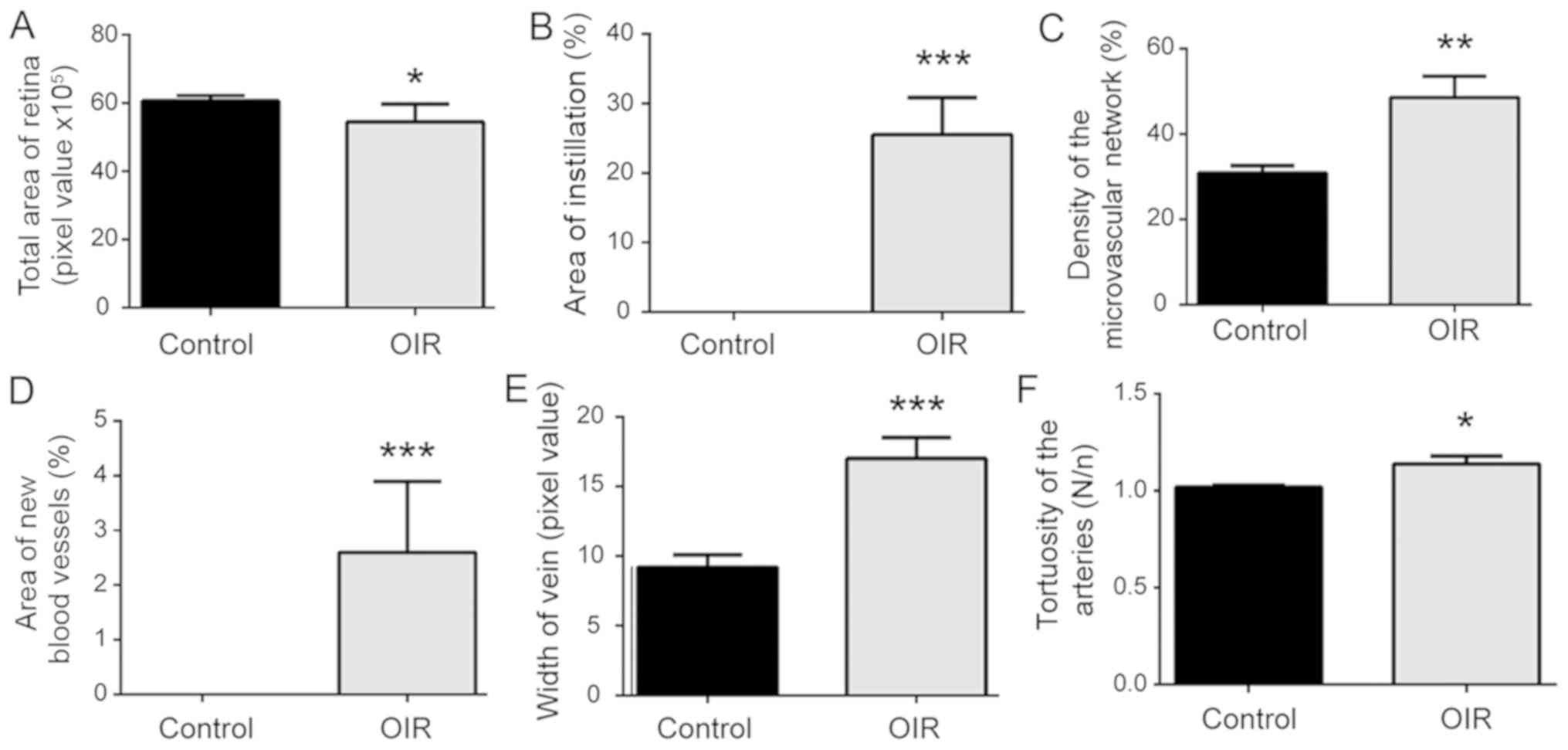
OIR group compared with the control group (Fig. 1D, E). Mice in the OIR group presented
smaller total retina areas, a larger area of instillation, a larger
area of new blood vessels, and a higher microvascular network
density compared with the control PBS group. Marked retinal vein
dilatation and arterial tortuosity were identified in the OIR
group. The number of endotheliocyte nuclei of new vessels beyond
the inner limiting membrane was larger in the OIR group compared
with the control group. The results suggest that there was impaired
retinal vascular development (Fig.
