Introduction
Cytomegalovirus (CMV) is known as a ubiquitous viral
agent that affects 40–100% of adults worldwide (1). Most cases of CMV infections among
immunocompetent individuals are asymptomatic and establish a
long-term latent infection within the hosts (2). However, serious pathological conditions
due to CMV are frequently observed as opportunistic infections in
immunocompromised hosts, such as patients with acquired
immunodeficiency syndrome (AIDS), malignancies, and those receiving
steroids and/or immunosuppressants (3,4).
Recently, a high prevalence of CMV infection of the
colon has been reported in patients with inflammatory bowel disease
(IBD), including Crohn's disease (CD) and ulcerative colitis (UC)
(5–7). Most cases of CMV colitis in patients
with IBD can be attributed to the reactivation of a latent CMV
infection; active colonic infection induced by a primary CMV
infection is rare (5,8,9). In
addition, CMV infection of the colon exacerbates IBD, particularly
in association with severe or refractory cases under steroid and/or
immunosuppressive therapies (5,6,8,10–12). The
use of steroids tends to delay the diagnosis of CMV infection by
masking abdominal symptoms (13,14).
Herein, we present a rare case of acute mononucleosis and colitis
caused by a primary CMV infection. Notably, the features of acute
CMV infection were observed in an immunocompetent patient with UC
without concurrent use of steroids and immunosuppressants.
Case report
Patient information
A 28-year-old man was diagnosed with active UC
(pancolitis type) when he was 20 years old and received remission
induction therapy with oral prednisolone (loading dose, 40 mg/day)
and 5-aminosalicylic acid (5-ASA) (4,000 mg/day) for 8 weeks. Since
then, he had been on oral 5-ASA (4,000 mg/day) treatment for over 7
years. Annual colonoscopy revealed slight inflammation from the
rectum to the sigmoid colon; however, no subjective symptoms were
noted during the course of the treatment. On September 30, 2016, he
developed a headache, rhinorrhea, and myalgia with high-grade fever
(38°C). He was treated with oral acetaminophen (400 mg twice a day)
for 5 days; however, his symptoms did not improve. On October 7,
2016, he presented with bloody diarrhea (5–10 times a day) and
lower abdominal discomfort. In addition, computed tomography showed
an edematous change in the large intestine that progressed from the
rectum to the sigmoid colon. Blood tests also revealed an elevated
inflammatory response, as indicated by an increase in the number of
