Introduction
The immune system of a child has a degree of
immaturity that is maintained from the time the child is a new-born
until 6–7 years of age. This immaturity may be due to age-related
functional disorders in the immune response (1). Therefore, the child's immune response
is considered ‘hypo-inflammatory’ (2). Throughout this period, a healthy child
can contract a series of infections, which contribute to the
strengthening of the immune system, expanding immunological memory
and increasing the immune response. These conditions contribute to
the maturation of the immune system during the pre-pubertal period.
If repeated or prolonged infections with significant clinical
expression (pulmonary and cutaneous) and possible complications,
occur during childhood, the presence of a humoral or cellular
immunodeficiency (ID) should then be considered (3).
Generally, IDs can be classified in quantitative ID,
when the components of the immune system are numerically
diminished, or functional ID (FID) when, although cellular elements
are numerical within normal limits, cellular activation mechanisms
are disrupted, clonal expansion is defective, and/or intercellular
cooperation and effector mechanisms are deficient. The quantitative
ID (primary or secondary) consists of diminishing the immune
response efficiency caused by the absence of any major components
of the immune system: Lymphocytes, phagocytes, immunoglobulins, and
complement system elements (4). The
consequence of ID is the impossibility of providing a full defence
of the organism against infectious agents.
Much more frequent than primary ID (PID) are
recurrent infections (RIs). These are less severe, occurring in the
absence of PID, under the conditions of an immune system with no
apparent major defects (5). This
requires finding the causes that may produce functional imbalances
in the immune system or conditions that induce a secondary or
acquired form of ID. Sometimes, a FID may be suspected based on
minor symptoms related to the respiratory system (oro-pharyngeal)
or the appearance of the skin symptoms (allergic syndromes, minor
infections, and mycosis). RIs are the most frequent expression of
FID, and a persistent and repeated infectious syndrome can generate
changes or delays in the overall development of the child. The
causes of this pathology are multiple, being congenital or
acquired, but also at the local or general level.
Immune deficiencies are considered as underlying
conditions predisposing to RIs (6).
Taking into account that a child's immune system is still
developing until the age of 12–14 years, and that infections can
halt this development, it is crucial to identify the weak links of
anti-infective defence in children and, if possible, to correct
them. Sometimes, the maturation of the immune system corrects some
functional deficiencies; however, if they are not corrected
spontaneously, a diagnosis and an appropriate therapy are
required.
Respiratory RIs (RRIs) are more common in children
and frequently involve the upper respiratory tract (7). The number of infections per year also
depends on the exposure to pathogens, living standards, day-care
attendance, complications, the geographical area and second-hand
smoke (8). According to data of the
World Health Organization (WHO), a child can present with 4 to 8
episodes of RIs within a year, during the first 5 years of its life
(9). The average duration of
infection is 8 days and up to 2 weeks; if a child presents with 3
episodes of acute infections over a period of 6 months, the
respiratory infections are then considered recurrent (6).
As RRIs (in the absence of PID) may be associated
with an altered cellular immune response, we it can be considered
that in such situations, an extended immunophenotyping with the
investigation of immunological parameters, such as T and B cell
subtypes, is useful.
In this study, we investigated a group of children
with RRIs (in the absence of PID) positive for respiratory
syncytial virus (RSV) to observe the immunological changes that
could lead to this clinical syndrome. The serum levels of
immunoglobulins (IgGs, IgA, IgM) were examined and lymphocyte
immunophenotyping was performed. The currently investigated T, B
and natural killer (NK) lymphocyte populations and subpopulations
were completed with some subsets not usually quantified:
T-double-negative (T-DN) cells [cluster of differentiation
(CD)45+CD3+CD1d−CD4−CD8−],
mature/naive B cells
(CD45+CD19+CD20+CD27−,
IgD+), memory B cells
(CD45+CD19+CD20+CD27+),
switched memory B cells IgD−IgM−,
non-switched memory B cells IgM/IgD+, non-switched
IgD+IgM+ memory B cells, plasmablasts/plasma
cells
(CD45+CD19+CD10−CD27+CD38bright),
NKT cells
(CD45+CD3+CD16+CD56+CD1d+).
This comprehensive cellular investigation could complete the
clinical diagnosis and may further guide treatment.
Materials and methods
Patients
A group of 48 children diagnosed with RRIs, having
at least 6 episodes of respiratory infections during a year were
considered. The diagnosis was established at ‘Grigore Alexandrescu’
Children's Emergency Clinical Hospital in Bucharest, Romania based
on a clinical evaluation and the patient's history. The target
group selection was made depending on the presence of IgG
antibodies against RSV in serum (ELISA quantitative determination
of serum IgG antibodies against RSV–IBL International GmbH). At the
time of testing, the children had surpassed the acute respiratory
episode and no therapy was administered at least 2 weeks before
actual testing.
In order to evaluate the peripheral blood (PB)
lymphocytes, 2 groups of children were considered: i) the RRI
group, which included 30 children (19 boys and 11 girls, aged 1–7
years) presenting a positive titre of anti-RSV IgG; and ii) the
control group, which included 10 healthy children, with a normal
nutritional status, with no fever, no history of the use of
medications, no immunizations during the past 4 weeks, and no
evidence of infectious diseases and haematological or immunological
disorders. Subjects with atopic disorders were excluded. For all
patients and the control group, an informed consent was signed from
each subject's legally authorized representative. The study was
approved by the Ethics Committee from Victor Babes National
Institute (no. 17/17.05.2016). Subjects were recruited regardless
of race or ethnic background, and their home status was from a
medium to high family income.
Blood sampling
Peripheral blood samples from the RRI and control
group were collected by venipuncture during the morning hours in
K2-EDTA coated tubes and in blood clot activator tubes (Vacutest
Kima). Blood collection was carried out at the ‘Grigore
Alexandrescu’ Children's Emergency Clinical Hospital. Serum
samples, separated by centrifugation (1,500 × g, 10 min, room
temperature) within 4 h of blood collection, were used for
quantitative determination of immunoglobulins. Peripheral blood
samples collected in K2-EDTA coated tubes were stained for flow
cytometry as described below on the day of blood collection.
Nephelometry
Serum levels of immunoglobulins were determined by
nephelometry (Minineph). The determination of the immunoglobulin
concentration by nephelometry involved an antigen-antibody reaction
in order to form insoluble immune complexes. Briefly, the principle
of the test is that when a luminous ray is passing through the
formed suspension, the dispersed fraction can be measured by a
photodiode. The degree of light scattering is directly proportional
to the concentration of the protein in the sample. Concentrations
are automatically calculated by reference to a calibration curve
stored in the device memory. As quality control, we used high and
low controls provided with the kits. The levels of IgG (Minineph
Human IgG kit), IgA (Minineph Human IgA kit) and IgM (Minineph
Human IgM kit) were quantified for all the participants included in
this study.
