Introduction
Thrombocytopenia is a frequent complication in
cirrhotic patients and its severity correlates with the disease
severity (1). In addition to
hypersplenism secondary to portal hypertension, impaired production
of endogenous thrombopoietin (TPO) in the liver is a major cause of
thrombocytopenia (2). Until
recently, the combination of pegylated interferon (pegIFN) α and
ribavirin (RBV) was the standard treatment for chronic hepatitis C
and compensated cirrhosis (3).
However, thrombocytopenia interferes with the initiation and
continuation of interferon (IFN) therapy owing to its
myelosuppressive effects, especially in patients with cirrhosis
(2).
The recently developed direct-acting antivirals
(DAAs) have revolutionized the treatment of hepatitis C virus (HCV)
infections, with highly sustained virological response (SVR) rates
and better tolerability than the IFN-based therapy (4,5).
Nevertheless, DAAs have several potential limitations, including
the issue of resistance-associated variants and their high cost.
Furthermore, the long-term effects of DAAs remain unclear. Certain
DAAs have been associated with the worsening of dyslipidemia
(6,7). Although some recent reports have
suggested that an SVR by DAA therapy reduces the risk of developing
hepatocellular carcinoma (HCC), the role of DAAs in the recurrence
of HCC in patients treated for hepatic decompensation remains
controversial, and thus, warrants further elucidation and follow-up
in the HCC high-risk group (8).
Moreover, DAAs have not been approved worldwide as they have
limited access, and their high price creates a barrier, especially
in low-income countries with high prevalence of HCV as well as in
some developed countries due to payer restrictions on the DAA
therapy (3,8). In addition, IFN-based therapy is still
one of recommendations for patients with chronic infection of HCV
that has multiple drug resistance mutation (9).
For the aforementioned reasons, IFN-based therapy
still remains a feasible treatment strategy in countries where DAAs
are not accessible or affordable. As thrombocytopenia may restrict
the initiation or continuation of IFN-based therapy, its control
has clinical importance. The clinical management of
thrombocytopenic patients with HCV infection receiving IFN-based
therapy primarily depends on IFN dose reductions, which may
preclude patients from achieving an SVR.
Eltrombopag is an orally bioavailable, small
molecule thrombopoietin receptor (TPO-R) agonist that has been
approved for the treatment of chronic immune thrombocytopenic
purpura (ITP). It significantly increases the platelet counts in a
dose-dependent manner in thrombocytopenic patients with chronic
hepatitis C [Eltrombopag to initiate and maintain interferon
antiviral treatment to benefit subjects with hepatitis C-related
liver disease (ENABLE)-1 and ENABLE-2 studies] (10), as well as in those with chronic ITP.
However, eltrombopag has been associated with an increased risk of
thrombosis in patients with chronic liver disease (CLD) (11–13),
there are reports of portal vein thrombosis associated with the use
of eltrombopag (14). In this study,
we assessed the ability of eltrombopag in increasing the platelet
counts in HCV-infected patients with thrombocytopenia to a
sufficient level to initiate and maintain pegIFN/RBV therapy
without dose modifications of pegIFN and development of
thrombosis.
Patients and methods
Study design
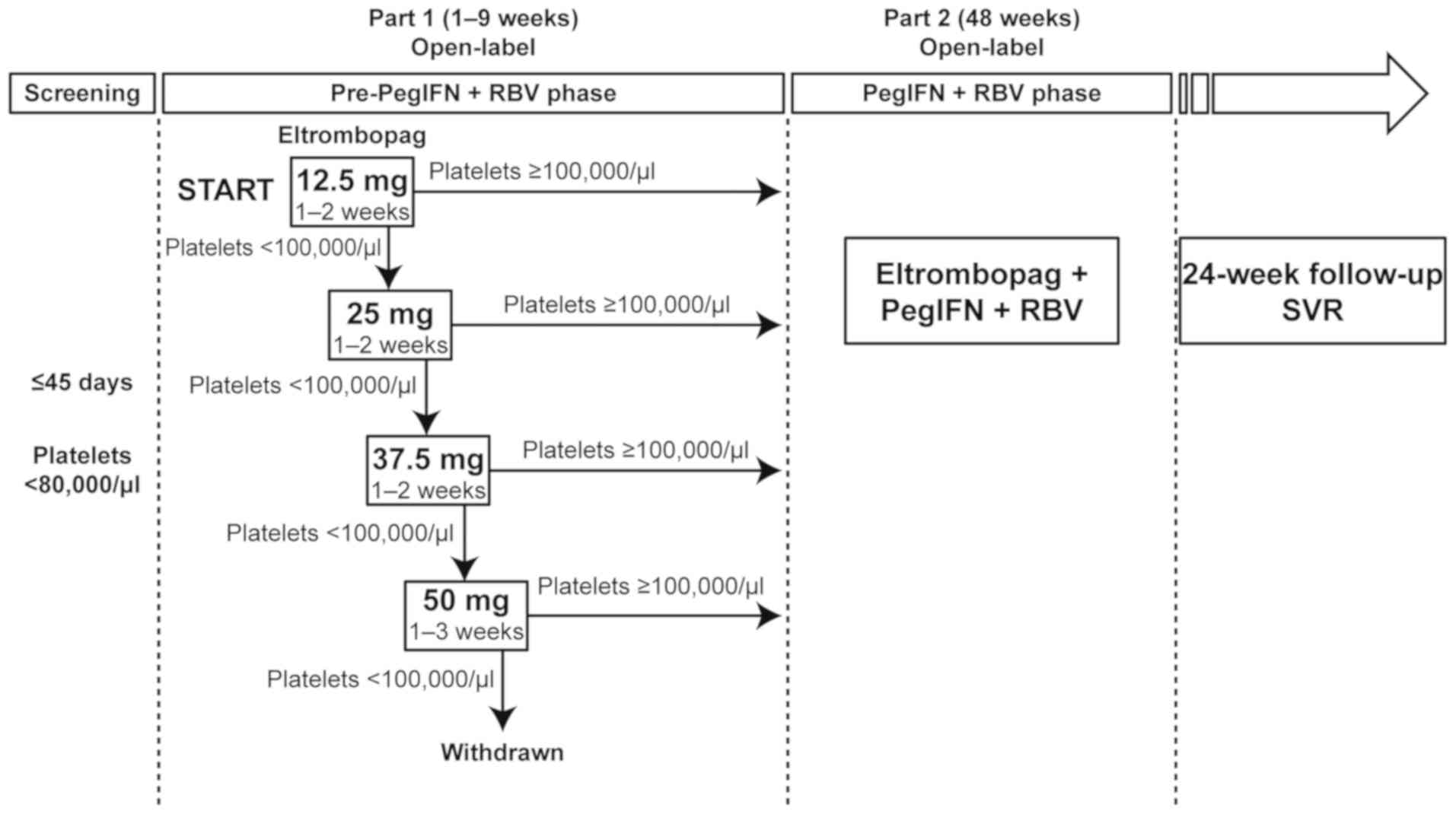
This was a phase II, single-arm, open-label study
comprising three phases: A pre-antiviral phase (up to 9 weeks), an
antiviral phase (48 weeks, with possible extension to 72 weeks in
slow or late responders), and a 24-week follow-up phase (Fig. 1). The study was approved by the
National Hospital Organization Central Review Board of four
national hospitals and institutional review boards of 12 other
participating centers; the study was performed in accordance with
the Declaration of Helsinki, Good Clinical Practice guidelines, and
local laws and regulations. All participating patients provided
written informed consent prior to their inclusion in the study.
