Introduction
The central nervous system (CNS) does not
spontaneously regenerate following injury, a theory that has been
widely accepted since it was initially devised in the early 20th
century, by the Spanish neuroanatomist Santiago Ramon y Cajal
(1). The lack of axonal regeneration
in the adult mammalian CNS, particularly in the spinal cord, is due
to a combination of factors, including demyelination and
injury-induced glial scars (2).
Following spinal cord injury, the microenvironment of the spinal
cord and brain contains endogenous neural stem cells (NSCs);
however, there is no evidence of de novo neurogenesis upon
injury (3). Numerous studies have
demonstrated that the fate of NSCs is regulated locally by the
niche microenvironment and that this is influenced by a number of
factors, including specific hormones, such as dexamethasone, growth
hormone and prolactin (4,5). Numerous compounds and hormones have
been identified to affect NSC differentiation and proliferation
(6,7), which suggests that regeneration of the
injured spinal cord may be possible.
NSCs are multipotent cells capable of self-renewal
and differentiation into multiple cell types, including neurons,
astrocytes and oligodendrocytes (8).
As such, they are a powerful research tool for examining the
mechanisms underlying CNS diseases, as well as therapeutic
strategies. Mature NSCs contribute to new memory formation and
mediate the response to neural injury (9,10).
Therefore, NSC proliferation and differentiation serve an important
role in neural development, maintenance and regeneration. However,
there is a tendency for NSCs to differentiate into astrocytes,
which may form glial scars that suppress spontaneous remyelination
and neural recovery (11).
Therefore, a compound that promotes endogenous NSC proliferation
and differentiation is likely to also promote neuronal repair in
the CNS.
Clobetasol propionate (Clo), which has been approved
by the U.S. Food and Drug Administration, is a corticosteroid and a
member of the glucocorticoid family that is widely used to treat a
number of skin disorders, including herpes labialis, psoriasis and
lichen sclerosus (12). Clo is also
a potential remyelinating agent that has been demonstrated to
promote differentiation in oligodendrocyte precursor cell (OPC)
cultures and remyelination in vivo (12–15). Clo
enhances the differentiation of ectodermal stem cell-derived OPCs
into oligodendrocytes, which has been validated in vivo
(16,17).
Clo promotes the proliferation of primary neuronal
precursor cells alone and synergistically in the presence of sonic
hedgehog (SHH) protein (18).
Therefore, Clo may function as an important modulator for the
proliferation and differentiation of NSCs; however, to the best of
the authors' knowledge, no study to date has examined the direct
effect of Clo on cultured NSCs.
The aim of the present study was to determine the
effect of Clo on the differentiation of NSCs into neurons,
oligodendrocytes and astrocytes in vitro. The results of the
current study demonstrate that Clo has potential as a
disease-modifying therapy that may have applications in
neurological disorders that affect neurons and
oligodendrocytes.
Materials and methods
Cell culture
Pregnant (E14) adult Sprague-Dawley (SD) rats (n=5;
weight, 250 g; age, 12 weeks; supplied by the Animal Center of
Jiangsu University) were used in the present study. The present
study, including all animal experimental protocols, was approved by
the Jiangsu University Animal Care Committee (Jiangsu, China;
protocol number 2016-08-07). Pregnant (E14) SD rats were sacrificed
by the intraperitoneal injection of pentobarbital (200 mg/kg).
Embryonic rats were dissected from the uterine horn under sterile
conditions and placed in PBS. The embryonic brains were immediately
harvested by microdissection, and maintained in PBS prior to
dissociation. Briefly, cells were dissociated by mechanical
shearing followed by filtration through a 40-µm mesh. Cell
suspensions were prepared, and primary embryonic neurospheres
cultured as previously described (19). The cells were seeded in
ultra-low-attachment culture plates (Corning Inc.) and cultured in
serum-free DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc)
supplemented with 2 mM L-glutamine (Invitrogen; Thermo Fisher
Scientific, Inc.), 20 ng/ml basic fibroblast growth factor, 20
ng/ml epidermal growth factor (both PeproTech, Inc.), 2% B27
(Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin (Beijing Solarbio Science & Technology
Co., Ltd.). All cultures were maintained at 37°C in a humidified
atmosphere of 5% CO2. After passage 3, NSCs were
identified by their morphological characteristics and nestin
expression, as described below. The subcultured neurospheres were
characterized by immunofluorescence staining with anti-nestin
antibody. Neurospheres formed from NSCs of passage three were used
for subsequent experiments.
Cell viability assay
The cell viability of NSCs was assessed using the
MTT colorimetric assay (Beijing Solarbio Science & Technology
Co., Ltd.). Briefly, dissociated neurosphere-derived cells from
primary cultures were seeded onto non-treated 96-well plates
(Corning Inc.) at a density of 5×103 cells/well and
grown for 48 h following treatment with different concentrations
(2.5, 5, 7.5, 10, 12.5 and 15 µM) of Clo (Selleck Chemicals),
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA)
to a final concentration of 0.01%, or DMSO alone as a control.
After the 48-h treatment, the Clo-containing medium was removed,
the cells were gently washed twice with PBS and then 200 µl MTT
(0.5 mg/ml) in PBS was added to each well. Following incubation
with MTT for 4 h at 37°C, the formazan dye in DMSO produced by
viable cells was quantified by measurement of the absorbance at 460
nm using a microplate reader. Five independent experiments were
conducted. In each experiment, 15 replicates were measured for each
concentration.
Neurosphere counting
Separated neurosphere-derived cells were seeded onto
ultra-low-attachment 96-well culture plates at a density of
5×103 cells/well. Cells were treated with different
concentrations (2.5, 5, 7.5, 10, 12.5 and 15 µM) of Clo dissolved
in DMSO or with DMSO alone as a control (each treatment condition
repeated in 15 wells). Cells were cultured in serum-free DMEM/F12
(Gibco; Thermo Fisher Scientific, Inc) supplemented with 2 mM
L-glutamine (Invitrogen; Thermo Fisher Scientific, Inc.), 20 ng/ml
basic fibroblast growth factor, 20 ng/ml epidermal growth factor
(both PeproTech, Inc.), 2% B27 (Gibco; Thermo Fisher Scientific,
Inc.) for 5 days. The total number of neurospheres with a diameter
>50 µm was counted in each well using an inverted light
microscope (Zeiss Axio Observer; Zeiss GmbH).
NSC differentiation
In all experiments NSCs were cultured in serum-free
DMEM/F12 for 12 h, unless otherwise stated. For NSC differentiation
assays, 100-µm-diameter neurospheres were collected by
centrifugation (1,000 × g for 10 min at 4°C) and 10–15 neurospheres
were seeded onto a 48-well plate (BD Biosciences) in stem cell
differentiation induction medium [DMEM/F12 containing 4% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 2% B27 and 2%
N2]. Following 12-h culture, differentiating NSCs were treated with
Clo (5 or 10 µM) or DMSO (control) at 37°C and differentiation was
evaluated after culture in stem cell differentiation induction
medium after 7 days of treatment.