white blood cells (10,770 cells/µl) and elevated C-reactive protein
level (4.38 mg/dl) and erythrocyte sedimentation rate (19 mm/h). A
slight elevation of serum aspartate aminotransferase (36 U/l) was
observed, but clinically evident liver dysfunction and
thrombocytopenia were not. Based on these clinical observations,
the patient was suspected to have either infectious colitis or
acute exacerbation of UC. Oral levofloxacin (500 mg/day) was
administered from October 7 to 11, 2016; however, the patient's
symptoms did not improve. He was then admitted to our Hospital on
October 11, 2016, for further examination of his condition.
Upon admission, the patient had bloody diarrhea (12
times a day) with a high fever of 38.5°C and lower abdominal
discomfort. Physical examination revealed slight tenderness at the
left lower abdomen and mild hepatosplenomegaly. His chest
radiograph and urinalysis results were normal. Blood tests revealed
increased numbers of total and atypical lymphocytes (Table I). Mildly elevated aspartate
aminotransferase, alanine aminotransferase, and lactate
dehydrogenase levels were observed; however, the results of
serological tests for hepatitis virus A, B, and C and Epstein-Barr
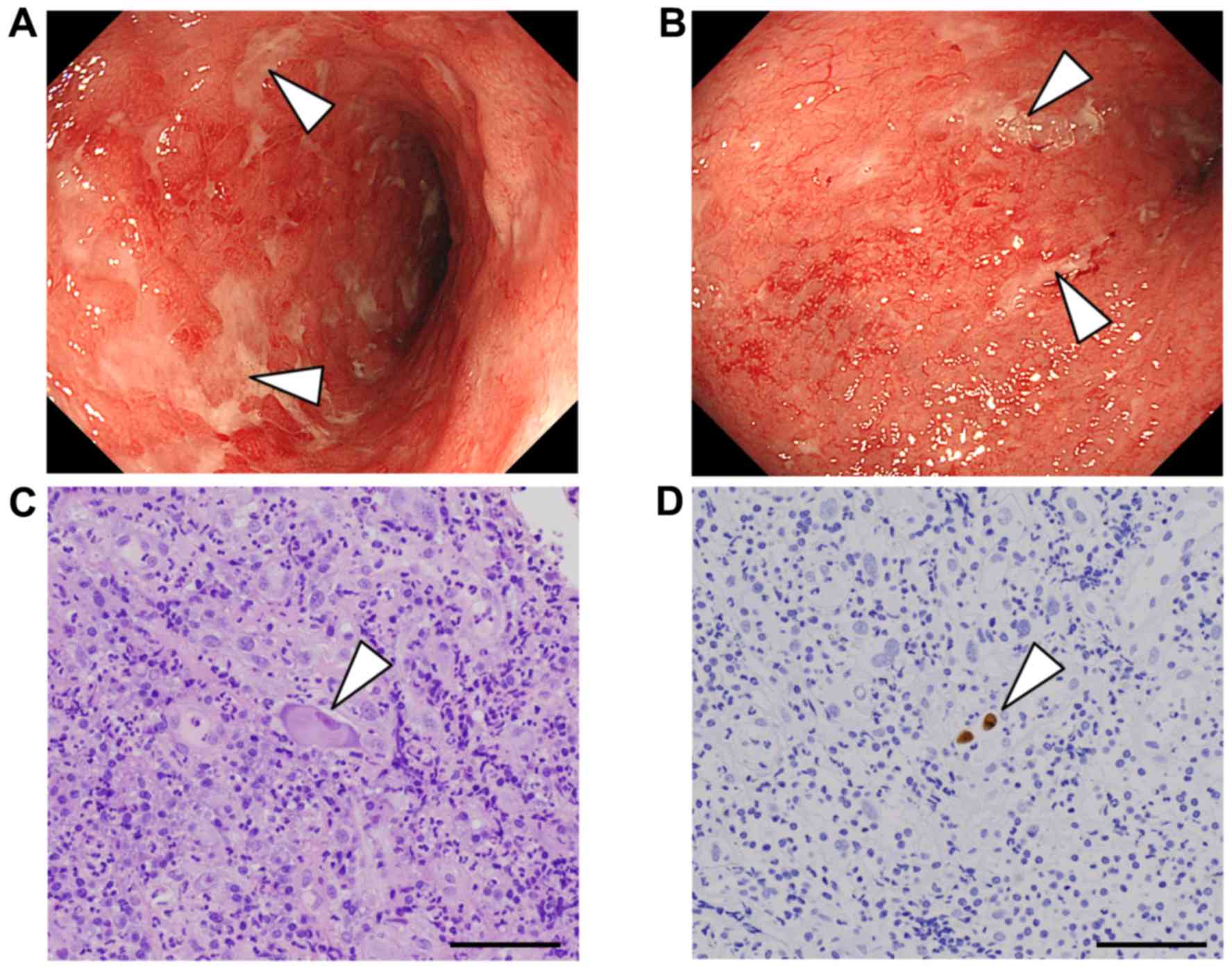
virus were negative. Colonoscopy showed edematous and erosive
changes in the entire mucosa from the rectum to the sigmoid colon
(Fig. 1A and B), while colonic
mucosa from the cecum to the descending colon was normal.
Endoscopic findings from the rectum to the sigmoid colon were
consistent with moderately active UC. The bacterial culture results
of the patient's blood and stool samples were negative.
Clostridium difficile toxins A and B were absent. Notably,
the CMV antigenemia assay (CMV pp65 antigen), which is commonly
used to detect CMV infection in Japan (4), showed 65 positive cells per 50,000
cells. In addition, the test for anti-CMV IgM antibodies was
positive, and the titer of anti-CMV IgG antibodies, which was
negative on admission, had increased on the tenth day after
admission. Furthermore, hematoxylin and eosin staining of the
biopsy specimens from the inflamed colonic mucosa showed cells with
inclusion bodies (Fig. 1C).
Immunohistochemical examination of these specimens showed CMV
positivity (Fig. 1D). Notably, no
CMV-positive cells were observed in the biopsy specimens previously
collected at the outpatient department. On the basis of these
clinical findings, the patient was diagnosed as having acute CMV
mononucleosis and colitis on October 14, 2016.
 | Table I.Laboratory data on admission. |
Table I.
