Introduction
With the rapid increase in the number of motor
vehicles, the air quality in cities is increasingly deteriorating
(1). Fine particles [particulate
matter with an aerodynamic diameter smaller than 2.5 µm
(PM2.5)] cause ozone pollution, which leads to secondary
pollution by oxidized pollutants under specific meteorological
conditions, which in turn directly influence the health of most
inhabitants in a polluted environment (1,2). Due to
the characteristics of road traffic distribution, a considerable
part of the population is under long-term exposure to serious
PM2.5 pollution in such a polluted environment (3,4). Thus,
the health risks of PM2.5 pollution cannot be ignored
(1,2). Therefore, research on the effects of
exhaust pollution on the human body, as well as the development of
effective protection technologies, is necessary.
Diesel exhaust is not a single component, but a
mixture of hundreds of gaseous and solid pollutants (5–7). Among
them, the fine solid particles are mainly responsible for chronic
lung injury. Pollutants in the range from PM2.5 to
PM10 have been demonstrated to induce respiratory burst
in RAW264.7 cells and could induce chronic lung injury (8–10).
Previous studies showed that numerous acute toxicological
experiments using single components of diesel exhaust confirmed
that a variety of exhaust components can lead to an increased
production of oxygen free radicals, thus directly damaging lung
cells, and influencing digestive function, growth and development
by negatively affecting Ca2+ balance (11,12).
In vivo studies have revealed that the expression of
transforming growth factor (TGF)-β1 is positively correlated with
the duration of exposure to and the amount of atmospheric
particulate matter, such as PM2.5, in the environment
(13–17). It was also demonstrated that the
serum levels of interleukin (IL)-4 and tumor necrosis factor
(TNF)-α are positively correlated with the duration of exposure to
air pollution, and that IL-4 levels were correlated with gaseous
pollutants, such as NOx, while TNF-α levels were
correlated with particulate matter, such as PM2.5
(18,19). In a study of the morphological
changes in chronic lung injury of rats caused by gasoline exhaust
pollution, inflammatory reactions were observed in the first 2
weeks, followed by lung fibroblast hyperplasia after 4 weeks
(20). The hyperplasia of
fibroblasts was increasingly evident with time and has been
associated with chronic inflammatory reaction (21–23).
However, the detailed changes in macrophage morphology and the
macrophage M1-M2 balance remain unclear.
The air quality in the Central Laboratory of the
First Affiliated Hospital of Sun Yat-sen University used by our
group meets the requirements of GB3095-2012 standard class II of
China (24), while the pollution
levels exceed the national standard in underground parking lots. In
the current study, the authors evaluated the levels of three
representative indicators of pollution: CO, NOx and
PM2.5, in an underground parking lot and the laboratory.
After living in the environment for 3 months, the ultrastructure in
the lungs of mice was examined by electron microscopy, and
morphological structures were detected by hematoxylin and eosin
(H&E) staining. To study the changes in the macrophage M1-M2
balance, double fluorescence staining of adhesion G protein-coupled
receptor E1 (F4/80) with human leukocyte antigen (HLA)-DR or
cluster of differentiation (CD)163 was further performed in lung
tissue sections.
Materials and methods
Animals
A total of 20 female Kunming mice, 8–10 weeks old,
weighing 22–25 g, were provided and approved by the Experimental
Animal Ethics Committee Center of The First Affiliated Hospital of
Sun Yat-sen University (Guangzhou, China; animal certificate no.
4408500931). The mice were randomly divided into two groups:
Control and polluted (10 mice per group).
Feeding location and exposure to
pollution
The mice were housed as previously described
(25). In brief, the mice were
housed at the Central Laboratory of the First Affiliated Hospital
of Sun Yat-sen University in a controlled environment (food and
water available ad libitum, 21±1°C, 60% humidity and 12-h
light/dark cycle). The mice in the polluted group were fed at the
Second underground passage in a parking lot at the southeast corner
of the First Affiliated Hospital of Sun Yat-sen University: At this
location, there was a high flux of cars, and it was far from the
exit and exhaust air outlets, therefore it had a relatively high
level of pollution.
As described previously (26), PM2.5 samples were
collected using a Thermo Anderson G-2.5 air sampler (FH62C14, Model
GV 2630 Series; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
that was installed in glass fiber filters. Firstly, the filters
were heated at 200°C for 24 h prior to sampling. Then, the filters
were cut into small pieces, immersed in 0.9% saline and sonicated.
The PM2.5 suspension was vacuum-freeze dried and diluted
with sterilized 0.9% physiological saline.
As described previously (27), the mice in the polluted group were
treated with PM2.5 at 20 mg/kg body weight for 3 days
(once every other day). The mice in the control group were fed in
the animal laboratory in The First Affiliated Hospital of Sun
Yat-sen University. During the feeding period, all mice were free
to eat and drink. The mice were treated and fed as described for 3
months. Live mice were injected with inhalational anesthetics, and
then were sacrificed humanely using carbon dioxide asphyxiation.
The mice were necropsied soon following the completion of the
exposure protocol. During the study the body weight was assessed
every 3 days.
Determination of the concentration of
pollutants
Measurements of the concentration of pollutants in
the cages in the underground garage and in the laboratory were
performed using three ambient air quality monitoring instruments.
The concentration of NOx was measured at the designated
time-points using a 42C chemiluminescence
NO-NO2-NOx analyzer (Thermo Environmental
Instruments; Thermo Fisher Scientific, Inc.). For the determination
of PM2.5 concentration (28), a β-ray particle monitoring instrument
(model FH62C14; Thermo Fisher Scientific, Inc.) was used to measure
the attenuation of β-rays through the particles suspended in the
air. CO concentration was determined using a gas correlation
infrared CO analyzer (model 48C; Thermo Environmental Instruments;
Thermo Fisher Scientific, Inc.). The concentrations of pollutants
at the two feeding locations were measured 12 times a day.
Electron microscopy examination
Lung tissues samples were examined using electron
microscopy as previously described (29,30). In
brief, the mice were sacrificed, and the lung tissues were removed.
Lung tissue samples were fixed with 2.5% glutaraldehyde for 2 h at
4°C followed by 1% osmium tetroxide for 1 h at room temperature.
The samples were dehydrated at room temperature using a series of
acetone solutions of increasing concentration. Subsequently, they
were counterstained in a saturated solution of uranyl acetate for 2
h at room temperature, followed by lead citrate treatment for 40
min at room temperature. Finally, sections (60 nm) were examined
using a transmission electron microscope at a magnification of
×50,000. In addition, the morphological changes in type II alveolar
epithelial cells and macrophages from the lungs were also observed
by electron microscopy. The morphological features of type II
alveolar epithelial cells include large nuclei and multiple
vacuoles, and mitochondria and rough endoplasmic reticulum in the
cytoplasm (31). The surfaces of
macrophages were covered with multiple irregular folds, microvilli
and pseudopods; the cytoplasm contained a large number of
lysosomes, phagosomes and vesicles; and there were many
microfilaments and microtubules near the cell membrane (32).
Hematoxylin and eosin staining
All samples were treated with 4% paraformaldehyde
(cat. no. #P6148, Sigma-Aldrich; Merk KGaA) for 24 h at 4°C. The
samples were then treated with hematoxylin (cat. no. ZLI-9609;
OriGene Technologies, Inc., Rockville, MD, USA) for 10 min, 70%
ethanol and 1% hydrochloric acid at 25°C. The sections (4-µm-thick)
were stained with 0.1% eosin (Surgipath®; Leica
Microsystems, Ltd., Milton Keynes, UK) for 30 sec, and dehydrated
in 80%, 90 and 100% ethanol at 25°C. Ethanol was used to dehydrate
the sections at 25°C and the results were examined under an optical
microscope (Eclipse E100; Nikon Corporation, Tokyo, Japan;
magnification, ×100).