SHH inhibition
To establish whether the SHH signaling pathway is
involved in NSC differentiation, the SHH signaling pathway
inhibitor cyclopamine (CYC; Sigma-Aldrich; Merck KGaA) was used to
treat NSCs. Prior to inhibition experiments, NSCs were cultured in
stem cell differentiation induction medium and treated with Clo (10
µM) for 7 days, as described above. Cells were treated with 10 µM
CYC diluted in stem cell differentiation induction medium for 7
days.
5′-AMP-activated protein kinase (AMPK)
inhibition
To establish whether the AMPK signaling pathway is
involved in NSC differentiation, the AMPK signaling pathway
inhibitor Compound C (CC; Sigma-Aldrich; Merck KGaA) was used to
treat NSCs. NSCs were cultured in stem cell differentiation
induction medium and treated with Clo (10 µM) for 7 days, as
described above. Cells were treated with 5 µM CC diluted in stem
cell differentiation induction medium for 7 days.
Immunocytochemistry and western blot
analysis
Immunocytochemistry was used to determine the
expression pattern of differentiated cell markers in NSCs. Cells
were washed three times with PBS and fixed with 4% paraformaldehyde
at 4°C for 12 h. Subsequently, cells were blocked with 0.1% Triton
X-100 in PBS supplemented with 3% bovine serum albumin (Beijing
Solarbio Science & Technology Co., Ltd.) at 37°C for 45 min.
Cells were incubated with the following primary antibodies:
Anti-growth associated protein 43 (GAP-43; rabbit polyclonal;
1:200; cat. no. PA-1037), anti-myelin basic protein (MBP; rabbit
polyclonal; 1:200; cat. no. BA0094), anti-nestin (1:200; cat. no.
PB0920), anti-glial fibrillary acidic protein (GFAP; mouse
monoclonal; 1:200; cat. no. PB0046; all Boster Biological
Technology), anti-SHH (1:200; rabbit monoclonal; cat. no. ab53281;
Abcam), anti-phosphorylated adenosine 5-monophosphate
(p-AMP)-activated protein kinase (p-AMPK; rabbit polyclonal; 1:200;
cat. no. sc-25792) and anti-phosphorylated mitogen-activated
protein kinase (p-ERK; rabbit polyclonal; 1:200; cat. no.
sc-514302; both Santa Cruz Biotechnology, Inc.) for 12 h at 4°C.
Following primary antibody incubation, cells were washed three
times with PBS and then incubated with secondary antibodies,
Cy3-labeled goat anti-mouse/rabbit IgG (1:200; cat. no. C5838/C2821
Sigma-Aldrich; Merck KGaA) and Alexa Fluor 488-conjugated goat
anti-mouse IgG (1:200; cat. no. SAB4600388; Sigma-Aldrich; Merck
KGaA) for 3 h at room temperature. The nuclei were counterstained
with DAPI (Sigma-Aldrich) for 30 min at room temperature and images
were acquired using a fluorescent microscope (Zeiss Axio Observer;
Zeiss GmbH).
For western blot analysis, total protein from
adherent cells in each experimental group was harvested. Total
protein was extracted from cells using radioimmunoprecipitation
assay buffer (Beijing Solarbio Science & Technology Co., Ltd.)
containing PMSF, phosphatase inhibitor cocktail and EDTA (all
Beijing Solarbio Science & Technology Co., Ltd.). Total protein
was quantified using a Bradford protein assay and 15 µg
protein/lane was separated via SDS-PAGE on a 10% gel. The separated
proteins were subsequently transferred onto PVDF membranes (EMD
Millipore) and blocked for 1 h at room temperature with 3% non-fat
dried milk powder in TBS at room temperature. The membranes were
incubated with primary antibodies against: GAP-43 (rabbit
polyclonal; 1:200; cat. no. PA-1037), GFAP (mouse monoclonal;
1:200; cat. no. PB0046), MBP (MBP; rabbit polyclonal; 1:200; cat.
no. BA0094), brain derived neurotrophic factor (BDNF; rabbit
polyclonal; 1:200; cat. no. PB0013), neurotrophins-3 (NT-3; mouse
polyclonal; 1:200; cat. no. PB0886) and β-tubulin (1:200; cat. no.
BM1453) all from Wuhan Boster Biological Technology, Ltd.; SHH
(1:200; rabbit monoclonal; cat. no. ab53281) and SMO (1:200; rabbit
monoclonal; cat. no. ab236465) both from Abcam; and p-AMPK/AMPK
(rabbit polyclonal; 1:200; cat. nos. sc-25792 and sc-74461,
respectively) and p-ERK/ERK (rabbit polyclonal; 1:200; cat. nos.
sc-81492 and sc-514302, respectively) from Santa Cruz
Biotechnology, Inc., overnight at 4°C. Following primary antibody
incubation, membranes were incubated with HRP-conjugated secondary
antibodies (1:200; cat. nos. BA1050 and BA1056; Wuhan Boster
Biological Technology, Ltd.) for 1 h at room temperature. Protein
bands were visualized using an ECL kit (EMD Millipore).
Chemiluminescent signals were captured digitally using a
chemiluminescence imaging system. The images were analyzed using
ImageJ software (version 1.51 k; National Institutes of
Health).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. All statistical analyses were performed using SPSS
software (version 22.0; IBM Corp.,). One-way analysis of variance
followed by protected Fisher's least significant difference post
hoc test was used to analyze differences among multiple groups.
P<0.05 were considered to indicate a statistically significant
difference.
Results
Characterization of NSCs
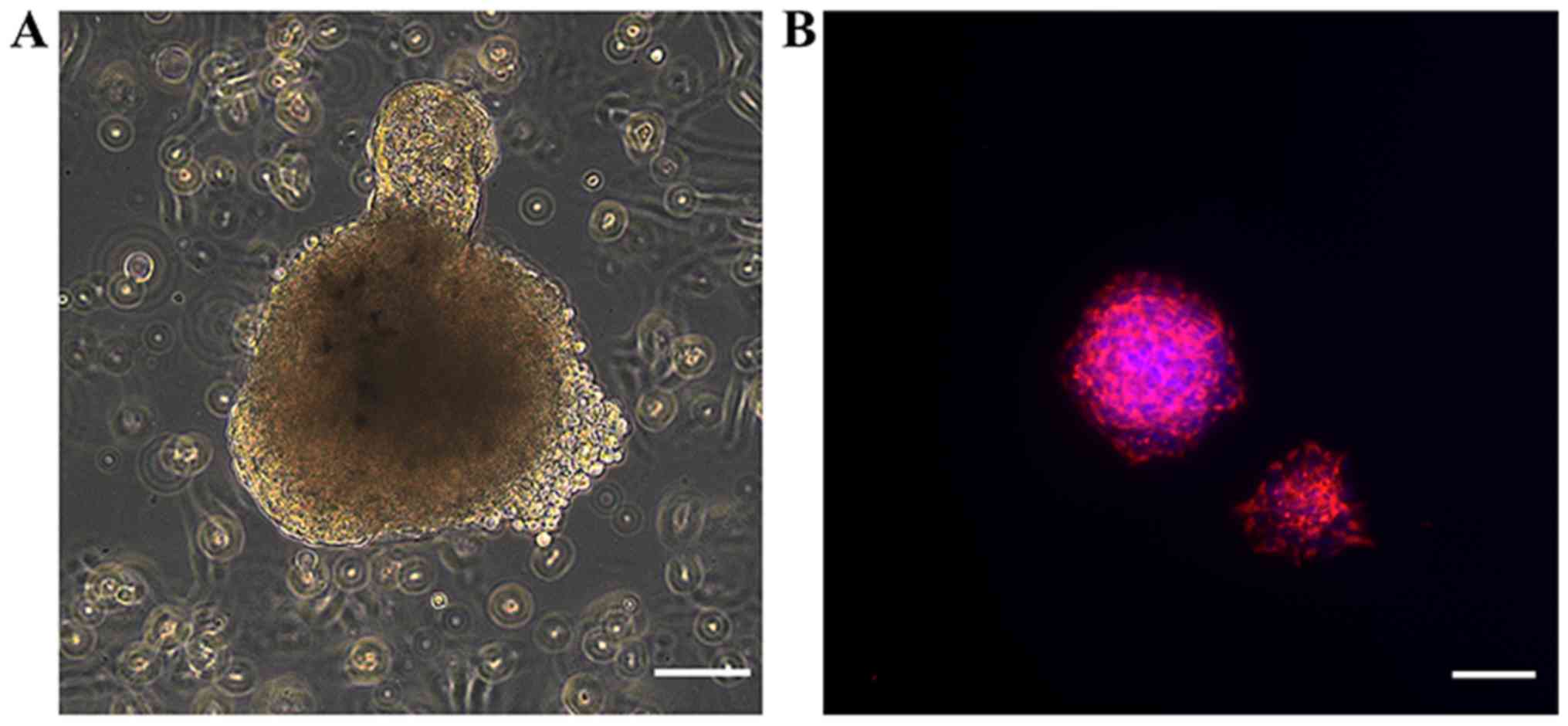
Primary NSCs formed neurospheres of various sizes,
with diameters of 50–100 µm after 7 days of culture in serum-free
medium (Fig. 1A). To characterize
NSCs, immunocytochemical staining of nestin, a commonly used NSC
marker, was performed (Fig. 1B).