Laboratory data on admission.
| Parameter | Data | N.R. |
|---|
| WBC (cells/µl) | 11,230a | 3,300–8,600 |
| Neut (%) | 22b | 38–74 |
| Lym (%) | 50a | 16.5–49.0 |
| Aty-lymph (%) | 24a | 0–1 |
| RBC
(×106/µl) | 5.29 | 4.35–5.55 |
| Hb (g/dl) | 13.8 | 13.7–16.8 |
| Ht (%) | 41.5 | 40.7–50.1 |
| Plt
(×103/µl) | 272 | 158–348 |
| TP (g/dl) | 6.7 | 6.6–8.1 |
| Alb (g/dl) | 3.5b | 4.1–5.1 |
| AST (U/l) | 87a | 15–30 |
| ALT (U/l) | 96a | 10–42 |
| LDH (U/l) | 554a | 124–222 |
| ALP (U/l) | 330a | 106–322 |
| γ-GTP (U/l) | 52 | 13–64 |
| ChE (U/l) | 137b | 201–421 |
| T.Bil (mg/dl) | 0.4 | 0.4–1.5 |
| CK (U/l) | 35b | 59–248 |
| Amy (U/l) | 73 | 44–132 |
| T-Chol (mg/dl) | 139b | 142–248 |
| TG (mg/dl) | 108 | 30–117 |
| UN (mg/dl) | 9.7 | 8.0–20.0 |
| Cr (mg/dl) | 0.83 | 0.65–1.07 |
| Na (mmol/l) | 136 | 134–145 |
| K (mmol/l) | 4.4 | 3.6–4.8 |
| Cl (mmol/l) | 103 | 101–108 |
| FBS (mg/dl) | 94 | 73–109 |
| HbA1c (%) | 5.3 | 4.9–6.0 |
| Cortisol
(µg/dl) | 6.8 | 6.2–18.0 |
| ACTH (pg/ml) | 18 | 7.2–63.3 |
| CRP (mg/dl) | 7.83a | <0.14 |
| ESR |
|
|
| 30 min (mm) | 4 | N/A |
| 60 min (mm) | 23a | 3–15 |
| CD4 (%) | 38.4 | 35–65 |
| CD8 (%) | 35.4 | 20–38 |
| CD4/CD8 | 1.09 | 0.4–2.3 |
| C3 (mg/dl) | 99 | 73–138 |
| CH50 (U/ml) | 55.5 | 31.6–57.6 |
| IgG (mg/dl) | 1,587 | 861-1,747 |
| IgA (mg/dl) | 221 | 93–393 |
| IgM (mg/dl) | 116 | 33–183 |
| IgM-HA Ab | (−) | (−) |
| IgM-HBc Ab | (−) | (−) |
| HBs-Ag | (−) | (−) |
| HCV Ab | (−) | (−) |
| EBV |
|
|
| VCA-IgM | <10 | <10 |
| VCA-IgG | x80a | <10 |
| EBNA | x40a | <10 |
| IgM-CMV Ab | (+) | (−) |
| IgG-CMV Ab | (−) | (−) |
| CMV
antigenemia | (+) | (−) |
| HIV-1/2 Ab | (−) | (−) |
| HTLV-1 Ab | (−) | (−) |
| T-SPOT.TB | (−) | (−) |
| β-D-glucan | <5.0 | <5.0 |
| CD toxin A/B | (−)/(−) | (−)/(−) |
Following the diagnosis of acute CMV mononucleosis
and colitis, intravenous ganciclovir (9-[1,3-dihydroxy-2-propoxy]
methyl)-guanine (DHPG) (550 mg/day) was administered from October
14 to 19, 2016 (Fig. 2A and B). On
the third day, after the initiation of ganciclovir, the hepatic
enzymes and atypical lymphocyte levels gradually improved. In
addition, the patient's symptoms, including high-grade fever,
bloody diarrhea and abdominal discomfort, had subsided. After
intravenous ganciclovir was stopped, oral valganciclovir was
administered from October 19 to 22, 2016. However, on October 22,
2016, serum CMV pp65 antigen results were found to be negative, and
the Seo and Rachmilewitz indices that were used to assess clinical
and endoscopic disease activity for UC (15,16),
respectively, had also improved from those seen at admission
(Fig. 2C). Therefore, the antiviral
treatment for CMV was ended and the patient was discharged on
October 22, 2016. Since then, he has been on oral 5-ASA (4,000
mg/day) treatment, and no recurrence of CMV infection has been
observed.