ELISA
At the end of treatment in mice, blood was collected
from mice and rapidly centrifugated at 5,000 × g for 10 min at 4°C.
The supernatants were stored at −20°C until analysis. ELISA kits
for mouse IL-4 (cat. no. EK0405; Boster Biological Technology,
Pleasanton, CA, USA), TNF-α (CSB-E04741m) and TGF-β1 (CSB-E4726m;
both Cusabio Technology LLC, Wuhan, China) were used according to
the manufacturers' protocol.
Double immunofluorescence
staining
Lung tissues collected from mice were fixed, cut
into sections and stained using the standard procedure (33). The slides were examined and
photographed using fluorescence microscopy (ECLIPSE TI-SR; Nikon
Corporation) using a magnification of ×100. The slides were blocked
with 5% bovine serum albumin (cat. no. A7638; Sigma Aldrich; Merck
KGaA, Darmstadt, Germany) for 1 h at room temperature. The slides
were incubated with primary antibodies at 4°C overnight. The
following day, the slides were incubated with
fluorescence-conjugated secondary antibodies (1:200; cat. no.
A-11029; Alexa Fluoro, Molecular Probes; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. The fluorescence intensities of
CD68, F4/80, CD163 and HLA-DR were calculated using Image Pro Plus
6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). The
following primary antibodies were used: Anti-CD68 (1:25; cat. no.
ab201844), anti-F4/80 (1:100; cat. no. ab100790), anti-CD163 (1:10;
cat. no. ab17051) and anti-HLA-DR (1:500; cat. no. ab226820; all
Abcam, Cambridge, UK).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The RNA was extracted from the lung tissues by using
TRIzol reagent (cat. no. #9109; Takara Bio Inc., Otsu, Japan) and
its concentration was measured using the Nanodrop 2000
Spectrophotometer (Thermo Fisher Scientific, Inc.). RNA was reverse
transcribed into cDNA by using the Reverse Transcription kit (cat.
no. RR037A; Takara Bio Inc.) according to the manufacturer's
protocol. The qPCR was performed using the TB Green Premix Ex TaqII
kit (cat. no RR820A; Takara Bio Inc.). The reaction system was
performed in a volume of 20 µl and the thermocycling conditions
were as follows: Initial denaturation at 95°C for 30 sec, and 40
cycles of 95°C for 5 sec and 60°C for 34 sec.
Gene expression was normalized to the level of GAPDH
within each sample using the 2−ΔΔCq methods (34). Gene expression was shown as
expression relative to the indicated controls. The specific primers
were: IL-4: 5′-AACGAGGTCACAGGAGAAGG-3′ (forward),
5′-TCTGCAGCTCCATGAGAACA-3′ (reverse); TNF-α:
5′-AGGTCCAGCTCTTTTCCTCC-3′ (forward), 5′-TGGGGCTGAAGTGTAGATGG-3′
(reverse); TGF-β1: 5′-TCGCTTTGTACAACAGCACC-3′ (forward),
5′-ACTGCTTCCCGAATGTCTGA-3′ (reverse); and GAPDH:
5′-ATGGTGAAGGTCGGTGTGAA-3′ (forward), 5′-TGGAAGATGGTGATGGGCTT-3′
(reverse).
Western blotting
Protein extraction and blotting were performed as
previously described (35). Proteins
were extracted using RIPA buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Following the determination of
protein concentration by a Protein Determination kit (cat. no.
704002; Cayman Chemical Company, Inc., Ann Arbor, MI, USA), equal
amounts of protein samples (20 µg loaded per lane) were
size-fractioned using SDS-PAGE (8% gels), electrotransferred onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were blocked with 5% skimmed milk
(BD Biosciences), and then were hybridized with primary antibodies
overnight at 4°C. After washing, the membranes were incubated with
horseradish peroxidase conjugated secondary antibodies (1:10,000;
cat. no. #W4011; Promega Corporation, Madison, WI, USA) for 2 h at
room temperature. Finally, the enhanced chemiluminescent reagents
(MILLIPORE, WBKLS0500) was used to treat the membranes.
Densitometric quantification of bands was performed using the
ImageJ software (version 1.50; National Institutes of Health,
Bethesda, MD, USA). The following primary antibodies were used:
Anti-GAPDH (cat. no. ab9485; 1:2,500), anti-IL-4 (cat. no. ab9811),
anti-TNF-α (cat. no. ab6671) and anti-TGF-β1 (cat. no. ab92486; all
1:1,000; all Abcam).
Statistical analysis
All data were analyzed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA) with Student's t-test and reported
as mean ± standard error of the mean (n=10). P<0.05 indicated
that the difference between groups was statistically
significant.
Results
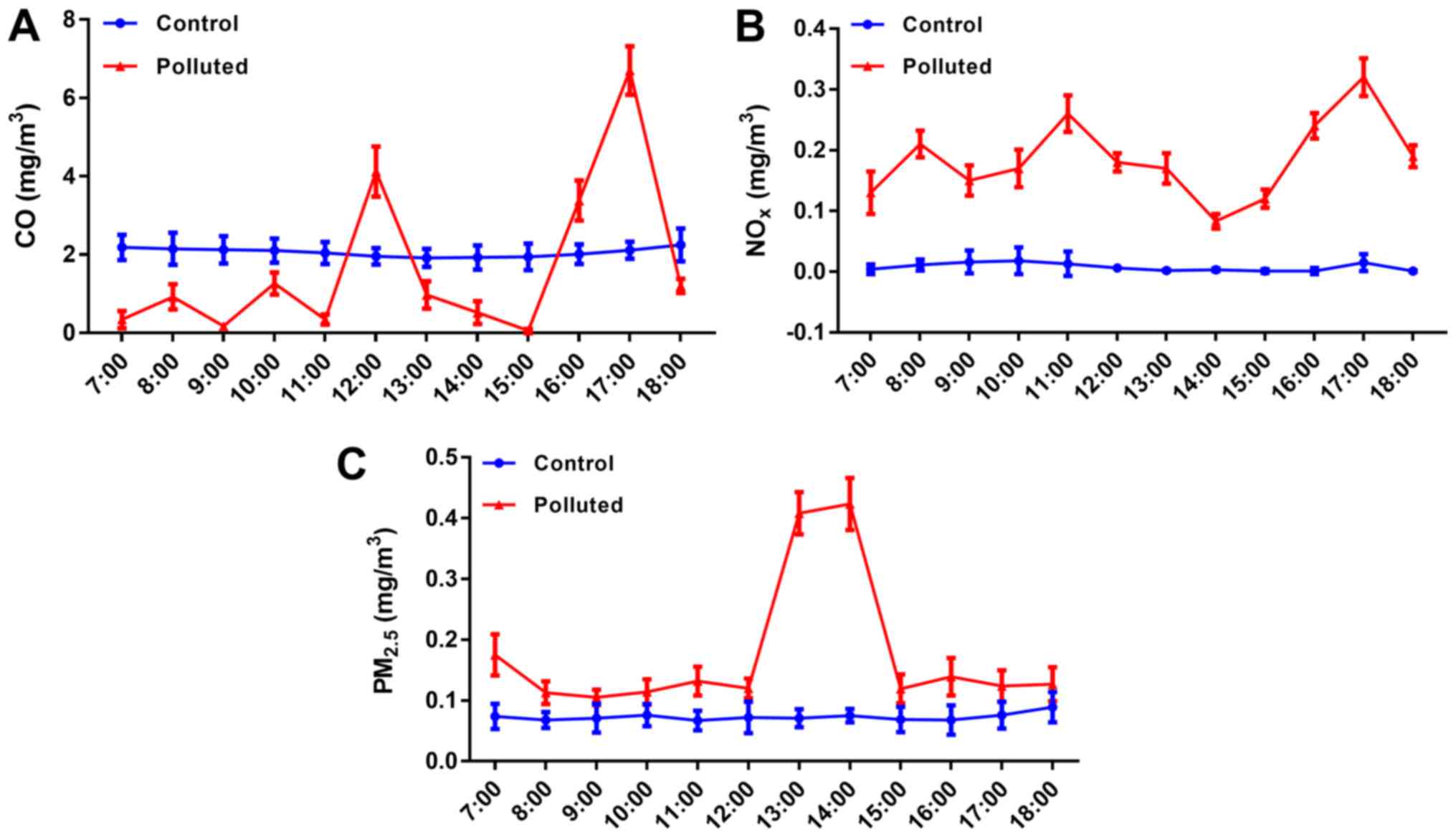
NOx and PM2.5
concentrations are markedly high, and CO concentrations markedly
fluctuate in the underground parking lot
The atmospheric pollutants, CO, NOx and
PM2.5, were examined (Fig.