Strong staining of nestin was observed in the cultured
neurospheres.
Effect of Clo on NSC
proliferation
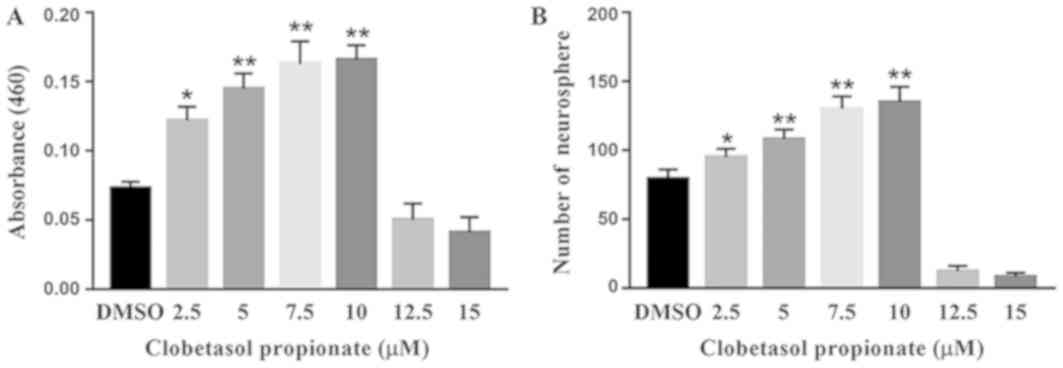
To determine the effect of Clo on NSCs, cell
viability was examined using the MTT assay following treatment with
different concentrations (2.5, 5, 7.5, 10, 12.5 and 15 µM) of Clo.
Treatment with Clo significantly increased NSC viability at lower
concentrations (2.5, 5, 7.5 and 10 µM) compared with DMSO (Fig. 2A). However, cell death was observed
following treatment with Clo at concentrations ≥12.5 µM. These data
indicate that treatment with Clo increases NSC proliferation at low
concentrations. Similarly, treatment with Clo significantly
increased neurosphere formation at lower concentrations, with a
peak number of neurospheres obtained with a Clo concentration of 10
µM (Fig. 2B).
Effect of Clo on NSC
differentiation
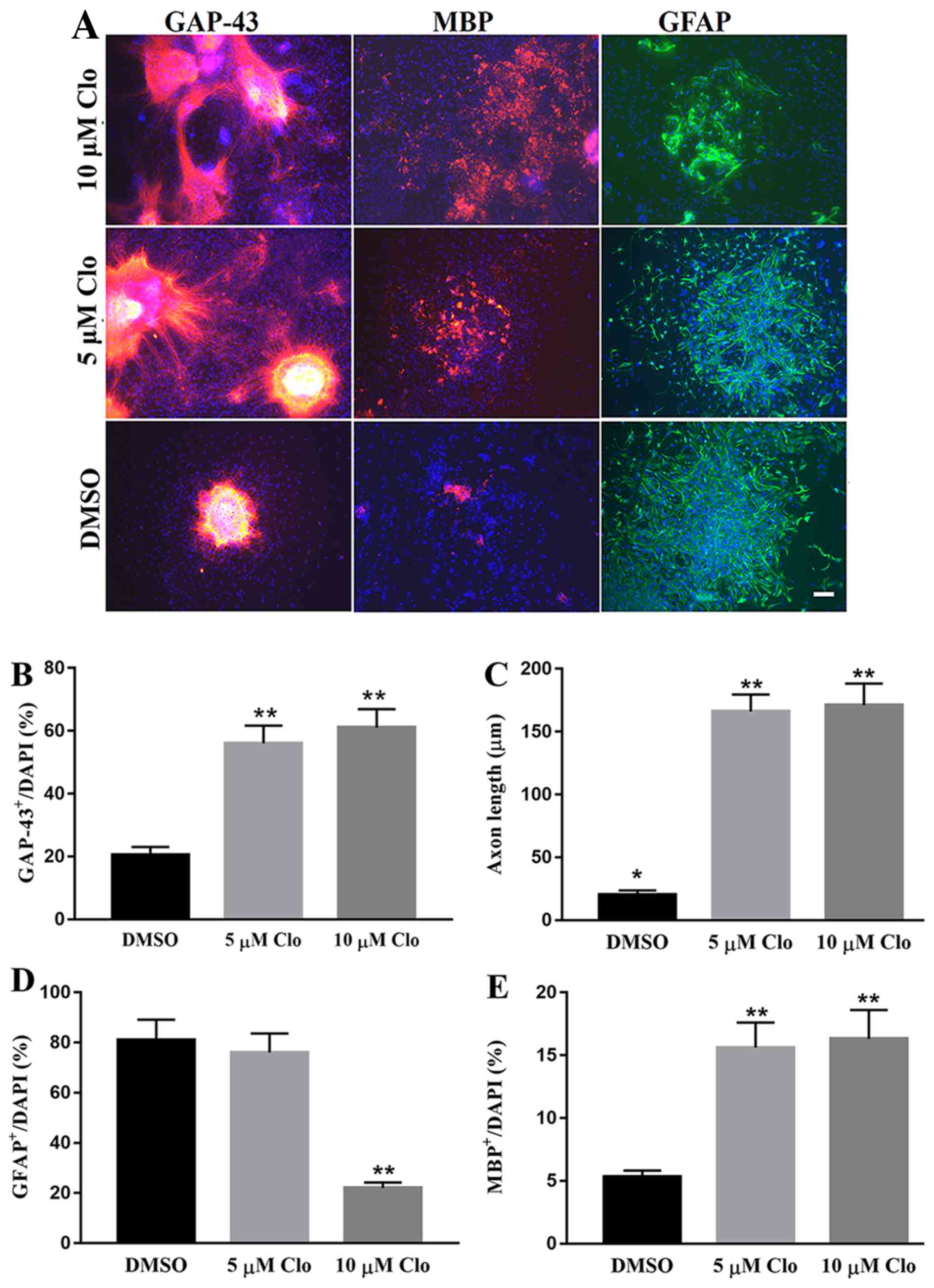
To study the effect of Clo on NSC differentiation,
NSCs were cultured in specific differentiation induction medium
with or without Clo (5 and 10 µM). NSC differentiation was
confirmed by immunocytochemistry using neural cell lineage-specific
antibodies, which included GAP-43 (neurons), GFAP (astrocytes) and
MBP (oligodendrocytes; Fig. 3A).