This study is registered at ClinicalTrials.gov (NCT01636778; July 05, 2012).
Patients who achieved the required platelet count
threshold (≥100,000/µl) in the pre-antiviral phase entered the
antiviral phase and received antiviral therapy in combination with
eltrombopag for 48 weeks. Extended treatment with pegIFN/RBV for up
to 72 weeks was allowed based on the investigator's judgment;
however, the duration of treatment with eltrombopag was limited to
48 weeks. During the follow-up period, the SVR was also evaluated
in addition to monitoring the safety of eltrombopag at 4, 12, and
24 weeks after the treatment.
Evaluations
The primary endpoints included the proportion of
patients who achieved a platelet count >100,000/µl during the
pre-antiviral phase and those who maintained a platelet count
>50,000/µl throughout the antiviral phase.
Secondary efficacy endpoints included the median
platelet counts throughout the study, the proportion of patients
who had to undergo dose reductions or discontinuation of pegIFN
and/or RBV, and the SVR rate. The SVR rate was defined as the
proportion of patients with undetectable HCV RNA at the end of the
treatment and all subsequent planned visits up to 24 weeks after
completing the treatment. Rapid virological response (RVR), early
virological response (EVR), complete EVR (cEVR), and
end-of-treatment response (ETR) were also assessed at weeks 4, 12,
and 48, respectively.
Adverse events (AEs) and their severity and
causality were assessed by the individual investigator and
summarized by incidence and severity. Since one of major
complications of eltrombopag is portal vein thrombosis, doppler
ultrasound of abdomen was performed at screening or baseline and
every 6 months thereafter to assess portal vein thrombosis. In
addition, abdominal image (e.g., CT, MRI) was performed whenever
any symptoms of portal vein thrombosis are observed. The severity
of AEs was graded using the Division of Acquired Immunodeficiency
Syndrome (DAIDS) Table for Grading the Severity of Adult and
Pediatric Adverse Events (version 1.9, dated December 2004).
Patients
Patients aged between 20 and 75 years who were
diagnosed with hepatitis C or compensated liver cirrhosis and
thrombocytopenia (baseline platelet count, <80,000/µl) were
eligible.
Patients who had a Child-Pugh score of 6 or less
(Child-Pugh class A) without hepatic encephalopathy or ascites and
adequate hepatic, renal, and hematologic function to receive
antiviral therapy were appropriate candidates for the pegIFN/RBV
therapy. Those who had received prior treatment with pegIFN/RBV but
had stopped the treatment because of disease- or treatment-related
thrombocytopenia were also eligible.
The exclusion criteria were based on the ENABLE
studies (10). In brief, patients
who did not respond to previous pegIFN/RBV therapy for reasons
other than thrombocytopenia or had decompensated liver disease,
serious medical complications (including serious cardiac,
cerebrovascular, or pulmonary disease, a history of arterial or
venous thrombosis, hepatitis B virus or human immunodeficiency
virus infection, or platelet aggregation abnormalities), active
bleeding, or a history of clinically significant bleeding from
esophageal or gastric varices were excluded from the study.
Patients with HCC or a history of previous HCC within 5 years
before enrollment were also excluded.
Treatment
In view of the differences in the pharmacokinetics
of eltrombopag caused by inter-ethnic differences, lower doses of
eltrombopag (12.5 mg as the starting dose and 50 mg as the maximum
dose) were used compared with those used in the ENABLE studies
(10). All doses were administered
orally once daily in the fasting state.
During the pre-antiviral phase, all patients
initially received 12.5 mg of eltrombopag. The dose was increased
by 12.5 mg every 2 weeks up to 50 mg until the platelet count
increased to the required threshold (≥100,000/µl) to initiate
antiviral therapy. Patients who did not achieve the platelet count
threshold after 3 weeks of treatment with eltrombopag at 50 mg once
daily discontinued the treatment and entered the 24-week follow-up
phase. Patients achieving the platelet count threshold initiated
antiviral therapy (pegIFN α-2a/RBV or pegIFN α-2b/RBV; the
treatment regimen was selected by the investigator) in combination
with eltrombopag per the package insert of each drug (15–18).
During the antiviral phase, dose adjustments of
eltrombopag were based on individual platelet response to maintain
the platelet counts between 50,000/µl and 150,000/µl, which was a
lower threshold (more cautious on platelet increases) than that
applied in the ENABLE studies (50,000-200,000/µl) (10). Patients showing a trend toward
greater increases in platelet counts were allowed a dose reduction
of eltrombopag per the investigator's decision, even if their
platelet counts were lesser than 150,000/µl. The platelet counts
were monitored weekly until week 8 of treatment and every 4 weeks
thereafter.
Pharmacokinetics
Serial blood samples were collected prior to dose
administration (prior to the morning administration of eltrombopag
on day 14) and at 1, 2, 4, 6, 8, and 24 h after the dose
administration in five patients who received 12.5 mg of
eltrombopag. Pharmacokinetic parameters [maximum plasma
concentration (Cmax), time to maximum plasma
concentration (Tmax), area under the plasma
concentration-time curve up to the last measurable concentration
(AUC0-t), and area under the plasma concentration curve
to the end of the dosing period (AUC0-τ)] were
calculated at actual sampling timings using non-compartmental
analysis. Summary statistics were used to summarize the
pharmacokinetic parameters. If patients withdrew from the study
because of abnormal liver function tests (LFTs), plasma eltrombopag
concentrations were to be measured within 3 days after
withdrawal.
Statistical analysis
The proportion of patients who maintained the
platelet counts at >50,000/µl during the antiviral phase was
assumed to be 70% on the basis of the results [68.9%, 95%
confidence interval (CI): 64.4–73.1] of a previous phase III study
(unpublished data). Based on the analysis of binomial distribution,
50 evaluable patients were needed to provide 95% or more power to
detect a response rate of at least 60%. Considering the proportion
of patients who would not complete the pre-antiviral phase, the
planned sample size for this study was 52 evaluable patients.
However, the study was terminated early by the sponsor because of
introduction of DAAs and low recruitment rates.
