Introduction
Cerebral infarction, also known as acute ischemic
stroke, is a common acute cerebrovascular disease that is harmful
to the health of the elderly and the young (1). It is the characterized by high rates of
recurrence (26% within 5 years and 39% within 10 years), disability
(19% of patients within 3 months and 38% within 12 months) and
mortality (114.8/100,000) in China (2,3), which
seriously affects the quality of life of patients, as well as
causing substantial emotional and economic burden to both the
patients and their families. Developed countries worldwide,
including China, have a rapidly aging society; therefore, effective
detection techniques for the early screening, risk prediction,
diagnosis and prognosis of cerebral infarction are urgently needed
(3,4). Clinical and epidemiological data have
confirmed that homocysteine (Hcy) is an important risk factor for
cardiovascular diseases and is closely associated with the
pathogenesis of cerebral infarction (5). The level of Hcy in the plasma of
patients with cerebral infarction is significantly higher compared
with that of the healthy population (6,7).
Therefore, efficient methods of monitoring Hcy levels provides a
potentially useful avenue for the early diagnosis, prevention and
treatment, as well as prognosis, of patients affected by cerebral
infarction.
Materials and methods
Synthesis of the probe
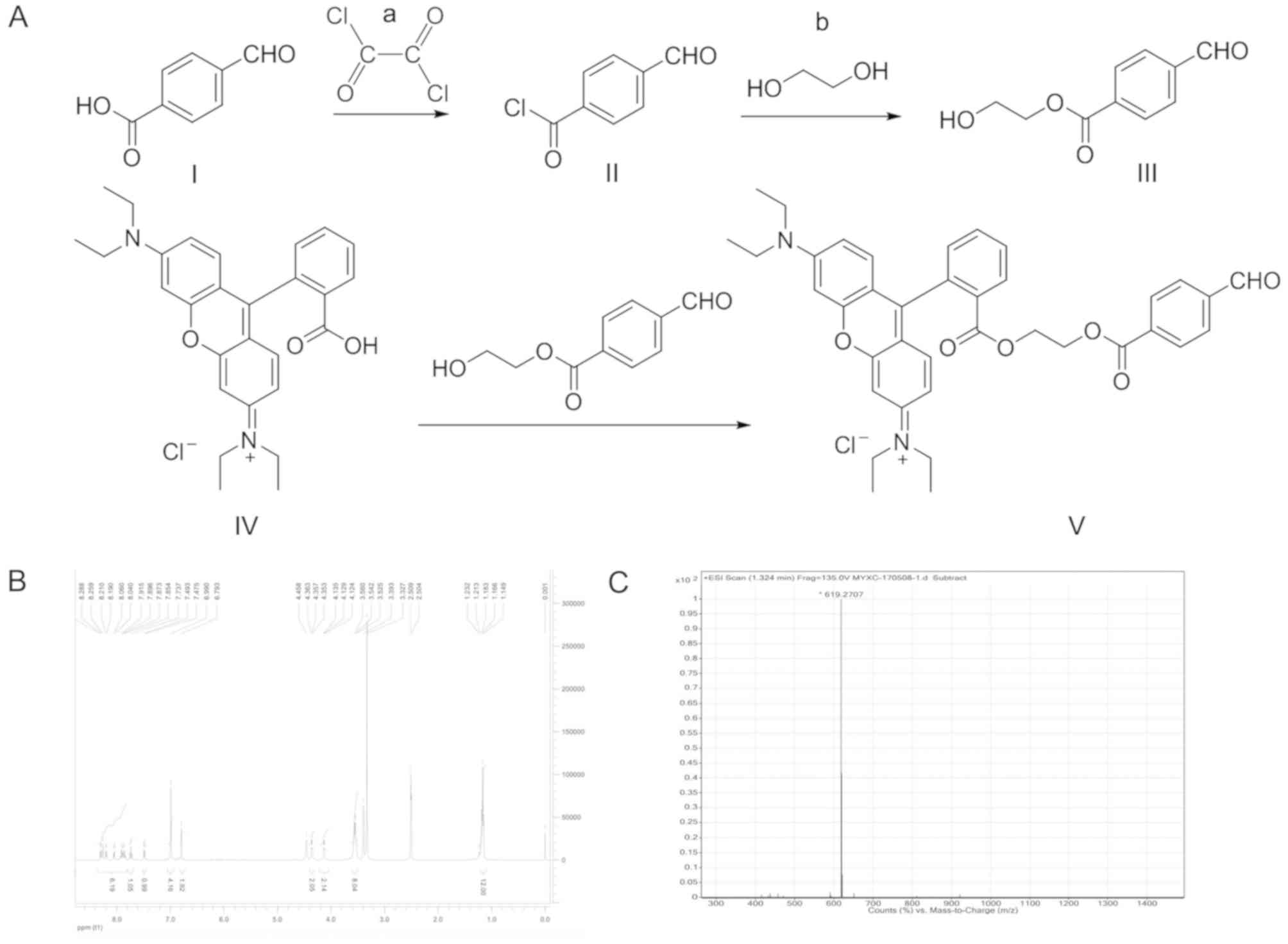
The Hcy probe was synthesized according to the
pathway illustrated in Fig. 1.