2).
Treatment with sTie2-Fc alleviates RNV
via regulating the Ang/Tie2 pathway
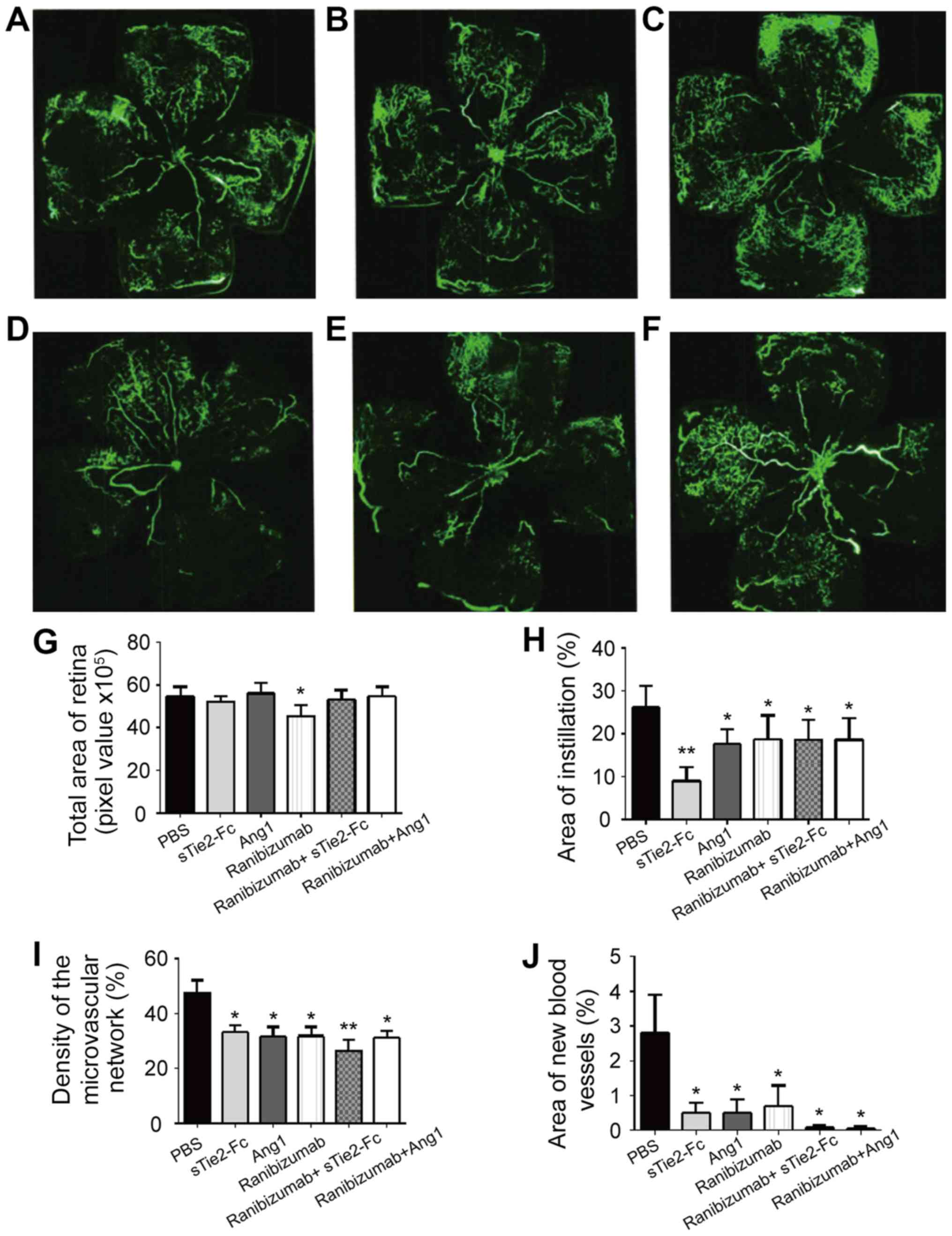
Mice received intravitreal injection of PBS,
sTie2-Fc, Ang1, ranibizumab, ranibizumab + sTie2-Fc or ranibizumab
+ Ang1. Compared with the PBS group, mice injected with sTie2-Fc,
Ang1 or ranibizumab presented less endotheliocyte nuclei of new
vessels beyond the inner limiting membrane (Fig. 3A-F). The results demonstrated that
individual administration of sTie2-Fc, Ang1 and ranibizumab did not
affect retinal vascular development which would manifest as a
larger area of FITC-dextran instillation compared with PBS group
(Fig. 3H). In addition, it was
determined that sTie2-Fc administration may promote retinal
vascular development compared with the PBS group (Fig. 3G-J) which suggested that sTie2-Fc may
be a preferred option for RNV treatment. Ranibizumab + sTie2-Fc