Continuous variables were summarized using
descriptive statistics and categorical variables were summarized
using frequency counts and percentages. The primary endpoints for
the pre-antiviral and antiviral phases were analyzed using point
estimates and two-sided 95% CI. For analysis of the SVR and other
virologic endpoints, a patient with missing HCV RNA data at the
assessment for any reason was regarded as a non-responder. A
patient with missing data due to early discontinuation of the
antiviral therapy and being treated with antiviral therapy beyond
48 weeks was regarded as a non-responder for all subsequent
assessments.
Results
Pre-antiviral phase
Patient demographic and baseline
characteristics
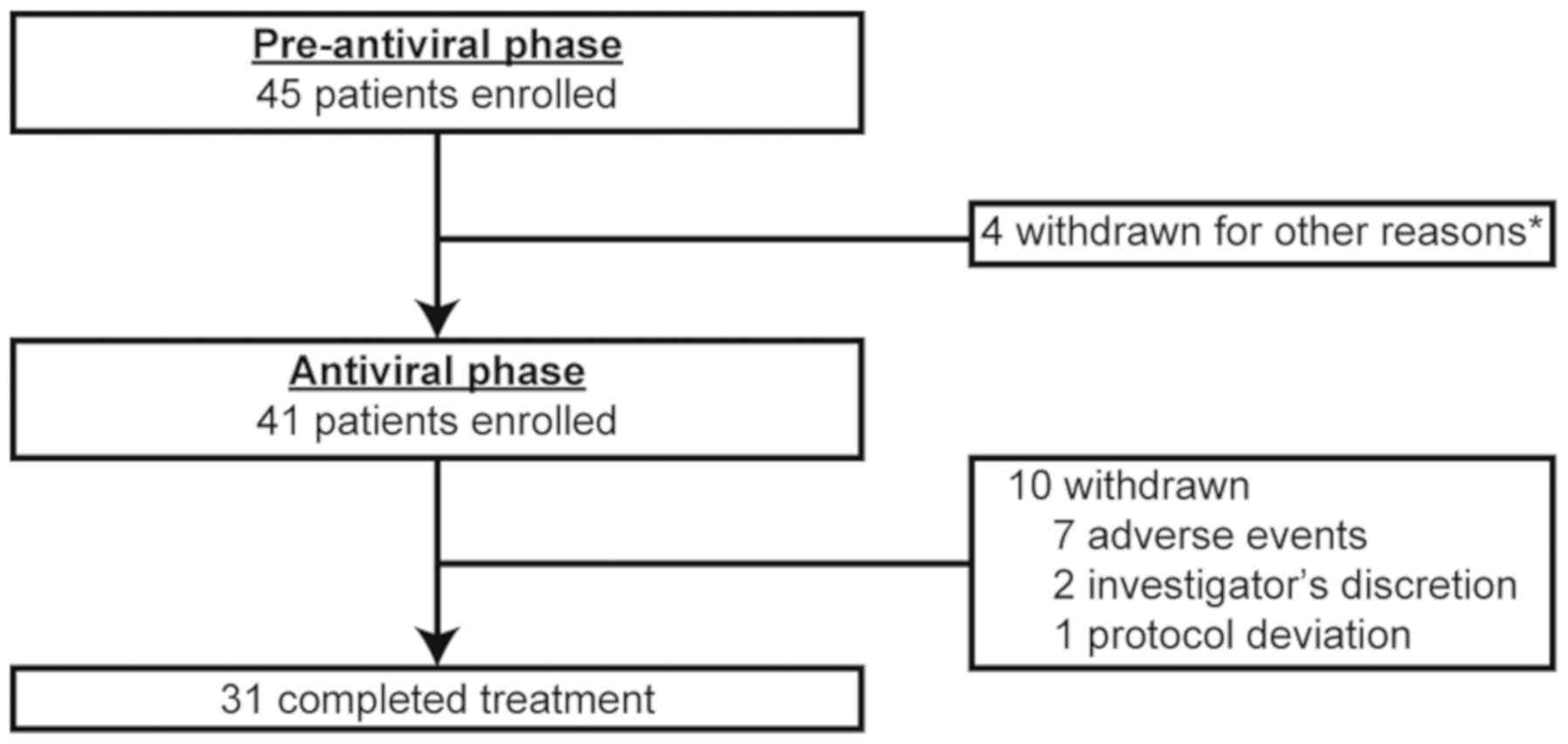
A total of 45 patients with HCV infection were
enrolled from 16 centers in Japan between July 2012 and April 2014.
Of these, four patients were withdrawn in the pre-antiviral phase,
three because of violation of the eligibility criteria and one due
to lack of achievement of the pre-defined platelet count threshold
(Fig. 2). The baseline patient
demographic and disease characteristics in the pre-antiviral phase
are shown in Table I.
 | Table I.Patient demographics and baseline
disease characteristics during the pre-antiviral phase. |
Table I.
Patient demographics and baseline
disease characteristics during the pre-antiviral phase.
| Patient
demographics | Pre-antiviral
treatment phase N=45 |
|---|
| Age (years), median
(range) | 59 (31–72) |
| Sex, n (%) |
|
Female | 30 (67) |
|
Male | 15 (33) |
| Body mass index
(kg/m2), mean ± SD | 23.3±3.5 |
| Diagnosis, n
(%) | 23/22 (51/49) |
| Chronic
hepatitis C | 23 (51) |
|
Cirrhosis | 22 (49) |
| HCV genotype, n
(%) |
| 1a | 0 |
| 1b | 33 (73) |
| 2a | 6 (13) |
| 2b | 5 (11) |
|
Othera | 1 (2) |
| Child-Pugh
classification, n (%) |
| A
(score 5–6) | 44 (98) |
| B
(score 7–9) | 1 (2) |
| Platelet count,
median (range) | 63,000 |
|
|
(34,000–78,000) |
| HCV RNA (Log
IU/ml), mean ± SD | 6.4±0.7 |
| ALT (U/l), mean ±
SD | 78.2±47.8 |
At baseline, the median age of patients was 59 years
(range, 31–72 years), and 67% of the patients were female. The
median platelet count at baseline was 63,000/µl. In total, 98% of
the patients had Child-Pugh class A and 73% had genotype b HCV
infection with high viral load (≥5 log IU/ml). Approximately half
of the patients (24 patients, 53%) had undergone antiviral therapy
at least once and 71% (17 patients) of them had experienced
treatment failure.
Efficacy and safety
Of the 45 enrolled patients, 43 (96%) achieved a
platelet count ≥100,000/µl, which was the platelet count threshold
required to initiate the antiviral combination therapy with pegIFN
α/RBV. The median time to response was 2.14 weeks (range, 1.0–9.6
weeks), and 36 patients (84%) achieved the threshold within 4 weeks
after starting eltrombopag. A total of 41 patients, almost all of
whom were receiving 12.5 mg or 25 mg of eltrombopag, entered the
antiviral phase (Fig. 2). The
remaining two responders did not meet the eligibility criteria
after starting eltrombopag and were withdrawn from the study in the
pre-antiviral phase.