Flow cytometric analysis
[fluorescence-activated cell sorting (FACs) analysis]
The immunophenotyping of PB lymphocytes was
performed by flow cytometry with an 8-color system setup, based on
the expression of surface markers. As negative controls, we used
unlabelled cells and the compensation of a fluorescence signal
overlapping was performed (Anti-Mouse Ig, κ/Negative Control (FBS)
Compensation Particles Set, BD Biosciences). Data acquisition and
analysis were performed on a BD FACSCanto II cytometer with BD
FACSDiva v.6.1 software (BD Biosciences). Cytometer performances
were checked properly using CST beads (BD Cytometer Setup &
Tracking Beads kit; BD Biosciences).
EDTA-anticoagulated whole blood samples were split
into 2 different custom panels: The T- and NK-panel, in order to
identify T-cell subsets and NK cells, respectively B-panel, for
B-cell subsets. For each panel, 100 µl whole blood was incubated
with specific monoclonal antibodies for the targeted cell
populations, for 20 min at room temperature in the dark. Surface
staining was followed by red blood cell lysis with BD FACS Lysing
Solution (BD Biosciences) for 10 min at room temperature in the
dark and spun down for 5 min at 350 × g and room temperature. The
cells were washed twice with Cell Staining Buffer (BioLegend) and
analysed by flow cytometry.
For the T- and NK-panel, the following monoclonal
antibodies conjugated with fluorochromes were used: mouse
anti-human CD3-FITC (isotype IgG1, clone UCHT1, cat. no.
1F-514-T025, 20 µl/100 µl whole blood), mouse anti-human CD16-PE
(isotype IgG1, clone 3G8, cat. no. 1P-646-T025, 20 µl/100 µl whole
blood), mouse anti-human CD56-PE (isotype IgG2a, clone LT56, cat.
no. 1P-789-T025, 10 µl/100 µl whole blood), mouse anti-human
CD4-PE-Cy7 (isotype IgG1, clone MEM-241, cat. no. T7-359-T025, 4
µl/100 µl whole blood) and mouse anti-human CD8- allophycocyanin
(APC)-Cy7 (isotype IgG2a, clone MEM-31, cat. no. T4-207-T025, 4
µl/100 µl whole blood) (Exbio), mouse anti-human CD45-PerCP-Cy5.5
(isotype IgG1, clone HI30, cat. no. 564106, 5 µl/100 µl whole
blood, BD Pharmingen-BD Biosciences) and mouse anti-human
CD1d-Brilliant Violet 510 (isotype IgG2b κ, clone 51.1, cat. no.
350314, 5 µl/100 µl whole blood, BioLegend). The defined
populations were as follows: T-CD3+ lymphocytes
(CD45+CD3+) and T-CD4+
(CD45+CD3+CD4+), T-CD8+
(CD45+CD3+CD8+) and T-double
negative (T-DN;
CD45+CD3+CD1d−CD4−CD8−)
subsets, NK cells
(CD45+CD3−CD16+CD56+)
and NKT cells
(CD45+CD3+CD16+CD56+CD1d+).
For the B-panel, the following monoclonal antibodies
conjugated with fluorochromes were used: Mouse anti-human IgD-FITC
(isotype IgG2b κ, clone IA6-2, cat. no. 348205, 5 µl/100 µl whole
blood), mouse anti-human CD38-PE (isotype IgG1 κ, clone HIT2, cat.
no. 303505, 5 µl/100 µl whole blood), mouse anti-human CD10-PE-Cy7
(isotype IgG1 κ, clone HI10a, cat. no. 312213, 5 µl/100 µl whole
blood) and mouse anti-human CD19-PerCP-Cy5.5 (isotype IgG1 κ, clone
HIB19, cat. no. 302229, 5 µl/100 µl whole blood) (BioLegend), mouse
anti-human IgM-APC (isotype IgG1, clone CH2, cat. no. 1A-320-C025,
10 µl/100 µl whole blood EXBIO, Praha), mouse anti-human
CD20-APC-H7 (isotype IgG2b κ, clone 2H7, cat. no. 560734, 5 µl/100
µl whole blood), mouse anti-human CD27-Brilliant Violet 421
(isotype IgG1 κ, clone M-T271, cat. no. 562514, 5 µl/100 µl whole
blood) and mouse anti-human CD45-Brilliant Violet 510 (isotype IgG1
κ, clone HI30, cat. no. 563204, 5 µl/100 µl whole blood) (BD
Biosciences). The following subpopulations of B cells were gated: B
cells (CD45+CD19+ and
CD45+CD19+CD20+), mature/naive B
cells
(CD45+CD19+CD20+CD27−,
IgD+), memory B cells
(CD45+CD19+CD20+CD27+),
switched memory B cells IgD−IgM−,
non-switched memory B cells IgM/IgD+, non-switched
IgD+IgM+ memory B cells, plasmablasts/plasma
cells
(CD45+CD19+CD10−CD27+CD38bright).
Statistical analysis
Data were analysed using Microsoft Excel
(Microsoft). The results are presented as the means ± SD of the
cell percentage. The Student's t-test (two-tailed, assuming unequal
variance) was used for the statistical evaluation of the
differences between the experimental groups. The threshold of
significance was set at a P-value <0.05.
Results
Serum levels of immunoglobulins
The serum immunoglobulin values in the children with
RRIs were normal in 70% of the cases; 30% of the cases exhibited a
decreases or increase in the immunoglobulin levels (data not
shown).