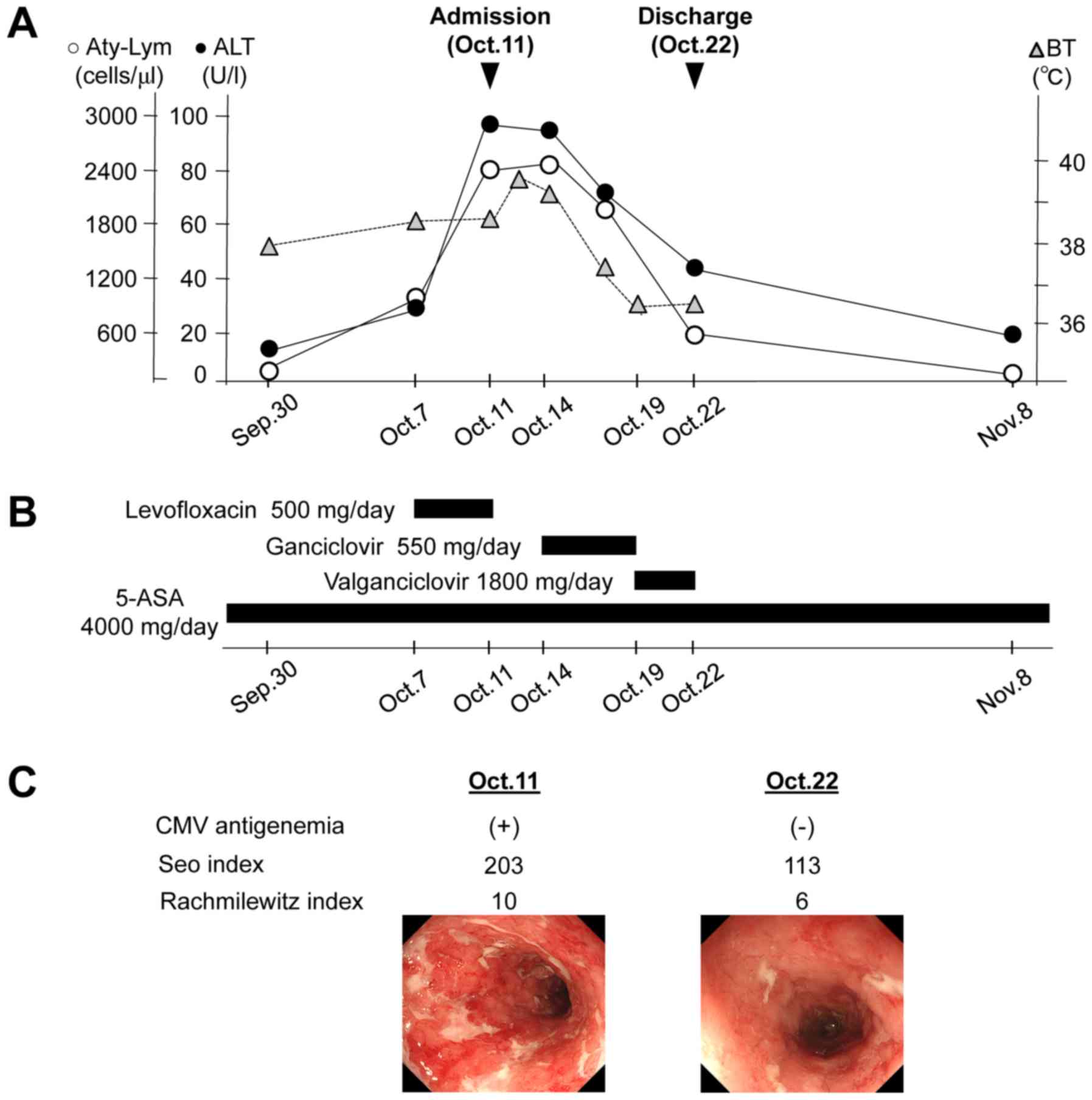 | Figure 2.Clinical course of illness of the
patient. (A) Clinical course of this patient. (B) Treatment
schedule. (C) Comparison of CMV antigenemia and the clinical and
endoscopic disease activities at the time of admission and
discharge. The Seo index was evaluated by the total score of the
patient's symptoms (the presence of bloody stool and bowel
movements per day) and laboratory data (ESR, Hb and Alb),
indicating the clinical disease activity of UC; total score
<150, mild; 150–220, moderate; >220, severe. A score <120
indicates clinical remission (15).
The Rachmilewitz endoscopic index is calculated from the total
score of colonic mucosal findings [granulation scattering reflected
light, visible vascular pattern, vulnerability of mucosa, and
mucosal damage (mucus, fibrin, exudate, erosion and ulcer)],
indicating the endoscopic disease activity of UC; a total score
<4 is defined as endoscopic remission (16). Endoscopic findings in the rectum on
admission (left) and at the time of discharge (right) are shown.
Alb, albumin; ALT, alanine aminotransferase; 5-ASA,
5-aminosalicylic acid; Aty-Lym, atypical lymphocyte; BT, body
temperature; CMV, cytomegalovirus; ESR, erythrocyte sedimentation
rate; Hb, hemoglobin; Nov., November; Oct., October; Sep.,
September; UC, ulcerative colitis. |
Hematoxylin and eosin staining and
immunohistochemistry
Histological examination was performed on biopsy
specimens fixed with 10% formalin for 24 h at room temperature.