During the pre-antiviral phase, two patients did not
achieve platelet counts ≥100,000/µl. One patient withdrew from the
study 1 week after starting eltrombopag because the severity of
liver cirrhosis was re-classified as Child-Pugh class B. The other
patient withdrew for lack of achievement of the platelet count
threshold at week 9 despite dose escalation to the maximum dose of
eltrombopag (50 mg).
Thirteen patients (29%) experienced at least one AE
during the pre-antiviral phase. All AEs reported were grade 1 in
severity. Headache and vomiting were the most common AEs and
occurred in two patients each. AEs considered to be drug related
were reported by four patients (9%); no serious AEs (SAEs) were
reported during the pre-antiviral phase (Table II).
 | Table II.AEs during the pre-antiviral
phase. |
Table II.
AEs during the pre-antiviral
phase.
| AEs | Antiviral phase
N=45 |
|---|
| AEs occurring in ≥2
patients, n (%) | 4 (8%) |
|
Vomiting | 2 (4%) |
|
Headache | 2 (4%) |
| Grade ≥3 adverse
events, n (%) | 0 |
| Serious adverse
events, n (%) | 0 |
Antiviral phase
Patient demographic and baseline
disease characteristics
A total of 41 patients who achieved the platelet
count threshold entered the antiviral phase. The patient
demographic and baseline disease characteristics are consistent
with those in the pre-antiviral phase (Table III).
 | Table III.Patient demographic and baseline
disease characteristics during the antiviral phase. |
Table III.
Patient demographic and baseline
disease characteristics during the antiviral phase.
| Patient
demographics | Antiviral phase
N=41 |
|---|
| Age (years), median
(range) | 61 (31–72) |
| Sex, n (%) |
|
Female | 28 (68) |
|
Male | 13 (32) |
| Body mass index
(kg/m2), mean ± SD | 23.2±3.6 |
| Diagnosis, n
(%) |
| Chronic
hepatitis | 22 (54) |
|
Cirrhosis | 19 (46) |
| HCV genotype, n
(%) |
| 1a | 0 |
| 1b | 31 (76) |
| 2a | 6 (15) |
| 2b | 3 (7) |
|
Othera | 1 (2) |
| Child-Pugh
classification |
| A
(score 5–6) | 40 (98) |
| B
(Score 7–9) | 1 (2) |
| Baseline platelet
counts, median (range) | 63,000 |
|
|
(37,000–78,000) |
| HCV RNA (Log
IU/ml), mean ± SD | 6.4±0.7 |
| ALT (IU/l), mean ±
SD | 82.0±48.0 |
Efficacy
During the antiviral phase, eltrombopag doses were
adjusted to maintain platelet counts ≥50,000/µl, which exceeded the
threshold for dose reductions/interruptions of pegIFN. Of the 41
patients who entered the antiviral phase, 36 (88%) maintained the
platelet count threshold throughout the antiviral phase. Although
the five remaining patients experienced a transient decrease in
platelet counts (<50,000/µl), no patient had a platelet count
<25,000/µl (threshold for dose discontinuation of pegIFN) during
the antiviral phase. Following the initiation of the antiviral
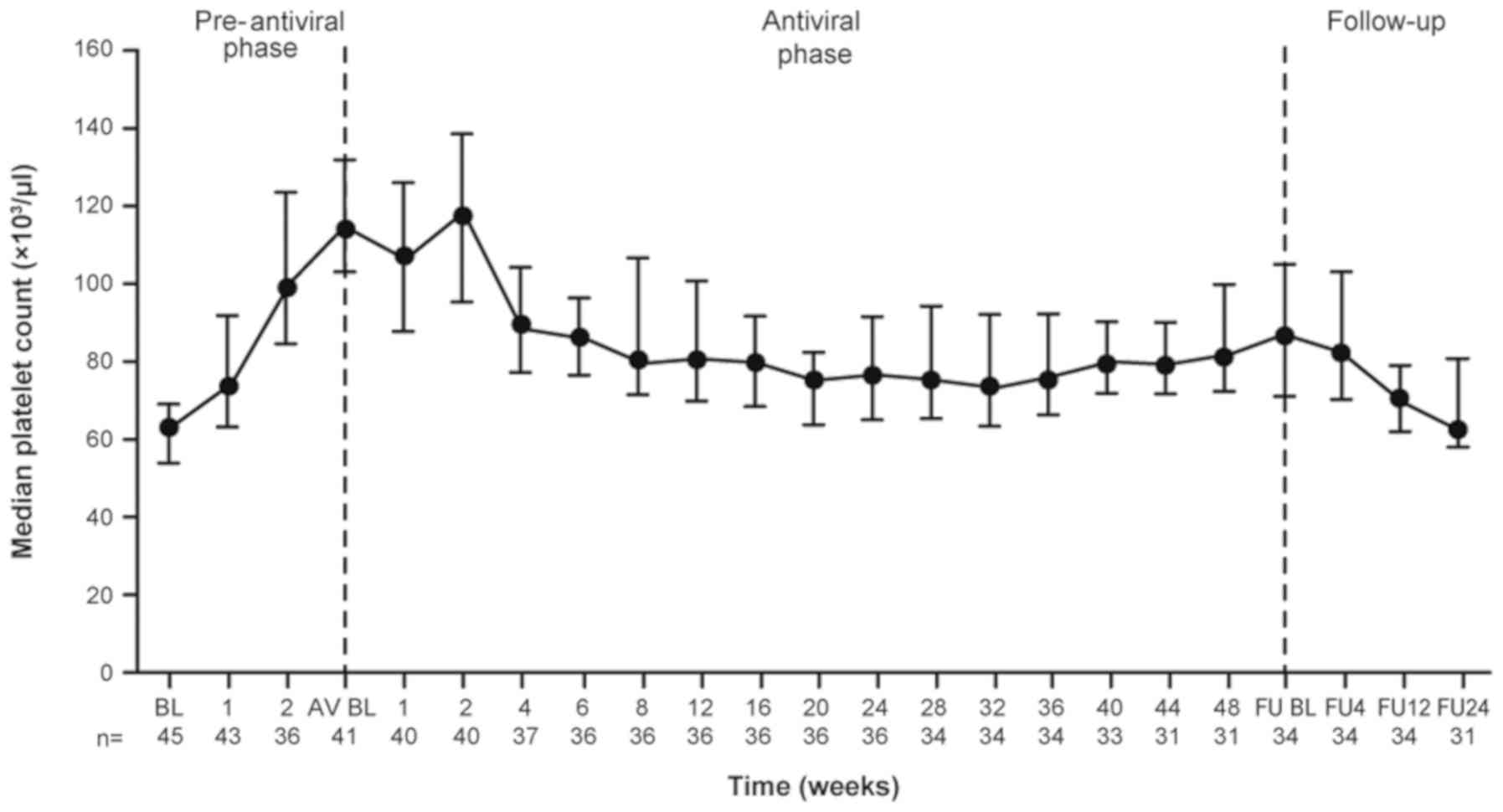
combination therapy, the median platelet counts gradually decreased
by 4 weeks and remained at around 80,000/µl thereafter (Fig. 3).