treatment markedly inhibited retinal vascular development,
presenting as a smaller area of instillation and lower density of
the microvascular network when compared with the PBS group
(Fig. 3H and I). By contrast,
ranibizumab + Angl administration did not affect retinal vascular
development compared with the PBS group (Fig. 3G-J). Therefore ranibizumab + Angl
treatment was considered the better option when compared with
Ranibizumab + sTie2-Fc for treating RNV via the combined regulation
of the Ang/Tie2 and VEGF/VEGFR pathways.
Combination of sTie2-Fc or Ang1 with
ranibizumab alleviates RNV
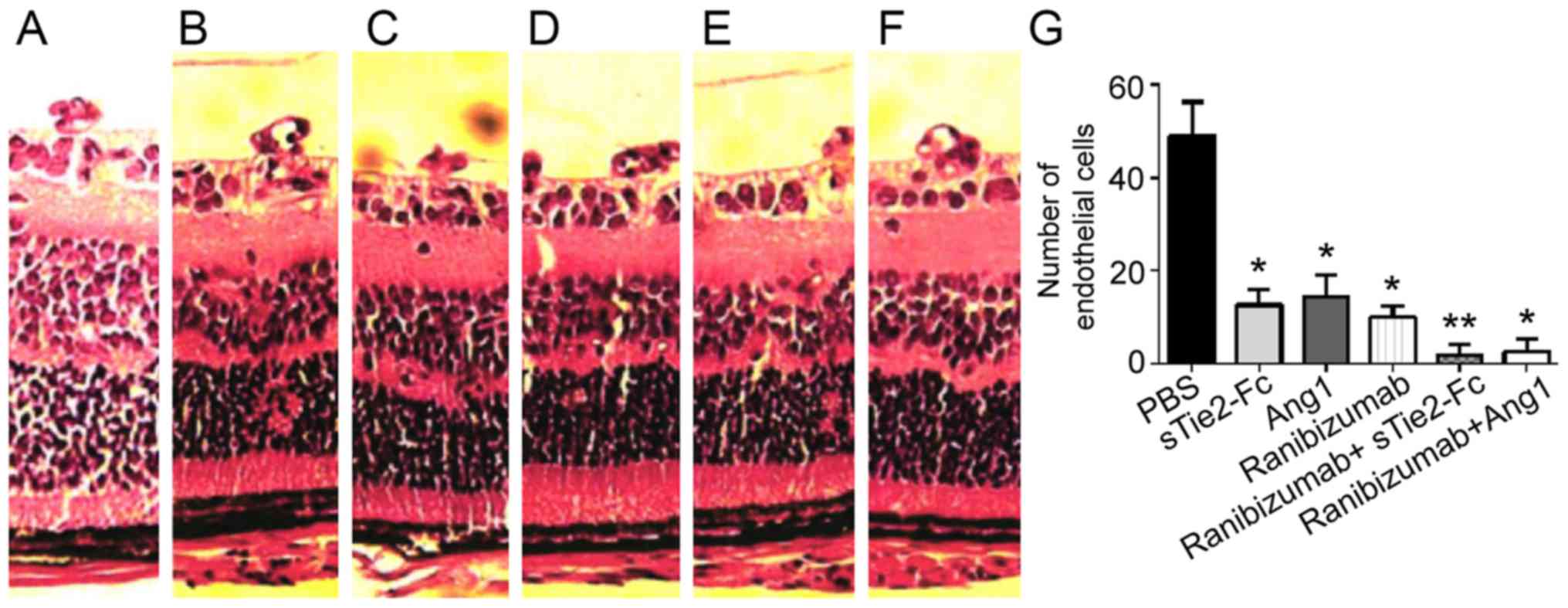
By comparing the HE staining amongst different
groups, it was identified that intravitreal injection of sTie2-Fc,
Ang1, ranibizumab, ranibizumab + sTie2-Fc and ranibizumab + Angl
reduced the amount of endotheliocyte nuclei of new vessels beyond
the inner limiting membrane compared with the PBS group.