Briefly, 4-formylbenzoic acid (‘I’; 15.0 g, 0.1 M) was dissolved in
100 ml of dichloromethane, and 5 drops of N,N-dimethylformamide was
added as a catalyst. The reaction was cooled in an ice bath to
approximately 0°C, before oxalyl chloride (‘a’; 19.05 g, 0.15 M)
was added dropwise. The reaction continued under rotary
distillation at room temperature for 2 h to remove the solvent and
excess oxalyl chloride. The 4-formylbenzoyl chloride (‘II’)
obtained was used directly for the next step without purification.
In a reaction flask, 50 ml of ethylene glycol (‘b’) was added and
cooled to approximately 0°C in an ice bath. 4-Formylbenzoyl
chloride was gradually added to the ethylene glycol, and the
reaction was continued under rotary distillation at room
temperature for 2 h to remove excess ethylene glycol. Crude
2-hydroxyethyl 4-formylbenzoate (‘III’) was then purified by silica
gel column chromatography and eluted using petroleum ether/ethyl
acetate (2:1) as the eluent, and a colorless, waxy solid (15.7 g,
80.93%) was obtained. Rhodamine B (‘IV’; 2.39 g, 0.005 M) was
dissolved in 30 ml of dichloromethane before the sequential
addition of 2-hydroxyethyl 4-formylbenzoate (1.16 g, 0.006 M),
N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (0.96
g, 0.005 M) and 4-(dimethylamino)pyridine (0.12 g, 0.001 M). The
reaction was carried out at room temperature for ~28 h, and was
monitored using thin-layer chromatography with
dichloromethane/methanol (10:1) as the solvent. This reaction
system was subjected to rotary distillation to remove the solvent
dichloromethane. The compound was dissolved in 2 ml dichloromethane
and chromatography for purification was performed using a vitreous
silica gel column (column diameter, 1.5 cm; column length, 70 cm;
HuaSheng Chromatography, Jiangyin, China at a flow rate of 2 ml/min
at room temperature. Dichloromethane/methanol (50 ml; 10:1) was
used as the eluent. Finally,
N-(6-(diethylamino)-9-(2-((2-(4-formylbenzoyloxy)ethoxy)carbonyl)phenyl)-3H-xanthen-3-ylidene)-N-ethylethanaminium
chloride (‘V’ also known as S1-4; 0.471 g, 14.4%) was obtained,
named thereafter as S1-4. All reagents mentioned above were
purchased from Sigma-Aldrich (Merck KGaA). The compound structure
‘V’ was dissolved by deuterium oxide and analyzed using
1H-Nuclear magnetic resonance (1H-NMR; 400-MR
DD2; 400 MHz; Agilent Technologies, Inc.) and an electron
ionization-high resolution mass spectrometry (EI-HRMS;
Micromass® GCT Premier™; Waters Corporation).
Determination of Hcy probe
fluorescence linearity
The Hcy probe (10 µM) was prepared in PBS (Beijing
Solarbio Science & Technology Co., Ltd.). In a 96-well black
microplate, 0, 1, 2, 5, 10, 20, 50 and 100 µM Hcy (Beijing Solarbio
Science & Technology Co., Ltd.), 100 µl in human-based serum
(cat. no. LUN5048; Randox Laboratories, Ltd.), was mixed with 100
µl of the working solution of the Hcy probe. Following incubation
at 37°C for 30 min, fluorescence intensity was measured at the
excitation wavelength of 280 nm and emission wavelength of 590 nm
using a microplate reader (Thermo Fisher Scientific Inc.), from
which a standard curve of concentration vs. fluorescence was
generated.
Effect of fluid viscosity and pH on
the response of the probe
The working solution of the probe (10 µM) was
prepared in PBS. In a 96-well black microplate, 100 µl of 20 µM Hcy
with different fluid viscosities (glycerol/water=1.005–219 cp) were
added to 100 µl of the working solution of the probe. Following
incubation at 37°C for 30 min, fluorescence intensity was measured
at an excitation wavelength of 280 nm and emission wavelength of
590 nm to analyze the effect of fluid viscosity on probe
responsiveness using a microplate reader (Thermo Fisher Scientific
Inc.). To measure the effect of pH on the response of the probe,
buffers at pH 6.5–8.5 were prepared using HCl, NaOH and PBS before
10 µM of the probe was added to 20 µM Hcy. Following incubation at
37°C for 30 min, probe response was measured by the same method
using a microplate reader (Thermo Fisher Scientific, Inc.).
Determination of probe
selectivity
Considering the complex intracellular environment,
various interfering substances, such as the main amino acids and
other small molecules, were added to the probe mixture, including
various amino acids derivatives [cysteine (Cys); Hcy;
N-acetylcysteine; glutathione (GSH); arginine; asparagine; aspartic
acid; β-alanine; dithiothreitol; glutamine; glutamic acid; glycine;
histidine; isoleucine; L-alanine; leucine; lysine; methionine;
phenylalanine; proline; serine; threonine; tryptophan; tyrosine;
and valine; all 1 mM], metal ions [potassium (K+);
sodium (Na+); calcium (Ca2+); magnesium
(Mg2+); zinc (Zn2+); aluminum
(Al3+); cadmium (Cd2+); manganese
(Mn2+)], and Na2SO3 and
H2S (made from Na2S; 1 mM for both). The
working solution of the probe (10 µM) was prepared in PBS. In a
96-well black microplate, 100 µl of each of the substances
mentioned above were added to the working solution of the probe.