No dose reductions of pegIFN were required in
approximately half of the patients (20 patients, 48%) while
administering eltrombopag in addition to the pegIFN therapy, 25%
with pegIFN α-2a and 64% with pegIFN α-2b. However, one or no dose
reductions of pegIFN were required in 63 and 72% of patients,
respectively.
Overall, eight patients (20%) discontinued the
pegIFN therapy prematurely because of AEs (six patients) and
insufficient antiviral response (two patients). The proportions of
patients who achieved the RVR, EVR, and ETR were 15% (six
patients), 68% (28 patients), and 46% (19 patients), respectively.
Nine patients (22%) achieved an SVR at the 24-week follow-up, 25%
with pegIFN α-2a and 20% with pegIFN α-2b. Patients with
non-genotype 1 HCV infection had a higher SVR rate compared with
those having the genotype 1 HCV (60% vs. 10%, respectively).
Patients aged <65 years had a higher SVR rate compared with
those aged ≥65 years (27% vs. 9%, respectively). Viral breakthrough
during the pegIFN/RBV therapy and relapse after the end of therapy
were observed in two and eight patients (42% of patients achieving
the ETR), respectively.
Safety
During the antiviral phase, all 41 patients
experienced at least one AE or drug-related AE, with the majority
being grade 2 or grade 3 in severity. The most common AEs were
pyrexia, anemia, neutropenia, alopecia, pruritus, headache, and
rash, which occurred in at least 30% of patients.
Grade 3 or higher AEs occurred in 25 patients (61%)
during the treatment. Only four patients discontinued eltrombopag
because of these AEs.
A 64-year-old man experienced a hepatic function
abnormality on day 56 (42 days after starting the antiviral
treatment): Increase in alanine aminotransaminase (ALT)
concentration to >5 times the upper limit of normal (ULN) (417
U/l) and aspartate transaminase (AST) concentration to >3 times
the ULN (301 U/l), in conjunction with a total bilirubin
concentration two times the ULN (2.4 mg/dl). The patient withdrew
from the study based on the pre-defined criteria. Treatment with
pegIFN and eltrombopag was discontinued on day 50 and 57,
respectively. Although the abnormality seemed to improve with
treatment discontinuation and use of conservative therapy, he was
hospitalized due to grade 3 acute liver injury on day 63 (6 days
after discontinuation of eltrombopag). The event was considered to
be related to all three study treatments (eltrombopag, pegIFN, and
RBV). On day 70, he developed mild hepatic encephalopathy due to
hepatic failure. His platelet count began to increase after
discontinuation of pegIFN and eltrombopag and reached the peak
level (646,000/µl) on day 89; this was reported as a drug-related
SAE (thrombocytosis). However, this patient did not present with a
TEE. Using conservative therapy, the liver injury associated with
the study treatment improved by day 152.
Another 37-year-old man experienced a liver disorder
(grade 3 AE) on day 64 (29 days after starting the antiviral
treatment), which manifested as increased ALT concentration to
>5 times the ULN (363 U/l) and AST concentration to >3 times
the ULN (308 U/l), in conjunction with a total bilirubin
concentration two times the ULN (3.8 mg/dl). He was withdrawn from
the study based on the pre-defined liver stopping criteria. The
event was considered to be related to the study treatment and it
resolved by day 99 (36 days after the discontinuation of the study
treatment) without any treatment.
A 53-year-old woman experienced an increase in the
percentage of myeloblasts (grade 3 SAE) on day 78 (3 days after
starting the antiviral treatment), which was considered to be
related to the antiviral treatment as it developed immediately
after treatment initiation. Although eltrombopag was discontinued
on day 80, the abnormality remained thereafter. The possibility of
fibrosis was excluded based on a bone marrow biopsy performed on
day 81.
Another 67-year-old woman experienced a decrease in
body weight (grade 4 AE) by day 56 (41 days after starting the
antiviral treatment), which was considered to be related to the
study treatment. Although her weight decreased from 48.2 kg at
baseline to 45.0 kg on day 56, the study treatment was continued
thereafter. By day 323, her weight decreased to 37.8 kg and the
treatment was discontinued. The decrease in body weight did not
subside with treatment discontinuation.
During the antiviral phase, 20 patients (49%)
experienced hepatobiliary AEs, with the majority being grade 1 or
grade 2 in severity. Other than the above grade 3 hepatobiliary AEs
(liver injury and liver disorder), one case of hyperbilirubinemia
was reported as a grade 3 AE that resolved without dose
modifications of the study drug. Only one patient presented with
hepatic decompensation (grade 1 ascites) during the antiviral
phase, and there was one case of hepatic encephalopathy during the
follow-up phase. No deaths, TEEs, or cataract progression occurred
in this study.
A total of eight SAEs were seen in five patients
(12%) during the study (Table IV).
In addition to the three SAEs (liver injury and thrombocytosis in
the same patient and increase in the percentage of myeloblasts in
another) described above, five other SAEs were reported in five
patients, including the three patients with HCC and one patient
each with herpes zoster and trigeminal neuralgia. None of these
events were considered to be related to eltrombopag treatment. All
of these other SAEs except one case of HCC resolved/recovered
during the study.
 | Table IV.AEs during the antiviral treatment
phase. |
Table IV.
AEs during the antiviral treatment
phase.