Treatment with Clo significantly increased the number of GAP-43
positive neurons compared with the DMSO control group (Fig. 3B). In addition, treatment with Clo
significantly increased the axon length in GAP-43 positive neurons
compared with the DMSO control group (Fig. 3C). No significant difference in axon
length was observed between the 5 and 10 µM groups (Fig. 3C). No significant difference was
detected in astrocyte differentiation between the 5 µM Clo group
and the DMSO control group (Fig.
3D). However, treatment with a higher concentration (10 µM) of
Clo significantly decreased the number of differentiated GFAP
positive astrocytes compared with that in the 5 µM Clo or DMSO
control group (Fig. 3D). Treatment
with Clo significantly increased the number of MBP positive
oligodendrocytes compared with that in the DMSO control group
(Fig. 3E). No significant difference
was observed between the 5 and 10 µM groups (Fig. 3E).
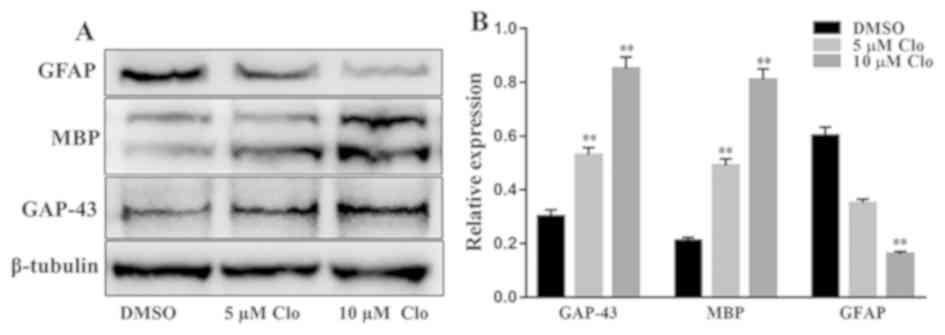
Furthermore, the protein expression levels of
GAP-43, MBP and GFAP were determined by western blot analysis in
differentiated NSCs following treatment with Clo (5 or 10 µM) or
DMSO (Fig. 4A). Treatment with Clo
significantly increased GAP-43 and MBP protein expression levels,
and significantly decreased GFAP protein expression levels compared
with the corresponding levels in the DMSO control group (Fig. 4B).
Effect of Clo on p-AMPK protein
expression
Given the role of ERK and AMPK signaling pathways in
stem cell proliferation and differentiation (20,21), the
expression of SHH, p-AMPK and p-ERK proteins was examined in NSCs
cultured in specific differentiation induction medium with or
without Clo (5 and 10 µM) using immunocytochemistry (Fig. 5A) and western blotting (Fig. 5B and C). No significant difference in
the level of p-ERK was observed between the Clo and DMSO treatment
groups (P>0.05; Fig. 5B and C).
However, western blot analysis demonstrated that the level of
p-AMPK was significantly increased in differentiating NSCs
following treatment with Clo compared with the DMSO control group
(P<0.05; Fig. 5B and C).
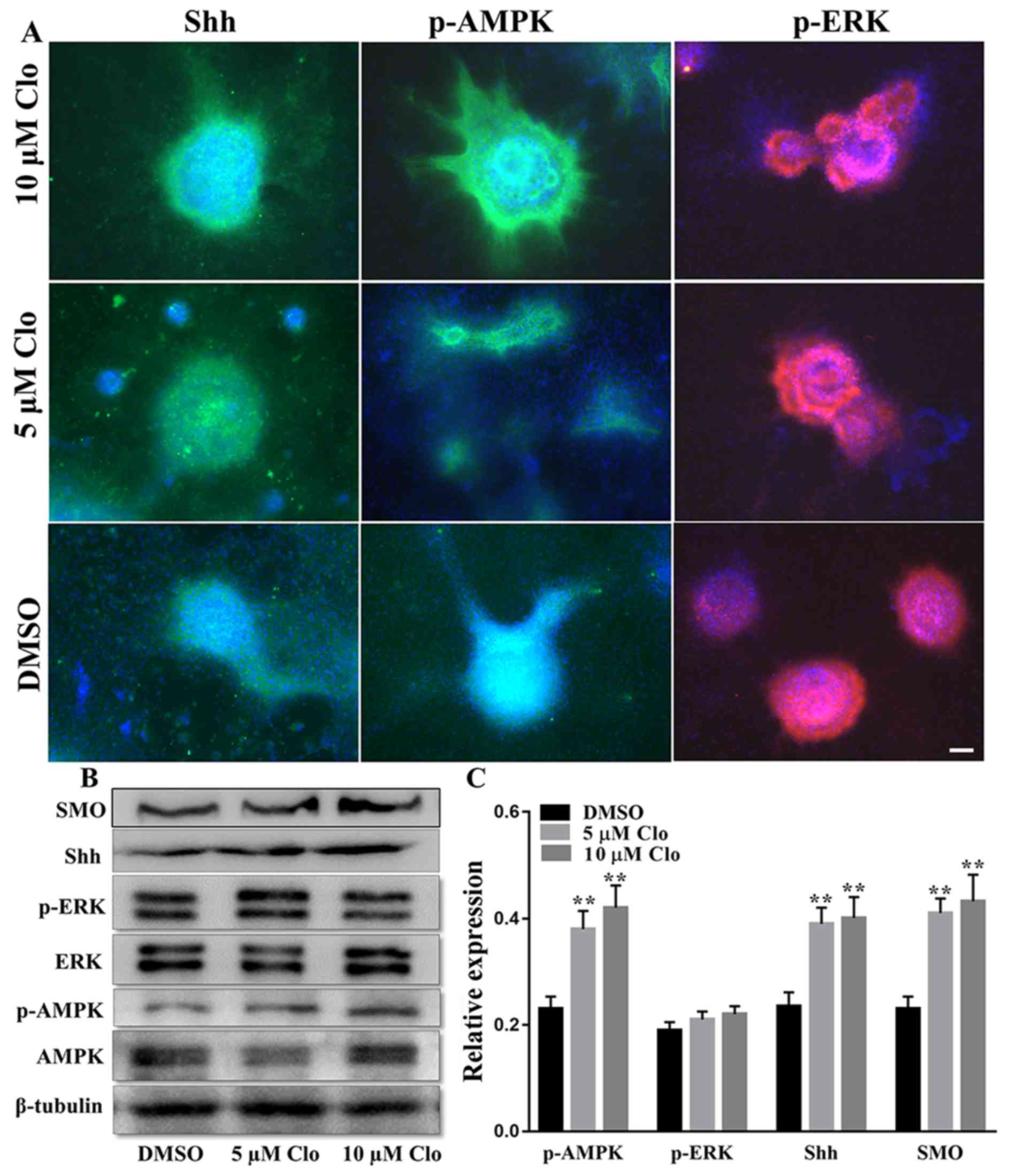 | Figure 5.Effect of Clo on SHH, AMPK and ERK
signaling pathways in NSCs. (A) Immunostaining of SHH, p-AMPK or
p-ERK with DAPI nuclear staining in differentiated NSCs following
treatment with Clo (5 or 10 µM) or DMSO (control). Scale bar=25 µm.