Following incubation at 37°C for 30 min, fluorescence intensity was
measured at an excitation wavelength of 280 nm and emission
wavelength of 590 nm to analyze probe selectivity in the presence
of interfering substances. All reagents mentioned above were
purchased from Beijing Solarbio Science & Technology Co.,
Ltd.
Detection of Hcy in the serum using
the probe and a biochemistry analyzer
A total of 50 patients with cerebral infarction (CI
group) and 50 patients with transient cerebral ischemia (TIA group)
diagnosed in Tianjin Huanhu Hospital (Tianjin, China) between May
2016 and November 2017 were enrolled into the present study. A
total of 50 healthy volunteers were classified into the normal
(Norm) group. All participants in the experiments were matched for
age (TIA, 60.1±11.2; CI, 62.7±9.1; Norm, 61.1±9.6) and sex (25
males and 25 females in each group). This study was approved by the
medical ethics committee of Tianjin Huanhu Hospital, and followed
the principles according to the Declaration of Helsinki; all
patients provided informed consent before the experiments.
The inclusion criteria were as follows: i) Patients
in the CI and TIA group were diagnosed within 24 h following the
onset of symptoms according to the standard clinical criteria for
CI and TIA, with supporting brain images produced either by
computed tomography or magnetic resonance imaging and magnetic
resonance angiography. The venous blood of Norm, CI and TIA groups
were collected within 24 h of admission into hospital into an
sterile and impermeable container before being frozen immediately
at −80°C after collection; and ii) asymptomatic patients undergoing
physical examinations who did not suffer from previous or current
cardiovascular and cerebrovascular diseases were enrolled into the
healthy control (Norm) group. The exclusion criteria were as
follows: i) Patients with intracerebral hemorrhage or other brain
injuries, atherosclerosis, heart failure, respiratory failure,
renal failure, severe liver dysfunction, malignant tumors,
autoimmune disease, diabetes mellitus or any other metabolic
diseases, intestinal disorders, or other malignant disease; and ii)
admission >24 h after stroke onset.
A total of 5 ml venous blood was collected from
patients in each of the groups and centrifuged at 300 × g for 10
min at room temperature. Hcy content in the serum was measured
using a Biochemistry Analyzer (Beckman Coulter, Inc.).
Dithiothreitol (1 M, 5 µl; Sigma-Aldrich; Merck KGaA) was added to
95 µl of the serum and incubated at 37°C for 30 min, followed by
the addition of the probe solution (10 µM) and another round of
incubation at 37°C for 30 min. Fluorescence intensity was measured
at the excitation wavelength of 280 nm and emission wavelength of
590 nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Using the fluorescence intensity data, a correlation and receiver
operating characteristic (ROC) curve was produced to compare the
accuracy, specificity and sensitivity of probe in biological
samples.
Statistical analysis
Data are represented as mean ± SD and were analyzed
using SPSS 11.0 software (SPSS, Inc.). One-way ANOVA and the Tukey
post hoc test was used for general measurement data. Pearson's
correlation coefficient was used for correlative analysis. ROC
curve was calculated using GraphPad Prism 7.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
The structural identification of the
probe
To illustrate the structure of the probe, the
following results were obtained using 1H-NMR and EI-HRMS
spectroscopy: 1H NMR (400 MHz, DMSO-D6) δ 10.07 (s, 1H,
-CHO), 8.30–7.90 (m, 6H), 7.74 (t, J=7.6 Hz, 1H), 7.49 (d, J=7.2
Hz, 1H), 6.99 (m, 4H), 6.79 (s, 2H), 4.35 (t, J=2.4Hz, 2H), 4.13
(t, J=2.4 Hz, 2H), 3.55 (q, J=7.2 Hz, 8H), 1.17 (t, J=6.8 Hz, 12H;
Fig. 1B); EI-HRMS
(C38H39N2O6+;
Fig. 1C). Molecular weight:
619.2707. Thus, S1-4 was identified as the target compound.
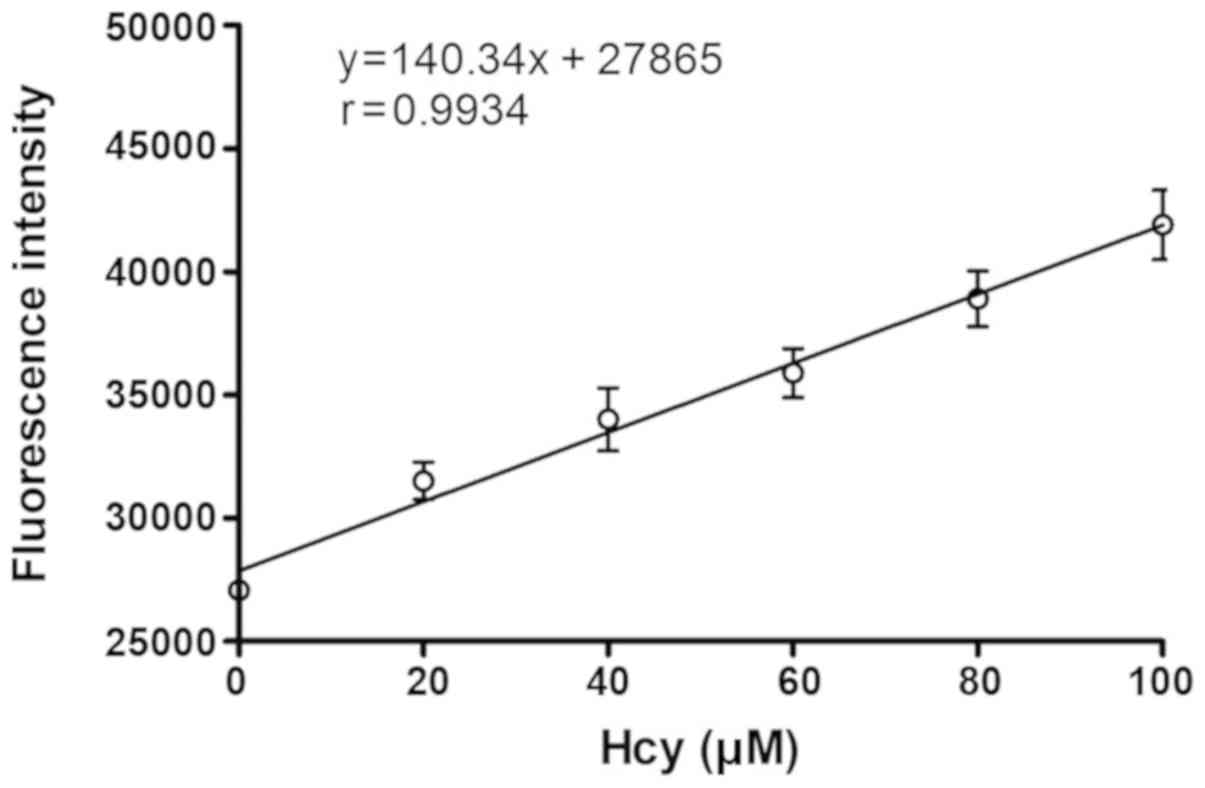
Linearity of Hcy probe detection
Following probe synthesis, to explore the
correlation between fluorescence intensity and Hcy concentration,
the fluorescence intensity of the S1-4 probe in the presence of
ascending concentrations of Hcy was measured. A plot displaying
fluorescence intensity of the probe vs. Hcy concentration was
obtained (Fig. 2). At the
concentration range of 0–100 µM, fluorescence intensity of the
probe exhibited a good linear relationship with Hcy concentration
(r=0.9934).
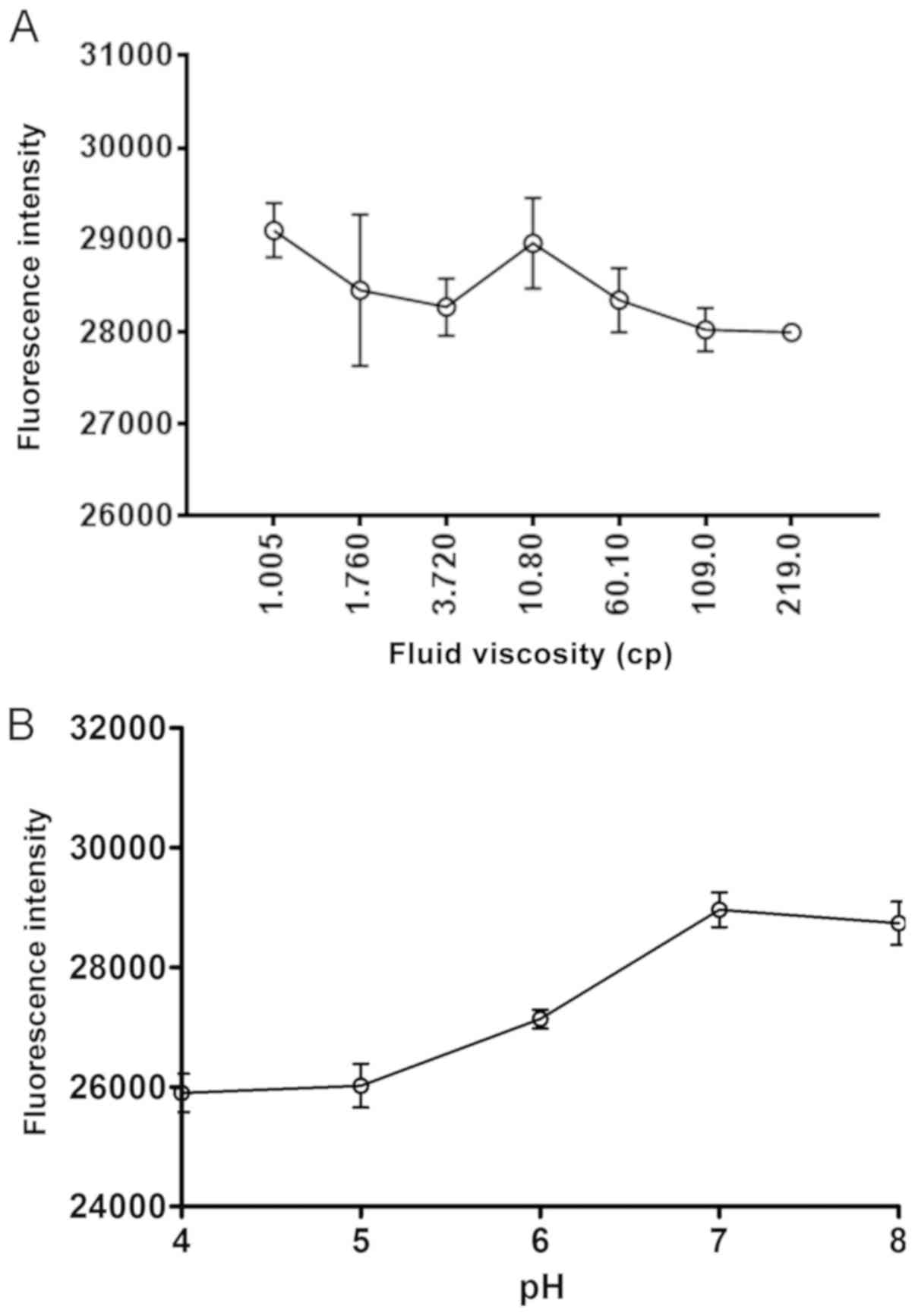
Effect of fluid viscosity and pH on
the responsiveness of the probe
Hcy at a concentration of 20 µM was selected as it
is the concentration found in the majority of healthy individuals
(8). To characterize the probe under
physiological conditions in the human bloodstream (including blood,
pH and viscosity), following reaction with 20 µM Hcy, the
fluorescence intensity of the S1-4 probe did not appear to be
affected by the increasing viscosities of the reaction mixture
(glycerol/water, 1.005–219 cp; Fig.
3A). The viscosity range was selected as it spans the normal