Paraffin-embedded tissues were cut into 4 µm-thick sections and
deparaffinized. These sections were stained with hematoxylin and
eosin or used for immunohistochemistry. For hematoxylin and eosin
staining, the sections were stained with 0.1% hematoxylin solution
for 4 min at room temperature and then stained with 0.1% eosin Y
(cat. no. 058-00062; Wako Pure Chemical Industries, Ltd., Osaka,
Japan) solution for 2 min at room temperature. For
immunohistochemistry, the deparaffinized sections were placed in a
citrate buffer (pH 6.0), and then autoclaved at 121°C for 1 min to
retrieve the antigen. The sections were then rinsed and blocked
with 3% hydrogen peroxide in methanol for 10 min to remove
endogenous peroxidase activity. Non-specific binding sites were
blocked in 0.01 M phosphate-buffered saline (PBS) containing 2%
bovine serum albumin (BSA; cat. no. 019-07494; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) for 30 min. Anti-CMV antibody
(mouse IgG, cat. no. M0854; Dako, Glostrup, Denmark) diluted to
1:200 in 2% BSA/PBS was added to the slides and incubated overnight
at 4°C. Anti-CMV antibody was detected using a biotinylated
anti-mouse IgG (1:300, cat. no. E0433; Dako, Glostrup, Denmark) for
30 min at room temperature, followed by incubation with
avidin-coupled peroxidase (Vectastain ABC kit; Vector Laboratories,
Burlingame, CA, USA) for 30 min. The peroxidase binding sites were
visualized by staining with 3,3′-diaminobenzidine (DAB) in 50 mM
Tris-EDTA buffer and counterstained with hematoxylin.
Discussion
CMV is a ubiquitous member of the Herpesviridae
family that can present with a variety of clinical manifestations,
including encephalitis, retinitis, interstitial pneumonia, and
colitis (17). These serious
symptoms are generally observed as opportunistic infections in
immunocompromised hosts, including patients with AIDS and those
receiving steroids and/or immunosuppressants (9). Patients with severe or uncontrollable
UC often require administration of a steroid or immunosuppressant
to decrease disease activity. Therefore, patients with UC are
recognized to be at a high risk for symptomatic CMV infections. In
the present case, acute mononucleosis and colitis due to a primary
CMV infection had been observed in a patient with UC without
concurrent immunosuppressive treatments, which is a rare occurrence
among patients with UC.
It was previously reported that most cases of CMV
colitis observed among patients with IBD can be attributed to the
reactivation of a latent CMV infection (8). In this case, CMV-positive cells were
observed in biopsy specimens of the colonic mucosa (Fig. 1C and D), indicating the presence of
an active colonic infection. Active colonic infection due to CMV is
likely to occur by either a primary CMV infection or reactivation
of a latent CMV infection. Another study reported that patients
with UC, regardless of their disease activity, have a higher
prevalence of anti-CMV IgG than normal control patients (18), indicating a high prevalence of
persistent or latent CMV infection among patients with UC. However,
in this case, not only were serum anti-CMV IgG and CMV antigenemia
present, CMV-positive cells in the biopsy specimens had been
negative before admission. In addition, acute elevation of the
anti-CMV IgM titer and positive conversion of anti-CMV IgG were
also observed following admission. A primary CMV infection is one
that occurs in seronegative subjects without prior specific
immunity against CMV (19).
Therefore, we concluded that the CMV colitis observed in this
patient had resulted from a primary CMV infection and not from the
reactivation of a latent CMV infection.