(B) Representative western blots of SMO, SHH, p-ERK, ERK, p-AMPK
and AMPK in differentiated NSCs following treatment with Clo (5 or
10 µM) or DMSO (control). (C) Quantification of SMO, SHH, p-ERK/ERK
and pAMPK/AMPK protein levels. Data are presented as the mean ±
standard error of the mean. n=5. *P<0.05 and **P<0.01 vs.
DMSO. Clo, clobetasol propionate; SMO, smoothened homolog; SHH,
sonic hedgehog; AMPK, AMP-activated protein kinase; ERK,
mitogen-activated protein kinase 1; p, phosphorylated; NSCs, neural
stem cells. |
Effect of Clo on SHH and SMO protein
expression
The protein expression levels of SHH and SMO were
determined in differentiated NSCs following treatment with Clo (5
or 10 µM) or DMSO (Fig. 5).
Immunocytochemistry indicated that treatment with Clo markedly
increased SHH protein expression levels (Fig. 5A), and western blotting confirmed
that SHH and SMO were significantly increased in the Clo treatment
groups compared with the DMSO control group (Fig. 5B and C). These results suggest that
treatment with Clo affects SHH signaling and this may provide a
mechanistic explanation for the enhanced differentiation of NSCs
into neurons and oligodendrocytes in the presence of Clo.
Inhibition assays
To further examine the involvement of AMPK and SHH
signaling pathways in NSC differentiation, the SHH pathway
inhibitor CYC and the AMPK pathway inhibitor CC were used to block
these pathways and NSC differentiation was examined. The protein
expression levels of GAP-43, MBP, GFAP, SHH and SMO were determined
by western blot analysis in differentiated NSCs following treatment
with 10 µM Clo and 10 µM CYC (Fig.
6A). Treatment with Clo alone significantly increased GAP-43,
MBP, SHH and SMO protein expression levels, whilst GFAP protein
expression levels were significantly decreased compared with those
in the DMSO control group (Fig. 6B).
However, these Clo-induced changes were attenuated by treatment
with CYC, with no significant difference from the DMSO control
group being detected (Fig. 6B).
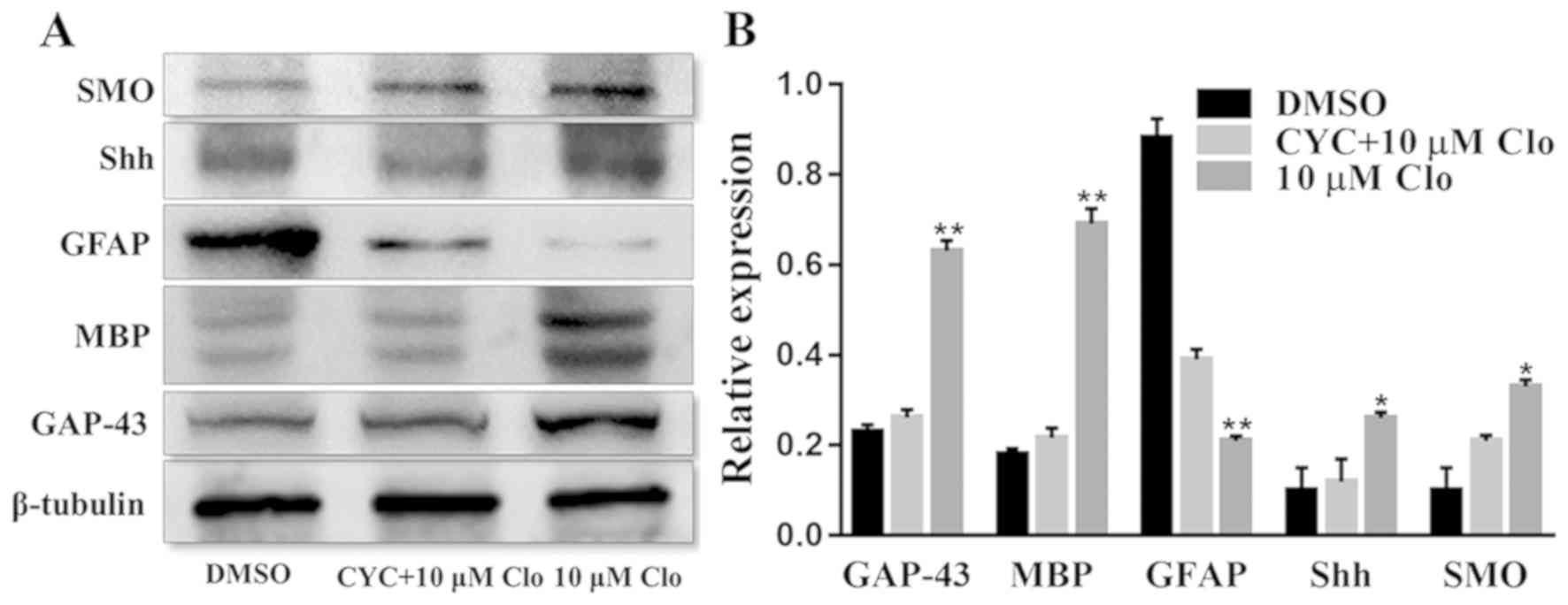 | Figure 6.CYC inhibits Clo-induced NSC
differentiation. (A) Representative western blots of GAP-43, MBP,
GFAP, SHH and SMO in differentiated NSCs following treatment with
10 µM Clo and 10 µM CYC, or 10 µM Clo alone. (B) Quantification of
GAP-43, MBP, GFAP, SHH and SMO protein expression. Data are
presented as the mean ± standard error of the mean. n=5. *P<0.05
and **P<0.01 vs. DMSO. CYC, cyclopamine; Clo, clobetasol
propionate; NSC, neural stem cell; GAP-43, growth associated
protein 43; MBP, myelin basic protein; GFAP, glial fibrillary
acidic protein; SHH, sonic hedgehog; SMO, smoothened homolog. |
Furthermore, the protein expression levels of
GAP-43, MBP, GFAP, AMPK and p-AMPK were determined by western blot
analysis in differentiated NSCs following treatment with 10 µM Clo
and 5 µM CC (Fig. 7A). Treatment
with Clo alone significantly increased GAP-43, MBP and p-AMPK
protein expression levels, whilst GFAP protein expression levels
were significantly decreased compared with those in the DMSO
control group (Fig. 7B). However,
these Clo-induced changes were attenuated by treatment with CC,
with no significant differences detected compared with the DMSO
control group (Fig. 7B).
