Introduction
Steroids are widely used to inhibit inflammation in
a variety of immune-mediated diseases, including systemic lupus
erythematosus and arthritis (1–3).
However, continuous oral steroid therapy for 3–6 months or longer
is associated with bone loss and fractures as the major adverse
events (4–6). The fracture risk is positively
correlated with the daily and cumulative glucocorticoid dose in
patients with glucocorticoid-induced osteoporosis (GIOP) (6–8). A
clinical survey indicated that calcium plus vitamin D
supplementation has a limited effect on the treatment of GIOP,
while specific anti-osteoporotic medicines, including
bisphosphonates, alendronate (ALN) and teriparatide, are effective
for the management of GIOP (6).
Immunologic thrombocytopenic purpura (ITP) is a
hematologic disorder characterized by accelerated platelet
consumption, shortened platelet survival and impaired platelet
production (9). In the last two
decades, splenectomy, rituximab and thrombopoietin-receptor
agonists have been introduced for the treatment of patients with
ITP (3). Furthermore,
corticosteroids are considered as the first-line and mainstay of
treatment and are recommended by the American Society of Hematology
and the international consensus report on the investigation and
management of primary ITP (10,11). For
instance, prednisone at 1 mg/day for 3–4 weeks followed by tapering
for another 2–3 weeks is a general standard approach for ITP
treatment (3). Unfortunately, a
glucocorticoid-induced decrease in bone mineral density (BMD) is
observed in patients with ITP (12).
Therefore, ALN was co-administered to prevent bone loss in patients
with ITP in the present study.
Previous studies have validated that treatment with
ALN reduces bone loss and the risk of fracture in patients with
GIOP (13), normocalcemic primary
hyperparathyroidism (14),
ankylosing spondylitis (15),
orthotopic liver transplantation (16) and post-menopausal osteoporosis
(17). The present study aimed to
evaluate the efficacy and safety of ALN (70 mg) weekly combined
with caltrate D (CalD; Ca, 1,200 mg and vitamin D3, 250
IU) daily to prevent bone loss in comparison to CalD treatment
alone in ITP patients under glucocorticoid treatment. The BMD at
the lumbar vertebrae (L1-L4), femoral neck and total hip, as well
as bone metabolic parameters were assessed in patients with ITP. At
the 9-month follow up, the results demonstrated that ALN prevented
urinary Ca excretion and loss in BMD, and reduced bone resorption
markers in patients with ITP.
Materials and methods
Patients
A total of 105 ITP patients were recruited from the
First People's Hospital of Huzhou (Huzhou, China) and Huzhou
Central Hospital (Huzhou, China) between January 2014 and June
2017. The patients were between 18 and 50 years of age and provided
written informed consent to participate in the study. The inclusion
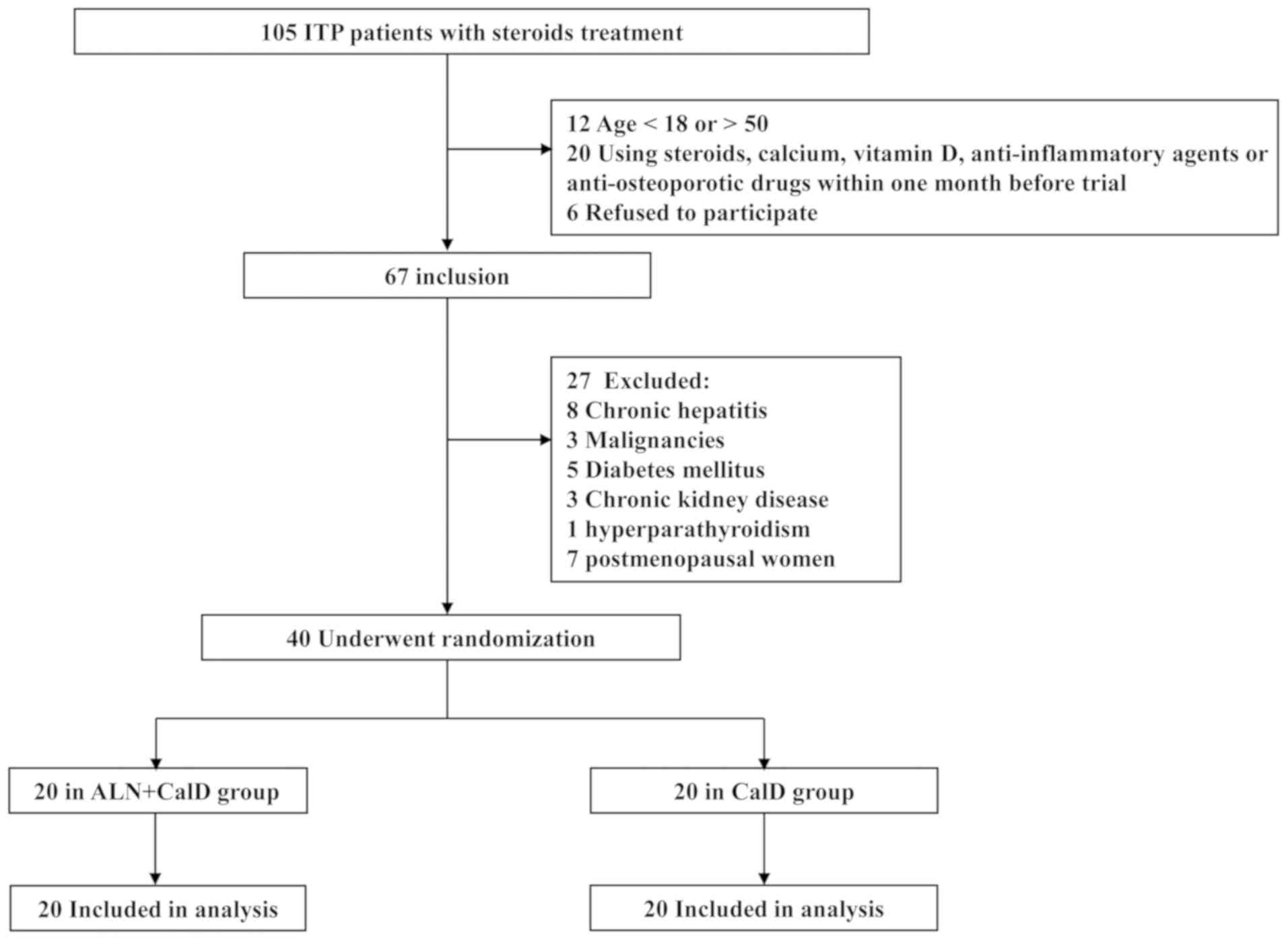
and exclusion criteria are presented in Fig. 1. Among the candidate ITP patients, 7
post-menopausal women were excluded due to their clinical
presentation, pelvioscopy results and serum levels of
follicle-stimulating hormone (>10 U/l) and estradiol (<20
pg/ml). The baseline physiological and biochemical parameters of
all patients are provided in Tables
I and II. The effect of
steroids on bone loss in ITP patients mainly depends on the
accumulated dose. Before the commencement of the present trial, all
ITP patients received steroid therapy for one month. At 1 month
prior to the start of the trial, the steroid treatment was
discontinued for all ITP patients, observation or replacement
therapy with gamma globulin.