1). In the cage in the laboratory, the levels of CO,
NOx and PM2.5 were stable at ~2, 0.01, and
0.08 mg/m3, respectively. In the cage in the underground
garage, NOx and PM2.5 fluctuated considerably
and were markedly higher compared with those levels in the
laboratory at all time-points (7:00 am to 6:00 pm), whereas CO
levels were increased in the underground garage compared with the
laboratory at 12:00, 16:00 and 17:00 pm.
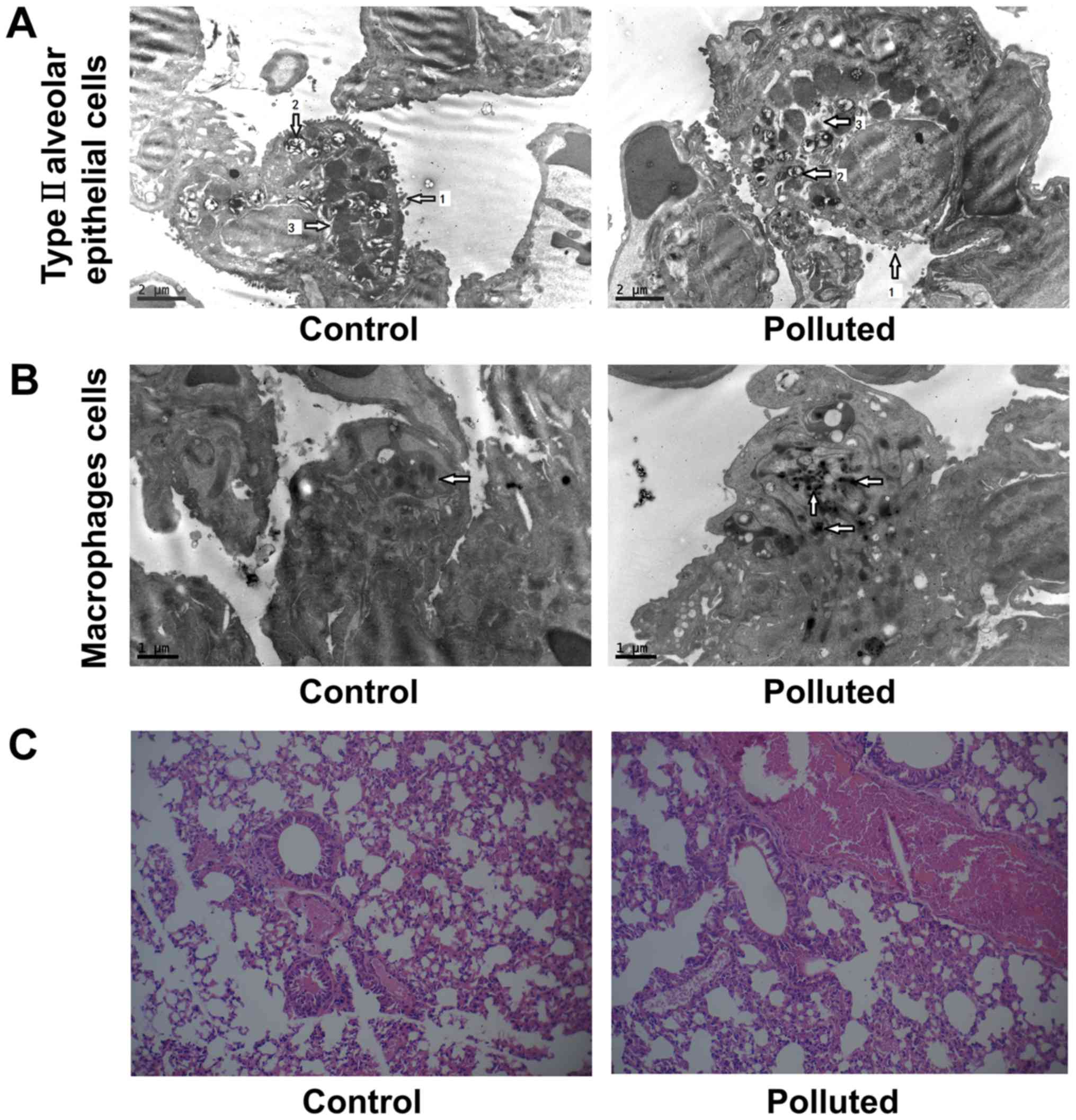
Atmospheric pollutants induce lung
damage
The morphological changes in type II alveolar
epithelial cells and macrophages from the lungs were examined by
electron microscopy (Fig. 2A and B).
In the control mice, microvilli and lamellar bodies were abundant
in type II alveolar epithelial cells, which did not exhibit
intracellular edema (Fig. 2A). In
mice fed in the underground garage, the microvilli and the lamellar
bodies were markedly reduced in type II alveolar epithelial cells.
The size of lamellar bodies also decreased. In addition,
intracellular edema was present in the type II alveolar epithelial
cells. These findings indicated functional impairment of the
cells.
In the control mice, macrophages contained a few
low-density particles, but not the high-density particles of
inorganic substances (Fig. 2B). The
observed particles could have been bacteria or products of
decomposition, such as remnants of cell necrosis. In the polluted
group, there was a large number of high-density inorganic particles
in the lung macrophages. The particles deposited in the lungs were
PM2.5 from diesel exhaust pollution, which may have
activated the macrophages.
Thus, the changes in the morphology of type II
alveolar epithelial cells and macrophages suggested that diesel
exhaust induced lung damage. During the study, no changes in the
body weight or clinical parameters of the mice were observed (data
not shown).
Furthermore, morphological structures of lung tissue
were assessed by H&E staining in the control and polluted
groups (Fig. 2C). The tissues were
comparatively complete and the nuclei were clearly visible in the
control group, while the tissues were damaged in the polluted
group. The number of inflammatory cells, including neutrophils,
polylymphocytes and eosinophils, increased in the polluted group
compared with the control group (data not shown).