Ranibizumab + sTie2-Fc and ranibizumab + Ang1 treatment groups
displayed a smaller quantity of endotheliocyte nuclei of new
vessels beyond the inner limiting membrane compared with the
sTie2-Fc, Ang1 and ranibizumab groups (Fig. 4). Combined administration of
ranibizumab + sTie2-Fc and ranibizumab + Angl demonstrated better
treatment efficacy than individual administration (Fig. 4G). However, no significant difference
in the amount of endotheliocyte nuclei of new vessels beyond the
inner limiting membrane was demonstrated between ranibizumab +
sTie2-Fc and ranibizumab + Angl groups. These results indicated
that a combination of sTie2-Fc or Ang1 with ranibizumab alleviated
RNV.
Ranibizumab affects normal development
of OIR mice
Subsequently, the potential mechanism of ranibizumab
in regulating RNV development was investigated. Results
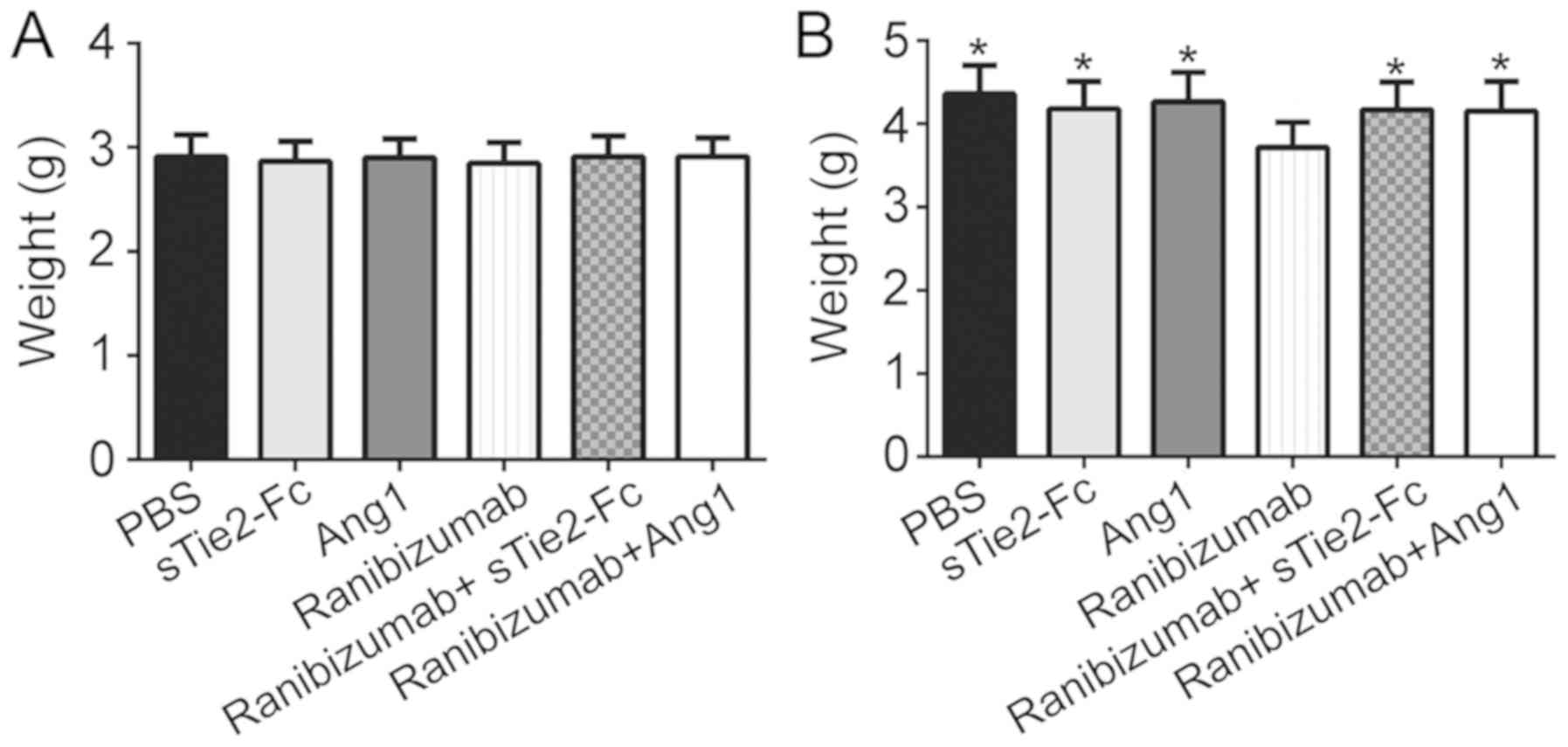
demonstrated that there was no significant difference in mouse
weight between any groups following 5 days of experimentation
(Fig. 5A). Treatment with sTie2-Fc
or Ang1 + ranibizumab had no effect on body weight following 5 days
of experimentation. However, body weight was significantly
decreased in the ranibizumab group compared with the other groups
following 10 days of experimentation (Fig. 5B). This result suggested that
ranibizumab may affect the normal development of OIR mice.
Discussion
The OIR animal model simulates RNV symptoms, in
particular ROP, and therefore has been commonly utilized to
investigate the underlying mechanism of ROP (7–9). RNV
development is considered to be the result of the imbalance between
local angiogenesis promoting and inhibitory factors (23,24).
VEGF and VEGFR are considered to be the most important angiogenesis