 | Table I.Baseline clinicopathological
characteristics of patients with immunologic thrombocytopenic
purpura. |
Table I.
Baseline clinicopathological
characteristics of patients with immunologic thrombocytopenic
purpura.
| Parameter | ALN+CalD (n=20) | CalD (n=20) | P-value |
|---|
| Gender (M/F) | 15/5 | 13/7 | 0.490 |
| Age (years) | 37.6±9.2 | 35.5±8.1 | 0.449 |
| BMI
(kg/m2) | 23.7±2.2 | 22.8±2.2 | 0.215 |
| Steroid dosage
(mg/day) | 13.15±6.70 | 12.15±5.86 | 0.619 |
| Steroid therapy
duration (days) | 479±187 | 505±177 | 0.646 |
| Platelet count
(×104/mm3) | 13.7±4.3 | 14.2±3.9 | 0.673 |
| Bone status (%) |
|
| 0.609 |
| Normal
(T-score >-1 SD) | 4 (20) | 5 (25) |
|
|
Osteopenia (−2.5 SD <
T-score ≤-1 SD) | 6 (30) | 7 (35) |
|
|
Osteoporosis (T-score ≤-2.5
SD) | 10 (50) | 8 (40) |
|
| Bone mineral density
(g/cm2) |
|
|
|
| Lumbar
L1-L4 | 0.816±0.052 | 0.832±0.061 | 0.375 |
| Femoral
neck | 0.752±0.059 | 0.741±0.065 | 0.592 |
| Total
hip | 0.656±0.067 | 0.670±0.054 | 0.461 |
 | Table II.Baseline biochemical parameters of
patients with ITP. |
Table II.
Baseline biochemical parameters of
patients with ITP.
| Parameter | ALN+CalD
(n=20) | CalD (n=20) | P-value |
|---|
| Serum Ca
(mg/dl) | 10.21±0.58 | 9.79±0.71 | 0.517 |
| 24-h urinary Ca
(mg/day) | 215±137 | 184±91 | 0.438 |
| Serum P
(mg/dl) | 4.17±0.56 | 4.28±0.48 | 0.715 |
| Serum intact-PTH
(pg/ml) | 46.1±18.4 | 50.9±20.5 | 0.563 |
| 25(OH) vitamin D
(ng/ml) | 19.5±8.3 | 21.4±9.2 | 0.279 |
| BAP (ng/ml) | 21.6±8.2 | 18.5±9.7 | 0.288 |
| P1NP (ng/ml) | 53.9±21.7 | 60.3±24.4 | 0.189 |
| Osteocalcin
(ng/ml) | 31.6±5.9 | 28.7±6.5 | 0.419 |
| TRACP-5b (U/l) | 567±173 | 515±164 | 0.663 |
| Serum CTX
(ng/ml) | 0.457±0.163 | 0.406±0.154 | 0.408 |
Patient randomization was performed using the SAS
9.0 system (SAS Institute, Cary, NC, USA). The 40 enrolled ITP
patients were randomized into CalD or CalD + ALN treatment groups
at a pre-determined proportion of 1:1. The randomization process
was an unsupervised classification. Before the start of the trial,
the participants were given a fixed prescription and received study
medications from pharmacy staff according to the enrollment
sequence. The medication for the CalD and CalD + ALN groups had the
same appearance, shape, color and packaging, so that the research
physicians and participants were not distinguish them. The research
physicians and participants were not informed about any individual
treatment details until the end of the treatment course and after
receiving the laboratory test results. The Huzhou Central Hospital
(Huzhou, China) approved the study protocol (approval no.
201401A006). Informed consent forms were signed by all patients.
None of the patients were included in the current study prior to
obtaining ethical approval.
Treatment
The patients in the CalD group (n=20) received 1,200
mg calcium and 250 IU vitamin D3 daily (Caltrate D;
Pfizer, Inc., New York, NY, USA). Patients in the CalD + ALN group
(n=20) received 1,200 mg calcium and 250 IU vitamin D3
daily with ALN (70 mg; Merck & Co., Darmstadt, Germany) weekly
for 9 months.
Measurement of BMD
The BMD from the first to fourth lumbar vertebrae
(L1-L4), femoral neck and total hip were measured by dual-energy
X-ray absorptiometry (Hologic DQR-4500W; Hologic, Inc., Bedford,
MA, USA), according to the manufacturer's protocol (16). Osteoporosis and osteopenia were
defined according to international guidelines: Osteoporosis was
classified by a t-score of <-2.5 standard deviation (SD) in
either lumbar spine or femur, and osteopenia by a t-score between
−2.5 and −1 SD (18).
Bone metabolism markers
Serum and urine samples for determination of Ca and
P were obtained at baseline levels and after 1, 3, 6 and 9 months,
using an Olympus AU 2700 automated multichannel analyzer (Olympus,
Tokyo, Japan). Bone formation and resorption markers in the serum
were determined at the baseline and after 1, 3, 6 and 9 months.