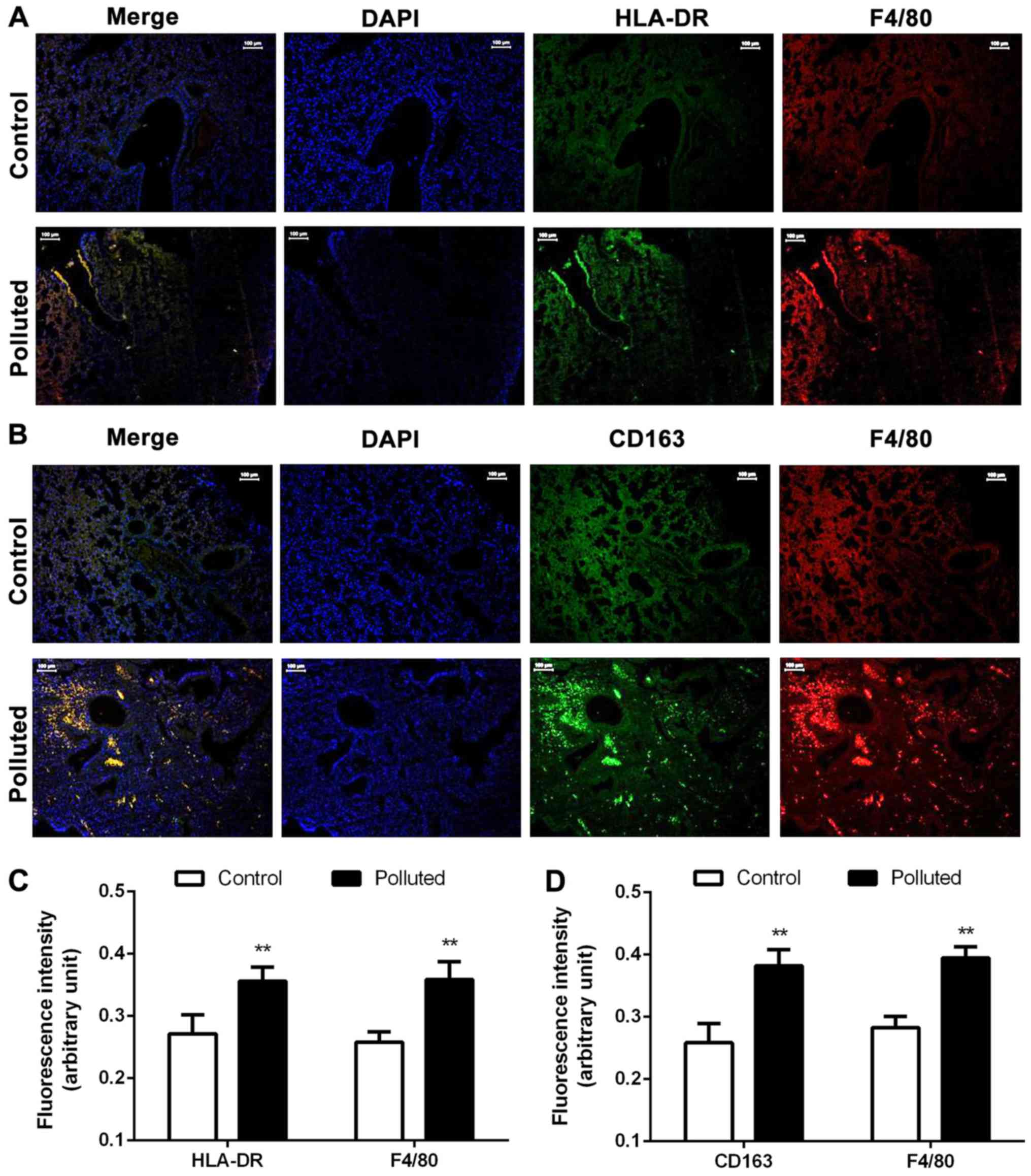
HLA-DR, CD163 and F4/80 protein
expression increases in the lungs of the polluted group
To further confirm the lung injury, double
immunofluorescence staining for HLA-DR and F4/80, which are
expressed in M1 polarized macrophages (36), and for CD163 and F4/80, which are
expressed in M2 polarized macrophages, was performed (Fig. 3). In the polluted group, HLA-DR and
F4/80 expression levels were markedly increased, while CD163 and
F4/80 expression levels were also markedly increased (Fig. 3A and B). The protein expression
levels of HLA-DR and F4/80 were significantly increased in the
polluted group compared with the control group, suggesting an
increased number of M1 polarized macrophages, which express HLA-DR
(Fig. 3C). Similarly, CD163 and
F4/80 protein expression levels were significantly increased in the
polluted group, suggesting an increased number of M2 polarized
macrophages, which express CD163 (Fig.
3D). Thus, the numbers of M1 and M2 macrophages were increased,
and M1 and M2 macrophages infiltrated the tissue. CD163 and HLA-DR
could thus be used for monitoring the extent of lung injury in a
polluted environment.
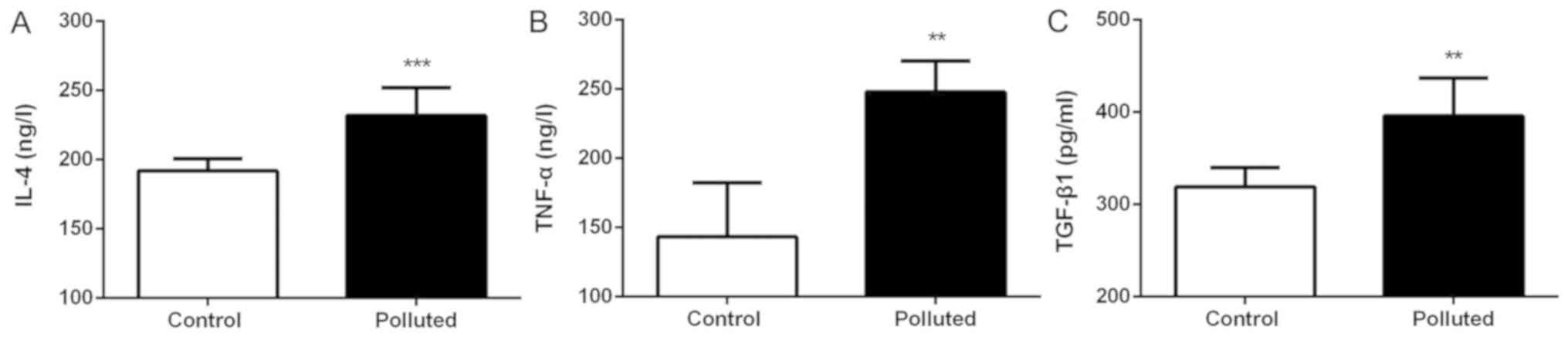
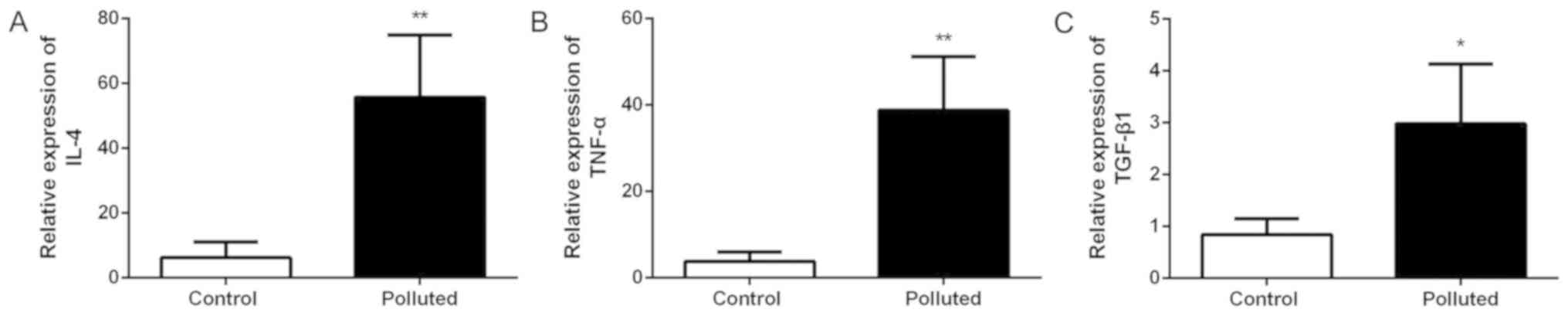
IL-4, TNF-α and TGF-β1 serum levels
increase in the polluted group
The serum levels of the cytokines, IL-4, TNF-α and
TGF-β1, were examined (Fig. 4). In
the mice fed in the polluted environment, the serum levels of IL-4
(Fig. 4A), TNF-α (Fig. 4B) and TGF-β1 (Fig. 4C) were significantly increased
compared with those mice fed in the laboratory.