promoting factors (4,5). Intravitreal injections of various VEGF
antagonists are used for anti-neovascular therapy (34–36).
Ranibizumab is a recombinant human monoclonal antibody that binds
to all isoforms of VEGF with high affinity, does not contain a
fragment crystallizable region and inhibits the VEGF/VEGFR
signaling pathway (25). Ranibizumab
received Food and Drug Administration certification in 2006 and is
currently approved for clinical ophthalmology in China (25).
Previous studies have identified severe limitations
of anti-VEGF therapy for ROP (10–12);
therefore, research has focused on the development of more
effective medication for this condition. In recent years, the
Ang/Tie2 pathway has been identified to inhibit angiogenesis
(26–30). Ang1 administration inhibits
angiogenesis by enhancing the interaction between endothelial cells
and pericytes (37,38). The Ang/Tie2 pathway mainly affects
vascular plasticity and maturation. In Ang2 heterozygous knockout
mice, it was determined that Ang2 modulates hypoxia-induced
neovascularization by regulating MMP activity (39).
In the present study, an OIR mouse model was first
established. Then mice received intravitreal injection of PBS,
sTie2-Fc, Ang1, ranibizumab, ranibizumab + sTie2-Fc or ranibizumab
+ Ang1. Results demonstrated that individual administration of Ang1
and ranibizumab both markedly alleviated RNV without affecting
retinal vascular development. Intravitreal injection of sTie2-Fc
promoted retinal vascular development, manifesting as a smaller
instillation retina area compared with the PBS group. Therefore, it
was hypothesized that sTie2-Fc may be a preferred option compared
with ranibizumab for treating RNV.