Serum intact parathyroid hormone (PTH; cat. no. 11972219122) and
osteocalcin (cat. no. 12149133122) were measured using
chemiluminescence immunoassays (Roche Diagnostics, Mannheim,
Germany). Serum 25(OH) vitamin D was determined using a DiaSorin
kit on a LIAISON automated immunoassay analyzer (DiaSorin,
Saluggia, Italy). Serum bone-specific alkaline phosphatase (cat.
no. 48262; BAP; Access Ostase, Beckman Coulter, Inc., Brea, CA,
USA), type 1 procollagen N-terminal propeptide (cat. no.
E-EL-H0185c; P1NP; Elabscience Biotechnology Co., Ltd, Wuhan,
China), tartrate resistant acid phosphatase 5b (cat. no.
CSB-E08490h; TRACP-5b; CUSABIO Technology LCC, Wuhan, Hubei, China)
and C-terminal telopeptides of type 1 collagen (cat. no.
E-EL-H0960c; CTX; Elabscience Biotechnology Co., Ltd, Wuhan, China)
were measured with a commercial ELISA. All tests were performed
according to the manufacturers' instructions in a routine
laboratory.
Statistical analysis
Continuous data are expressed as the mean ± standard
deviation. A two-tailed, unpaired Student's t-test for independent
samples or an χ2 test was used to compare baseline
variables between the two groups. The differences between
quantitative variables were analyzed using a Student's t-test for
unpaired data. Inter-group differences were analyzed by one-way
analysis of variance, followed by Tukey's post-hoc test. SPSS
(v.17; SPSS, Inc., Chicago, IL, USA) for Windows was used for
statistical analysis and GraphPad Prism software (version 7.0;
GraphPad Software, Inc., La Jolla, CA, USA) was used to draw the
histograms. P<0.05 was considered to indicate statistically
significant differences.
Results
Study population and baseline
characteristics
Initially, 105 patients with a clinical diagnosis of
ITP and who had received steroids treatment were recruited for the
present study. Among these patients, 67 were included based on the
following: i) An age of 18–50 years; ii) no use of steroids,
calcium, vitamin D, anti-inflammatory agents or anti-osteoporotic
drugs within one month prior to the trial; iii) the patients
provided written informed consent form. In addition, 27 patients
were excluded due to co-morbidities or post-menopausal status, and
the remaining 40 patients were randomized into two groups (20 in
the ALN + CalD group and 20 in the CalD group; Fig. 1).
The baseline clinicopathological characteristics and
biochemical parameters of the patients with ITP are presented in
Tables I and II, respectively. At the baseline,
clinicopathological characteristics, including the gender ratio,
age, BMI, steroid dosage and therapy duration, platelet count, as
well as the BMD of lumbar vertebrae, femoral neck and total hip,
were not significantly different between the ALN + CalD and CalD
groups (P>0.05; Table I).
Furthermore, the baseline levels of serum Ca, P, intact PTH, 25(OH)
vitamin D, BAP, P1NP, osteocalcin, TRACP-5b, CTX and 24-h urinary
Ca were not significantly different between the two groups
(Table II).
Outcomes
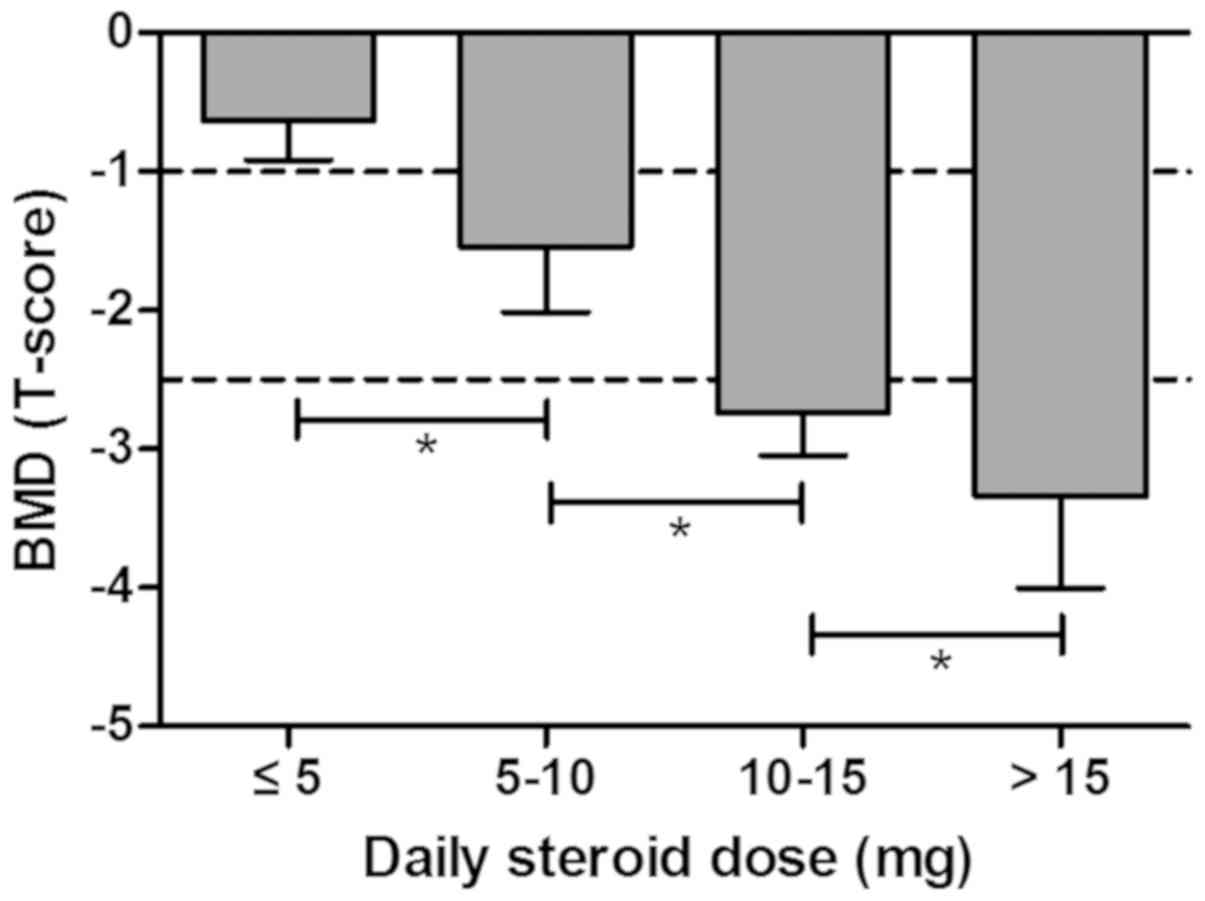
First, the association between BMD (T-score) and the
average daily steroid dose was demonstrated in patients with ITP.