Thus far, primary CMV infections have only rarely
been reported in patients with UC without concurrent
immunosuppressive treatments. To our knowledge, only 5 case reports
have been published since February 1998 (Table II) (20–24), and
all cases presented the co-occurrence of CMV colitis. The features
of CMV mononucleosis, including fever, atypical lymphocytosis,
elevated liver enzymes, and hepatosplenomegaly, were observed in 3
cases (20,21,23). One
of the remaining 2 cases showed protein-losing gastroenteropathy
(22), whereas the other had toxic
megacolon (24). One case had
previously undergone short-term steroid treatment for remission
induction therapy (21). There were
no underlying diseases except UC. Neither was host defense impaired
nor were steroids or immunosuppressants administered at the onset
of acute CMV infections in all cases. The mechanism of onset of CMV
infection has been unknown in most cases (20–22,24),
although the immunosuppressive effects of leukocytapheresis (LCAP)
have been suggested to be a cause of CMV infection in one study
(23). In the present case,
prednisolone was previously used for remission induction therapy
for UC; however, the patient had not taken any steroids for over 7
years. Indeed, the serum levels of cortisol and
adrenocorticotrophic hormone (ACTH) were within the normal range on
admission (Table I), suggesting
intact adrenal cortex function. In addition, no abnormal values
were observed in both humoral and cellular immunity, as shown by
the amount of immunoglobulin and the CD4/CD8 ratio, respectively
(Table I). Moreover, comorbidities
that may be associated with a degree of immune dysfunction, such as
diabetes mellitus or renal failure, were not observed in this
patient. Here, immunocompetency was defined as the exclusion of
individuals with profound loss of immune function, including
patients with AIDS, pharmacologically immunosuppressed patients,
and chemotherapy recipients (25).
According to this definition and the above-mentioned clinical data,
this patient was considered immunocompetent at the onset of acute
CMV infection. In addition, the patient had not undergone any
procedures such as LCAP. Therefore, consistent with previous
reports (20–22,24), the
detailed mechanism of the onset of primary CMV infection is also
unknown in this case. However, a previous report has shown that a
decrease in the seroprevalence of CMV was found among young adults
in Japan, suggesting a possible association with increased
susceptibility to primary CMV infection in this population
(26). Given that this patient's
anti-CMV IgG had been negative before admission, this might also,
in part, be associated with the onset of primary CMV infection.
 | Table II.Reported cases of primary CMV
infection in patients with ulcerative colitis without concurrent
steroid or immunosuppressant use. |
Table II.
Reported cases of primary CMV
infection in patients with ulcerative colitis without concurrent
steroid or immunosuppressant use.
| Author, year | Age
(years)/sex | Manifestation | Past steroid or
immunosuppressant use (agent, duration) | Gancyclovir | Outcome | (Refs.) |
|---|
| Moonka et
al, 1998 | 29/F | Pouchitis,
mononucleosis | No | Yes | Survived | (20) |
| Rachima et
al, 1998 | 38/F | Rectosigmoid
colitis, mononucleosis | Yes (Steroid,
unspecified) | No | Survived | (21) |
| Kraus et al,
2000 | 76/M | Pancolitis,
protein-losing syndrome | No | Yes | Survived | (22) |
| Osaki et al,
2008 | 32/M | Pancolitis,
mononucleosis | No | Yes | Survived | (23) |
| Inoue et al,
2012 | 57/M | Pancolitis, toxic
megacolon | No | Yes |
Surgery/survived | (24) |
| Present study | 28/M | Rectosigmoid
colitis, mononucleosis | Yes (Steroid, 8
weeks) | Yes | Survived | – |
Of the 5 cases described in Table II, 3 patients who were treated with
the antiviral agent ganciclovir showed marked improvements in all
disease manifestations (Table II)
(20,22,23).
Another case showed recovery with 5-ASA treatment alone (21), and the last case underwent surgical
colectomy due to the onset of toxic megacolon (24). It is known that primary CMV infection
in immunocompetent individuals is usually asymptomatic or causes a
mild mononucleosis-like syndrome, typically resulting in a
spontaneous recovery. Therefore, symptomatic treatment is primarily
used, and antiviral treatment is not recommended for these
populations (27). However, severe
tissue-invasive CMV infection with a wide range of manifestations,
including colitis, vascular thrombosis, pneumonia, and myocarditis,
is occasionally observed, even in immunocompetent adults. Notably,
because of the low incidence of such severe CMV infections, usually
referred to as CMV disease, its diagnosis and the initiation of
targeted therapy tend to be delayed, which leads to high morbidity
and mortality due to disease progression. Accordingly, targeted
antiviral therapy with ganciclovir or valganciclovir is considered
appropriate for the treatment of CMV disease in healthy adults
(25). Indeed, 4 patients with UC
including the present case have presented with a manifestation of
CMV disease, such as colitis, and experienced a dramatic
improvement in all disease manifestations after antiviral treatment
with ganciclovir (Table II)
(20,22,23).
CMV infection in the colon has been reported to
exacerbate UC symptoms, resulting in a high incidence of toxic
megacolon, colonic perforation, and death (28,29). In
fact, a case presented in Table II
underwent surgical colectomy due to the onset of toxic megacolon
after admission (24). Currently,
there is no consensus on how to manage UC patients diagnosed with
an active CMV infection. However, considering the role of CMV as an
exacerbating factor of UC, the determination of a therapeutic
strategy to combat CMV is warranted for this patient population. A
recent publication reported that both the mortality and surgical
rates of patients with UC have improved since the use of
ganciclovir and have suggested that ganciclovir is a clearly
beneficial therapy for CMV in patients with UC (30). Indeed, 4 cases of UC, including the
present case, have been cured by ganciclovir treatment without any
complications (Table II) (20,22,23).