| AEs | N=41 |
|---|
| AEs in ≥20% of
patients, n (%) | 41 (100) |
|
Pyrexia | 24 (59) |
|
Anemia | 22 (54) |
|
Neutropenia | 17 (41) |
|
Alopecia | 16 (39) |
|
Pruritus | 14 (34) |
|
Headache | 13 (32) |
|
Rash | 13 (32) |
|
Stomatitis | 12 (29) |
|
Malaise | 11 (27) |
|
Arthralgia | 9 (22) |
|
Decreased appetite | 9 (22) |
|
Nasopharyngitis | 9 (22) |
|
Thrombocytopenia | 8 (20) |
| Grade ≥3 AEs in ≥2
patients, n (%) | 25 (61) |
|
Neutropenia | 10 (24) |
|
Anemia | 4 (10) |
| Weight
decreased | 4 (10) |
|
Hemoglobin level
decreased | 3 (7) |
|
Neutrophil count
decreased | 3 (7) |
| WBC
decreased | 3 (7) |
|
Leucopenia | 2 (5) |
|
Thrombocytopenia | 2 (5) |
| Serious AEs, n
(%) | 5 (12) |
| Liver
injury | 1a (2) |
|
Myeloblast percentage
increased | 1 (2) |
|
Hepatocellular carcinoma | 3a,b
(2) |
|
Thrombocytosis | 1a (2) |
| Herpes
zoster | 1b (2) |
|
Trigeminal neuralgia | 1 (2) |
Pharmacokinetics
The geometric mean Cmax was 2.23
µg/ml (95% CI, 1.56–3.22) for the 12.5 mg dose of eltrombopag at
approximately 5 h [Tmax, 4.94 (95% CI,
3.38–7.24)] after drug administration and the geometric mean of the
AUC0-τ was 44.54 µg h/ml (95% CI, 26.2–67.6). In this
study, two patients withdrew from the study because of
hepatobiliary AEs; their plasma eltrombopag concentrations were
11.8 and 10.1 µg/ml at 14.5 and 12.5 h after the last dose (25 mg
doses) of eltrombopag, respectively.
Discussion
The present study demonstrated that eltrombopag
treatment ameliorates thrombocytopenia in Japanese patients with
HCV-related cirrhosis and facilitates the initiation and subsequent
maintenance of the pegIFN-based antiviral therapy for subjects who
would otherwise be ineligible or poor candidates for the
pegIFN-based antiviral therapy.
Although the median age of patients enrolled was
relatively higher compared with the age in the ENABLE studies, our
results show that eltrombopag in combination with the antiviral
therapy enabled 22% of cirrhotic Japanese patients to achieve a
clinically meaningful SVR, which was consistent with the previous
findings of ENABLE-1 and ENABLE-2 (23 and 19%, respectively)
(10).
In the present study, owing to the effect of
inter-ethnic differences on the pharmacokinetics of eltrombopag, a
lower starting dose of 12.5 mg, ie, half the daily dose in the
ENABLE studies and a more cautious dose titration regimen, was
chosen. However, the AUC0-τ in Japanese patients with
HCV receiving 12.5 mg eltrombopag once daily was estimated to be
44.54 µg h/ml, which was unexpectedly lower than that in patients
receiving 25 mg eltrombopag once daily in the ENABLE studies (118
µg h/ml) (19). In addition, the
mean daily dose of eltrombopag during the antiviral phase was 22
mg/day, which was much lower than the dose used in the ENABLE
studies (data not shown). Despite the lower dose, the magnitude of
platelet response observed in the pre-antiviral and antiviral
phases was similar to that observed in the ENABLE studies.
Furthermore, the ability of this lower mean daily dose of
eltrombopag has also been confirmed in this population, as observed
in previous Japanese ITP studies (20,21).
Consistent with this platelet response, the proportion of patients
who did not require dose reduction of pegIFN in this study was
similar to that found in the pooled analysis from the ENABLE
studies (49 and 58%, respectively) (19).
The primary goal of treatment in chronic hepatitis C
patients is to achieve an SVR and prevent progression to HCC.
Although the SVR rate of 22% may seem low, this finding is in line
with the observation in Japanese cirrhotic patients receiving
pegIFN/RBV (22). Furthermore, it is
clinically meaningful that eltrombopag treatment could facilitate
the complete eradication of HCV, as these patients had no other
available treatment options. At the time of initiation of this
study, triple therapies, consisting of protease inhibitors
(telaprevir or simeprevir) plus pegIFN/RBV therapy, were approved
in Japan, and this treatment option showed a considerable
improvement in the SVR rates in genotype 1 HCV patients (23–26).
Further evaluation of the appropriate use of eltrombopag in
combination with triple therapy will help in improving the SVR
rates in cirrhotic patients with thrombocytopenia.
In this study, eltrombopag was generally well
tolerated in patients with HCV infection and thrombocytopenia and
the findings were similar to those observed in the previous ENABLE
studies (10). TEEs are a frequent
complication in patients with CLD, especially liver cirrhosis
(2). In the recent studies, the
incidence of portal vein thrombosis in patients with cirrhosis was
reported to be 4.5–16.4% (27). In a
post hoc analysis of a previous eltrombopag study in patients with
CLD, it was found that increased platelet counts (>200,000/µl)
were associated with the development of TEEs (18). In our study, a lower platelet count
threshold for dose reduction than that in the ENABLE studies was
considered in order to minimize the risk of thrombosis; the
threshold used in this study was 150,000/µl compared with
200,000/µl in the ENABLE studies (10). If any symptoms or ultrasound findings
of portal vein thrombosis were observed, abdominal scanning (e.g.,
CT, MRI) was performed. No TEEs were observed in the present study,
whereas a higher incidence of TEEs was observed in the
eltrombopag-treated group compared with the placebo-treated group
in the antiviral phase of the ENABLE studies (10). However, a low baseline albumin level
of ≤35 g/l or patient age >60 years, rather than high platelet
counts, has been reported to be associated with the development of
TEEs (19). Nevertheless, we
recommend maintaining the platelet counts between 50,000 and
150,000/µl in Japanese HCV patients with thrombocytopenia based on
the following findings: the platelet response was similar to that
observed in the ENABLE studies (10)
despite the different platelet count threshold; the increased risk
of TEEs by platelets or platelet-amplified inflammation cannot be
ruled out (28); more cautious
criteria (dose reduction if platelet counts >100,000/µl) were
recommended in the European Union Summary of Product
Characteristics of eltrombopag (29). Following the discontinuation of
eltrombopag and pegIFN therapy, one patient developed
thrombocytosis. Elevation of platelet counts following
discontinuation of eltrombopag was observed in this study, similar
to other studies of eltrombopag in patients with CLD and
thrombocytopenia (18,30). The increased risk of TEEs should
therefore be carefully evaluated, and platelet counts should be
closely monitored during and after treatment with eltrombopag.
PegIFN therapy, as well as eltrombopag, has been
associated with hepatobiliary laboratory abnormalities. Adjunctive
treatment with eltrombopag can help avoid dose reduction or
interruption of pegIFN, resulting in a greater exposure to pegIFN,
which may contribute to an increased risk of pegIFN-related side
effects. In our study, approximately half of the patients
experienced hepatobiliary AEs. The majority of the events were
grade 1 or 2 in severity and only one patient presented with
hepatic decompensation (grade 1 ascites) during the antiviral
phase, except for one hepatic encephalopathy during the follow-up
phase. However, the plasma eltrombopag concentrations in two
patients who withdrew from the study because of abnormal LFTs were
2.2- and 2.6-fold higher than those estimated from the
Cmax and Tmax values after
administration of the 12.5 mg dose in five patients without
abnormal LFTs. Furthermore, it has recently been reported that a
higher incidence of hepatic decompensation was observed in patients
receiving the pegIFN therapy with eltrombopag compared with those
receiving placebo (10), and a low
baseline albumin level of ≤ 35 g/l or Model for End-Stage Liver
Disease (MELD) score ≥10 was found to be associated with a higher
risk of hepatic decompensation (18). Thus, hepatic enzymes should be
carefully monitored during the treatment, and eltrombopag treatment
should be administered with caution in patients with advanced liver
disease.