The BMD T-score was −0.64±0.28 in the group receiving >5 mg/day,
−1.54±0.48 in the group treated with 5–10 mg/day, −2.74±0.30 in the
group receiving 10–15 mg/day and −3.34±0.66 in the group receiving
>15 mg/day, suggesting that average daily steroid dose was
positively correlated with bone loss in ITP patients with steroids
treatment prior to clinical trial (Fig.
2).
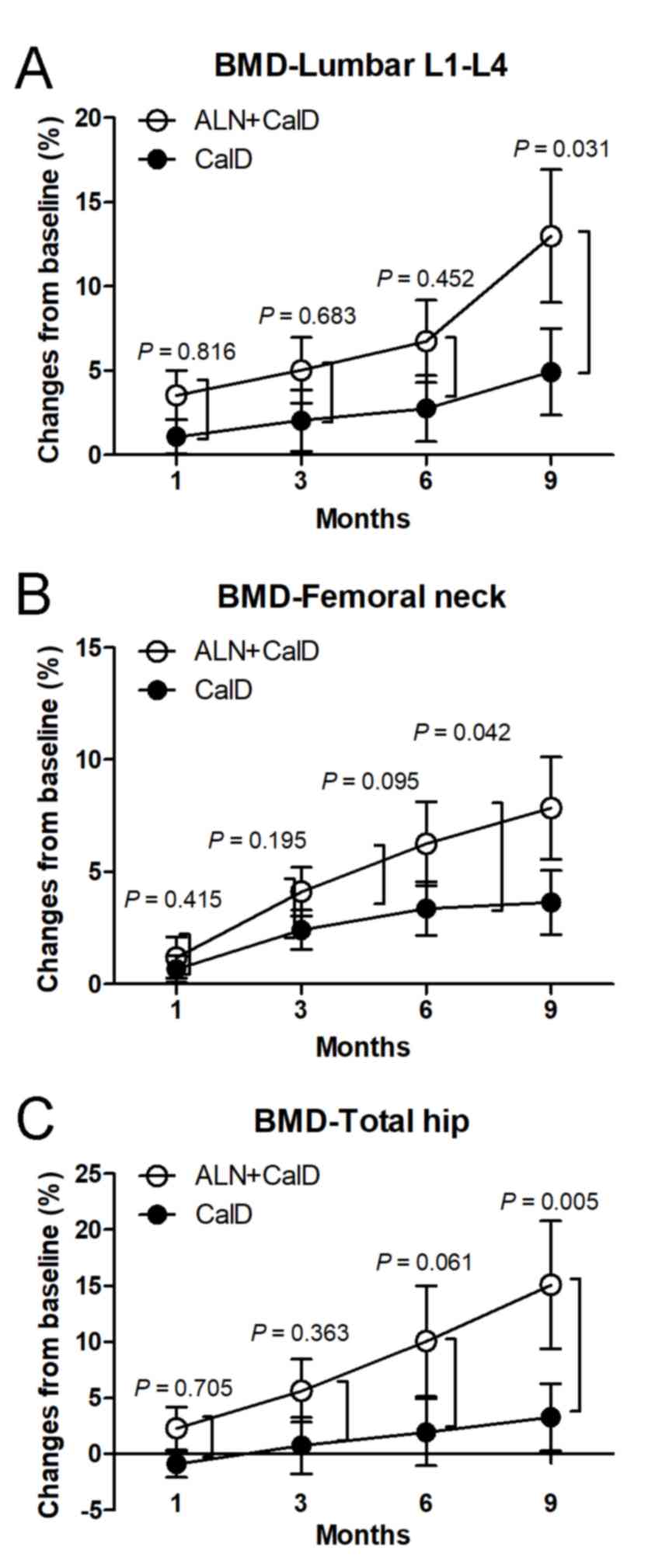
The BMD of the lumbar vertebrae L1-L4 was
significantly increased between month 3 and 9 of ALN + CalD
treatment compared with that at the baseline. Compared with the
baseline levels, CalD treatment alone also increased the BMD of the
lumbar vertebrae L1-L4 at month 9 in patients with ITP. These
results suggest that ALN + CalD and CalD have a beneficial effect
to improve the BMD of the lumbar vertebrae L1-L4 in patients with
ITP. Of note, the BMD of the lumbar vertebrae L1-L4 in the ALN +
CalD group was significantly higher than that in the CalD group at
month 9 (P<0.05; Table III;
Fig. 3A). In addition, the BMD of
the femoral neck and total hip was significantly increased in the
ALN + CalD group compared with that at the baseline at month 6 and
9. However, CalD treatment alone had no significant effect on the
BMD of the femoral neck and total hip in patients with ITP. Of
note, ALN combined with CalD markedly increased the BMD of the
femoral neck and total hip as compared with that in the CalD group
at month 9 (P<0.05; Table III;
Fig. 3B and C). These results
indicate that ALN combined with CalD is superior to CalD treatment
alone in protecting against steroid-associated bone deterioration
in patients with ITP.
 | Table III.BMD of lumbar, femoral neck and hip
in the two patient groups at different time-points within the study
period. |
Table III.