Immunophenotyping of PB
lymphocytes
In order to assess the immune cell status in
children with RRIs, different subtypes of circulating T and B
lymphocytes were analysed by flow cytometry, using two different
custom panels: The T and NK-panel, to identify T-cell subsets and
NK cells, respectively and B-panel for B-cell subsets. The results
are presented below:
i) T cell subsets and NK cells
The percentages of PB T cell subsets and NK cells (T
cells, T-CD4+ cells, T-CD8+ cells, T-DN
cells, NK cells and NKT cells) for 30 children with RRIs were
compared with the data obtained from 10 healthy children (control
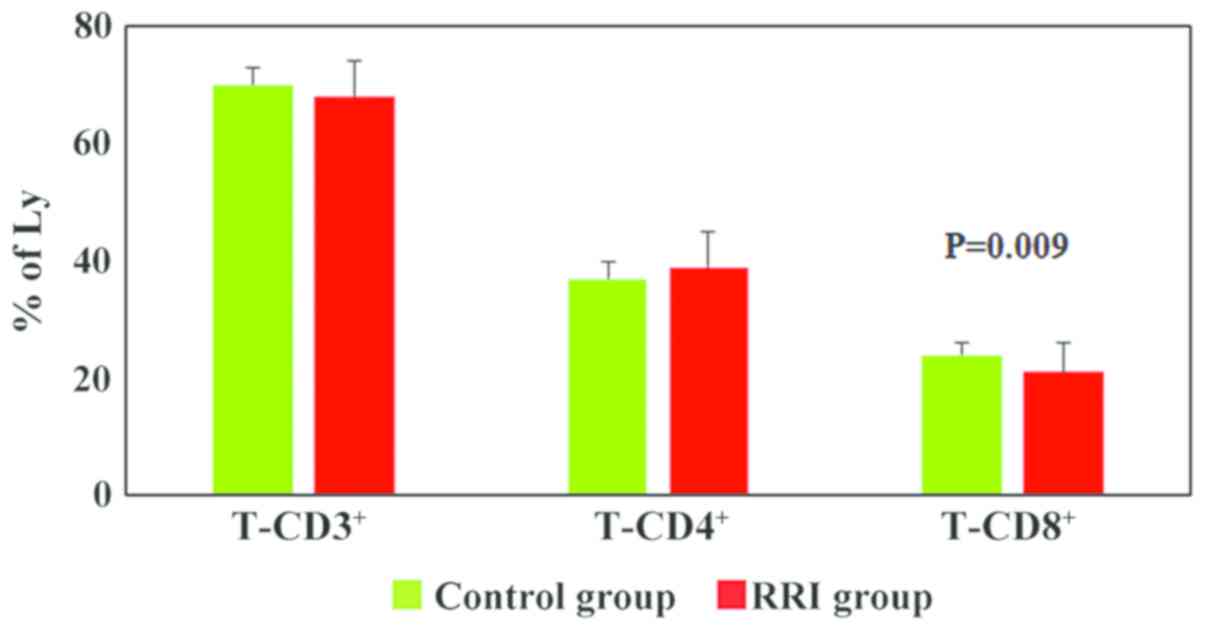
group). The analysis of T cell subpopulations revealed a
significant decrease in the T-CD8+ lymphocytes percentages
(P=0.009) in children with RRIs (21±5% cells as compared to 24±2%
in the control group). The percentages of T-CD3+ and
T-CD4+ lymphocytes were similar between the patients and
the control group (Fig. 1).
Most of the children with RRIs exhibited changes in
the relative number of T lymphocytes. Thus, a significant number of
children (67%) exhibited a decrease in the numbers of
T-CD8+ lymphocytes; 47% of the cases presented an
increased value for T-CD4+ lymphocytes, and 40% of the
children with RRIs presented a decreased percentage of total
T-CD3+ lymphocytes (Table
I).
 | Table I.Incidence of T cell subsets and NK
cells deregulations in the group diagnosed with RRIs. |
Table I.
Incidence of T cell subsets and NK
cells deregulations in the group diagnosed with RRIs.
|
| Incidence in the
RRI group (%) |
|---|
|
|
|
|---|
| Parameter | Decreased values
(%) | Increased values
(%) |
|---|
| T cells
(CD45+CD3+) | 40 | 13 |
| T-CD4+
cells (CD45+CD3+CD4+) | 17 | 47 |
| T-CD8+
cells (CD45+CD3+CD8+) | 67 | 10 |
| T-DN cells
(CD45+CD3+CD1d−CD4−CD8−) | 0 | 23 |
|
T-CD4+/T-CD8+ | 10 | 63 |
| NK cells
(CD45+CD3−CD16+CD56+) | 7 | 57 |
| NKT cells
(CD45+CD3+CD16+CD56+CD1d+) | 0 | 0 |
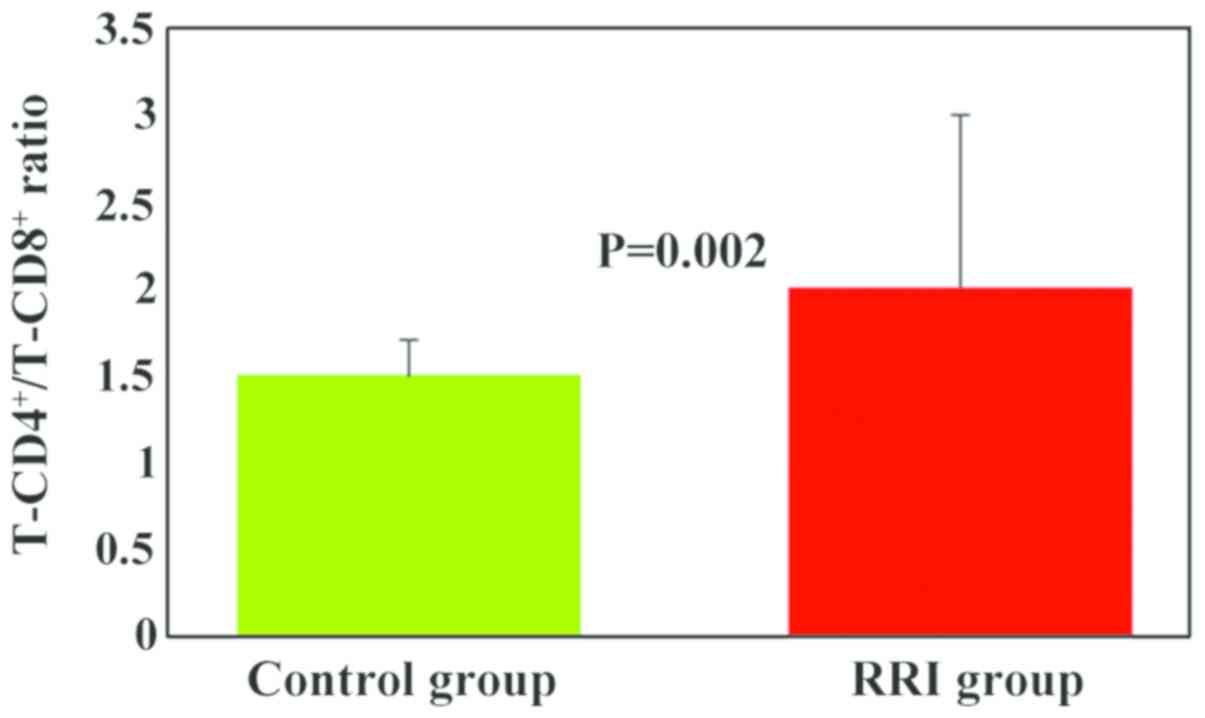
The T-CD4+/T-CD8+ ratio was
higher in the RRI group (2±1) as compared to the control group
(1.5±0.2), and the differences between the experimental groups were
statistically significant (P=0.002; Fig.
2). A significant number of children with RRIs (63%) exhibited
an increased value for the T-CD4+/T-CD8+
ratio (Table I).