and abnormally high viscosities observed in healthy and patients
with diseases, respectively (9).
This suggested that probe activity is unlikely to be affected by
changes in blood viscosity induced by changes to physiological
functions or diseases (Fig. 4A). The
probe exhibited the most fluorescence intensity (responsiveness) to
Hcy at pH 7, which suggested that the activity of the probe is
unlikely to be affected by changes in blood pH induced by changes
to physiological functions or diseases (Fig. 3B).
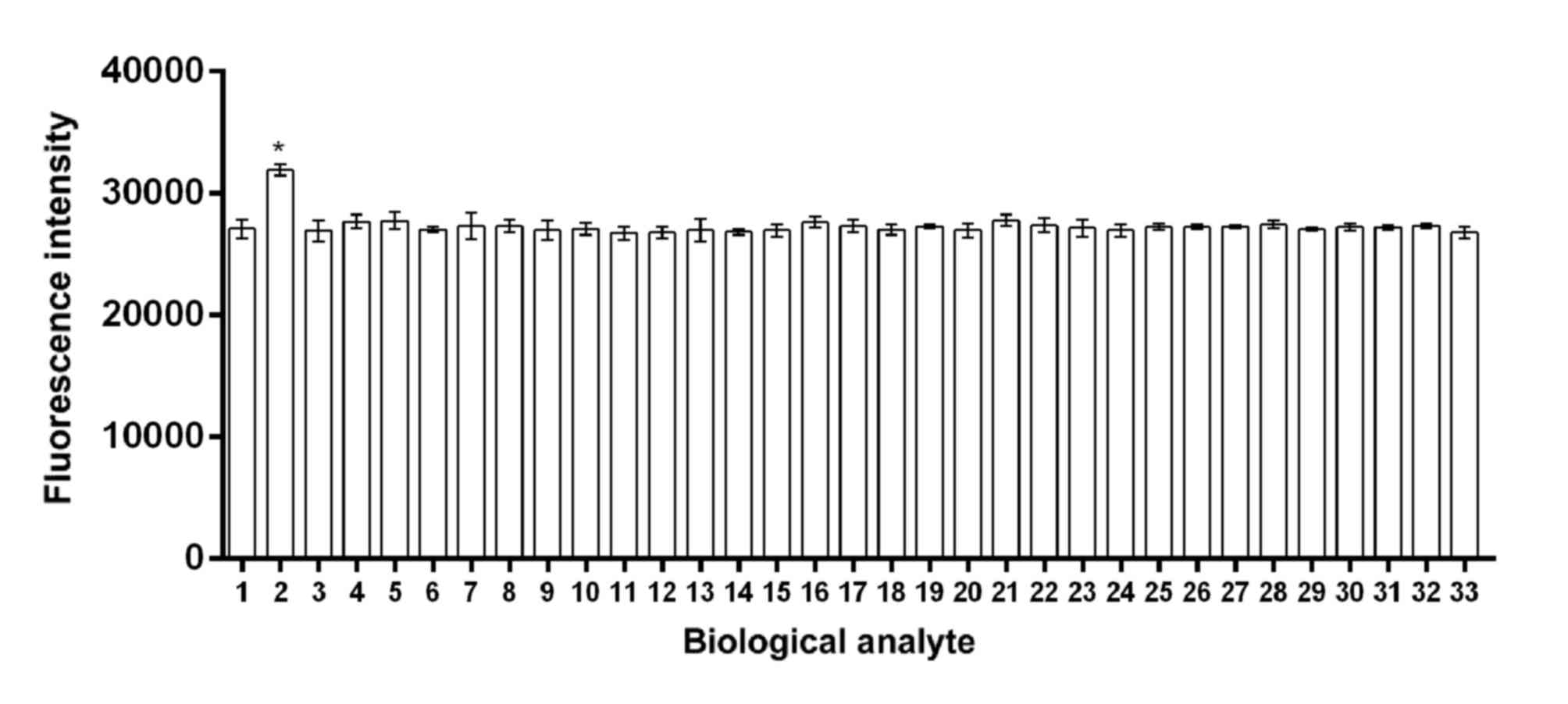 | Figure 4.Selectivity of the probe in the
presence of different biological analytes. The graph illustrates
that there was a significantly increased fluorescence intensity of
the probe in response to Hcy compared with other biological
analytes. *P<0.05 vs. the other biological analytes. 1,
cysteine; 2, Hcy; 3, N-acetylcysteine; 4, glutathione; 5, arginine;
6, asparagine; 7, aspartic acid; 8, β-alanine; 9, dithiothreitol;
10, glutamine; 11, glutamic acid; 12, glycine; 13, histidine; 14,
isoleucine; 15, L-alanine; 16, leucine; 17, lysine; 18, methionine;
19, phenylalanine; 20, proline; 21, serine; 22, threonine; 23,