BMD of lumbar, femoral neck and hip
in the two patient groups at different time-points within the study
period.
| Site/group | Baseline | 1 month | 3 months | 6 months | 9 months |
|---|
| Lumbar L1-L4
(g/cm2) |
|
|
|
|
|
|
ALN+CalD | 0.816±0.052 | 0.845±0.057 |
0.857±0.063a |
0.871±0.069b |
0.922±0.073c,d |
|
CalD | 0.832±0.061 | 0.841±0.051 | 0.849±0.060 | 0.855±0.064 |
0.873±0.065a |
| Femoral neck
(g/cm2) |
|
|
|
|
|
|
ALN+CalD | 0.752±0.059 | 0.761±0.054 | 0.783±0.060 |
0.799±0.062a |
0.811±0.065b,d |
|
CalD | 0.741±0.065 | 0.746±0.061 | 0.759±0.055 | 0.766±0.060 | 0.768±0.064 |
| Total hip
(g/cm2) |
|
|
|
|
|
|
ALN+CalD | 0.656±0.067 | 0.671±0.065 | 0.693±0.068 |
0.722±0.070b |
0.755±0.072c,e |
|
CalD | 0.670±0.054 | 0.664±0.050 | 0.675±0.055 | 0.683±0.057 | 0.692±0.060 |
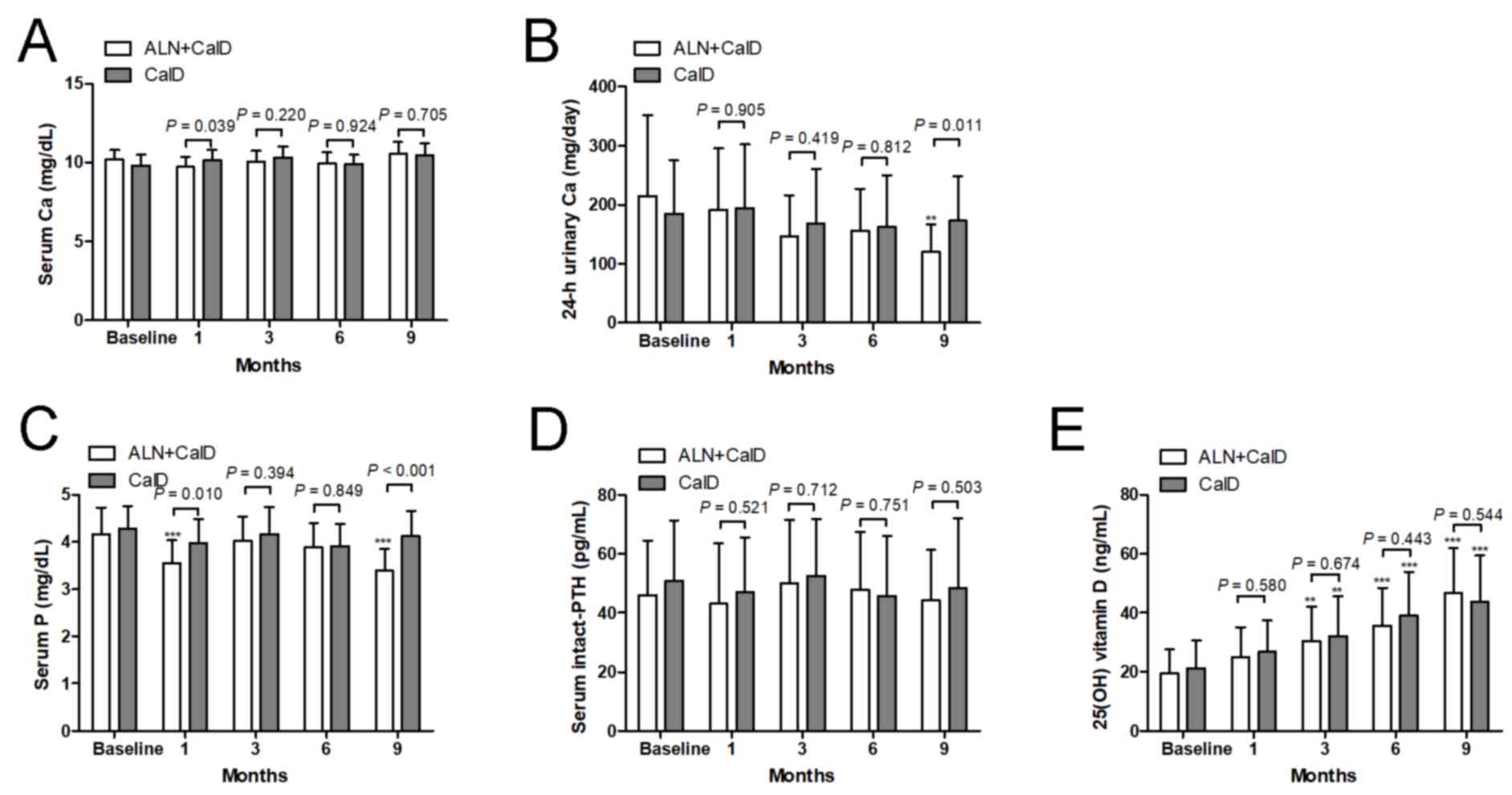
No change of serum Ca from the baseline was observed
at each time-point in each of the two treatment groups (Fig. 4A). There was no difference in serum
Ca between the CalD and ALN + CalD groups, except at month 1. Of
note, after 9 months of ALN + CalD treatment, the 24-h urinary Ca
excretion was significantly suppressed compared with the baseline
levels. Compared with CalD treatment alone, ALN + CalD caused a
significant decrease in 24-h urinary Ca at 9 months. However, CalD
treatment alone did not significantly change the 24-h urinary Ca at
any time-point (Fig. 4B). Compared
with the baseline levels or CalD treatment alone, ALN + CalD
treatment markedly decreased serum P at month 1 and 9. CalD
treatment alone had on significant effect on serum P at any
time-point (Fig. 4C). Neither CalD
treatment alone nor CalD combined with ALN had any effect on PTH at
any time-point (Fig. 4D). An
increase of 25(OH) vitamin D levels was detected in the CalD and
CalD combined with ALN groups after 3 months of treatment; however,
no significant difference in 25(OH) vitamin D levels was observed
between the two groups (Fig.
4E).