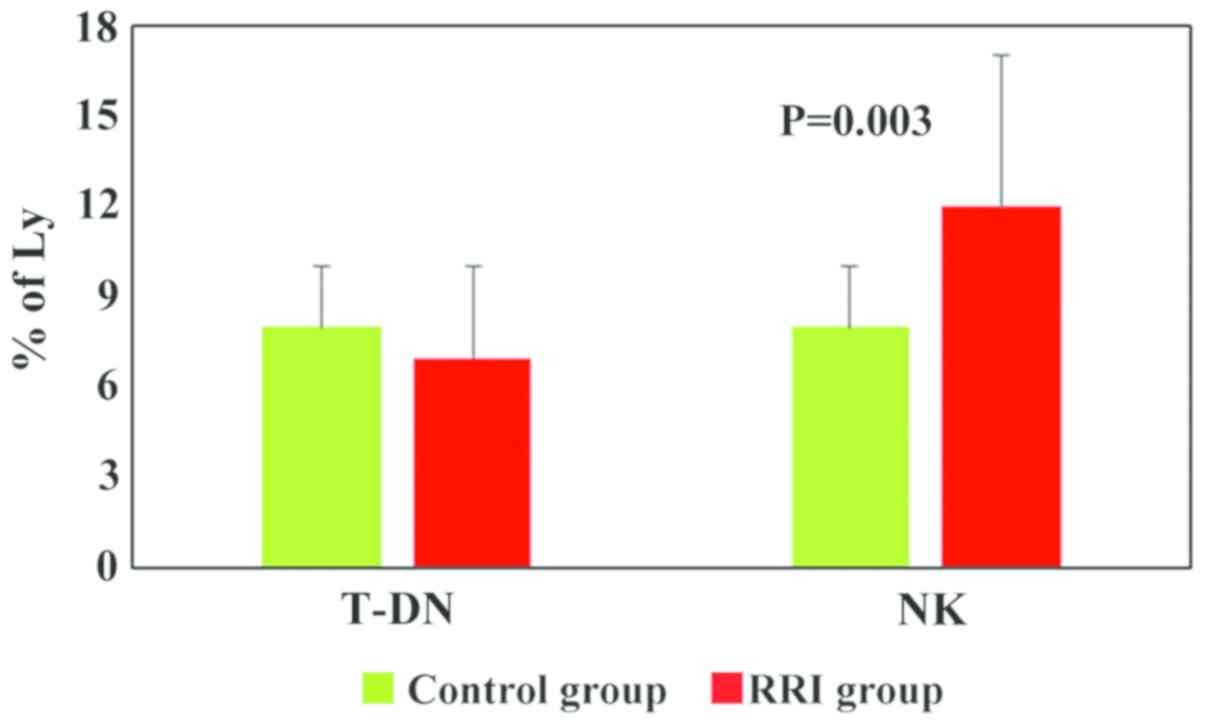
The values obtained for T-DN for both experimental
groups were within normal limits, only 23% of RRI children had an
increased value (>10) (Fig. 3 and
Table I). For all the lymphocyte
subpopulations described above, the ranges obtained for the control
group were similar with the normal values described in the
literature (10). Only for the NK
cells, the range of normal values (6–10%) was below the values
described in the literature (8–15%). Although the NK cell values
were significantly higher in the RRI group (12±5, P=0.003) as
compared to the healthy children (8±2), and 57% of children with
RRIs exhibited increases, these values were within the normal range
for NK cells (Fig. 3 and Table I) (10). NKT cell analysis (% of lymphocytes)
revealed very low values for both groups (0.01±0.02 for RRI group
and 0.01±0.01 for control group) (graph not shown).
In terms of overall T and NK circulatory
populations, in Table I, the
incidence of peripheral deregulations is presented in the group of
children with RRIs. It can be easily seen that most of the
decreased values were registered in the T-CD8+
subpopulation, followed by the total T-CD3+ population.
As regards the incidence of increased values, the highest incidence
was observed for the T-CD4+ subpopulation. The
consequence is that the T-CD4+/T-CD8+ ratio
was increased in the majority of the children tested.
ii) B cell subsets
Peripheral blood B cell subsets (total B cells,
mature/naive B cells, memory B cells, switched memory B cells
IgD−IgM−, non-switched memory B cells
IgM/IgD+, non-switched IgD+IgM+
memory B cells, plasmablasts/plasma cells) were evaluated for 30
children with RRIs and the obtained data were compared with the
children in the control group.
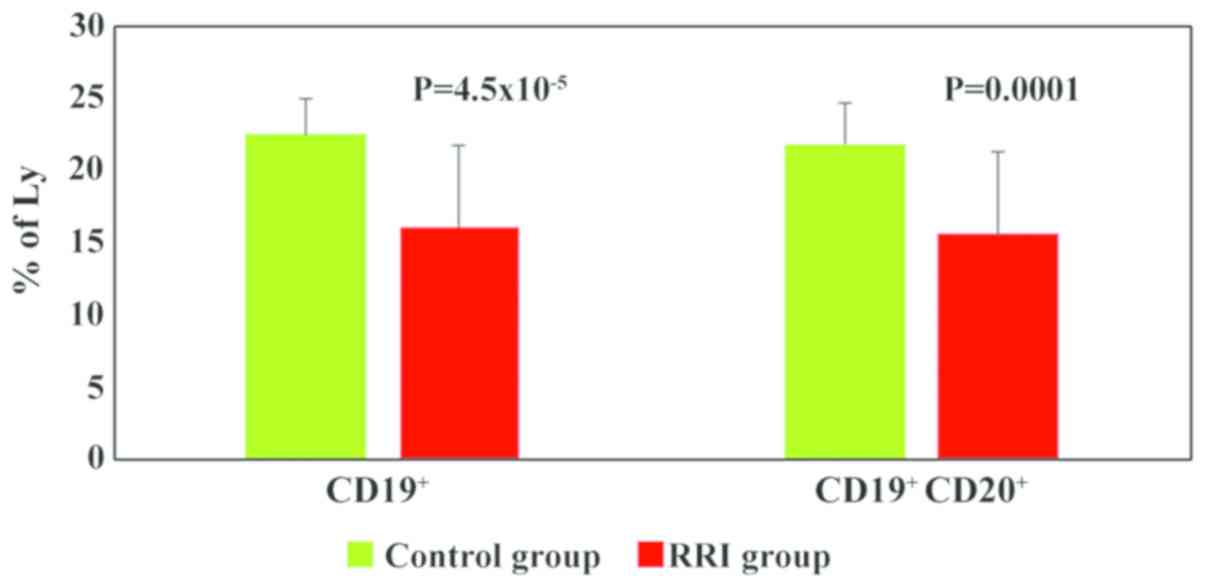
The main change observed in panel B analysis was the
decrease in the total B cell population percentages in most of the
children with RRIs (86%) (Table
II). The mean values of total B cells were significantly lower
in the children with RRIs (16.14±6, P=4.5×10−5) as
compared to the control group (22.56±2) (Fig. 4).
 | Table II.Incidence of B cell subsets
deregulations in the group diagnosed with RRIs. |
Table II.
