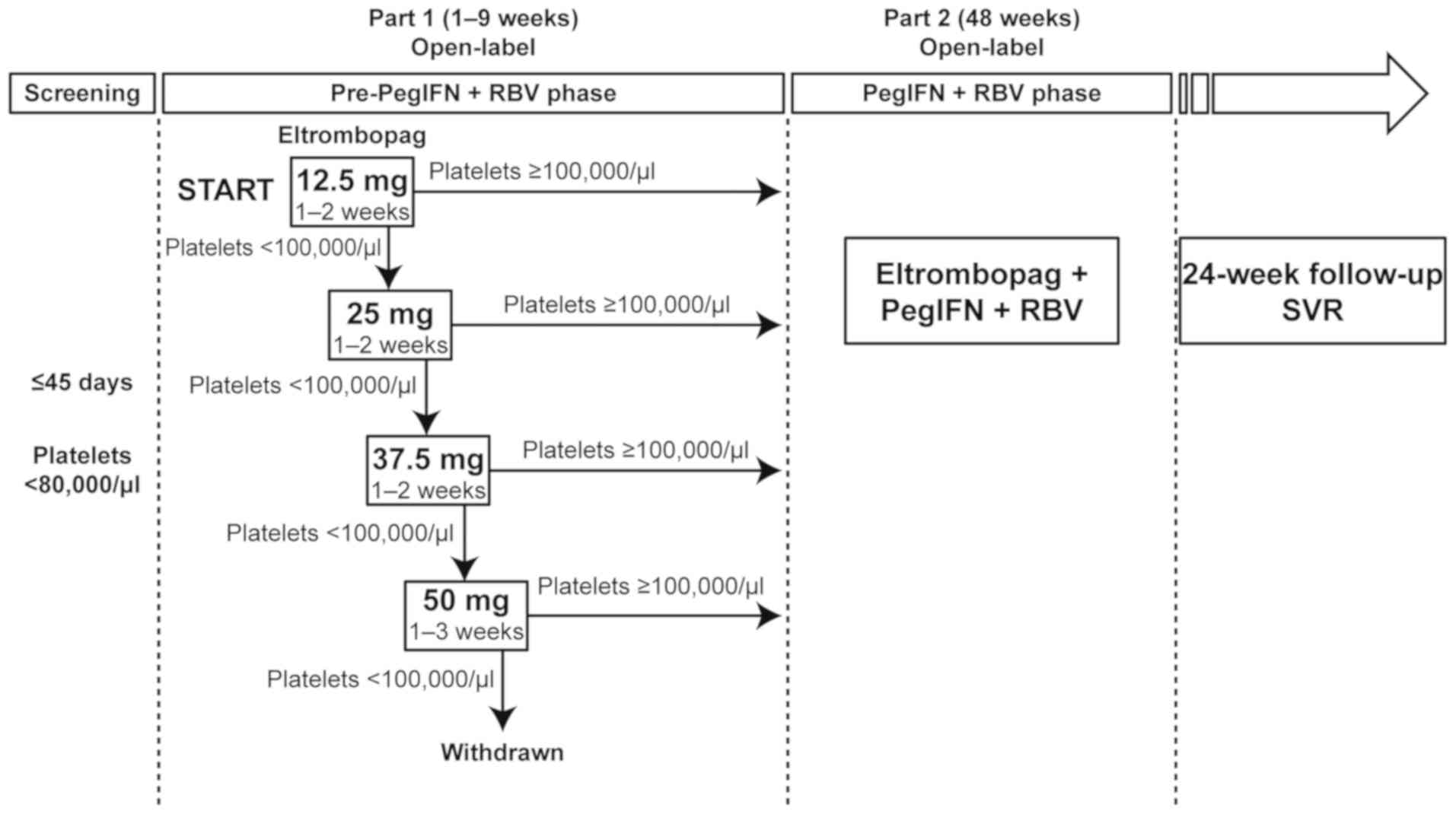
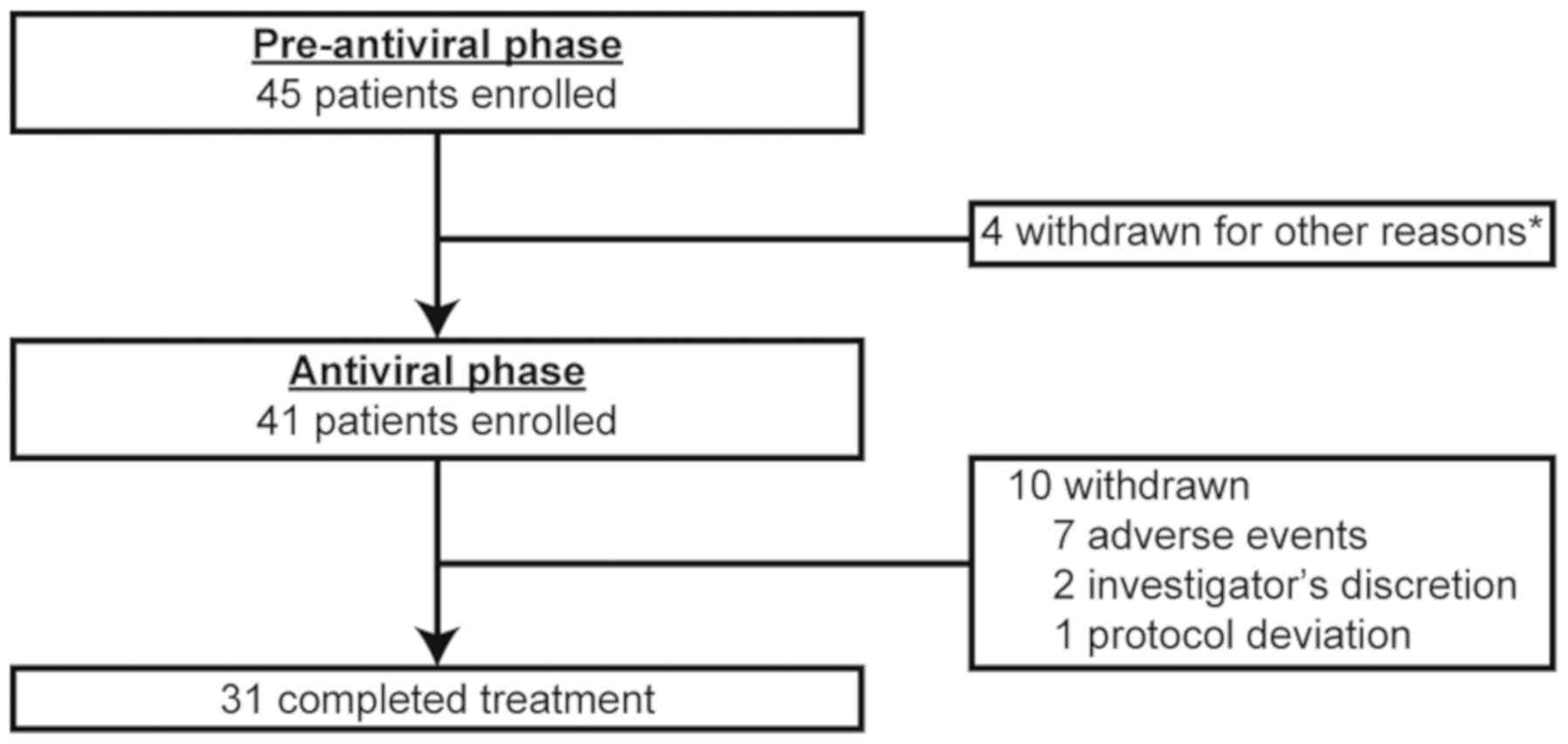
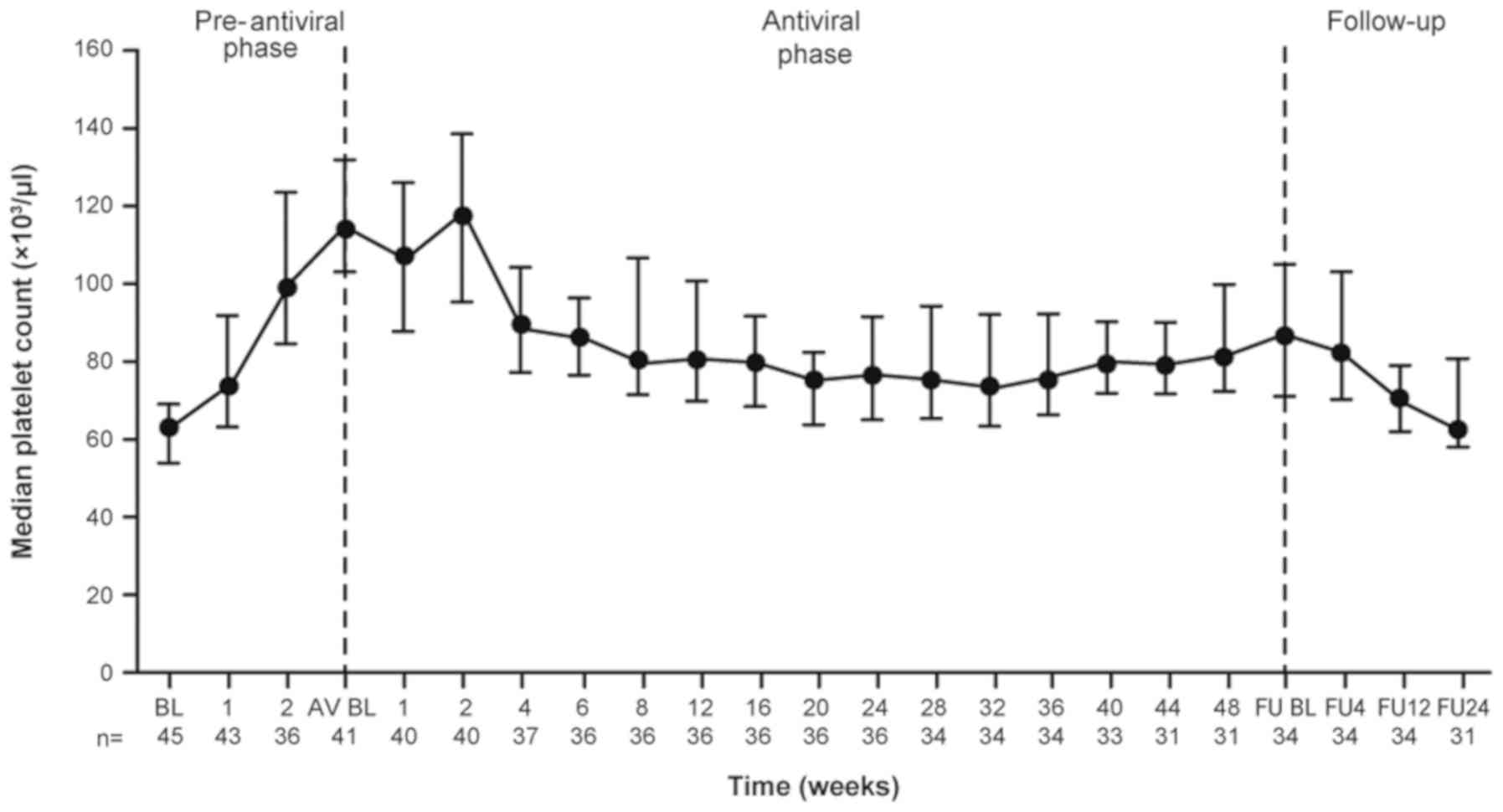
|
1
|
Latorre R, Vaquero J, Rincón D, Puerto M,
Ponce MD, Sarnago F, Matamoros JA, Ramón E, Elizaga J, Bañares R
and Ripoll C: Determinants of platelet count are different in
patients with compensated and decompensated cirrhosis. Liver Int.