The present study also indicated that CalD treatment
alone or combined with ALN provided no significant improvement in
the bone formation markers BAP, P1NP and osteocalcin (Fig. 5A, B and C, respectively), while the
bone absorption markers TRACP-5b and CTX were inhibited by CalD
combined with ALN treatment (Fig. 5D and
E). Compared with the baseline levels, ALN + CalD treatment
resulted in a significant decrease in TRACP-5b levels after 3
months of treatment. TRACP-5b was significantly lower in the ALN +
CalD group compared with that in the CalD group at month 6 and 9
(Fig. 5D). Compared with the
baseline levels, ALN + CalD treatment led to a reduction of CTX
levels in the serum after 9 months of treatment. The levels of CTX
were significantly decreased in the ALN + CalD group compared with
those in the CalD group. These results suggested that ALN had a
specific effect to inhibit bone resorption markers in patients with
ITP.
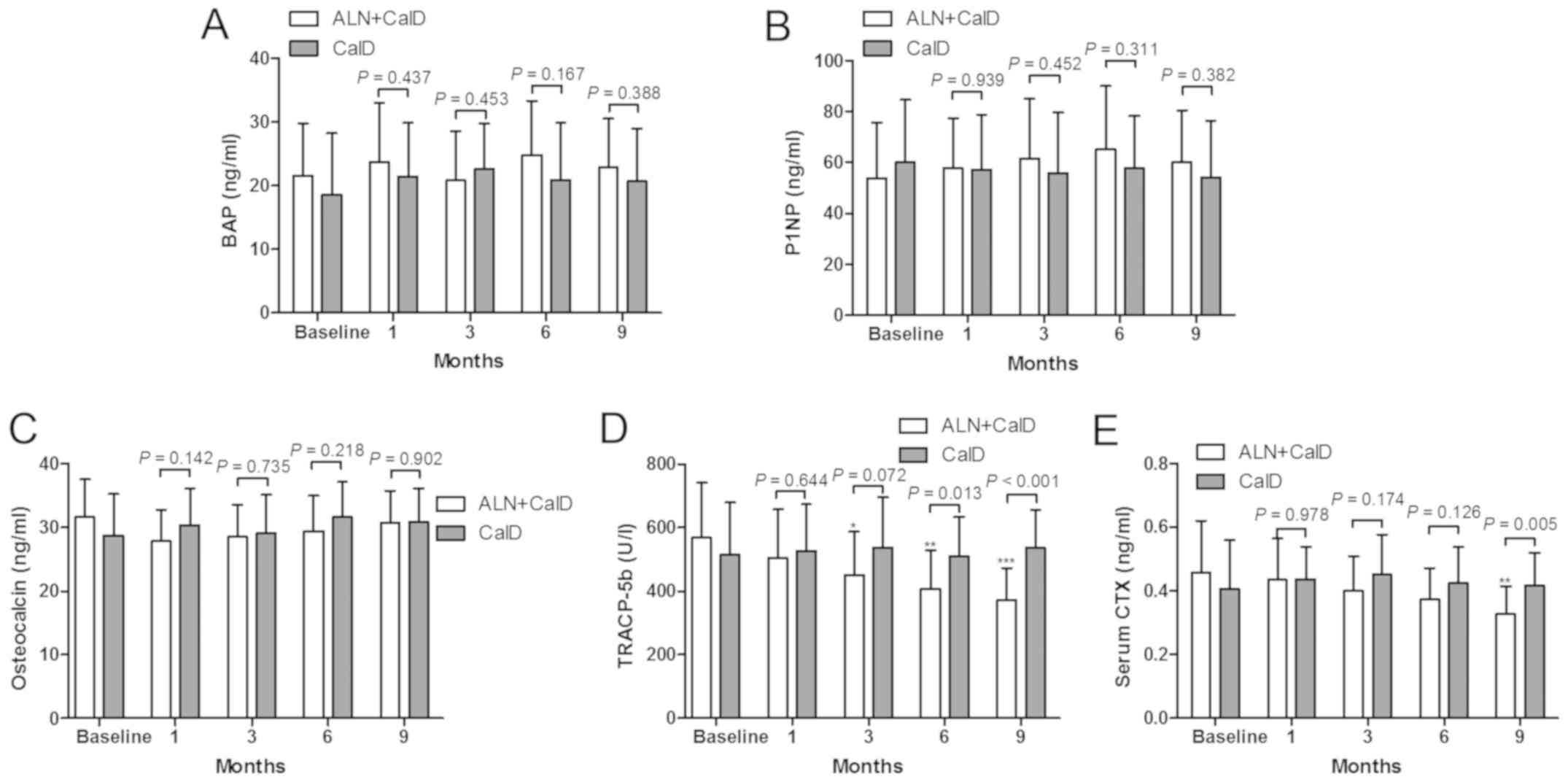 | Figure 5.Impact of ALN on bone metabolism
markers. (A-C) Bone formation markers (A) BAP, (B) P1NP and (C)
osteocalcin, and (D and E) bone resorption markers (D) TRACP-5b and
(E) CTX, were measured at baseline levels and after 1, 3, 6 and 9
months of treatment with CalD or CalD + ALN. *P<0.05,
**P<0.01 and ***P<0.001 vs. baseline levels. ALN,
alendronate; CalD, caltrate D; BAP, bone-specific alkaline
phosphatase; P1NP, type 1 procollagen N-terminal propeptide;
TRACP-5b, tartrate resistant acid phosphatase 5b; CTX, C-terminal
telopeptides of type 1 collagen. |
Discussion
Emerging evidence has indicated that ITP patients
commonly present with osteopenia or osteoporosis, which may be
attributed to a prolonged use of steroid drugs in numerous
refractory cases (12,19). Nomura et al (12) reported that administration of
bisphosphonate, a novel anti-metabolic osteopathy drug, increased
the BMD of lumbar vertebrae and decreased urinary levels of
collagen type 1 cross-linked N-telopeptides, a bone resorption
marker. In the present study, ALN (70 mg), an anti-osteoporosis
medication, combined with CalD (Ca, 1,200 mg and vitamin
D3, 250 IU) was demonstrated to increase the BMD at the
lumbar vertebrae (L1-L4), femoral neck and total hip, inhibit
urinary Ca excretion and the activity of bone resorption markers
TRACP-5b and CTX, compared with the baseline. The results also
suggested that ALN combined with CalD is superior to CaldD alone in
the prevention of bone loss in ITP patients with steroid
administration. A significant increase in the BMD of lumbar
vertebrae, but not in the femoral neck and total hip, and serum
25(OH) vitamin D levels were identified with CaldD treatment alone
for 9 months, compared with the baseline levels.