Incidence of B cell subsets
deregulations in the group diagnosed with RRIs.
| Parameter | Decreased values
(%) | Increased values
(%) |
|---|
| B cells
(CD45+CD19+ and
CD45+CD19+CD20+) | 86 | 7 |
| Mature/naive B
cells
(CD45+CD19+CD20+CD27−IgD+) | 61 | 18 |
| Memory B cells
(CD45+CD19+CD20+CD27+) | 21 | 50 |
| Non-switched
IgD+IgM+ memory B cells | 14 | 43 |
| Non-switched memory
B cells IgM/IgD+ | 18 | 39 |
| Switched memory B
cells IgD−IgM− | 25 | 29 |
| Plasma cells
(CD45+CD19+CD10−CD27+CD38bright) | 0 | 7 |
The PB total B lymphocytes were divided into 2
subsets based on CD27 expression: Mature/naive B cells
(CD27−) and memory B cells (CD27+). Both
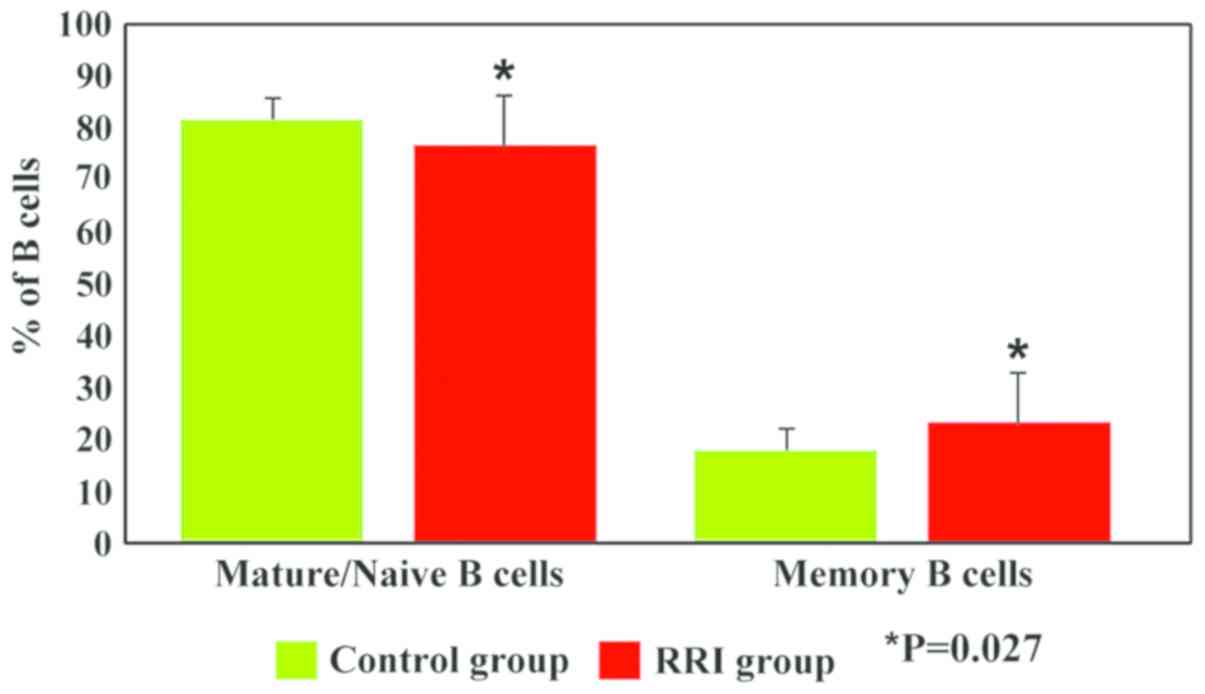
subsets exhibited significant differences (P=0.027) between the
children with RRIs and the controls. Thus, our data revealed a
decrease in the percentage of mature/naive B cells (76.8±10 vs.
81.95±4, P=0.027) and an increase in the percentage of memory B
cells (23.2±10 vs. 18.05±4, P=0.027) in children with RRIs as
compared to the control group (Fig.
5).
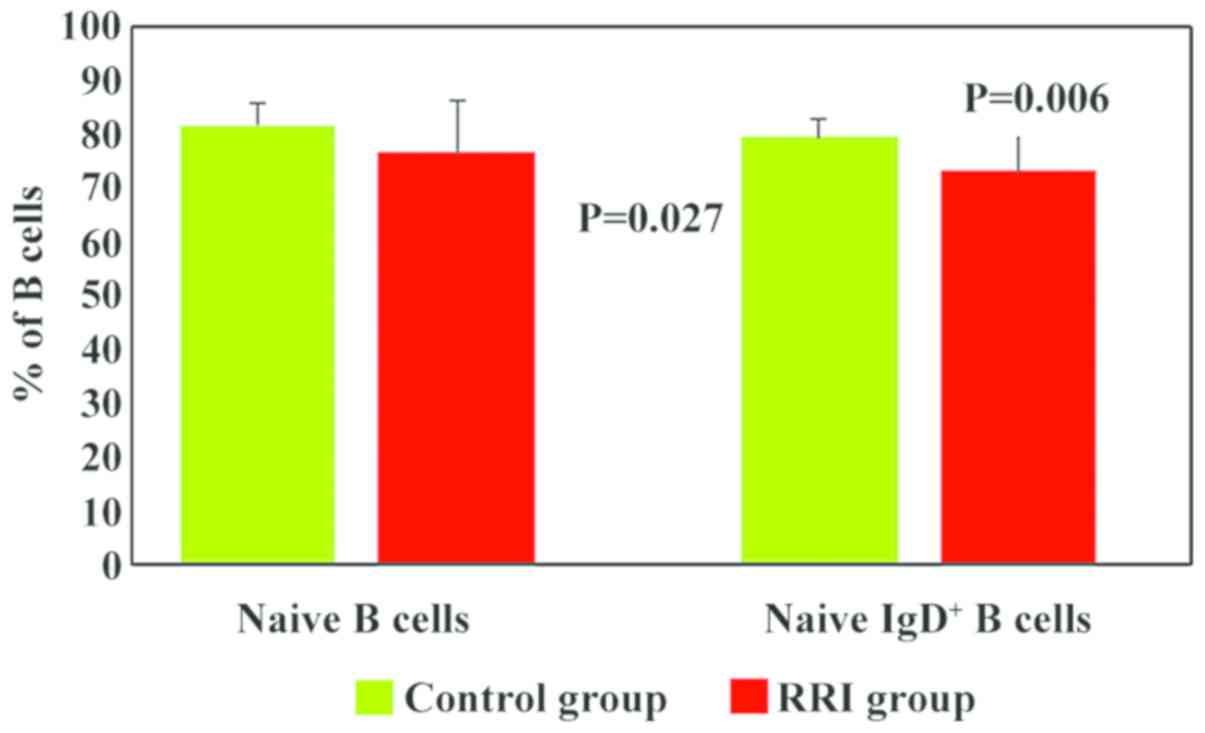
Mature/naive B cells were analysed based on the
expression of IgD. In the children with RRIs, the numbers of naive
IgD+ B cells were significantly lower (73.3±10, P=0.006)
than those in the control group (79.59±4) (Fig. 6). In addition, 61% of the children
with RRIs exhibited low values for naive IgD+ B cells
(Table II). We hypothesised that
the decrease in the numbers of total B lymphocytes was mainly due
to the decrease in mature/naive IgD+ B lymphocytes.