The TPO-R is expressed not only on megakaryocytes
but also on hepatic progenitor cells and hepatic sinusoidal
endothelial cells, which plays a crucial role in HCC development
and progression. TPO accelerates the proliferation of these cell
types (31,32). Theoretically, there is a possibility
that eltrombopag may increase the risk of HCC. In the present
study, the incidence of HCC was 7% (three of 41 patients), similar
to the annual rate in Japanese cirrhotic patients with HCV
infection (33). A recent report of
a randomized open-label study indicated that short-term eltrombopag
treatment does not accelerate the progression of HCC in
HCV-infected patients with cirrhosis (30). Furthermore, it has been found that
the level of TPO-R expression in hematoma cell lines was lower than
that in hepatocytes (34) and that
TPO does not accelerate the proliferation of Huh7 cells both in
vitro and in vivo (35).
Thus, treatment with eltrombopag may not accelerate tumor
progression in HCV-infected patients with liver cirrhosis.
Our study lacked a direct comparison with placebo
and this could be considered a limitation of the study. However, a
lower starting dose (12.5 mg) and a lower platelet count threshold
(150,000/µl) for dose adjustment of eltrombopag than those used in
the ENABLE studies were helpful to determine the minimum effective
dose of eltrombopag without clinically important complications
(hepatic decompensation and HCC progression).
In conclusion, eltrombopag (at doses of 12.5–50 mg)
increased the platelet counts in chronic HCV-infected patients and
enabled these patients to initiate and complete the IFN-based
antiviral therapy. Thus, eltrombopag could be useful in the
treatment of HCV-infected patients with thrombocytopenia who would
be ineligible or poor candidates for the pegIFN-based antiviral
therapy.
Acknowledgements
Medical writing service was provided by Miss Keyra
Martinez Dunn, and Dr Pranitha Manchanapalli. The authors would
like to thank the patients, physicians, nurses, and study
coordinators who participated in the study.
Funding
The present study was sponsored by GlaxoSmithKline
(GSK), Tokyo, Japan. All study activities (the design of the study,
collection, analysis, and interpretation of data) were conducted by
GSK Japan. Medical writing service was funded by Novartis Pharma
K.K.
Availability of data and materials
The datasets generated during and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors' contributions
TK, AK, KF, SN, MK, HT, YT, KN, EM, HN, MSh, KT, and
MSa helped in the recruitment of patients in the clinical study and
interpreted the clinical data. MSa, TH, and KK contributed to the
design of the clinical study. TK and TH wrote and edited the
manuscript. TH contributed to the data analysis. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the National Hospital
Organization Central Review Board of four national hospitals and
institutional review boards of 12 other participating centers. It
was performed in accordance with the Declaration of Helsinki, Good
Clinical Practice guidelines, and local laws and regulations. All
participating patients provided written informed consent prior to
their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
KT received lecture fees from Bristol-Myers Squibb
K.K. (Japan), Abbvie (Japan), and AstraZeneca K.K (Japan). TH is
currently an employee of Novartis Pharma K.K. and was an employee
of GSK (Japan) during the conduct of the study. TK received lecture
fees from Mitsubishi Tanabe Pharma Corporation (Japan), MSD K.K.
Japan and Otsuka Pharmaceuticals Co., Ltd. (Japan). All other
authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
AE
|
Adverse event
|
|
ALT
|
alanine transaminase
|
|
AST
|
aspartate transaminase
|
|
AUC0-t
|
area under the plasma
concentration-time curve up to the last measurable
concentration
|
|
AUC0-τ
|
area under the plasma concentration
curve to the end of the dosing period
|
|
BL
|
baseline
|
|
cEVR
|
complete early virological
response
|
|
CI
|
confidence interval
|
|
CLD
|
chronic liver disease
|
|
Cmax
|
maximum plasma concentration
|
|
DAA
|
direct-acting antiviral
|
|
DAIDS
|
Division of Acquired Immunodeficiency
Syndrome;
|
|
ETR
|
end-of-treatment response
|
|
EVR
|
early virological response
|
|
FU
|
follow-up
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
IFN
|
interferon
|
|
ITP
|
immune thrombocytopenic purpura
|
|
LFT
|
liver function test
|
|
MELD
|
Model for End-Stage Liver Disease
|
|
PegIFN
|
pegylated interferon
|
|
RBV
|
ribavirin
|
|
RVR
|
rapid virological response
|
|
SAE
|
serious adverse event
|
|
SVR
|
sustained virological response
|
|
TEE
|
thromboembolic event
|
|
Tmax
|
time to maximum plasma
concentration
|
|
TPO
|
thrombopoietin
|
|
TPO-R
|
thrombopoietin receptor
|
|
ULN
|
upper limit of normal
|
References
|
1
|
Latorre R, Vaquero J, Rincón D, Puerto M,
Ponce MD, Sarnago F, Matamoros JA, Ramón E, Elizaga J, Bañares R
and Ripoll C: Determinants of platelet count are different in
patients with compensated and decompensated cirrhosis. Liver Int.
36:232–239. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitchell O, Feldman DM, Diakow M and Sigal
SH: The pathophysiology of thrombocytopenia in chronic liver
disease. Hepat Med. 8:39–50. 2016.PubMed/NCBI
|
|
3
|
Jayasekera CR, Barry M, Roberts LR and
Nguyen MH: Treating hepatitis C in lower-income countries. N Engl J
Med. 370:1869–1871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lawitz E, Lalezari JP, Hassanein T,
Kowdley KV, Poordad FF, Sheikh AM, Afdhal NH, Bernstein DE, Dejesus
E, Freilich B, et al: Sofosbuvir in combination with peginterferon
alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients
with genotypes 1, 2 and 3 hepatitis C infection: A randomised,
double-blind, phase 2 trial. Lancet Infect Dis. 13:401–408. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kronenberger B and Zeuzem S: New
developments in HCV therapy. J Viral Hepat. 19 (Suppl 1):S48–S51.
2012. View Article : Google Scholar
|
|
6
|
Casey LC and Lee WM: Hepatitis C virus
therapy update 2013. Curr Opin Gastroenterol. 29:243–249.
2013.PubMed/NCBI
|
|
7
|
Nkuize M, Sersté T, Buset M and Mulkay JP:
Combination ledipasvir-sofosbuvir for the treatment of chronic
hepatitis C virus infection: A review and clinical perspective.