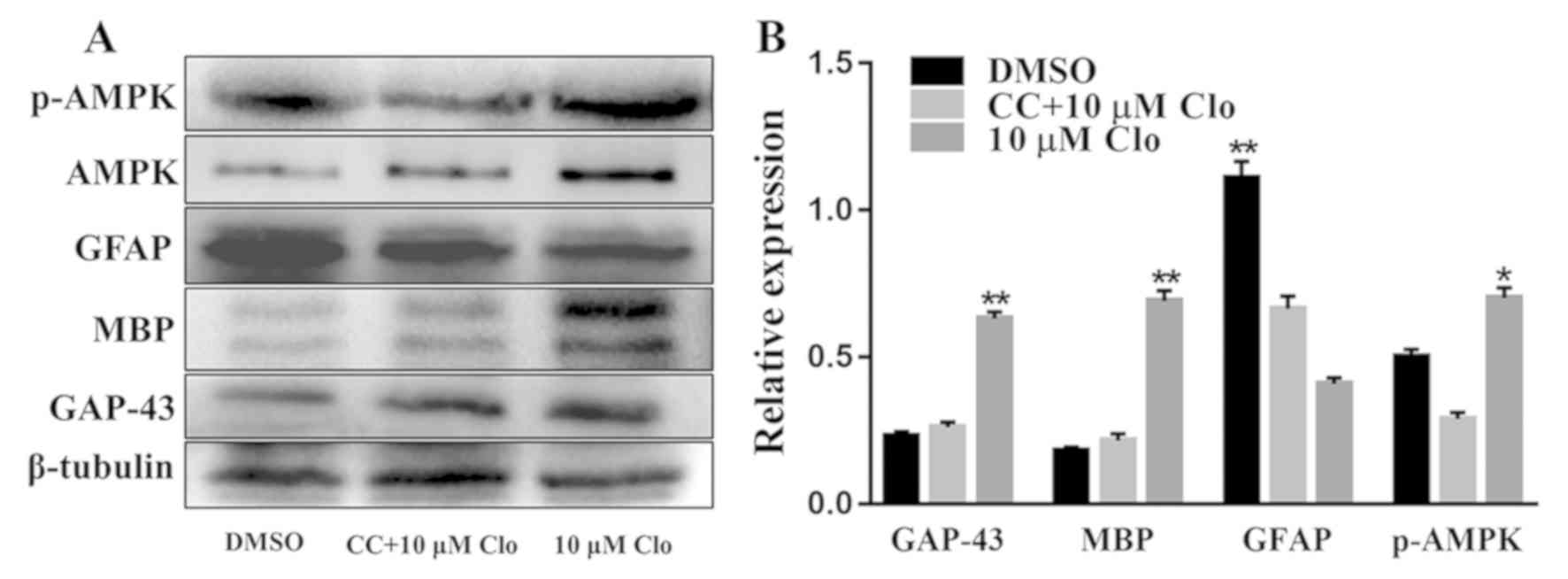 | Figure 7.CC inhibits Clo-induced NSC
differentiation. (A) Representative western blots of GAP-43, MBP,
GFAP, AMPK and p-AMPK in differentiated NSCs following treatment
with 10 µM Clo and 5 µM CC. (B) Quantification of GAP-43, MBP,
GFAP, AMPK and p-AMPK proteins. Data are presented as the mean ±
standard error of the mean. n=5. *P<0.05 and **P<0.01 vs.
DMSO. CC, Compound C; Clo, clobetasol propionate; NSC, neural stem
cell; GAP-43, growth associated protein 43; MBP, myelin basic
protein; GFAP, glial fibrillary acidic protein; AMPK, AMP-activated
protein kinase; p, phosphorylated. |
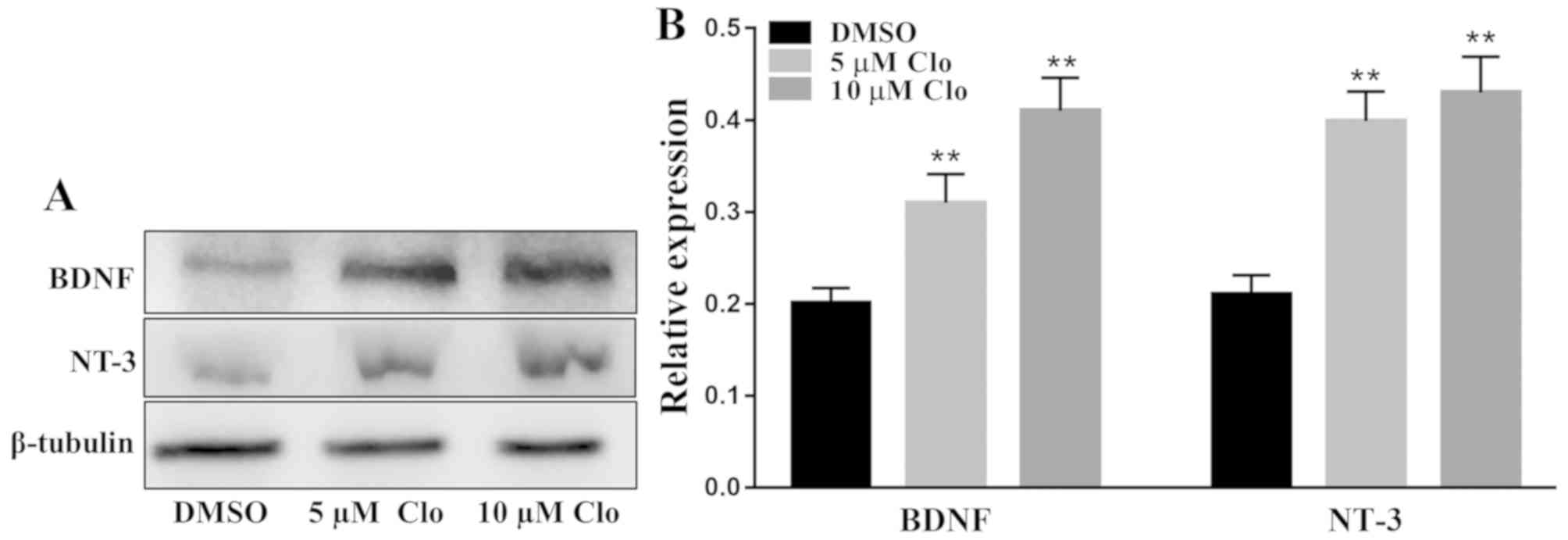
Effect of Clo on brain-derived
neurotrophic proteins
The protein expression levels of the neurotrophic
factors NT-3 and BDNF were determined by western blot analysis in
differentiated NSCs following treatment with Clo (5 or 10 µM) or
DMSO (Fig. 8A). Treatment with Clo
significantly increased NT-3 and BDNF protein expression levels
compared with those in the DMSO control group (Fig. 8B).
