Introduction
Parkinson's disease (PD) is a progressive
neurodegenerative disorder characterized by selective loss of
dopaminergic neurons in the substantia nigra. This process leads to
dopamine (DA) depletion in the striatum affecting several million
patients worldwide, notably older adults (1). Although the biochemical and molecular
pathogenesis of the loss of dopaminergic neurons in PD have not yet
been fully characterized, a number of biochemical processes and
molecular mechanisms have been identified as mediators of neuronal
cell death in PD, including the overproduction of oxidative stress,
the increase in neuroinflammation and the activation of the
apoptotic cascade (2). Oxidative
stress and apoptosis have been shown to play central roles in the
degeneration of dopaminergic neurons in PD (2–4).
Therefore, the exploration of novel molecular mechanisms involved
in oxidative stress and apoptosis is of great significance for the
treatment of PD.
MicroRNAs (miRNAs/miRs), are a class of endogenous
highly conserved noncoding RNA molecules, which are ~22 nucleotides
in length (5). By binding directly
to the 3′-UTR (3′-untranslated region) of target mRNAs, miRNAs are
involved in a series of physiological and pathological processes
(6). Accumulating evidence suggests
that the dysregulation of miRNA plays an important role in the
pathogenesis of neurodegenerative disorders, including PD (7,8). Fu
et al (9) demonstrated that
docosahexaenoic acid upregulated the expression of Peroxisome
Proliferator Activated Receptor α by inhibiting miR-21 in SH-Y5Y
cells. Moreover, a recent study showed that the levels of miR-21 in
PD models were significantly higher than those in normal controls
in SH-SY5Y cells (10). This
evidence suggests that miR-21 may play an important role in PD,
while the underlying mechanism remains unclear.
In the present study, the classic neurotoxin
1-methyl-4-phenylpyridinium (MPP+) was used to produce a
PD cell model in vitro. This compound is selectively
transported into dopaminergic neurons via the DA transporter and
localizes in the mitochondria (11).
The aim of the current study was to investigate the role of miR-21
in MPP+-induced neurotoxicity of MES23.5 cells and to
prove the mechanism of miR-21 in PD to provide novel approaches for
clinical treatment.
Materials and methods
Cell culture and treatment
MES23.5 cells were cultured in DMEM/F12
(Sigma-Aldrich; Merck KGaA) supplemented with 5% fetal bovine serum
(Thermo Fisher Scientific, Inc.), 2% of 50X of Sato's solution
(Thermo Fisher Scientific, Inc.) at 37°C in a humidified (70–80%)
atmosphere containing 5% CO2. The cells were seeded in
96-well plates at a density of 1×105 cells/well and
treated with different concentrations of MPP+ (100, 200
or 300 µM) for 3, 6, 12 or 24 h to optimize the experimental
conditions.
Experimental groups were as follows: Control group,
MES23.5 cells with no treatment and the model group, MES23.5 cells
with 200 µM MPP+ treatment for 24 h.
Transfection
miR-21 inhibitor (100 nM) and negative control (NC,
100 nM) sequences were obtained from GenePharma and were
transfected into MES23.5 cells using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The sequences of the inhibitor and the NC samples were as
follows: miR-21 inhibitor (5′-UCAACAUCAGUCUGAUAAGCUA-3′), and NC
(5′-CAGUACUUUUGUGUAGUACAA-3′). At 24-h following transfection, the
cells were treated with 200 µM of MPP+ for an additional
24 h at 37°C. Subsequently, the cells were harvested for further
experiments. Experimental groups were as follows: Control group,
MES23.5 cells with 200 µM MPP+ treatment and no
transfection; NC group, MES23.5 cells with 200 µM MPP+
treatment and inhibitor NC and miR-21 inhibitor group, MES23.5
cells with 200 µM MPP+ treatment and miR-21
inhibitor.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay was used to assess cell viability.
The cells were seeded in 96-well plates (2×104
cells/well) and incubated at 37°C for 24 h. At 3, 6, 12 and 24-h
following MPP+ treatment (100, 200 and 300 µM) or
MPP+ treatment (200 µM) for 24, 48 and 72-h, the cell
proliferation indices were measured using a CCK-8 kit (cat. no.