tryptophan; 24, tyrosine; 25, valine; 26, potassium
(K+); 27, sodium (Na+); 28, calcium
(Ca2+); 29, magnesium (Mg2+); 30, zinc
(Zn2+); 31, aluminum (Al3+); 32, cadmium
(Cd2+); 33, manganese (Mn2+). Hcy,
homocysteine. |
Selectivity of the probe in the
presence of different biological analytes
None of the interfering substances tested, including
amino acids and high concentrations of various metal ions (see
Materials and methods), appeared to have adversely affected the
fluorescence intensity of the S1-4 probe in response to 20 µM Hcy
(Fig. 4). These observations
suggested that the responsiveness of the probe has good selectivity
for Hcy as the fluorescence intensity of Hcy was significant
greater compared with cysteine and glutathione.
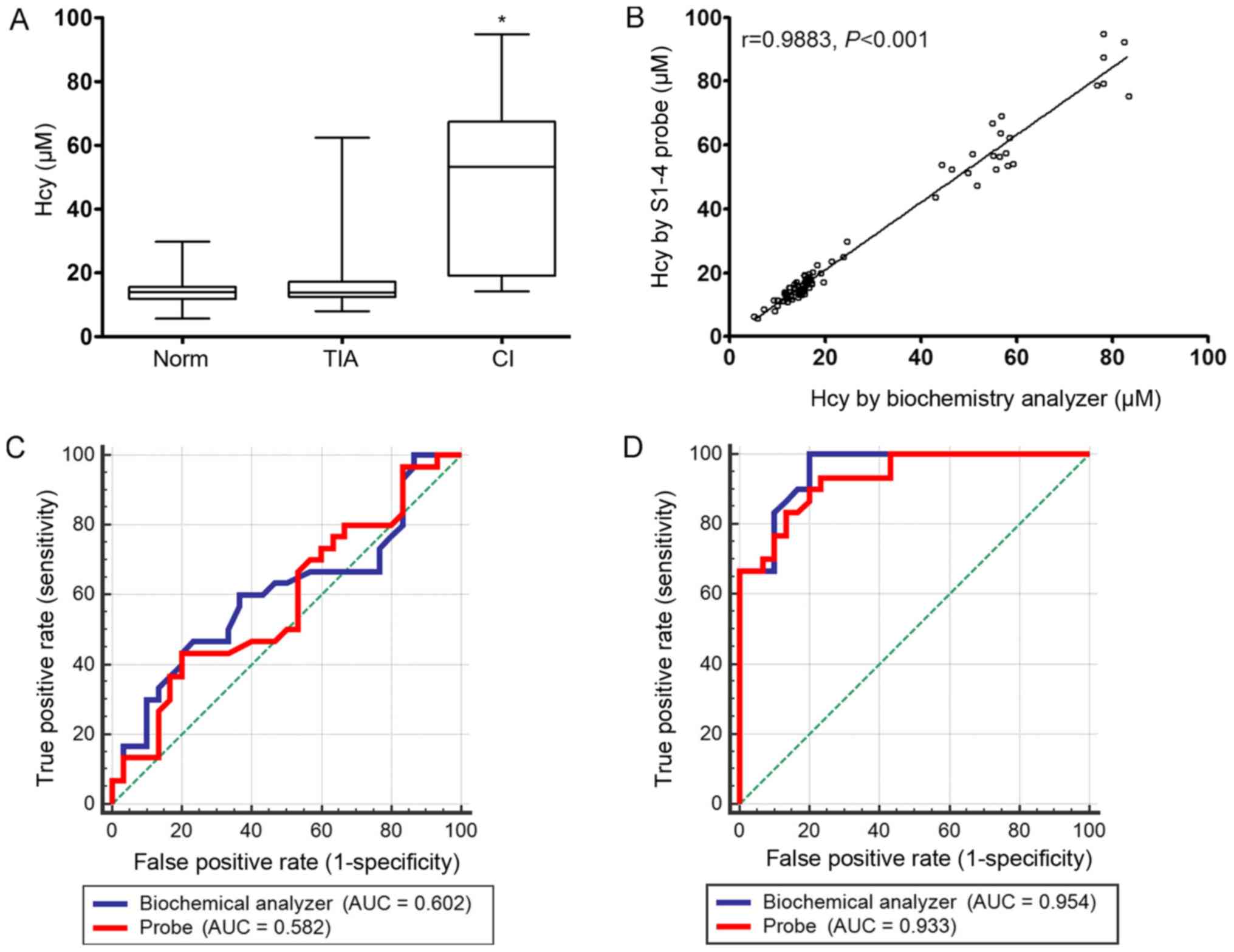
Measurement of Hcy in human serum
The probe was used to determine if it was able
measure serum Hcy levels in healthy people, in addition to patients
in the TCI and CI groups. The levels of Hcy between the three
groups could be distinguished by the probe. The serum levels of
Hcy, as measured by the probe, was significantly higher in the CI
group compared with the Norm group (Fig.