36:232–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitchell O, Feldman DM, Diakow M and Sigal
SH: The pathophysiology of thrombocytopenia in chronic liver
disease. Hepat Med. 8:39–50. 2016.PubMed/NCBI
|
|
3
|
Jayasekera CR, Barry M, Roberts LR and
Nguyen MH: Treating hepatitis C in lower-income countries. N Engl J
Med. 370:1869–1871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lawitz E, Lalezari JP, Hassanein T,
Kowdley KV, Poordad FF, Sheikh AM, Afdhal NH, Bernstein DE, Dejesus
E, Freilich B, et al: Sofosbuvir in combination with peginterferon
alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients
with genotypes 1, 2 and 3 hepatitis C infection: A randomised,
double-blind, phase 2 trial. Lancet Infect Dis. 13:401–408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kronenberger B and Zeuzem S: New
developments in HCV therapy. J Viral Hepat. 19 (Suppl 1):S48–S51.
2012. View Article : Google Scholar
|
|
6
|
Casey LC and Lee WM: Hepatitis C virus
therapy update 2013. Curr Opin Gastroenterol. 29:243–249.
2013.PubMed/NCBI
|
|
7
|
Nkuize M, Sersté T, Buset M and Mulkay JP:
Combination ledipasvir-sofosbuvir for the treatment of chronic
hepatitis C virus infection: A review and clinical perspective.
Ther Clin Risk Manag. 12:861–872. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shahid I, AlMalki WH, Hassan S and Hafeez
MH: Real-world challenges for hepatitis C virus medications: A
critical overview. Crit Rev Microbiol. 44:143–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Treatment guidelines for hepatitis C from
Japan Society of Hepatology. http://www.jsh.or.jp/files/uploads/Brief_C_v6.2_Oct15.pdfMarch
14th–2019(In Japanese).
|
|
10
|
Afdhal NH, Dusheiko GM, Giannini EG, Chen
PJ, Han KH, Mohsin A, Rodriguez-Torres M, Rugina S, Bakulin I,
Lawitz E, et al: Eltrombopag increases platelet numbers in
thrombocytopenic patients with HCV infection and cirrhosis,
allowing for effective antiviral therapy. Gastroenterology.
146:442–452.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
COPEGUS (ribavirin) product information.
Roche products Ltd., . https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021511s023lbl.pdfMarch
14th–2019
|
|
12
|
PEGASYS (peginterferon alfa-2a) Product
Information. Roche Products Ltd., . https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103964s5204lbl.pdfMarch
14th–2019
|
|
13
|
PEGINTRON (peginterferon alfa-2b) product
information. Merck & Co, Inc., . https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103949s5306lbl.pdfMarch
14th–2019
|
|
14
|
Loffredo L and Violi F: Thrombopoietin
receptor agonists and risk of portal vein thrombosis in patients
with liver disease and thrombocytopenia: A meta-analysis. Dig Liver
Dis. 51:24–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
REBETOL (ribavirin) product information.
Schering-plough. Merck &Co, Inc., . https://www.merck.com/product/usa/pi_circulars/r/rebetol/rebetol_pi.pdfMarch
14th–2019
|
|
16
|
Danish FI and Yasmin S: The role of
eltrombopag in the management of hepatitis C virus-related
thrombocytopenia. Hepat Med. 5:17–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galli L, Gerdes VE, Guasti L and Squizzato
A: Thrombosis associated with viral hepatitis. J Clin Transl
Hepatol. 2:234–239. 2014.PubMed/NCBI
|
|
18
|
Afdhal NH, Giannini EG, Tayyab G, Mohsin
A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH,
et al: Eltrombopag before procedures in patients with cirrhosis and
thrombocytopenia. N Engl J Med. 367:716–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burness CB: Eltrombopag: A review of its
use in the treatment of thrombocytopenia in patients with chronic
hepatitis C. Drugs. 74:1961–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomiyama Y, Miyakawa Y, Okamoto S,
Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S,
Ozaki K, et al: A lower starting dose of eltrombopag is efficacious
in Japanese patients with previously treated chronic immune
thrombocytopenia. J Thromb Haemost. 10:799–806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katsutani S, Tomiyama Y, Kimura A,
Miyakawa Y, Okamoto S, Okoshi Y, Ninomiya H, Kosugi H, Ishii K,
Ikeda Y, et al: Oral eltrombopag for up to three years is safe and
well-tolerated in Japanese patients with previously treated chronic
immune thrombocytopenia: An open-label, extension study. Int J
Hematol. 98:323–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Editors of the Drafting Committee for
Hepatitis Management Guidelines, . The Japan Society of Hepatology:
Guidelines for the management of hepatitis C virus infection: First
edition, May 2012, The Japan society of hepatology. Hepatol Res.