Discussion
The present study demonstrated that Clo
significantly enhanced NSC viability, and promotes the
differentiation of NSCs into neurons and oligodendrocytes whilst
inhibiting astrocyte differentiation. Furthermore, the present
study indicated that Clo may promote NSC differentiation through
the SHH and AMPK signaling pathways, providing a potentially novel
mechanism underlying the therapeutic effect of Clo in CNS
injury.
Previous studies demonstrated that Clo enhances
differentiation in OPC cultures (17) and reduces myelin loss (22). However, whether Clo also affects NSC
differentiation was unknown. Therefore, the aim of the present
study was to investigate the effect of Clo on the differentiation
of NSCs in vitro. NSCs were isolated from mouse embryonic
brain tissue and expanded in vitro. It was observed that the
NSCs, cultured as neurospheres, expressed nestin, a widely used
marker of NSCs (23). Neurosphere
formation can reflect the self-renewal capacity of cells (4). The MTT assay in the present study
revealed that treatment with Clo significantly increased NSC
viability at low concentrations (2.5, 5, 7.5 and 10 µM), consistent
with a previous study (18).
However, cell death was observed following treatment with Clo at
concentrations ≥12.5 µM, indicating that high concentrations Clo
may be toxic to NSCs. In addition, a recent study revealed that Clo
activates the SHH signaling pathway, and as high concentrations of
SHH inhibit cellular proliferation (24), high concentrations of Clo may inhibit
NSC viability in a similar manner.
A previous study identified four glucocorticoids
(halcinonide, fluticasone propionate, Clo and fluocinonide) as SMO
agonists via a high-throughput screen of cells containing an SMO
and β-arrestin-GFP reporter that binds to activated SMO (18). SMO is a component of the SHH
signaling pathway (25), which can
promote the expression of HH target genes by the Gli family of
transcription factors (26). SHH is
a morphogen produced by the notochord and the ventral floor plate
that induces the development of various populations of ventral
neurons and oligodendrocytes, and the proliferation of embryonic
spinal cord OPCs and NSCs (27). SHH
also serves a role in the development and maintenance of the
nervous system (28). The current
study demonstrated that treatment with Clo upregulates the
expression of SMO and SHH proteins in differentiating NSCs.
Although the molecular mechanism of Clo remains unknown, it can be
hypothesized that Clo may activate the SHH signaling pathway by
targeting SMO. Najm et al (17) reported that the glucocorticoid
receptor may be involved in the process that regulates NSC
differentiation into oligodendrocytes; however, the underlying
molecular mechanism requires further investigation.
A previous study has demonstrated that Clo acts as a
SMO agonist to promote myelin gene expression (18). Therefore, the Clo-associated
enhancement of NSC viability and differentiation observed in the
present study may be due to Clo having SHH-like and SMO
agonist-like effects. Given that Clo is able to pass through the
blood-brain barrier (16), Clo may
exert a direct effect on the CNS. To further investigate whether
Clo promotes the differentiation of NSCs through SHH signaling, SHH
signaling was blocked in differentiating NSCs using CYC (29). Blocking the SHH signaling pathway
exhibited a marked effect on Clo-induced NSC differentiation.
Inhibition experiments further confirmed that Clo enhances NSC
differentiation through the SHH signaling pathway.
Furthermore, the present study indicated that the
AMPK signaling pathway may be involved in NSC proliferation and
differentiation, as it was significantly upregulated following
treatment with Clo. It is thought that AMPK maintains cellular
energy homeostasis through the regulation of glucose and lipid
metabolism (30). Although AMPK
activation may inhibit cell proliferation (31,32), the
current study revealed that treatment with Clo enhances NSC
viability. AMPK was previously demonstrated to be expressed in
nerve cells (33). In the current
study, p-AMPK was detected at elevated levels in differentiated
NSCs. Inhibition experiments confirmed that the involvement of the
AMPK signaling pathway in the regulation of NSC differentiation. A
previous study reported that the administration of glucocorticoids
may cause hyperphagia via the AMPK-neuropeptide Y (NPY) signaling
pathway (34). NPY is involved in
the modulation of the dynamics of NSC niches in the dentate gyrus
and subventricular zone. Therefore, it may be hypothesized that
AMPK-NPY is a target for Clo in NSCs. SHH signaling promotes
polyamine biosynthesis in cerebellar granule cell precursors and
this process is governed by AMPK (35). Although the mechanism underlying the
activation of AMPK signaling by Clo was not elucidated in the
current study, it is possible that SHH may be involved.
Furthermore, it was previously revealed that Clo is able to
upregulate the neural cell expression of NT-3 and BDNF, to promote
neural differentiation and proliferation (36). Hence, Clo may enhance NSC
differentiation and proliferation via the upregulation of
neurotrophic factors.
The present study demonstrated that a high
concentration (10 µM) of Clo significantly suppressed the
differentiation of NSCs into astrocytes. This was likely caused by
the ability of corticosteroids to inhibit the inflammatory response
in cells (37). Neuroinflammation is
a feature associated with CNS disorders, including spinal cord
injury and neurodegeneration, in which astrocytes serve critical
roles (38). The reduced astrocyte
differentiation capacity of Clo-treated NSCs may be a potential
benefit towards the reduction of astrogliosis, the main cause of
glial-scar formation, which limits axonal outgrowth and
regeneration.
The current study has several limitations. In order
to validate the results obtained in the current study, relevant
in vivo studies and further mechanistic studies are
required. In addition, human-derived stem cells should be used to
ensure there are no species-specific differences.
In conclusion, the current study identified a novel,
straightforward and efficient method to obtain differentiated cells
with the typical morphological characteristics associated with
NSC-derived neurons and oligodendrocytes. Furthermore, the
well-known pharmacokinetic and pharmacodynamic properties of Clo
combined with the results of the present study support the
potential therapeutic application of Clo in human regenerative
medicine.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81571830),
Jiangsu Provincial Development Fund Project: Clinical Application
of 3D Printing Technology in Complex Pelvic Fractures (grant no.
YKK16228), Clinical Access Development Fund of Jiangsu University
School of Medicine (grant nos. JLY20160185 and JLY20180040) and
Social Development Project of Zhenjiang (grant no. 5561280012) from
the Program for Scientific Research Innovation Team in Colleges and
Universities of Jiangsu Province.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS and ZZ designed the study. SB, YD and KY
performed the experiments. WS and YZ prepared the manuscript. ZZ
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feder G, Murgai R and Quizon JB:
Degeneration & regeneration of the nervous system. Oxford
University Press; 1928
|
|
2
|
Vismara I, Papa S, Rossi F, Forloni G and
Veglianese P: Current options for cell therapy in spinal cord
injury. Trends Mol Med. 23:831–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johansson CB, Momma S, Clarke DL, Risling
M, Lendahl U and Frisén J: Identification of a neural stem cell in
the adult mammalian central nervous system. Cell. 96:25–34. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sundberg M, Savola S, Hienola A, Korhonen
L and Lindholm D: Glucocorticoid hormones decrease proliferation of
embryonic neural stem cells through ubiquitin-mediated degradation
of cyclin D1. J Neurosci. 26:5402–5410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pathipati P, Gorba T, Scheepens A, Goffin
V, Sun Y and Fraser M: Growth hormone and prolactin regulate human
neural stem cell regenerative activity. Neuroscience. 190:409–427.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koutmani Y and Karalis KP: Neural stem
cells respond to stress hormones: Distinguishing beneficial from
detrimental stress. Front Physiol. 6:772015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghareghani M, Sadeghi H, Zibara K, Danaei
N, Azari H and Ghanbari A: Melatonin increases oligodendrocyte
differentiation in cultured neural stem cells. Cell Mol Neurobiol.