Compared with individual administration of sTie2-Fc,
ranibizumab or Ang1, the inhibitory effect of ranibizumab +
sTie2-Fc and ranibizumab + Ang1 on RNV was more pronounced. The
present results indicated that a combination treatment targeting
the Ang/Tie2 and VEGF/VEGFR pathways could better alleviate RNV.
Takagi et al (29)
demonstrated that the combined inhibition of Tie2 and VEGF
signaling may be effective in preventing pathologic angiogenesis in
ischemic retinal disorders, which is in agreement with our
results.
In particular, the present study identified that
mice injected with ranibizumab demonstrated a decreased total
retina area and lower body weight. In clinical research, baby
weight gain is closely related to the development of multiple
organs, especially the lungs (40,41).
Anti-VEGF therapy has been reported to inhibit multiple organ
development in infants (16).
Changes in blood VEGF levels have not been observed following
treatment with ranibizumab (42),
therefore the inhibition of organ development may not be associated
with ranibizumab. Further studies on the inhibitory effect of
ranibizumab are required to fully elucidate the potential side
effects. The present study demonstrated the changes in RNV on day
12 based on the preliminary experimental results; however, future
experiments should investigate the degree of neovascularization
beyond 12 days in the different experimental groups. In addition,
the investigation into the role of Ang2 in RNV using Ang2
inhibitors should be performed.
In conclusion, sTie2-Fc alleviated RNV without
affecting retinal vascular development by activating the Ang/Tie2
pathway. It was also determined that intravitreal administration of
ranibizumab may inhibit normal growth and development of mice.
Regulation of both the Ang/Tie2 and VEGF/VEGFR pathways markedly
increased the therapeutic efficacy, therefore may be the preferred
approach for treating RNV, in particular ROP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
WL and YL designed the study and performed the
experiments. WZ, CuZ and ChZ established the animal models. WZ and
XL collected the data. WL and YZ analyzed the data. WL and YL
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Liaocheng People's Hospital Animal Center.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kahn HA and Hiller R: Blindness caused by
diabetic retinopathy. Am J Ophthalmol. 78:58–67. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gibson DL, Sheps SB, Uh SH, Schechter MT
and McCormick AQ: Retinopathy of prematurity-induced blindness:
Birth weight-specific survival and the new epidemic. Pediatrics.
86:405–412. 1990.PubMed/NCBI
|
|
3
|
Jasani B, Nanavati R and Kabra N:
Mechanisms and management of retinopathy of prematurity. N Engl J
Med. 368:1161–1162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao RC and Dlouhy BJ: Mechanisms and
management of retinopathy of prematurity. N Engl J Med.
368:11612013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartnett ME and Penn JS: Mechanisms and
management of retinopathy of prematurity. N Engl J Med.
367:2515–2526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Connor KM, Krah NM, Dennison RJ, Aderman
CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL and Smith LE:
Quantification of oxygen-induced retinopathy in the mouse: A model
of vessel loss, vessel regrowth and pathological angiogenesis. Nat
Protoc. 4:1565–1573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Liang X, Xu C, Xie S, Kuang W and
Liu Z: Quantification of oxygen-induced retinopathy in the mouse.
Yan Ke Xue Bao. 22:103–106. 2006.(In Chinese). PubMed/NCBI
|
|
8
|
Ding X, Liang X, Xie S, Zhu X and Tang S:
A modified mouse model of oxygen-induced retinopathy. Yan Ke Xue
Bao. 22:98–102. 2006.(In Chinese). PubMed/NCBI
|
|
9
|
Smith LE, Wesolowski E, McLellan A, Kostyk
SK, D'Amato R, Sullivan R and D'Amore PA: Oxygen-induced
retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–111.
1994.PubMed/NCBI
|
|
10
|
Sato T, Wada K, Arahori H, Kuno N, Imoto
K, Iwahashi-Shima C and Kusaka S: Serum concentrations of
bevacizumab (avastin) and vascular endothelial growth factor in
infants with retinopathy of prematurity. Am J Ophthalmol.
153:327–333.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Käll A: Is Avastin the right choice of
treatment for retinopathy of prematurity? Acta Paediatr.