43:1–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi N, Seto C, Kato M, Komada Y and
Goto S: Once-daily simeprevir (TMC435) with peginterferon/ribavirin
for treatment-naïve hepatitis C genotype 1-infected patients in
Japan: The DRAGON study. J Gastroenterol. 49:138–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Izumi N, Hayashi N, Kumada H, Okanoue T,
Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C and Goto
S: Once-daily simeprevir with peginterferon and ribavirin for
treatment-experienced HCV genotype 1-infected patients in Japan:
The CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 49:941–953.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumada H, Sato K, Takehara T, Nakamuta M,
Ishigami M, Chayama K, Toyota J, Suzuki F, Nakayasu Y, Ochi M, et
al: Efficacy of telaprevir-based therapy for difficult-to-treat
patients with genotype 2 chronic hepatitis C in Japan. Hepatol Res.
45:745–754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumada H, Suzuki F, Kamiya N, Orihashi M,
Nakayasu Y and Yamada I: Efficacy and safety of telaprevir with
pegylated interferon α-2a and ribavirin in Japanese patients.
Hepatol Res. 47:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maruyama H, Okugawa H, Takahashi M and
Yokosuka O: De novo portal vein thrombosis in virus-related
cirrhosis: Predictive factors and long-term outcomes. Am J
Gastroenterol. 108:568–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boilard E, Nigrovic PA, Larabee K, Watts
GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E,
Farndale RW, Ware J and Lee DM: Platelets amplify inflammation in
arthritis via collagen-dependent microparticle production. Science.
327:580–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Revolade summary of product
characteristics. Novartis Europharm Limited. https://www.ema.europa.eu/en/documents/product-information/revolade-epar-product-information_en.pdf(last
updated January, 2015). March 14th–2019
|
|
30
|
Kawaguchi T, Komori A, Seike M, Fujiyama
S, Watanabe H, Tanaka M, Sakisaka S, Nakamuta M, Sasaki Y, Oketani
M, et al: Efficacy and safety of eltrombopag in Japanese patients
with chronic liver disease and thrombocytopenia: A randomized,
open-label, phase II study. J Gastroenterol. 47:1342–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cardier JE and Dempsey J: Thrombopoietin
and its receptor, c-mpl, are constitutively expressed by mouse
liver endothelial cells: Evidence of thrombopoietin as a growth
factor for liver endothelial cells. Blood. 91:923–929.
1998.PubMed/NCBI
|
|
32
|
Schmelzer E, Deiwick A, Bruns H, Fiegel HC
and Bader A: Thrombopoietin is a growth factor for rat hepatic
progenitors. Eur J Gastroenterol Hepatol. 20:209–216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hajarizadeh B, Grebely J and Dore GJ:
Epidemiology and natural history of HCV infection. Nat Rev
Gastroenterol Hepatol. 10:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erickson-Miller CL, Chadderton A, Gibbard
A, Kirchner J, Pillarisetti K, Baker K, Pandite L, El-Hariry I,
Mostafa Kamel Y, Liu Y, et al: Thrombopoietin receptor levels in
tumor cell lines and primary tumors. J Oncol 2010. 1353542010.
|
|
35
|
Nozaki R, Murata S, Nowatari T, Maruyama
T, Ikeda N, Kawasaki T, Fukunaga K and Ohkohchi N: Effects of
thrombopoietin on growth of hepatocellular carcinoma: Is
thrombopoietin therapy for liver disease safe or not? Hepatol Res.
43:610–620. 2013. View Article : Google Scholar : PubMed/NCBI
|