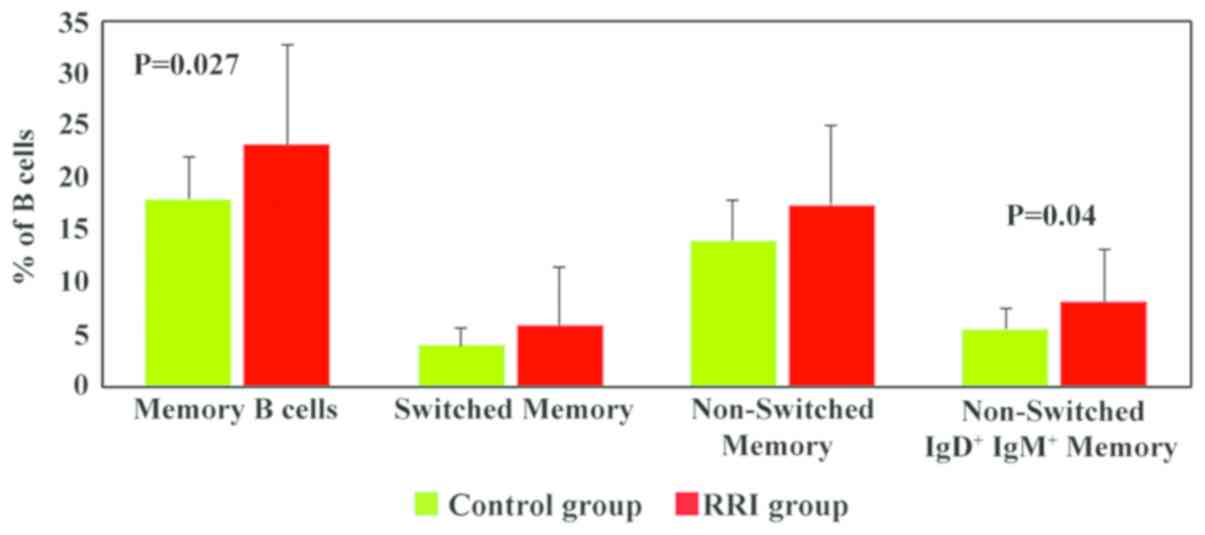
Memory B cells were analysed according to the
expression of IgD and IgM and divided into switched memory B cells
(IgD−IgM−), non-switched memory B cells
(IgM/IgD+) and non-switched
IgD+IgM+ memory B cells (Fig. 7). The numbers of memory B cells were
significantly increased in the RRI group (23.2±10 vs. 18.05±4 in
healthy children, P=0.027; Fig. 7).
All memory B cell subsets were increased in the children with RRIs;
however, statistically significant differences between the RRI
group and the control group were only observed for non-switched
IgD+IgM+ memory B cells (P=0.04).
Plasmablasts/plasma cells represent the most mature
subset of memory switched B cells. The values obtained for
plasmablasts/plasma cells were very small and there are no
differences between the 2 experimental groups (2.61±2 in RRI
children, 2.99±2 in the control group) (graph not shown).
In terms of overall B circulatory populations, in
Table II, the incidence of
peripheral deregulations is presented in the group of children with
RRIs. The results revealed that a great majority of the children
had decreased circulatory levels of B lymphocytes, this decrease
being based on the actually mature B cells. The equilibrium is set
by the fact that 50% of the children RRIs had high values of memory
B cells.
Discussion
In this study, children with confirmed with
respiratory infections with RSV were selected for the
identification of circulatory cellular immune populations in order
to depict an altered cellular immune response. In such cases, the
investigation of immunological parameters, such as T and B cell
subtypes may guide the treatment strategy.
The serum levels of immunoglobulins are generally
considered to be an indicator of B cell functionality and the
analysis of immunoglobulins is a screening test for the evaluation
of the humoral immunity. In this study, serum immunoglobulin values
in the children with RRIs tested were normal in 70% of cases and
30% of the cases exhibited decreases or increases in immunoglobulin
levels. These tests did not suggest changes in order to justify the
diagnosis of immunological recurrent infectious syndrome.
Considering clinical syndrome in children with normal
immunoglobulin values, it was necessary to complete the humoral
investigation with cellular tests. In order to assess the immune
cell status in children with RRIs, different subtypes of
circulating T and B lymphocytes were analysed by flow cytometry,
using two different custom panels: The T and NK-panel, to identify
T-cell subsets and respectively NK cells, and B-panel for B-cell
subsets.
The analysis of T cell subpopulations (T and
NK-panel) revealed a significant decrease in the T-CD8+
lymphocytes percentages (P=0.009) in children with RRIs, while the
percentages of T-CD3+ and T-CD4+ lymphocytes
were comparable in both experimental groups (patients and control
group). Most of the children with RRIs exhibited changes in the
relative number of T lymphocytes. Thus, a significant number of
children (67%) exhibited decreases in the numbers of
T-CD8+ lymphocytes; 47% of cases presented an increased
value for T-CD4+ lymphocytes, and 40% of children with
RRIs presented a decreased percentage of total T-CD3+
lymphocytes (Table I). Taking into
account that T-CD8+ lymphocytes are involved in
antiviral immune response, it can be presumed that there is a
decrease in the antiviral response in RRI children proven by the
reduced T-CD8+ mean values. In addition, there was also
an increase in T-CD4+ mean values as compared to the
control group, with no statistical significance, which suggests the
stimulation of this lymphocyte subpopulation during viral
respiratory infections.
The T-CD4+/T-CD8+ ratio was
significantly higher in the RRI group as compared to the control
group (P=0.002). A significant number of RRI children (63%)
exhibited an increased value for the
T-CD4+/T-CD8+ ratio (Table I). Increased values of the
T-CD4+/T-CD8+ ratio are explained by the
values obtained for each cell subpopulation (high values for
T-CD4+ in 47% of cases, low values for T-CD8+
in 67% of cases). This increased ratio reveals the tendency of the
cellular immune response to immuno-stimulation due to the presence
of infectious syndrome. Immuno-stimulation is supported by the
T-CD4+ subpopulation even in the case where
T-CD8+ is diminished.
T-DN lymphocytes are probably derived either from
double-positive T-immature cells (CD4+CD8+)
or mono-positive (CD4+CD8−,
CD4−CD8+) (13) and it has been shown that substantial
proportion of T-DN appears in the inflammatory response to
syncytial virus infection (11).
These cells appear to be involved in inflammation and autoimmune
diseases having a regulatory character (12), while their participation in
infectious syndromes has not yet been clarified. T-DN cells could
represent a complementary functional reserve for T-lymphocytes
under conditions of a decrease in the T-CD4+
subpopulation in cellular ID or HIV infection (13). In this study, the values obtained for
T-DN for both experimental groups were within normal limits, and
only 23% of RRI children had an increased value (>10). The
increase in T-DN did not occur in a significant number of children
with RRIs, but only in 23% (Table
I). This was probably due to the fact that there were few cases
where the ‘back-up’ role of T-DN was needed, namely in the cases
presenting with low values of T-CD4+ cells. The
‘reservoir’ T-DN role was met in only 2 cases with low
T-CD4+ numbers, and cannot be considered a rule in this
pathology. The mean values obtained for T-DN cells in both
experimental groups were similar.