101:796–798. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Avery RL: Bevacizumab (Avastin) for
retinopathy of prematurity: Wrong dose, wrong drug, or both? J
AAPOS. 16:2–4. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nuti E, Traversi C, Marigliani D,
Balestrazzi A, Alegente M, Martone G, Malandrini A, Romeo N,
Mazzotta C and Tosi GM: Treatment of macular edema because of
occlusive vasculitis with bevacizumab (avastin): Efficacy of three
consecutive monthly injections. Retina. 31:1863–1870. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu L, Arevalo JF, Roca JA, Maia M,
Berrocal MH, Rodriguez FJ, Evans T, Costa RA and Cardillo J;
Pan-American Collaborative Retina Study Group (PACORES), :
Comparison of two doses of intravitreal bevacizumab (Avastin) for
treatment of macular edema secondary to branch retinal vein
occlusion: Results from the Pan-American Collaborative Retina Study
Group at 6 months of follow-up. Retina. 28:212–219. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kook D, Wolf A, Kreutzer T, Neubauer A,
Strauss R, Ulbig M, Kampik A and Haritoglou C: Long-term effect of
intravitreal bevacizumab (avastin) in patients with chronic diffuse
diabetic macular edema. Retina. 28:1053–1060. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC and
Chen SN: An updated study of the use of bevacizumab in the
treatment of patients with prethreshold retinopathy of prematurity
in taiwan. Am J Ophthalmol. 155:150–158.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Bullock AJ, Zhang L, Wei L, Yu D,
Mahagaokar K, Alsop DC, Mier JW, Atkins MB, Coxon A, et al: The
role of angiopoietins as potential therapeutic targets in renal
cell carcinoma. Transl Oncol. 7:188–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saulle E, Guerriero R, Petronelli A,
Coppotelli E, Gabbianelli M, Morsilli O, Spinello I, Pelosi E,
Castelli G, Testa U and Coppola S: Autocrine role of angiopoietins
during megakaryocytic differentiation. PLoS One. 7:e397962012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas M and Augustin HG: The role of the
Angiopoietins in vascular morphogenesis. Angiogenesis. 12:125–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuo QH, Zeng H, Stinnett A, Yu H, Aschner
JL, Liao DF and Chen JX: Critical role of angiopoietins/Tie-2 in
hyperglycemic exacerbation of myocardial infarction and impaired
angiogenesis. Am J Physiol Heart Circ Physiol. 294:H2547–H2557.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hildbrand P, Cirulli V, Prinsen RC, Smith
KA, Torbett BE, Salomon DR and Crisa L: The role of angiopoietins
in the development of endothelial cells from cord blood CD34+
progenitors. Blood. 104:2010–2019. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plank MJ, Sleeman BD and Jones PF: The
role of the angiopoietins in tumour angiogenesis. Growth Factors.
22:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thurston G: Role of Angiopoietins and Tie
receptor tyrosine kinases in angiogenesis and lymphangiogenesis.
Cell Tissue Res. 314:61–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ellis LM, Ahmad S, Fan F, Liu W, Jung YD,
Stoeltzing O, Reinmuth N and Parikh AA: Angiopoietins and their
role in colon cancer angiogenesis. Oncology (Williston Park).
16:31–35. 2002.PubMed/NCBI
|
|
25
|
Campochiaro PA, Sophie R, Pearlman J,
Brown DM, Boyer DS, Heier JS, Marcus DM, Feiner L and Patel A:
Long-term outcomes in patients with retinal vein occlusion treated
with ranibizumab: The RETAIN study. Ophthalmology. 121:209–219.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung NA, Makin AJ and Lip GY: Measurement
of the soluble angiopoietin receptor tie-2 in patients with
coronary artery disease: Development and application of an
immunoassay. Eur J Clin Invest. 33:529–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Fu P, Li H, Gao R and Xiu R:
Soluble angiopoietin receptor Tie-2 in patients with acute
myocardial infarction and its effects on angiogenesis. Clin
Hemorheol Microcirc. 33:1–10. 2005.PubMed/NCBI
|
|
28
|
Singh N, Macnamara E, Rashid S, Ambati J,
Kontos CD, Higgins E and Ambati BK: Systemic soluble Tie2
expression inhibits and regresses corneal neovascularization.