A number of trials have reported on the beneficial
effect of ALN in the treatment of bone deteriorations in
post-menopausal women, as well as in GIOP and orthotopic liver
transplantation-associated bone loss (13,16,17,20). A
comparative study of ALN (10 mg/day), calcitriol or simple vitamin
D supplementation in 195 glucocorticoid-treated subjects with a
2-year follow-up reported a significant increase in the mean lumbar
vertebral BMD (+5.9%) with ALN treatment, compared with that
achieved by calcitriol (−0.7%) or vitamin D (−0.5%) treatment
(13). A meta-analysis of seven
studies comprising 1,111 patients with GIOP indicated that ALN
administration markedly elevated the BMD in lumbar vertebrae and
femoral neck (21). However, only
few randomized controlled trials have addressed the role of ALN in
the management of bone loss in patients with ITP. The present study
was the first to evaluate the effect of ALN and CalD in patients
with ITP. Consistent with previous results, the present study
demonstrated the ability of ALN + CalD to increase the BMD of
lumbar vertebrae (+6.74 and +12.99%), femoral neck (+6.25 and
+7.84%) and total hip (+10.06 and +15.09%) at 6 and 9 months
compared with the baseline levels. Compared to CaldD treatment
alone, ALN combined with CalD significantly elevated the BMD of the
lumbar vertebrae, femoral neck and total hip from +4.93 to +12.99%,
+3.64 to +7.84% and +3.28 to +15.09%, respectively, at 9 months.
These results confirmed the beneficial effects of ALN in improving
the BMD in GIOP patients.
Numerous studies have indicated that ALN blocks
osteoclast differentiation and osteoclastic bone resorption,
induces osteoclast precursor apoptosis and inhibits bone resorption
markers in vivo and in vitro (22–26). A
randomized, controlled trial on ALN in patients with glomerular
disease indicated that the serum levels of the bone formation
markers PINP and BAP, and the bone resorption marker TRACP-5b, were
markedly decreased by ALN treatment for 12 months compared with the
baseline levels (27). One previous
study in postmenopausal women with normocalcemic primary
hyperparathyroidism indicated that the bone formation marker
osteocalcin and the bone resorption marker CTX were continually
reduced over 6 months of ALN treatment (14). A randomized, double-blinded,
placebo-controlled trial of ALN treatment for fibrous dysplasia of
the bone reported a significant decrease in the bone resorption
marker N-terminal telopeptides of type 1 collagen, but no
significant effect on serum osteocalcin (28). The present results validated the
effects of ALN in increasing the BMD and decreasing bone resorption
markers, but no effect on bone formation markers was observed in
patients with ITP.
Of note, the present study had certain limitations.
The study population was small due to the low prevalence and
incidence of ITP. In these patients, the side effects of ALN were
not quantitatively evaluated. The bone microstructure was also not
evaluated in the ALN-treated ITP patients.
In conclusion, the present study indicated that in
ITP patients with steroid treatment, administration of ALN together
with CalD over 9 months significantly elevated the BMD at three
skeletal sites. Furthermore, treatment with ALN together with CalD
markedly inhibited the activity of bone resorption markers. These
results suggest that ALN may serve as an effective agent for the
prevention and treatment of steroid-induced bone deterioration in
patients with ITP scheduled for long-term steroid treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Huzhou
Science and Technology Plan Program (grant no. 2015GYB28).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was designed by XL and ZZ. Literature
research, data acquisition and data analysis were performed by XL,
HZ, LS and XS. The manuscript was prepared and edited by XL, HZ,
LS, XS and ZZ. Specimen collection and ELISA analysis were
performed by XL, HZ, LS and XS. The manuscript was reviewed by XL
and ZZ. All authors approved the final version of the
manuscript.
Ethical approval and consent to
participate
The Ethics Committee of Huzhou Central Hospital
(Huzhou, China) approved the study protocol (approval no.
201401A006). Written informed consent was obtained from all
patients.
Patient consent for publication
All patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sciascia S, Mompean E, Radin M, Roccatello
D and Cuadrado MJ: Rate of adverse effects of medium- to high-dose
glucocorticoid therapy in systemic lupus erythematosus: A
systematic review of randomized control trials. Clin Drug Investig.
37:519–524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Safy M, de Hair MJH, Jacobs JWG,
Buttgereit F, Kraan MC and van Laar JM: Efficacy and safety of
selective glucocorticoid receptor modulators in comparison to
glucocorticoids in arthritis, a systematic review. PLoS One.
12:e01888102017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodeghiero F and Ruggeri M: ITP and
international guidelines: What do we know, what do we need? Presse
Med. 43:e61–e67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rentero ML, Amigo E, Chozas N, Fernández
Prada M, Silva-Fernández L, Abad Hernandez MA, Rodriguez Barrera JM
and del Pino-Montes J; GHDP study group, : Prevalence of fractures
in women with rheumatoid arthritis and/or systemic lupus
erythematosus on chronic glucocorticoid therapy. BMC Musculoskelet
Disord. 16:3002015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson DE, Dennison EM, Cooper C, van
Staa TP and Dixon WG: A review of the methods used to define
glucocorticoid exposure and risk attribution when investigating the
risk of fracture in a rheumatoid arthritis population. Bone.
90:107–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rizzoli R and Biver E:
Glucocorticoid-induced osteoporosis: Who to treat with what agent?