However, spontaneous recovery of acute CMV infection has been shown
to occur in an immunocompetent patient with UC (21). Moreover, a recent study reported that
mild CMV colitis in patients with UC was cured without antiviral
therapy (31). Therefore, the use of
antiviral agents for acute CMV infection superimposed on
immunocompetent UC should be assessed carefully based on the
patient's condition, including disease activity and clinical
course. Further studies are required to clarify the role of
antiviral treatment in immunocompetent patients with UC.
In conclusion, we reported a rare case of acute
mononucleosis and colitis caused by primary CMV infection on an
immunocompetent patient with UC without concurrent steroids use.
Even if patients with UC are not treated with steroids and/or
immunosuppressants, clinicians should be aware of the possibility
of an acute CMV infection in the context of severe or persistent
colonic inflammation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TM and HS collaborated in the conception and design
of the study. TM, HS, NO, TS, JT, MK, TI, YS and HA performed the
case study, and acquired the data and images of the case. TM, HS
and MS performed data analysis and interpretation, and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Gifu University Hospital, Independent Ethics
Committee declares that a single case report does not require an
ethical review by the committee. The patient signed an informed
consent form for permission to use the clinical data and
images.
Patient consent for publication
Written informed consent was provided by the patient
for publication of this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de la Hoz RE, Stephens G and Sherlock C:
Diagnosis and treatment approaches of CMV infections in adult
patients. J Clin Virol. 25 (Suppl 2):S1–S12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gandhi MK and Khanna R: Human
cytomegalovirus: Clinical aspects, immune regulation, and emerging
treatments. Lancet Infect Dis. 4:725–738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berk T, Gordon SJ, Choi HY and Cooper HS:
Cytomegalovirus infection of the colon: A possible role in
exacerbations of inflammatory bowel disease. Am J Gastroenterol.
80:355–360. 1985.PubMed/NCBI
|
|
4
|
Ikeda K, Nakajima S, Tanji K, Hirai T,
Uomori K, Morimoto S, Tomita S, Fukunaga M, Tamura N and Sekigawa
I: Intestinal perforation due to hemorrhagic Cytomegalovirus
enteritis in a patient with severe uncontrolled lupus nephritis: A
case and review of the literature. Rheumatol Int. 37:1395–1399.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babyatsky MW, Keroack MD, Blake MA,
Rosenberg ES and Mino-Kenudson M: Case records of the Massachusetts
General Hospital. Case 35-2007. A 30-year-old man with inflammatory
bowel disease and recent onset of fever and bloody diarrhea. N Engl
J Med. 357:2068–2076. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimitroulia E, Spanakis N, Konstantinidou
AE, Legakis NJ and Tsakris A: Frequent detection of cytomegalovirus
in the intestine of patients with inflammatory bowel disease.
Inflamm Bowel Dis. 12:879–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galiatsatos P, Shrier I, Lamoureux E and
Szilagyi A: Meta-analysis of outcome of cytomegalovirus colitis in
immunocompetent hosts. Dig Dis Sci. 50:609–616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papadakis KA, Tung JK, Binder SW, Kam LY,
Abreu MT, Targan SR and Vasiliauskas EA: Outcome of cytomegalovirus
infections in patients with inflammatory bowel disease. Am J
Gastroenterol. 96:2137–2142. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan TV and Toms C: Cytomegalovirus
colitis and subsequent new diagnosis of inflammatory bowel disease
in an immunocompetent host: A case study and literature review. Am
J Case Rep. 17:538–543. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kishore J, Ghoshal U, Ghoshal UC,
Krishnani N, Kumar S, Singh M and Ayyagari A: Infection with
cytomegalovirus in patients with inflammatory bowel disease:
Prevalence, clinical significance and outcome. J Med Microbiol.
53:1155–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pillet S, Pozzetto B and Roblin X:
Cytomegalovirus and ulcerative colitis: Place of antiviral therapy.