Ther Clin Risk Manag. 12:861–872. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shahid I, AlMalki WH, Hassan S and Hafeez
MH: Real-world challenges for hepatitis C virus medications: A
critical overview. Crit Rev Microbiol. 44:143–160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Treatment guidelines for hepatitis C from
Japan Society of Hepatology. http://www.jsh.or.jp/files/uploads/Brief_C_v6.2_Oct15.pdfMarch
14th–2019(In Japanese).
|
|
10
|
Afdhal NH, Dusheiko GM, Giannini EG, Chen
PJ, Han KH, Mohsin A, Rodriguez-Torres M, Rugina S, Bakulin I,
Lawitz E, et al: Eltrombopag increases platelet numbers in
thrombocytopenic patients with HCV infection and cirrhosis,
allowing for effective antiviral therapy. Gastroenterology.
146:442–452.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
COPEGUS (ribavirin) product information.
Roche products Ltd., . https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021511s023lbl.pdfMarch
14th–2019
|
|
12
|
PEGASYS (peginterferon alfa-2a) Product
Information. Roche Products Ltd., . https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103964s5204lbl.pdfMarch
14th–2019
|
|
13
|
PEGINTRON (peginterferon alfa-2b) product
information. Merck & Co, Inc., . https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103949s5306lbl.pdfMarch
14th–2019
|
|
14
|
Loffredo L and Violi F: Thrombopoietin
receptor agonists and risk of portal vein thrombosis in patients
with liver disease and thrombocytopenia: A meta-analysis. Dig Liver
Dis. 51:24–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
REBETOL (ribavirin) product information.
Schering-plough. Merck &Co, Inc., . https://www.merck.com/product/usa/pi_circulars/r/rebetol/rebetol_pi.pdfMarch
14th–2019
|
|
16
|
Danish FI and Yasmin S: The role of
eltrombopag in the management of hepatitis C virus-related
thrombocytopenia. Hepat Med. 5:17–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galli L, Gerdes VE, Guasti L and Squizzato
A: Thrombosis associated with viral hepatitis. J Clin Transl
Hepatol. 2:234–239. 2014.PubMed/NCBI
|
|
18
|
Afdhal NH, Giannini EG, Tayyab G, Mohsin
A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH,
et al: Eltrombopag before procedures in patients with cirrhosis and
thrombocytopenia. N Engl J Med. 367:716–724. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burness CB: Eltrombopag: A review of its
use in the treatment of thrombocytopenia in patients with chronic
hepatitis C. Drugs. 74:1961–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomiyama Y, Miyakawa Y, Okamoto S,
Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S,
Ozaki K, et al: A lower starting dose of eltrombopag is efficacious
in Japanese patients with previously treated chronic immune
thrombocytopenia. J Thromb Haemost. 10:799–806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katsutani S, Tomiyama Y, Kimura A,
Miyakawa Y, Okamoto S, Okoshi Y, Ninomiya H, Kosugi H, Ishii K,
Ikeda Y, et al: Oral eltrombopag for up to three years is safe and
well-tolerated in Japanese patients with previously treated chronic
immune thrombocytopenia: An open-label, extension study. Int J
Hematol. 98:323–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Editors of the Drafting Committee for
Hepatitis Management Guidelines, . The Japan Society of Hepatology:
Guidelines for the management of hepatitis C virus infection: First
edition, May 2012, The Japan society of hepatology. Hepatol Res.
43:1–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi N, Seto C, Kato M, Komada Y and
Goto S: Once-daily simeprevir (TMC435) with peginterferon/ribavirin
for treatment-naïve hepatitis C genotype 1-infected patients in
Japan: The DRAGON study. J Gastroenterol. 49:138–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Izumi N, Hayashi N, Kumada H, Okanoue T,
Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C and Goto
S: Once-daily simeprevir with peginterferon and ribavirin for
treatment-experienced HCV genotype 1-infected patients in Japan:
The CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 49:941–953.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kumada H, Sato K, Takehara T, Nakamuta M,
Ishigami M, Chayama K, Toyota J, Suzuki F, Nakayasu Y, Ochi M, et
al: Efficacy of telaprevir-based therapy for difficult-to-treat
patients with genotype 2 chronic hepatitis C in Japan. Hepatol Res.
45:745–754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumada H, Suzuki F, Kamiya N, Orihashi M,
Nakayasu Y and Yamada I: Efficacy and safety of telaprevir with
pegylated interferon α-2a and ribavirin in Japanese patients.
Hepatol Res. 47:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maruyama H, Okugawa H, Takahashi M and
Yokosuka O: De novo portal vein thrombosis in virus-related
cirrhosis: Predictive factors and long-term outcomes. Am J
Gastroenterol. 108:568–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boilard E, Nigrovic PA, Larabee K, Watts
GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E,
Farndale RW, Ware J and Lee DM: Platelets amplify inflammation in
arthritis via collagen-dependent microparticle production. Science.
327:580–583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Revolade summary of product
characteristics. Novartis Europharm Limited. https://www.ema.europa.eu/en/documents/product-information/revolade-epar-product-information_en.pdf(last
updated January, 2015). March 14th–2019
|
|
30
|
Kawaguchi T, Komori A, Seike M, Fujiyama
S, Watanabe H, Tanaka M, Sakisaka S, Nakamuta M, Sasaki Y, Oketani
M, et al: Efficacy and safety of eltrombopag in Japanese patients
with chronic liver disease and thrombocytopenia: A randomized,
open-label, phase II study. J Gastroenterol. 47:1342–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cardier JE and Dempsey J: Thrombopoietin
and its receptor, c-mpl, are constitutively expressed by mouse
liver endothelial cells: Evidence of thrombopoietin as a growth
factor for liver endothelial cells. Blood. 91:923–929.
1998.PubMed/NCBI
|
|
32
|
Schmelzer E, Deiwick A, Bruns H, Fiegel HC
and Bader A: Thrombopoietin is a growth factor for rat hepatic
progenitors. Eur J Gastroenterol Hepatol. 20:209–216. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hajarizadeh B, Grebely J and Dore GJ:
Epidemiology and natural history of HCV infection. Nat Rev
Gastroenterol Hepatol. 10:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erickson-Miller CL, Chadderton A, Gibbard
A, Kirchner J, Pillarisetti K, Baker K, Pandite L, El-Hariry I,
Mostafa Kamel Y, Liu Y, et al: Thrombopoietin receptor levels in
tumor cell lines and primary tumors. J Oncol 2010. 1353542010.
|
|
35
|
Nozaki R, Murata S, Nowatari T, Maruyama
T, Ikeda N, Kawasaki T, Fukunaga K and Ohkohchi N: Effects of
thrombopoietin on growth of hepatocellular carcinoma: Is
thrombopoietin therapy for liver disease safe or not? Hepatol Res.
43:610–620. 2013. View Article : Google Scholar : PubMed/NCBI
|