5A) and correlated positively with corresponding biochemistry
data as measured using a Biochemistry Analyzer (Fig. 5B). However, according to the ROC
analysis, the specificity and sensitivity of the S1-4 probe was the
same as the analysis by the Biochemistry Analyzer, which suggested
that the S1-4 probe needs to be optimized in further studies in the
CI group (Fig. 5C and D).
Discussion
Hcy is a sulfur-containing amino acid that cannot be
synthesized in vivo and can only be produced by the
catabolism of methionine (10).
Under normal circumstances, the Hcy content in the blood is very
low, and high concentrations of Hcy have been found to be an
independent risk factor for coronary artery disease,
cerebrovascular disease and peripheral vascular disease; the risk
of developing these conditions are increased with the increase in
Hcy concentration (11–14). Some developed and developing
countries, including China, the United States and Japan have large
and rapidly aging populations. Hcy is an important diagnostic
marker for cerebral infarction (15); therefore, it is of great importance
to develop an effective Hcy detection method with potentially
beneficial values in clinical applications.
Existing Hcy detection methods, such as enzymatic
methods, high performance liquid chromatography, capillary
electrophoresis separation and immunoturbidimetry, all entail high
detection costs and produce ambiguous data, because Hcy, GSH and
Cys compounds all contain a sulfhydryl group. Therefore, new Hcy
detection technologies that are accurate and efficient are an
urgent need in the clinical setting.
In the present study, Rhodamine B was used as the
parent compound, and a new compound that has not been previously
reported was synthesized. It was found that the probe could
selectively react with the amino group and the thiol group in Hcy.
It was hypothesized that the fluorescent probe could measure the
levels of Hcy and may be applied as a potential clinical detection
method for cerebral infarction. The authors then found, that the
spatial structure of S1-4 caused a change in the fluorescence
intensity of the system, effectively reflecting the level of Hcy
in vitro. Therefore S1-4 was researched as a priority in the
present study. From the fluorescence intensity measurements, it was
effectively demonstrated that the probe can enhance the
fluorescence intensity of the reaction media with Hcy. This meant
that Hcy could be selectively detected without off-target responses
to cysteine and other amino acids, resulting in good
selectivity.
Significant structural homology of the small
molecule sulfhydryl-containing biological compounds meant that few
studies have been able to report the direct detection of various
sulfhydryl compounds (such as Cys and GSH) using small molecule
fluorescent probes (16). The probe
reported in the present study exhibited good water solubility and
linear relationship with Hcy concentration, and also demonstrated
good selectivity even in the presence of common amino acid and ions
found in serum. Importantly, it could discriminate Hcy from other
sulfhydryl compounds such as Cys and GSH, potentially avoiding
off-target interference.
Clinically, this probe also effectively reflected
the level of Hcy in serum of healthy people, as well as patients
with TCI and CI. The measurements from clinical specimens
demonstrated that the fluorescence signal of the probes in patients
with CI was significantly higher compared with those in the healthy
control group and in the TIA group; which were comparable with
those obtained from the Biochemistry Analyzer. This result
supported the notion that this probe may have potential clinical
application value.
In a recent study, Yang et al (17) reported a near-infrared fluorescent
probe for Cys and Hcy detection using the colorimetry method.
However, the probe proposed by that study is suitable for
monitoring and visualizing Cys and Hcy in cells, whereas the probe
synthesized by the present study is only suitable for monitoring
Hcy in blood sera. In addition, ROC curve analysis reported
unsatisfactory probe specificity and sensitivity compared with data
from the Biochemistry Analyzer, which indicated a need to optimize
this probe for further study, such as by improving the chemical
structure and linking to better vehicles. In the future, the number
of specimens will need to be increased further, with the detection
results compared with those obtained using existing established
detection methods to develop a new type of Hcy detection probe. As
diabetes mellitus, fatty liver disease and other metabolic diseases
are also risk factors for cerebral infarction, the efficacy of the
S1-4 probe in measurements of Hcy levels in patients with these
diseases will also be explored further.
Acknowledgements
Not applicable.