Nat Rev Rheumatol. 11:98–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Compston J: Glucocorticoid-induced
osteoporosis: An update. Endocrine. 61:7–16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanis JA, Johansson H, Oden A, Johnell O,
de Laet C, Melton III LJ, Tenenhouse A, Reeve J, Silman AJ, Pols
HA, et al: A meta-analysis of prior corticosteroid use and fracture
risk. J Bone Miner Res. 19:893–899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugiura T, Yamamoto K, Murakami K, Horita
S, Matsusue Y, Nakashima C and Kirita T: Immune thrombocytopenic
purpura detected with oral Hemorrhage: A case report. J Dent
(Shiraz). 19:159–163. 2018.PubMed/NCBI
|
|
10
|
Neunert C, Lim W, Crowther M, Cohen A,
Solberg L Jr and Crowther MA; American Society of Hematology, : The
American Society of Hematology 2011 evidence-based practice
guideline for immune thrombocytopenia. Blood. 117:4190–4207. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Provan D, Stasi R, Newland AC, Blanchette
VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB,
Godeau B, et al: International consensus report on the
investigation and management of primary immune thrombocytopenia.
Blood. 115:168–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nomura S, Kurata Y, Tomiyama Y, Takubo T,
Hasegawa M, Saigo K, Nishikawa M, Higasa S, Maeda Y and Hayashi K:
Effects of bisphosphonate administration on the bone mass in immune
thrombocytopenic purpura patients under treatment with steroids.
Clin Appl Thromb Hemost. 16:622–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sambrook PN, Kotowicz M, Nash P, Styles
CB, Naganathan V, Henderson-Briffa KN, Eisman JA and Nicholson GC:
Prevention and treatment of glucocorticoid-induced osteoporosis: A
comparison of calcitriol, vitamin D plus calcium, and alendronate
plus calcium. J Bone Miner Res. 18:919–924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cesareo R, Di Stasio E, Vescini F,
Campagna G, Cianni R, Pasqualini V, Romitelli F, Grimaldi F,
Manfrini S and Palermo A: Effects of alendronate and vitamin D in
patients with normocalcemic primary hyperparathyroidism. Osteoporos
Int. 26:1295–1302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li G, Lv CA, Tian L, Jin LJ and Zhao W: A
retrospective study of alendronate for the treatment of ankylosing
spondylitis. Medicine (Baltimore). 97:e107382018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Millonig G, Graziadei IW, Eichler D,
Pfeiffer KP, Finkenstedt G, Muehllechner P, Koenigsrainer A,
Margreiter R and Vogel W: Alendronate in combination with calcium
and vitamin D prevents bone loss after orthotopic liver
transplantation: A prospective single-center study. Liver Transpl.
11:960–966. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shapses SA, Kendler DL, Robson R, Hansen
KE, Sherrell RM, Field MP, Woolf E, Berd Y, Mantz AM and Santora AC
II: Effect of alendronate and vitamin D3 on fractional
calcium absorption in a double-blind, randomized,
placebo-controlled trial in postmenopausal osteoporotic women. J
Bone Miner Res. 26:1836–1844. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siris ES, Adler R, Bilezikian J, Bolognese
M, Dawson-Hughes B, Favus MJ, Harris ST, Jan de Beur SM, Khosla S,
Lane NE, et al: The clinical diagnosis of osteoporosis: A position
statement from the National Bone Health Alliance Working Group.
Osteoporos Int. 25:1439–1443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasegawa M, Nisikawa M, Nomura S, Takubo
T, Suehiro A, Saigo K, Hayashi K, Tamaki S, Mizutani M, Uemura Y
and Kurata Y; Kinki ITP Cooperative Study Group, : Effect of
glucocorticoids on bone mineral density in patients with idiopathic
thrombocytopenic purpura. Rinsho Ketsueki. 46:121–126. 2005.(In
Japanese). PubMed/NCBI
|
|
20
|
Liao EY, Zhang ZL, Xia WB, Lin H, Cheng Q,
Wang L, Hao YQ, Chen DC, Tang H, Peng YD, et al: Calcifediol
(25-hydroxyvitamin D) improvement and calcium-phosphate metabolism
of alendronate sodium/vitamin D3 combination in Chinese
women with postmenopausal osteoporosis: A post hoc efficacy
analysis and safety reappraisal. BMC Musculoskelet Disord.
19:2102018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Tian JH, He ZY, Tang XL and Yang
KH: A Meta-analysis of alendronate for the prevention and treatment
of glucocorticoid-induced osteoporosis. Zhonghua Nei Ke Za Zhi.
52:838–843. 2013.(In Chinese). PubMed/NCBI
|
|
22
|
D'Amelio P, Grimaldi A, Cristofaro MA,
Ravazzoli M, Molinatti PA, Pescarmona GP and Isaia GC: Alendronate
reduces osteoclast precursors in osteoporosis. Osteoporos Int.
21:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bradaschia-Correa V, Moreira MM and
Arana-Chavez VE: Reduced RANKL expression impedes osteoclast
activation and tooth eruption in alendronate-treated rats. Cell
Tissue Res. 353:79–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martins CA, Leyhausen G, Volk J and
Geurtsen W: Effects of alendronate on osteoclast formation and
activity in vitro. J Endod. 41:45–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Q, Badell IR, Schwarz EM, Boulukos
KE, Yao Z, Boyce BF and Xing L: Tumor necrosis factor prevents
alendronate-induced osteoclast apoptosis in vivo by stimulating
Bcl-xL expression through Ets-2. Arthritis Rheum. 52:2708–2718.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siebelt M, Waarsing JH, Groen HC, Müller
C, Koelewijn SJ, de Blois E, Verhaar JA, de Jong M and Weinans H:
Inhibited osteoclastic bone resorption through alendronate
treatment in rats reduces severe osteoarthritis progression. Bone.
66:163–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iseri K, Iyoda M, Watanabe M, Matsumoto K,
Sanada D, Inoue T, Tachibana S and Shibata T: The effects of
denosumab and alendronate on glucocorticoid-induced osteoporosis in
patients with glomerular disease: A randomized, controlled trial.
PLoS One. 13:e01938462018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boyce AM, Kelly MH, Brillante BA, Kushner
H, Wientroub S, Riminucci M, Bianco P, Robey PG and Collins MT: A
randomized, double blind, placebo-controlled trial of alendronate
treatment for fibrous dysplasia of bone. J Clin Endocrinol Metab.
99:4133–4140. 2014. View Article : Google Scholar : PubMed/NCBI
|