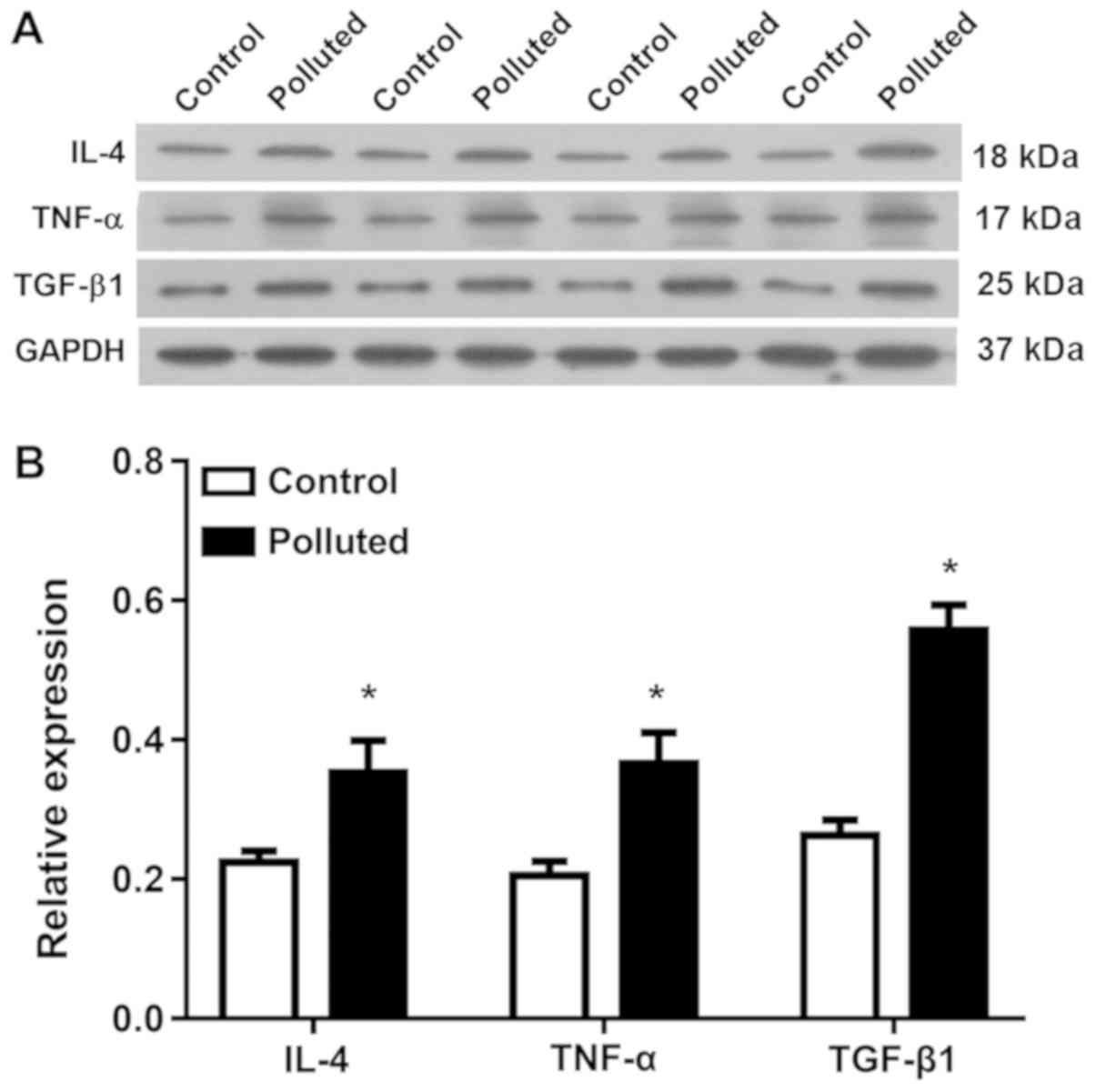
IL-4, TNF-α and TGF-β1 mRNA and
protein levels increase in the lungs of the polluted group
The mRNA and protein levels of the cytokines, IL-4,
TNF-α, and TGF-β1, in lung tissues were assessed by RT-qPCR
(Fig. 5) and western blotting
(Fig. 6). Atmospheric pollutants
significantly upregulated the mRNA levels of IL-4 (Fig. 5A), TNF-α (Fig. 5B) and TGF-β1 (Fig. 5C), as well as markedly (Fig. 6A) and significantly (Fig. 6A) upregulating the protein levels of
IL-4, TNF-α and TGF-β1. Thus, atmospheric pollutants increased the
levels of these cytokines in lung tissues.
Discussion
The authors of the current study demonstrated that
the levels of three representative indicators of pollution, CO,
NOx and PM2.5, in an underground parking lot
were significantly higher compared with those in the laboratory.
The air quality in the laboratory met the requirements of
GB3095-2012 standard class II of China, while, in the underground
parking lot, the pollution levels exceeded the national standard by
far (37).
Numerous acute toxicological experiments using
single components of diesel exhaust confirmed that a variety of
these components can lead to an increased production of oxygen free
radicals, thus directly damaging lung cells and influencing
digestive function, growth, and development by negatively affecting
Ca2+ balance (11). After
feeding in the polluted environment for 3 months, findings revealed
that atmospheric pollutants could induce lung damage. In addition,
it was determined that the number of inflammatory cells increased,
including neutrophils, lymphocytes and eosinophils. These results
support the hypothesis that lung injury can be induced by oxygen
free radicals. Previous research has shown that particulate matter
exposure could change macrophage morphology (8,38), which
is consistent with the observation of macrophages in lung tissue
using electron microscopy. The deposition of a large number of dust
particles in macrophages has been demonstrated to induce macrophage
apoptosis, which is also mediated by an increase in oxygen free
radicals and injury of mitochondria in macrophages (39–41). If
the deposition becomes more serious, macrophages are overburdened,
eventually leading to decreased phagocytosis, thus influencing
immune defense function (42). The
change in oxygen free radicals was not evaluated in the current
study.
Research has demonstrated that M1-polarized
macrophages expressing HLA-DR have a pro-inflammatory effect, while
M2-polarized macrophages expressing CD163 have the function of
immunosuppressive tissue repair (43). The present results indicated that the
number of M1 and M2 macrophages increased, and there was a large
number of macrophage aggregation and infiltration in lung tissues
of mice that were fed in the polluted environment. Therefore, the
increase of M1 and M2-polarized macrophages may be an important
cause of immune dysfunction caused by pollution. Particles/dust are
solid or liquid particulate matter in the atmosphere (44). According to particle size, particles
can be divided into total suspended particles and inhalable
particles. Inhalable particles can be divided into coarse particles
(PM10: aerodynamic diameter between 2.5–10 µm) and fine particles
(PM2.5 ≤2.5 µm) (45). As an
important air pollutant, particle size, shape and composition are
associated with health (46).