Biochem Biophys Res Commun. 332:194–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takagi H, Koyama S, Seike H, Oh H, Otani
A, Matsumura M and Honda Y: Potential role of the angiopoietin/tie2
system in ischemia-induced retinal neovascularization. Invest
Ophthalmol Vis Sci. 44:393–402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hangai M, Moon YS, Kitaya N, Chan CK, Wu
DY, Peters KG, Ryan SJ and Hinton DR: Systemically expressed
soluble Tie2 inhibits intraocular neovascularization. Hum Gene
Ther. 12:1311–1321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hohlbaum K, Bert B, Dietze S, Palme R,
Fink H and Thone-Reineke C: Impact of repeated anesthesia with
ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS
One. 13:e2035592018. View Article : Google Scholar
|
|
32
|
Shim HJ, Jung WB, Schlegel F, Lee J, Kim
S, Lee J and Kim SG: Mouse fMRI under ketamine and xylazine
anesthesia: Robust contralateral somatosensory cortex activation in
response to forepaw stimulation. Neuroimage. 177:30–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stewart KA, Wilcox KS, Fujinami RS and
White HS: Development of postinfection epilepsy after Theiler's
virus infection of C57BL/6 mice. J Neuropathol Exp Neurol.
69:1210–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
You JY, Chung H and Kim HC: Evaluation of
changes in choroidal neovascularization secondary to age-related
macular degeneration after anti-VEGF therapy using spectral domain
optical coherence tomography. Curr Eye Res. 37:438–445. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oishi A, Yamashiro K, Tsujikawa A, Ooto S,
Tamura H, Nakata I, Miyake M and Yoshimura N: Long-term effect of
intravitreal injection of anti-VEGF agent for visual acuity and
chorioretinal atrophy progression in myopic choroidal
neovascularization. Graefes Arch Clin Exp Ophthalmol. 251:1–7.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Introini U, Casalino G, Querques G, Gimeno
AT, Scotti F and Bandello F: Spectral-domain OCT in anti-VEGF
treatment of myopic choroidal neovascularization. Eye (Lond).
26:976–982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee J, Park DY, Park DY, Park I, Chang W,
Nakaoka Y, Komuro I, Yoo OJ and Koh GY: Angiopoietin-1 suppresses
choroidal neovascularization and vascular leakage. Invest
Ophthalmol Vis Sci. 55:2191–2199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Bi H, Teng D, Zou Y, Pan X, Guo D
and Cui Y: Potential protective effect of angiopoietin-1 on the
leakage of rat choroidal neovascularization. Saudi Med J.
34:584–590. 2013.PubMed/NCBI
|
|
39
|
Feng Y, Wang Y, Pfister F, Hillebrands JL,
Deutsch U and Hammes HP: Decreased hypoxia-induced
neovascularization in angiopoietin-2 heterozygous knockout mouse
through reduced MMP activity. Cell Physiol Biochem. 23:277–284.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Natarajan G, Johnson YR, Brozanski B,
Farrow KN, Zaniletti I, Padula MA, Asselin JM, Durand DJ, Short BL,
Pallotto EK, et al: Postnatal weight gain in preterm infants with
severe bronchopulmonary dysplasia. Am J Perinatol. 31:223–230.
2014.PubMed/NCBI
|
|
41
|
Turner S, Zhang G, Young S, Cox M,
Goldblatt J, Landau L and Le Souëf P: Associations between
postnatal weight gain, change in postnatal pulmonary function,
formula feeding and early asthma. Thorax. 63:234–239. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stahl A, Krohne TU, Eter N,
Oberacher-Velten I, Guthoff R, Meltendorf S, Ehrt O, Aisenbrey S,
Roider J, Gerding H, et al: Comparing alternative ranibizumab
dosages for safety and efficacy in retinopathy of prematurity: A
randomized clinical trial. Jama Pediatr. 172:278–286. 2018.
View Article : Google Scholar : PubMed/NCBI
|