The T and NK-panel was also used to quantify the NK
and NKT cells. While NKT cell analysis revealed very low values for
both experimental groups, for NK cells, the obtained values
differed significantly (P=0.003) in the RRI group as compared to
healthy children. Although the NK cell values were significantly
higher in the RRI group as compared to healthy children, and 57% of
RRI children exhibited increases, these values were within the
normal range for NK cells. We could not rule out the possibility
that by increasing the number of infected children, the standard
deviation of the mean would not decrease and hence, the difference
between the controls and ill children would become significantly
different. Although NK cells are involved in the antiviral
response, there are no peripheral NK cell changes in the RRI group,
indicating that this population remains within the normal
established ranges and sustains the antiviral defence.
In addition, peripheral blood B cell subsets,
including total B cells, mature/naive B cells, memory B cells,
switched memory B cells IgD−IgM−,
non-switched memory B cells IgM/IgD+, non-switched
IgD+IgM+ memory B cells and
plasmablasts/plasma cells were evaluated for RRI children and the
obtained data were compared with control group children. For the B
panel, the main change observed was the decrease in the total B
cell population percentages in most of the children with RRIs
(86%). The mean values of total B cells were also significant lower
in the children with RRIs (P=4.5×10−5) as compared to
the control group. Based on CD27 expression, the PB total B
lymphocytes were divided into 2 subsets: Mature/naive B cells
(CD27−) and memory B cells (CD27+). In this
study, both subsets differed significantly (P=0.027) between the
children with RRIs compared to the controls. Thus, our data
revealed a decrease in the percentage of mature/naive B cells
(P=0.027) and an increase in the percentage of memory B cells
(P=0.027) in children with RRIs as compared to the control group.
Mature/naive B cells were analysed based on the expression of IgD.
In the children with RRIs, the numbers of naive IgD+ B
cells were significantly lower (P=0.006) than in those in the
control group. In addition, 61% of the children with RRIs exhibited
low values for naive IgD+ B cells. We hypothesised that
the decrease in the numbers of total B lymphocytes was mainly due
to the decrease in mature/naive IgD+ B lymphocytes.
Memory B cells were analysed according to the
expression of IgD and IgM and divided into switched memory B cells
(IgD−IgM−), non-switched memory B cells
(IgM/IgD+) and non-switched
IgD+IgM+ memory B cells. These cells respond
to the thymus-dependent antigens and will generate long-term
immunological memory. These lymphocytes will circulate in the
peripheral blood regardless of the antigenic stimulation. In the
moment the cells meet their specific antigen, they will proliferate
and differentiate in plasma cells and will secrete high affinity
antibodies (IgG). In this study, memory B cells were significantly
increased in the RRI group (P=0.027). This finding is somewhat to
be expected as the virus is not at the first encounter with the
immune system of the children. All memory B cell subsets were
increased in children with RRIs; however, the results were
statistically significant (P=0.04) only for the non-switched
IgD+IgM+ memory B cells between the RRI group
and the controls. This can be explained by the mobilization in
peripheral circulation of memory B cells during infection, with the
predominance of the non-switched B cells, mainly non-switched
IgD+IgM+ memory B cells (P=0.04).
Plasmablasts/plasma cells represent the most mature
subset of memory switched B cells. The values obtained for this
subset were very small and there were no differences between the
RRI group and the controls. This finding is yet again to be
expected as the immunoglobulins levels are in normal ranges.
There are various reported results in children
infected with syncytial virus. While some reports have shown a Th2
predominance in serum (14–16), others have shown a decrease in the
Th1 response (17). These results in
fact indicate that the Th1/Th2 balance is not a golden standard for
this infection in children (18,19) and
that there are several immune populations involved (20). The findings of this study
demonstrated that, indeed, a decrease in the T-CD8+ and
total B cells percentages was balanced by an increase in
circulating memory B cells. The decrease in total B cells was
mainly due to the decrease in naive IgD+ B cells.
Throughout life, the immune system also evolves.
Human respiratory syncytial virus infections are responsible
worldwide for bronchiolitis, pneumonia and asthma, particularly in
children. Therefore, 70% of children will have been infected in
their first 12 months of life, and at 24 months, all children will
have been infected with this virus (21). Mild upper respiratory tract
symptomatology will be triggered, and around 3% will be rushed to
the ICU, where the mortality rate can reach up to 10% (22,23). As
this poses a serious health concern among small children (24), immunological parameters to follow
this type of infection in children and extended immunophenotyping
with the investigation of immunological parameters, such as T and B
cell subtypes, are mandatory (25).
In this study, we investigated several immune
parameters in a group of children with RRIs positive for RSV in
order to identify the immunological changes that could lead to this
clinical syndrome. We did not find alterations regarding the serum
levels of IgG, IgA, IgM, thus taking into account that these are
actually the routine tests carried out in clinical practice; the
results were not sufficient for an immunodiagnosis, and thus, we
considered that a cellular investigation is also necessary. We
performed several cellular assays regarding peripheral T, B, NK
cells, as well as several subtypes of these cells that are not
currently performed, such as T-DN cells, NKT cells, mature/naive B
cells, memory B cells and plasma cells. As the circulatory
immunoglobulin level was in the normal range, the peripheral blood
immune cells exhibited specific deregulations. Hence, a decrease in
the circulatory T-CD8+ and total B cells percentages and
an increase in memory B cells was observed. The decrease in total B
cells was mainly due to the decrease in naive IgD+ B
cells. It can thus be concluded that in respiratory recurrent
infections, the investigation of immunological parameters, such as
T and B cell subtypes could complete the clinical diagnosis and may
guide the treatment, thus increasing the quality of life of the
patients.
Acknowledgements
The present study will be integrated in the
original part of the PhD thesis of first author and PhD student
ANM.
Funding
This study was supported by the Core Program,
implemented with the support of NASR, projects PN 19.29.01.01;
PN19.29.02.03, Grant PN-III-P1-1.2-PCCDI-2017-0341/2018 and
7PFE/16.102018.