37:1319–1324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura M and Toyama Y: Transplantation
of neural stem cells into spinal cord after injury. Nihon Rinsho.
61:463–468. 2003.(In Japanese). PubMed/NCBI
|
|
9
|
Corsini NS, Sancho-Martinez I, Laudenklos
S, Glagow D, Kumar S, Letellier E, Koch P, Teodorczyk M, Kleber S,
Klussmann S, et al: The death receptor CD95 activates adult neural
stem cells for working memory formation and brain repair. Cell Stem
Cell. 5:178–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel S, Saha A, Buntz S, Kurtzberg J and
Balber A: Neuronal and glial cell composition in a mouse brain
slice culture model is useful in developing human cord blood
derived cellular therapies for neonatal hypoxic-ischemic brain
injury. Cytotherapy. 16 (Suppl):S612014. View Article : Google Scholar
|
|
11
|
Fawcett JW and Asher RA: The glial scar
and central nervous system repair. Brain Res Bull. 49:377–391.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Au WL, Skinner MF, Benfeldt E, Verbeeck
R-K and Kanfer I: Application of dermal microdialysis for the
determination of bioavailability of clobetasol propionate applied
to the skin of human subjects. Skin Pharmacol Physiol. 25:17–24.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olsen JA and Akirav EM: Remyelination in
multiple sclerosis: cellular mechanisms and novel therapeutic
approaches. J Neurosci Res. 93:687–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bove RM and Green AJ: Remyelinating
pharmacotherapies in multiple sclerosis. Neurotherapeutics.
14:894–904. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cole KLH, Early JJ and Lyons DA: Drug
discovery for remyelination and treatment of MS. Glia.
65:1565–1589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao X, Su T and Verkman AS: Clobetasol
promotes remyelination in a mouse model of neuromyelitis optica.
Acta Neuropathol Commun. 4:422016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Najm FJ, Madhavan M, Zaremba A, Shick E,
Karl RT, Factor DC, Miller TE, Nevin ZS, Kantor C, Sargent A, et
al: Drug-based modulation of endogenous stem cells promotes
functional remyelination in vivo. Nature. 522:216–220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Lu J, Bond MC, Chen M, Ren XR,
Lyerly HK, Barak LS and Chen W: Identification of select
glucocorticoids as Smoothened agonists: Potential utility for
regenerative medicine. Proc Natl Acad Sci USA. 107:9323–9328. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu P, Graham L, Wang Y, Wu D and Tuszynski
M: Promotion of survival and differentiation of neural stem cells
with fibrin and growth factor cocktails after severe spinal cord
injury. J Vis Exp. e506412014.PubMed/NCBI
|
|
20
|
Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan
Z, Song Z, Ding M and Deng H: MEK/ERK signaling contributes to the
maintenance of human embryonic stem cell self-renewal.
Differentiation. 75:299–307. 2010. View Article : Google Scholar
|
|
21
|
Zang Y, Yu LF, Nan FJ, Feng LY and Li J:
AMP-activated protein kinase is involved in neural stem cell growth
suppression and cell cycle arrest by
5-aminoimidazole-4-carboxamide-1-beta-D- ribofuranoside and glucose
deprivation by down-regulating phospho-retinoblastoma protein and
cyclin D. J Biol Chem. 284:6175–6184. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hampton T: Drugs that treat skin
conditions may help combat multiple sclerosis. JAMA. 313:21142015.
View Article : Google Scholar
|
|
23
|
Park D, Xiang AP, Mao FF, Zhang L, Di CG,
Liu XM, Shao Y, Ma BF, Lee JH, Ha KS, et al: Nestin is required for
the proper self-renewal of neural stem cells. Stem Cells.
28:2162–2171. 2011. View
Article : Google Scholar
|
|
24
|
Canettieri G, Coni S, Laura DM and
Sdruscia G: Non-canonical Hedgehog/AMPK-mediated control of
polyamine metabolism is required for medulloblastoma growth. Eur J
Cancer. 61 (Suppl):S722016. View Article : Google Scholar
|
|
25
|
Ye W, Shimamura K, Rubenstein JLR, Hynes
MA and Rosenthal A: FGF and SHH signals control dopaminergic and
serotonergic cell fate in the anterior neural plate. Cell.
93:755–766. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sinha S and Chen JK: Purmorphamine
activates the Hedgehog pathway by targeting smoothened. Nat Chem
Biol. 2:29–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Li Z, Deng W, He Q, Wang Q, Shi
W, Chen Q, Yang W, Spector M, Gong A, et al: Ectoderm mesenchymal
stem cells promote differentiation and maturation of
oligodendrocyte precursor cells. Biochem Biophys Res Commun.
480:727–733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scadden DT: The stem-cell niche as an
entity of action. Nature. 441:1075–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen JK, Taipale J, Cooper MK and Beachy
PA: Inhibition of Hedgehog signaling by direct binding of
cyclopamine to Smoothened. Genes Dev. 16:2743–2748. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krieg S, Lüscher B, Vervoorts J and Dohmen
M: Studying the role of AMPK in autophagy. Methods Mol Biol.
1732:373–391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
cellular energy sensing and restoration of metabolic balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ronnett GV, Ramamurthy S, Kleman AM,
Landree LE and Aja S: AMPK in the brain: Its roles in energy
balance and neuroprotection. J Neurochem. 109 (Suppl 1):S17–S23.
2009. View Article : Google Scholar
|
|
33
|
Rich B, Scadeng M, Yamaguchi M, Wagner PD
and Breen EC: Skeletal myofiber VEGF is required for the exercise
training-induced increase in dentate gyrus neuronal precursor
cells. J Physiol. 595:5931–5943. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Song Z, Jiao H and Lin H:
Glucocorticoids increase NPY gene expression via hypothalamic AMPK
signaling in broiler chicks. Endocrinology. 155:2190–2198. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di Magno L, Basile A, Coni S, Manni S,
Sdruscia G, D'Amico D, Antonucci L, Infante P, De Smaele E, Cucchi
D, et al: The energy sensor AMPK regulates Hedgehog signaling in
human cells through a unique Gli1 metabolic checkpoint. Oncotarget.
7:9538–9549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Marsh SE and Blurton-Jones M: Neural stem
cell therapy for neurodegenerative disorders: The role of
neurotrophic support. Neurochem Int. 106:94–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jauregui-Huerta F, Ruvalcaba-Delgadillo Y,
Gonzalez-Perez O, Gonzalez-Castañeda R, Garcia-Estrada J and Luquin
S: Responses of glial cells to stress and glucocorticoids. Curr
Immunol Rev. 6:195–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blancosuárez E, Caldwell AL and Allen NJ:
Role of astrocyte-synapse interactions in CNS disorders. J Physiol.
595:1903–1916. 2017. View Article : Google Scholar : PubMed/NCBI
|