C0038, Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. The optical density was measured at 450
nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies; Thermo Fisher Scientific, Inc.).
Single-stranded cDNA was synthesized using the TaqMan MicroRNA
Reverse Transcription Kit (Takara) with the special stem-loop
primers. The reverse transcription conditions were following 25°C
for 10 min, 45°C for 30 min and 5 min at 95°C. RT-qPCR was
performed using a Perfect Real Time SYBR Premix Ex Taq Kit (Takara
Bio) with an ABI 7500 thermocycler (Thermo Fisher Scientific,
Inc.). All procedures were performed according to the
manufacturer's protocol. U6 was used as control for the expression
levels of miR-21. The reaction conditions for PCR were as follows:
Pre-denaturation at 95°C for 3 min and 40 cycles of denaturation at
95°C for 30 sec and annealing at 60°C for 30 sec. The relative
expression levels of each gene were calculated by the
2−ΔΔCq method (12).
miRNA-specific reverse transcription primers and quantitative PCR
primers were obtained from RiboBio Co. Ltd. The following primer
sequences were used: miR-21 stem-loop primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA-3′ forward,
5′-CACGCACGCATAGCTTATCAGACT-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTA-3′. U6, stem-loop primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTGCACTGGATACGACAAAATATGG-3′, forward,
5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTA-3′.
Immunocytochemistry
MES23.5 cells (1×105 cells/well) were
fixed with 4% paraformaldehyde for 30 min at 4°C and permeabilized
with 0.5% Triton X-100 for 20 min at room temperature. Following
blocking with 5% goat serum (Gibco; Thermo Fisher Scientific, Inc.)
for 1-h at room temperature and incubation with mouse anti-tyrosine
hydroxylase (TH; cat. no. T2928; 1:500; Sigma-Aldrich; Merck KGaA)
at 4°C overnight, the cells were further incubated with
biotinylated goat anti-mouse secondary antibody (cat. no. A0286;
1,1000; Beyotime Institute of Biotechnology) for 15 min at 37°C.
Subsequently, the samples were washed with PBS three times for 15
min at 37°C and visualized by diaminobenzidine (DAB). TH positive
cells were counted in three randomly selected images
(magnification, ×200) using an Olympus Fluoview FV5000 microscope
(Thermo Fisher Scientific, Inc.).
Detection of ROS levels
The ROS levels were assessed using the fluorescent
dye 2′,7′-dichlorofluorescin diacetate (DCFDA; D6883,
Sigma-Aldrich; Merck KGaA). MES23.5 cells were seeded at a density
of 2×104 cells/well in 96-well plates. The cells were
incubated with 2.5 µM of DCFDA for 15 min at 37°C. DCFDA
fluorescence was acquired by confocal microscopy at excitation and
emission wavelengths of 485 and 535 nm, respectively (Carl Zeiss
LSM 700 Meta confocal microscope; Zeiss). The data were analyzed by
the Carl Zeiss confocal laser scanning microscopes with the ZEN
2008 software.
ELISA
The culture medium of MES23.5 cells was collected.
The levels of IL-6, IL-1β and tumor necrosis factor-α (TNF-α) were
measured by the Interleukin-1β ELISA kit (cat. no. ab100562; Abcam)
according to the manufacturer's protocol.
Cell apoptosis analysis
An Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection kit (NanJing KeyGen Biotech Co., Ltd) was used
to measure cell apoptosis according to the manufacturer's protocol.
The samples were analyzed by flow cytometry. A total of 100 nM
miR-21 inhibitor and NC sequences were transfected with MES23.5
cells for 24 h at 37°C then the cells were treated with
MPP+ for an additional 24 h. The cells were subsequently
harvested, washed twice with ice-cold PBS and suspended using
binding buffer (500 µl; Thermo Fisher Scientific, Inc.).
Subsequently, the cells were stained using (Annexin-V-FITC) reagent
and propidium iodide (PI). The cells were analyzed by a FACSCalibur
flow cytometer with the Cell Quest software (version 3.1; Becton
Dickinson).