World J Gastroenterol. 22:2030–2045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Römkens TE, Bulte GJ, Nissen LH and Drenth
JP: Cytomegalovirus in inflammatory bowel disease: A systematic
review. World J Gastroenterol. 22:1321–1330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohnuki Y, Moriya Y, Yutani S, Mizuma A,
Nakayama T, Ohnuki Y, Uda S, Inomoto C, Yamamoto S, Nakamura N and
Takizawa S: Eosinophilic granulomatosis with polyangiitis
(churg-strauss syndrome) complicated by perforation of the small
intestine and cholecystitis. Intern Med. 57:737–740. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ikeda K, Takasaki Y and Sekigawa I: Rapid
onset of small intestinal perforation after successful steroid
treatment in eosinophilic granulomatosis with polyangiitis. Mod
Pheumatol. 26:968–970. 2016.
|
|
15
|
Seo M, Okada M, Yao T, Ueki M, Arima S and
Okumura M: An index of disease activity in patients with ulcerative
colitis. Am J Gastroenterol. 87:971–976. 1992.PubMed/NCBI
|
|
16
|
Rachmilewitz D: Coated mesalazine
(5-aminosalicylic acid) versus sulphasalazine in the treatment of
active ulcerative colitis: A randomized trial. BMJ. 298:82–86.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eddleston M, Peacock S, Juniper M and
Warrell DA: Severe cytomegalovirus infection in immunocompetent
patients. Clin Infect Dis. 24:52–56. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farmer GW, Vincent MM, Fuccillo DA,
Horta-Barbosa L, Ritman S, Sever JL and Gitnick GL: Viral
investigations in ulcerative colitis and regional enteritis.
Gastroenterology. 65:8–18. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ho M: The history of cytomegalovirus and
its disease. Med Microbiol Immunol. 197:65–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moonka D, Furth EE, MacDermott RP and
Lichtenstein GR: Pouchitis associated with primary cytomegalovirus
infection. Am J Gastroenterology. 93:264–266. 1998. View Article : Google Scholar
|
|
21
|
Rachima C, Maoz E, Apter S, Thaler M,
Grossman E and Rosenthal T: Cytomegalovirus infection associated
with ulcerative colitis in immunocompetent individuals. Postgrad
Med J. 74:486–489. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kraus M, Meyenberger C and Suter W:
Generalized intestinal CMV infection with protein-losing syndrome
in ulcerative colitis. Schweiz Med Wochenschr. 130:1600–1605.
2000.PubMed/NCBI
|
|
23
|
Osaki R, Andoh A, Tsujikawa T, Ogawa A,
Koizumi Y, Nakahara T, Hata K, Sasaki M, Saito Y and Fujiyama Y:
Acute cytomegalovirus infection superimposed on
corticosteroid-naïve ulcerative colitis. Intern Med. 47:1341–1344.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inoue K, Wakabayashi N, Fukumoto K, Yamada
S, Bito N, Yoshida N, Katada K, Uchiyama K, Ishikawa T, Handa O, et
al: Toxic megacolon associated with cytomegalovirus infection in a
patient with steroid-naïve ulcerative colitis. Intern Med.
51:2739–2743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lancini D, Faddy HM, Flower R and Hogan C:
Cytomegalovirus disease in immunocompetent adults. Med J Aust.
201:578–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeda N, Isonuma H, Sekiya S, Ebe T,
Matsumoto T and Watanabe K: Studies of anti-cytomegalovirus IgG
antibody rate and cytomegalovirus mononucleosis in adults.
Kansenshogaku Zasshi. 75:775–779. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pokorska-Śpiewak M, Niezgoda A, Gołkowska
M, Czech-Kowalska J, Gruszfeld D, Dobrzańska A, Styczyński J and
Marczyńska M: Recommendations for the diagnosis and treatment of
CMV infections. Polish Society of epidemiology and infectious
disease. Przegl Epidemiol. 70:297–310. 2016.PubMed/NCBI
|
|
28
|
Cooper HS, Raffensperger EC, Jonas L and
Fitts WT Jr: Cytomegalovirus inclusions in patients with ulcerative
colitis and toxic dilation requiring colonic resection.
Gastroenterology. 72:1253–1256. 1977.PubMed/NCBI
|
|
29
|
Sidi S, Graham JH, Razvi SA and Banks PA:
Cytomegalovirus infection of the colon associated with ulcerative
colitis. Arch Surg. 114:857–859. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pfau P, Kochman ML, Furth EE and
Lichtenstein GR: Cytomegalovirus colitis complicating ulcerative
colitis in the steroid-naïve patient. Am J Gastroenterol.
96:895–899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kopylov U, Sasson G, Geyshis B, Oikawa MT,
Barshack I, Eliakim R and Ben-Horin S: Cytomegalovirus positive
ulcerative colitis: A single center experience and literature
review. World J Gastrointest Pathophysiol. 4:18–23. 2013.
View Article : Google Scholar : PubMed/NCBI
|