Previous research showed that inhalable particles are responsible
for chronic lung injury (47). A
large number of studies have confirmed that, due to their very
small size, PM10 particles easily enter the lungs
(48,49). With adsorption of heavy metals and
other toxic substances, the surface area of those particles becomes
relatively large (50,51). As a result, PM10 induces a
potent inflammatory effect by increasing intracellular
Ca2+, as well as the phenotypic transition of
macrophages to M1 macrophages (52).
The pollutants in the range from PM2.5 to
PM10 have been demonstrated to induce respiratory burst
in RAW264.7 cells, promoting the formation of a large number of
oxygen free radicals, the imbalance of the oxidant and antioxidant
system, and epithelial cell damage with increased epithelium
permeability, all eventually resulting in chronic lung injury
(8–10). The lung injury in turn promotes the
phenotypic transition of macrophages to M2 macrophages (53). The excessive increase in M2
macrophage numbers has been demonstrated to have negative
consequences, including pulmonary immune suppression, and an
increased incidence of pulmonary fibrosis and lung cancer (54,55).
The experimental results by ELISA demonstrated that
the serum levels of IL-4, TNF-α and TGF-β1 in the polluted group
were significantly higher compared with the control group. Similar
results were observed in the lung tissues. The mRNA and protein
levels of IL-4, TNF-α and TGF-β1 in the polluted group were also
significantly higher compared with the control group. The increase
in the level of proteins was evidently less compared with that of
mRNAs, which may be due to the regulation of the expression at the
translation level.
In vivo studies have revealed that the
expression of TGF-β1 is positively correlated with the duration of
exposure to and the amount of atmospheric particulate matter, such
as PM2.5, in the environment (13–17). It
was also demonstrated that the serum levels of IL-4 and TNF-α were
positively correlated with the duration of exposure to air
pollution and that IL-4 levels were correlated with gaseous
pollutants, such as NOx, while TNF-α levels were
correlated with particulate matter, such as PM2.5
(18,19). Those results are consistent with our
findings. The mechanism of increased levels of inflammatory
cytokines in mice may be associated with the oxidative damage of
the respiratory mucosa by gaseous pollutants, such as
NOx, the stimulation of the lung epithelial cells by
PM2.5 and transition metals present in PM2.5
(56).
IL-4 is an important cytokine in the regulation of T
lymphocytes and the synthesis of immunoglobulin (Ig)E by B
lymphocytes (57). An increase in
IL-4 promotes the secretion of IgE, which is key for the onset of
allergic diseases (58). This is
also consistent with epidemiological surveys that revealed that air
pollution results in an increase in IgE levels and a higher
incidence of chronic pulmonary diseases, including asthma and
chronic obstructive pulmonary disease (59,60).
TNF-α is an important inflammatory factor and immunoregulatory
factor; its overexpression has been determined to promote the
aggregation of a variety of inflammatory cells, leading to abnormal
inflammatory response, immune dysfunction or a variety of
inflammatory diseases (61). Many
studies have revealed that TGF-β1 is a potent chemokine for
fibroblasts (62–64). In lung tissues, TGF-β1 upregulates
the expression of collagen and fibronectin to promote an increase
of the extracellular matrix; meanwhile, it downregulates the
secretion of proteases, but increases the secretion of protease
inhibitors to inhibit the degradation of the extracellular matrix,
thus participating in lung fibrosis (65). In a study of the morphological
changes in the chronic lung injury of rats caused by gasoline
exhaust pollution, inflammatory reactions were observed in the
first 2 weeks, followed by lung fibroblast hyperplasia after 4
weeks. The hyperplasia of fibroblasts was increasingly evident with
time and has been associated with chronic inflammatory reaction
(21,22). Recent research has indicated that
TNF-α and TGF-β1 promotes the development of inflammation and
pulmonary fibrosis (66,67).
In conclusion, the sub-chronic exposure to a highly
polluted environment, such as that with high PM2.5
levels, can lead to lung injury in mice. Although ultrastructural
damage, and local and systemic immune imbalance was observed,
further studies should focus on the protection of lungs against
injury caused by polluted environments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JY, YC and ZM conceived and supervised the study;
YC, ZY and HD designed and performed experiments; JY, YC and ZM
analyzed data, wrote the manuscript and made manuscript revisions.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Experimental
Animal Ethics Committee Center of The First Affiliated Hospital of
Sun Yat-sen University (Guangzhou, China; animal certificate no.
4408500931).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown MJ, Williams MD, Nelson MA and
Werley KA: QUIC transport and dispersion modeling of vehicle
emissions in cities for better public health assessments. Environ
Health Insights. 9 (Suppl 1):S55–S65. 2016.
|
|
2
|
Guan WJ, Zheng XY, Chung KF and Zhong NS:
Impact of air pollution on the burden of chronic respiratory
diseases in China: Time for urgent action. Lancet. 388:1939–1951.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandey P, Patel DK, Khan AH, Barman SC,
Murthy RC and Kisku GC: Temporal distribution of fine particulates
(PM(2).(5):PM(1)(0)), potentially toxic metals, PAHs and
Metal-bound carcinogenic risk in the population of Lucknow City,
India. J Environ Sci Health A Tox Hazard Subst Environ Eng.
48:730–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Volk HE, Lurmann F, Penfold B,
Hertz-Picciotto I and McConnell R: Traffic-related air pollution,
particulate matter, and autism. JAMA Psychiatry. 70:71–77. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zielinska B, Sagebiel J, McDonald JD,
Whitney K and Lawson DR: Emission rates and comparative chemical
composition from selected in-use diesel and gasoline-fueled
vehicles. J Air Waste Manag Assoc. 54:1138–1150. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ezzati M, Lopez AD, Rodgers A, Vander
Hoorn S and Murray CJ; Comparative Risk Assessment Collaborating
Group, : Selected major risk factors and global and regional burden
of disease. Lancet. 360:1347–1360. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
IARC monographs on the evaluation of
carcinogenic risks to humans, . Diesel and gasoline engine exhausts
and some nitroarenes. International Agency for Research on Cancer.
IARC Monogr Eval Carcinog Risks Hum. 46:1–458. 1989.PubMed/NCBI
|
|
8
|
Su R, Jin X, Zhang W, Li Z, Liu X and Ren
J: Particulate matter exposure induces the autophagy of macrophages
via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere.
167:444–453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitkus RJ, Powell JL, Zeisler R and Squibb
KS: Comparative physicochemical and biological characterization of
NIST interim reference material PM2.5 and SRM 1648 in human A549
and mouse RAW264.7 cells. Toxicol In Vitro. 27:2289–2298. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XC, Li YJ, Wang YJ, Li Q, Yang Q, Weng
XG, Chen Ying, Cai WY, Guo Y, Kan XX, et al: Protection of Shenlian
extracts to PM2.5 infected RAW 264.7 cell damage. Zhongguo Zhong
Yao Za Zhi. 40:1977–1983. 2015.(In Chinese). PubMed/NCBI
|
|
11
|
Yanamala N, Hatfield MK, Farcas MT,
Schwegler-Berry D, Hummer JA, Shurin MR, Birch ME, Gutkin DW, Kisin
E, Kagan VE, et al: Biodiesel versus diesel exposure: Enhanced
pulmonary inflammation, oxidative stress, and differential
morphological changes in the mouse lung. Toxicol Appl Pharmacol.
272:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou H and Fan X: Changes of
Ca~(2+)-ATPase activity and oxygen free radicals in microsome
membranes of postharvest peach fruit. Acta Botanica
Boreali-Occidentalia Sinica. 27:1161–1166. 2007.
|
|
13
|
Libalova H, Uhlířová K, Kléma J, Machala
M, Šrám RJ, Ciganek M and Topinka J: Global gene expression changes
in human embryonic lung fibroblasts induced by organic extracts
from respirable air particles. Part Fibre Toxicol. 9:12012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dagher Z, Garçon G, Gosset P, Ledoux F,
Surpateanu G, Courcot D, Aboukais A, Puskaric E and Shirali P:
Pro-inflammatory effects of Dunkerque city air pollution
particulate matter 2.5 in human epithelial lung cells (L132) in
culture. J Appl Toxicol. 25:166–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie Y, Zhang X, Tian Z, Jiang R, Chen R,
Song W and Zhao J: Preexposure to PM2.5 exacerbates acute viral
myocarditis associated with Th17 cell. Int J Cardiol.
168:3837–3845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furuyama A, Hirano S, Koike E and
Kobayashi T: Induction of oxidative stress and inhibition of
plasminogen activator inhibitor-1 production in endothelial cells
following exposure to organic extracts of diesel exhaust particles
and urban fine particles. Arch Toxicol. 80:154–162. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Furuyama A and Mochitate K: Hepatocyte
growth factor inhibits the formation of the basement membrane of
alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol
Physiol. 286:L939–L946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nerriere E, Zmirou-Navier D, Blanchard O,
Momas I, Ladner J, Le Moullec Y, Personnaz MB, Lameloise P, Delmas
V, Target A and Desqueyroux H: Can we use fixed ambient air
monitors to estimate population long-term exposure to air
pollutants? The case of spatial variability in the Genotox ER
study. Environ Res. 97:32–42. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hystad P, Demers PA, Johnson KC, Carpiano
RM and Brauer M: Long-term residential exposure to air pollution
and lung cancer risk. Epidemiology. 24:762–772. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, et al: Study on the morphological
changes of the rats' lung caused by exhausting of gasoline engine.
Xian Dai Yu Fang Yi Xue. 39:1349–1354. 2012.(In Chinese).
|
|
21
|
Ali BH, Al Za'abi M, Shalaby A, Manoj P,
Waly MI, Yasin J, Fahim M and Nemmar A: The effect of thymoquinone
treatment on the combined renal and pulmonary toxicity of cisplatin
and diesel exhaust particles. Exp Biol Med (Maywood).
240:1698–1707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akopian AN, Fanick ER and Brooks EG: TRP
channels and traffic-related environmental pollution-induced
pulmonary disease. Semin Immunopathol. 38:331–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Chen Y, Yu Z, Ding H and Ma Z:
Changes in gene expression in lungs of mice exposed to
traffic-related air pollution. Mol Cell Probes. 39:33–40. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Shi M, Li S, Bai Z and Wang Z:
Combined use of land use regression and BenMAP for estimating
public health benefits of reducing PM 2.5 in Tianjin, China.
Atmospheric Environ. 152:16–23. 2017. View Article : Google Scholar
|
|
25
|
Chen Y, et al: A study on effect of
automobile exhaust pollutants in underground parking area on serum
inflammatory cytokines of mice. Zhongguo Zhong Xi Yi Ji Jiu Za Zhi
2013. 353–356. 2013.(In Chinese).
|
|
26
|
Wang G, Zhen L, Lü P, Jiang R and Song W:
Effects of ozone and fine particulate matter (PM2.5) on rat cardiac
autonomic nervous system and systemic inflammation. Wei Sheng Yan
Jiu. 42:554–560. 2013.(In Chinese). PubMed/NCBI
|
|
27
|
Pei Y, Jiang R, Zou Y, Wang Y, Zhang S,
Wang G, Zhao J and Song W: Effects of fine particulate matter
(PM2.5) on systemic oxidative stress and cardiac function in
ApoE(−/−) mice. Int J Environ Res Public Health. 13:E4842016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, An J, Cheng M, Shen L, Zhu B, Li
Y, Wang Y, Duan Q, Sullivan A and Xia L: One year online
measurements of water-soluble ions at the industrially polluted
town of Nanjing, China: Sources, seasonal and diurnal variations.
Chemosphere. 148:526–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao G, Li S, Hong G, Li M, Wu B, Qiu Q
and Lu Z: The effect of resveratrol on paraquat-induced acute lung
injury in mice and its mechanism. Zhonghua Wei Zhong Bing Ji Jiu Yi
Xue. 28:33–37. 2016.(In Chinese). PubMed/NCBI
|
|
30
|
Sun CZ, Shen H, He XW, Bao L, Song Y,
Zhang Z and Qin HD: Effect of dobutamine on lung aquaporin 5 in
endotoxine shock-induced acute lung injury rabbit. J Thorac Dis.
7:1467–1477. 2015.PubMed/NCBI
|
|
31
|
Ten Have-Opbroek AA, Otto-Verberne CJ and
Dubbeldam JA: Ultrastructural characteristics of inclusion bodies
of type II cells in late embryonic mouse lung. Anat Embryol (Berl).
181:317–323. 1990.PubMed/NCBI
|
|
32
|
Dale D: Neutropenia and neutrophilia.
Lichtman M, Beutler E and Kipps TJ: Williams Hematology. Seventh.
McGraw-Hill Professional; New York, NY: pp. 701–708. 2006
|
|
33
|
Bronkhorst IH, Ly LV, Jordanova ES,
Vrolijk J, Versluis M, Luyten GP and Jager MJ: Detection of
M2-macrophages in uveal melanoma and relation with survival. Invest
Ophthalmol Vis Sci. 52:643–650. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang
Y, Feng T, Wu J and Liu X: Sphingosine-1-phosphate induced
epithelial-mesenchymal transition of hepatocellular carcinoma via
an MMP-7/syndecan-1/TGF-β autocrine loop. Oncotarget.
7:63324–63337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schiechl G, Bauer B, Fuss I, Lang SA,
Moser C, Ruemmele P, Rose-John S, Neurath MF, Geissler EK, Schlitt
HJ, et al: Tumor development in murine ulcerative colitis depends
on MyD88 signaling of colonic F4/80+CD11bhighGr1low macrophages. J
Clin Invest. 121:1692–1708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen L, Shi M, Gao S, Li S, Mao J, Zhang
H, Sun Y, Bai Z and Wang Z: Assessment of population exposure to
PM2.5 for mortality in China and its public health
benefit based on BenMAP. Environ Pollut. 221:311–317. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Long JF, Waldman WJ, Kristovich R,
Williams M, Knight D and Dutta PK: Comparison of ultrastructural
cytotoxic effects of carbon and carbon/iron particulates on human
monocyte-derived macrophages. Environ Health Perspect. 113:170–174.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang YC, Soukup J, Harder S and Becker S:
Mitochondrial oxidant production by a pollutant dust and
NO-mediated apoptosis in human alveolar macrophage. Ajp Cell
Physiol. 284:C24–C32. 2003. View Article : Google Scholar
|
|
40
|
Lee KI, Whang J, Choi HG, Son YJ, Jeon HS,
Back YW, Park HS, Paik S, Park JK, Choi CH and Kim HJ:
Mycobacterium avium MAV2054 protein induces macrophage apoptosis by
targeting mitochondria and reduces intracellular bacterial growth.