Funding
The present study was supported by Tianjin Science
and Technology Key Project on Chronic Diseases Prevention and
Treatment (grant no. 16ZXMJSY-00020), Tianjin Municipal Special
Program of Talents Development for Excellent Youth Scholars, China
(grant no. TJTZJH-QNBJRC-2-9), Tianjin 131 Creative Talents
Cultivation Project (1st Class, 2016).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YZ and YM conceived and designed the study. XZ
acquired the data. XL performed the data analysis.
Ethics approval and consent to
participate
This study was approved by the medical ethics
committee of Tianjin Huanhu Hospital (Tianjin, China), in
accordance with the Declaration of Helsinki; all patients provided
informed consent prior to enrollment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhong C, Xu T, Xu T, Peng Y, Wang A, Wang
J, Peng H, Li Q, Geng D, Zhang D, et al: Plasma homocysteine and
prognosis of acute ischemic stroke: A gender-specific analysis from
CATIS randomized clinical trial. Mol Neurobiol. 54:2022–2030. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu J, Zhu Y, Wu Y, Liu Y, Teng Z and Hao
Y: Association of carotid atherosclerosis and recurrent cerebral
infarction in the Chinese population: A meta-analysis.
Neuropsychiatr Dis Treat. 13:527–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun LC, Chen R, Fu C, Chen Y, Wu Q, Chen
R, Lin X and Luo S: Efficacy and safety of botulinum toxin type a
for limb spasticity after stroke: A meta-analysis of randomized
controlled trials. Biomed Res Int. 2019:83293062019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai CF, Anderson N, Thomas B and Sudlow
CL: Comparing risk factor profiles between intracerebral hemorrhage
and ischemic stroke in Chinese and white populations: Systematic
review and meta-analysis. PLoS One. 11:e01517432016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu W, Guan Y, Xu K, Fu XJ, Lei XF, Lei LJ,
Zhang ZQ, Cheng Y and Li YQ: Plasma homocysteine levels predict the
risk of acute cerebral infarction in patients with carotid artery
lesions. Mol Neurobiol. 54:2510–2517. 2016. View Article : Google Scholar
|
|
6
|
Fox CK, Mackay MT, Dowling MM, Pergami P,
Titomanlio L and Deveber G; SIPS Investigators, : Prolonged or
recurrent acute seizures after pediatric arterial ischemic stroke
are associated with increasing epilepsy risk. Dev Med Child Neurol.
59:38–44. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cioni G, Marcucci R, Gori AM, Valente S,
Giglioli C, Gensini GF, Abbate R and Boddi M: Increased
homocysteine and lipoprotein(a) levels highlight systemic
atherosclerotic burden in patients with a history of acute coronary
syndromes. J Vasc Surg. 64:163–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar M and Sandhir R: Neuroprotective
effect of hydrogen sulfide in hyperhomocysteinemia is mediated
through antioxidant action involving Nrf2. Neuromolecular Med.
20:475–490. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Senturk B, Akdeniz B, Yilmaz MB, Ozcan
Kahraman B, Acar B, Uslu S and Birlik M: Whole blood viscosity in
systemic sclerosis: A potential biomarker of pulmonary
hypertension? Clin Rheumatol. May 26–2019.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ling-CongHong-ZhaoYu-WangYu-LiXin-Sui, .
The serum homocysteine level in patients with acute ischemic stroke
(AIS) after thrombolysis and its relationship with clinical
outcomes. Rev Assoc Med Bras (1992). 64:438–442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X, Zhang L, Miao Y, Yang J, Wang X,
Wang CC, Feng J and Wang L: Homocysteine causes vascular
endothelial dysfunction by disrupting endoplasmic reticulum redox
homeostasis. Redox Biol. 20:46–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Pyo S, Yoon DW, Lee S, Lim JY, Heo
JS, Lee S and Shin C: The co-existence of elevated high sensitivity
C-reactive protein and homocysteine levels is associated with
increased risk of metabolic syndrome: A 6-year follow-up study.
PLoS One. 13:e02061572018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu H, Wang C, Jin Y, Meng Q, Liu Q, Liu Z,
Liu K, Liu X and Sun H: Catalpol inhibits homocysteine-induced
oxidation and inflammation via inhibiting Nox4/NF-κB and GRP78/PERK
pathways in human aorta endothelial cells. Inflammation. 42:64–80.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saoji R, Das RS, Desai M, Pasi A, Sachdeva
G, Das TK and Khatkhatay MI: Association of high-density
lipoprotein, triglycerides, and homocysteine with bone mineral
density in young Indian tribal women. Arch Osteoporos. 13:1082018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Quan J, Li Y, Wu Y and Yang L:
Blood homocysteine levels could predict major adverse cardiac
events in patients with acute coronary syndrome: A STROBE-compliant
observational study. Medicine (Baltimore). 97:e126262018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W, Liu J, Yu Y, Han Q, Cheng T, Shen
J, Wang B and Jiang Y: A novel near-infrared fluorescent probe for
highly selective detection of cysteine and its application in
living cells. Talanta. 185:477–482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Wang Y, Zhao MX and Yang W: A
colorimetric and near-infrared fluorescent probe for cysteine and
homocysteine detection. Spectrochim Acta A Mol Biomol Spectrosc.
212:10–14. 2019. View Article : Google Scholar : PubMed/NCBI
|