Availability of data and materials
The datasets used and/or analysed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
ANM, MS, RIH, GI, CConstantin, IRP and CUrsaciuc
were responsible for the research creation and design, data
acquisition, analysis and interpretation of the data, statistical
analysis, manuscript drafting, and the critical revision of the
manuscript for important intellectual content. ANM, MS, IRP,
CChifiriuc, CUlmeanu and MN were responsible for the interpretation
of the data, manuscript drafting, and the critical revision of the
manuscript for important intellectual content. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
For all patients and the control group, an informed
consent was signed from each subject's legally authorized
representative. The study was approved by the Ethics Committee from
Victor Babes National Institute (no. 17/17.05.2016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APC
|
allophycocyanin
|
|
CD
|
cluster of differentiation
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FITC
|
fluorescein isothiocyanate
|
|
FID
|
functional immunodeficiency
|
|
ID
|
immunodeficiency
|
|
K2-EDTA
|
kalium 2
ethylenediaminetetraacetate
|
|
NK
|
natural killer cells
|
|
PE
|
phycoerythrin
|
|
PE/Cy
|
phycoerythrin complex with
cyanine
|
|
PerCP/Cy
|
peridinin chlorophyll protein complex
with cyanine
|
|
PB
|
peripheral blood
|
|
PID
|
primary immunodeficiency
|
|
RPMI
|
Roswell Park Memorial Institute
|
|
RRI
|
respiratory recurrent infections
|
|
RSV
|
respiratory syncytial virus
|
|
SD
|
standard deviation
|
References
|
1
|
Gervassi AL and Horton H: Is infant
immunity actively suppressed or immature? Virology (Auckl).
2014:1–9. 2014.PubMed/NCBI
|
|
2
|
Maddux AB and Douglas IS: Is the
developmentally immature immune response in paediatric sepsis a
recapitulation of immune tolerance? Immunology. 145:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feleszko W, Ruszczyński M and Zalewski BM:
Non-specific immune stimulation in respiratory tract infections.
Separating the wheat from the chaff. Paediatr Respir Rev.
15:200–206. 2014.PubMed/NCBI
|
|
4
|
McCusker C and Warrington R: Primary
immunodeficiency. Allergy Asthma Clin Immunol. 7 (Suppl 1):S112011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alkhater SA: Approach to the child with
recurrent infections. J Family Community Med. 16:77–82.
2009.PubMed/NCBI
|
|
6
|
El-Azami-El-Idrissi M, Lakhdar-Idrissi M,
Chaouki S, Atmani S, Bouharrou A and Hida M: Pediatric recurrent
respiratory tract infections: When and how to explore the immune
system? (About 53 cases). Pan Afr Med J. 24:532016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mammas IN, Koutsaftiki C, Nika E, Vagia F,
Zaravinos A, Priftis KN, Voyatzi A, Theodoridou M, Myriokefalitakis
N and Spandidos DA: Detection of human metapneumovirus in infants
with acute respiratory tract infection. Mol Med Rep. 4:267–271.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
American Academy of Pediatrics Committee
on Environmental Health, . Environmental tobacco smoke: A hazard to
children. Pediatrics. 99:639–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grüber C, Keil T, Kulig M, Roll S, Wahn U
and Wahn V; MAS-90 Study Group, : History of respiratory infections
in the first 12 yr among children from a birth cohort. Pediatr
Allergy Immunol. 19:505–512. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Erkeller-Yuksel FM, Deneys V, Yuksel B,
Hannet I, Hulstaert F, Hamilton C, Mackinnon H, Stokes LT,
Munhyeshuli V, Vanlangendonck F, et al: Age-related changes in
human blood lymphocyte subpopulations. J Pediatr. 120:216–222.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson JE, Gonzales RA, Olson SJ, Wright
PF and Graham BS: The histopathology of fatal untreated human
respiratory syncytial virus infection. Mod Pathol. 20:108–119.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hillhouse EE and Lesage S: A comprehensive
review of the phenotype and function of antigen-specific
immunoregulatory double negative T cells. J Autoimmun. 40:58–65.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sundaravaradan V, Mir KD and Sodora DL:
Double-negative T cells during HIV/SIV infections: Potential pinch
hitters in the T-cell lineup. Curr Opin HIV AIDS. 7:164–171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassan MA, Eldin AME and Ahmed MM:
T-helper2 /T-helper1 imbalance in respiratory syncytial virus
bronchiolitis in relation to disease severity and outcome. Egypt J
Immunol. 15:153–160. 2008.PubMed/NCBI
|
|
15
|
Qin L, Peng D, Hu C, Xiang Y, Zhou Y, Tan
Y and Qin X: Differentiation of Th subsets inhibited by
nonstructural proteins of respiratory syncytial virus is mediated
by ubiquitination. PLoS One. 9:e1014692014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dulek DE, Newcomb DC, Toki S, Goliniewska
K, Cephus J, Reiss S, Bates JT, Crowe JE Jr, Boyd KL, Moore ML, et
al: STAT4 deficiency fails to induce lung Th2 or Th17 immunity
following primary or secondary respiratory syncytial virus (RSV)
challenge but enhances the lung RSV-specific CD8+ T cell immune
response to secondary challenge. J Virol. 88:9655–9672. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Legg JP, Hussain IR, Warner JA, Johnston
SL and Warner JO: Type 1 and type 2 cytokine imbalance in acute
respiratory syncytial virus bronchiolitis. Am J Respir Crit Care
Med. 168:633–639. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pinto RA, Arredondo SM, Bono MR, Gaggero
AA and Díaz PV: T helper 1/T helper 2 cytokine imbalance in
respiratory syncytial virus infection is associated with increased
endogenous plasma cortisol. Pediatrics. 117:e878–e886. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Benten IJ, van Drunen CM, Koopman LP,
KleinJan A, van Middelkoop BC, de Waal L, Osterhaus AD, Neijens HJ
and Fokkens WJ: RSV-induced bronchiolitis but not upper respiratory
tract infection is accompanied by an increased nasal IL-18
response. J Med Virol. 71:290–297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mangodt TC, Van Herck MA, Nullens S, Ramet
J, De Dooy JJ, Jorens PG and De Winter BY: The role of Th17 and
Treg responses in the pathogenesis of RSV infection. Pediatr Res.
78:483–491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bueno SM, González PA, Pacheco R, Leiva
ED, Cautivo KM, Tobar HE, Mora JE, Prado CE, Zúñiga JP, Jiménez J,
et al: Host immunity during RSV pathogenesis. Int Immunopharmacol.
8:1320–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McNamara PS and Smyth RL: The pathogenesis
of respiratory syncytial virus disease in childhood. Br Med Bull.
61:13–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hervás D, Reina J, Yañez A, del Valle JM,
Figuerola J and Hervás JA: Epidemiology of hospitalization for
acute bronchiolitis in children: Differences between RSV and
non-RSV bronchiolitis. Eur J Clin Microbiol Infect Dis.
31:1975–1981. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mammas IN, Greenough A, Theodoridou M,
Kramvis A, Rusan M, Melidou A, Korovessi P, Papaioannou G,
Papatheodoropoulou A, Koutsaftiki C, et al: Paediatric Virology and
its interaction between basic science and clinical practice
(Review). Int J Mol Med. 41:1165–1176. 2018.PubMed/NCBI
|
|
25
|
Munteanu A, Surcel M, Constantin C and
Neagu M: Respiratory infection with syncitial virus in children -
immunologicalhighlights. Rev Biol Biomed Sci. (In Press).
|