Sci Rep. 6:378042016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suen YK, Fung KP, Lee CY and Kong SK:
Gliotoxin induces apoptosis in cultured macrophages via production
of reactive oxygen species and cytochrome c release without
mitochondrial depolarization. Free Radic Res. 35:1–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang X, Zhong W, Meng Q, Lin Q, Fang C,
Huang X, Li C, Huang Y and Tan J: Ambient PM2.5 exposure
exacerbates severity of allergic asthma in previously sensitized
mice. J Asthma. 52:785–794. 2015.PubMed/NCBI
|
|
43
|
He H, Buckley M, Britton B, Mu Y, Warner
K, Kumar S and Cory TJ: Polarized macrophage subsets differentially
express the drug efflux transporters MRP1 and BCRP, resulting in
altered HIV production. Antivir Chem Chemother.
26:2040206617745162018. View Article : Google Scholar
|
|
44
|
Liu D, Whitehead J, Alfarra MR, Villegas
ER, Spracklen DV, Reddington CL, Kong S, Williams P, Haslett S,
Taylor JW, et al: Black-carbon absorption enhancement in the
atmosphere determined by particle mixing state. Nat Geosci.
10:184–188. 2017. View Article : Google Scholar
|
|
45
|
Huang H, Gao L, Xia D, Qiao L, Wang R, Su
G, Liu W, Liu G and Zheng M: Characterization of short- and
medium-chain chlorinated paraffins in outdoor/indoor
PM10/PM2.5/PM1.0 in Beijing, China. Environ Pollut. 225:674–680.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sui BL, Chen X, YU Y, Costa M and Wu H:
Effect of particle size on particulate matter emissions during
biosolid char combustion under air and oxyfuel conditions.
232:251–256. 2018.
|
|
47
|
Gu LZ, Sun H and Chen JH: Histone
deacetylases 3 deletion restrains PM2.5-induced mice lung injury by
regulating NF-κB and TGF-β/Smad2/3 signaling pathways. Biomed
Pharmacother. 85:756–762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pirela S, Molina R, Watson C, Cohen JM,
Bello D, Demokritou P and Brain J: Effects of copy center particles
on the lungs: A toxicological characterization using a Balb/c mouse
model. Inhal Toxicol. 25:498–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Smith KR, Kim S, Recendez JJ, Teague SV,
Ménache MG, Grubbs DE, Sioutas C and Pinkerton KE: Airborne
particles of the california central valley alter the lungs of
healthy adult rats. Environ Health Perspect. 111:902–908. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mohammed MOA, Song WW, Ma YL, Liu LY, Ma
WL, Li WL, Li YF, Wang FY, Qi MY, Lv N, et al: Distribution
patterns, infiltration and health risk assessment of PM2.5-bound
PAHs in indoor and outdoor air in cold zone. Chemosphere.
155:70–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li M and Li YM: Fine particulate matter
and nonalcoholic fatty liver disease. Zhonghua Gan Zang Bing Za
Zhi. 24:713–715. 2016.(In Chinese). PubMed/NCBI
|
|
52
|
Lee CC and Kang JJ: Extract of motorcycle
exhaust particles induced macrophages apoptosis by
calcium-dependent manner. Chem Res Toxicol. 15:1534–1542. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Akbarshahi H, Menzel M, Posaric Bauden M,
Rosendahl A and Andersson R: Enrichment of murine CD68+ CCR2+ and
CD68+ CD206+ lung macrophages in acute pancreatitis-associated
acute lung injury. PLoS One. 7:e426542012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun L, Chen B, Jiang R, Li J and Wang B:
Resveratrol inhibits lung cancer growth by suppressing M2-like
polarization of tumor associated macrophages. Cell Immunol.
331:86–93. 2017. View Article : Google Scholar
|
|
55
|
Kimura Y and Sumiyoshi M: Resveratrol
prevents tumor growth and metastasis by inhibiting
lymphangiogenesis and M2 macrophage activation and differentiation
in tumor-associated macrophages. Nutr Cancer. 68:667–678. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Clarke RW, Catalano PJ, Koutrakis P,
Murthy GG, Sioutas C, Paulauskis J, Coull B, Ferguson S and
Godleski JJ: Urban air particulate inhalation alters pulmonary
function and induces pulmonary inflammation in a rodent model of
chronic bronchitis. Inhal Toxicol. 11:637–656. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Aversa G, Punnonen J and de Vries JE: The
26-kD transmembrane form of tumor necrosis factor alpha on
activated CD4+ T cell clones provides a costimulatory signal for
human B cell activation. J Exp Med. 177:1575–1585. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang CZ, Yang J, Qiao HL and Jia LJ:
Polymorphisms and haplotype analysis of IL-4Rα Q576R and I75V in
patients with penicillin allergy. Eur J Clin Pharmacol. 65:895–902.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nygaard UC, Samuelsen M, Aase A and Løvik
M: The capacity of particles to increase allergic sensitization is
predicted by particle number and surface area, not by particle
mass. Toxicol Sci. 82:515–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Samuelsen M, Nygaard UC and Lovik M:
Allergy adjuvant effect of particles from wood smoke and road
traffic. Toxicology. 246:124–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin ZM, Ma M, Li H, Qi Q, Liu YT, Yan YX,
Shen YF, Yang XQ, Zhu FH, He SJ, et al: Topical administration of
reversible SAHH inhibitor ameliorates imiquimod-induced
psoriasis-like skin lesions in mice via suppression of
TNF-α/IFN-γ-induced inflammatory response in keratinocytes and T
cell-derived IL-17. Pharmacol Res. 129:443–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang M, Su L, Yang L, Zhu L, Liu Z and
Duan R: Effect of exogenous TGF-β1 on the cadmium-induced
nephrotoxicity by inhibiting apoptosis of proximal tubular cells
through PI3K-AKT-mTOR signaling pathway. Chem Biol Interact.
269:25–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Labour MN, Riffault M, Christensen ST and
Hoey DA: TGFβ1-induced recruitment of human bone mesenchymal stem
cells is mediated by the primary cilium in a SMAD3-dependent
manner. Sci Rep. 6:355422016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhou X, Hu H, Balzar S, Trudeau JB and
Wenzel SE: MAPK regulation of IL-4/IL-13 receptors contributes to
the synergistic increase in CCL11/eotaxin-1 in response to TGF-β1
and IL-13 in human airway fibroblasts. J Immunol. 188:6046–6054.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ghavami S, Yeganeh B, Zeki AA, Shojaei S,
Kenyon NJ, Ott S, Samali A, Patterson J, Alizadeh J, Moghadam AR,
et al: Autophagy and the unfolded protein response promote
profibrotic effects of TGF-β1 in human lung fibroblasts. Am J
Physiol Lung Cell Mol Physiol. 314:L493–L504. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Puerta-Arias JD, Pino-Tamayo PA, Arango JC
and González Á: Depletion of neutrophils promotes the resolution of
pulmonary inflammation and fibrosis in mice infected with
paracoccidioides brasiliensis. PLoS One. 11:e01639852016.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zaafan MA, Zaki HF, El-Brairy AI and
Kenawy SA: Pyrrolidinedithiocarbamate attenuates bleomycin-induced
pulmonary fibrosis in rats: Modulation of oxidative stress,
fibrosis, and inflammatory parameters. Exp Lung Res. 42:408–416.
2016. View Article : Google Scholar : PubMed/NCBI
|