Western blot analysis
Total protein extraction was performed using RIPA
lysis buffer (Beyotime Institute of Biotechnology). The protein
concentration was measured using the bicinchoninic acid kit
(Bio-Rad Laboratories, Inc.). The protein samples (20 µg/lane) were
separated by SDS-PAGE (12% resolving gels, 4% stacking gels) and
blotted onto polyvinylidene difluoride membranes (EMD Millipore).
The membranes were blocked using 5% non-fat milk for 1-h at room
temperature, followed by overnight incubation at 4°C with the
indicated antibodies against Bax (cat. no. MAB4601, 1:200, EMD
Millipore) and Bcl-2 (cat. no. 05-826, 1:500, EMD Millipore).
Subsequently, the membranes were incubated with secondary rabbit
anti-mouse IgG-horseradish peroxidase antibodies (cat. no.
sc-358914, 1:5,000, Santa Cruz Biotechnology, Inc.) at room
temperature for a further 2-h. Then, membranes were washed with
Tris-buffered saline and Polysorbate 20 seven times (3 min per
wash). Chemiluminescent signals were visualized using the enhanced
chemiluminescence detection reagent (EMD Millipore). The results
were analyzed using ImageJ software 1.4 (National Institutes of
Health).
Dual-luciferase reporter assay
TargetScanHuman (www.targetscan.org) was used to predict the putative
target site of Bcl-2. The mutant type of the Bcl-2 3′UTR sequence
was constructed using a QuickChange Multi Site-Directed Mutagenesis
kit (Agilent Technologies, Inc.), according to the manufacturer's
protocol. The wild type or mutant types of the Bcl-2 3′UTR
sequences were cloned into the firefly luciferase reporter
pGL3-promoter vector (Promega Corp.) to generate the recombinant,
wild type (3′UTR-WT) or mutant type (3′UTR-MUT) pGL3-Bcl-2-3′UTR
luciferase plasmids. MES23.5 cells (1×105 cells/well)
were cultured in 24-well plates for 24 h at room temperature, and
the cells were co-transfected with 50 ng of 3′UTR-WT (or 3′UTR-MUT)
vector and 20 µM of miR-21 (or NC) mimics (Shanghai GenePharma Co.,
Ltd.) using Lipofectamine™ 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. At 24 h
following transfection, the Dual-Luciferase Reporter Assay System
(Promega Corp.) was used to determine the luciferase activity,
which was normalized to Renilla luciferase activity.
Statistical analysis
The data are presented as the mean ± standard error
of the mean. GraphPad Prism version 6 (GraphPad Software, Inc.) was
used to perform the statistical analyses. The differences between
two groups were carried out by a two-tailed Student's t-test.
One-way analysis of variance followed by Bonferroni's multiple
comparison tests was used to compare differences between means in
more than two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Downregulation of miR-21 enhances cell
survival in MPP+-treated MES23.5 cells
The optimal concentration of MPP+ was
determined based on neuronal viability, which was monitored by the
CCK-8 assay. MPP+ treatment demonstrated dose- and
time-dependent cytotoxicity of MES23.5 cells. These effects were
noted at the concentrations of 100, 200 and 300 µM for 0, 3, 6, 12
and/or 24-h time points (Fig. 1A).
The optimal concentration (200 µM) and time point (24 h) of
incubation exhibited approximately 50% cell viability following
MPP+ treatment (Fig. 1A).
The expression levels of miR-21 in MPP+-treated MES23.5
cells were assessed by RT-qPCR. The results indicated that the
expression levels of miR-21 were significantly higher in MES23.5
cells following MPP+ treatment compared with those of
the control group (Fig. 1B). To
further investigate the role of miR-21 in the development of PD,
MES23.5 cells were transfected with miR-21 inhibitor and NC
sequences, followed by treatment with 200 µM of MPP+ for
24 h. The expression levels of miR-21 were significantly decreased
following transfection with miR-21 inhibitor in MES23.5 cells
compared with those of the NC group (Fig. 1C). The CCK-8 assay indicated that the
transfection of the miR-21 inhibitor significantly increased the
cell survival of the MES23.5 cells following MPP+
treatment at the 24, 48 and 72-h time points compared with that
noted in the NC group (Fig. 1D).
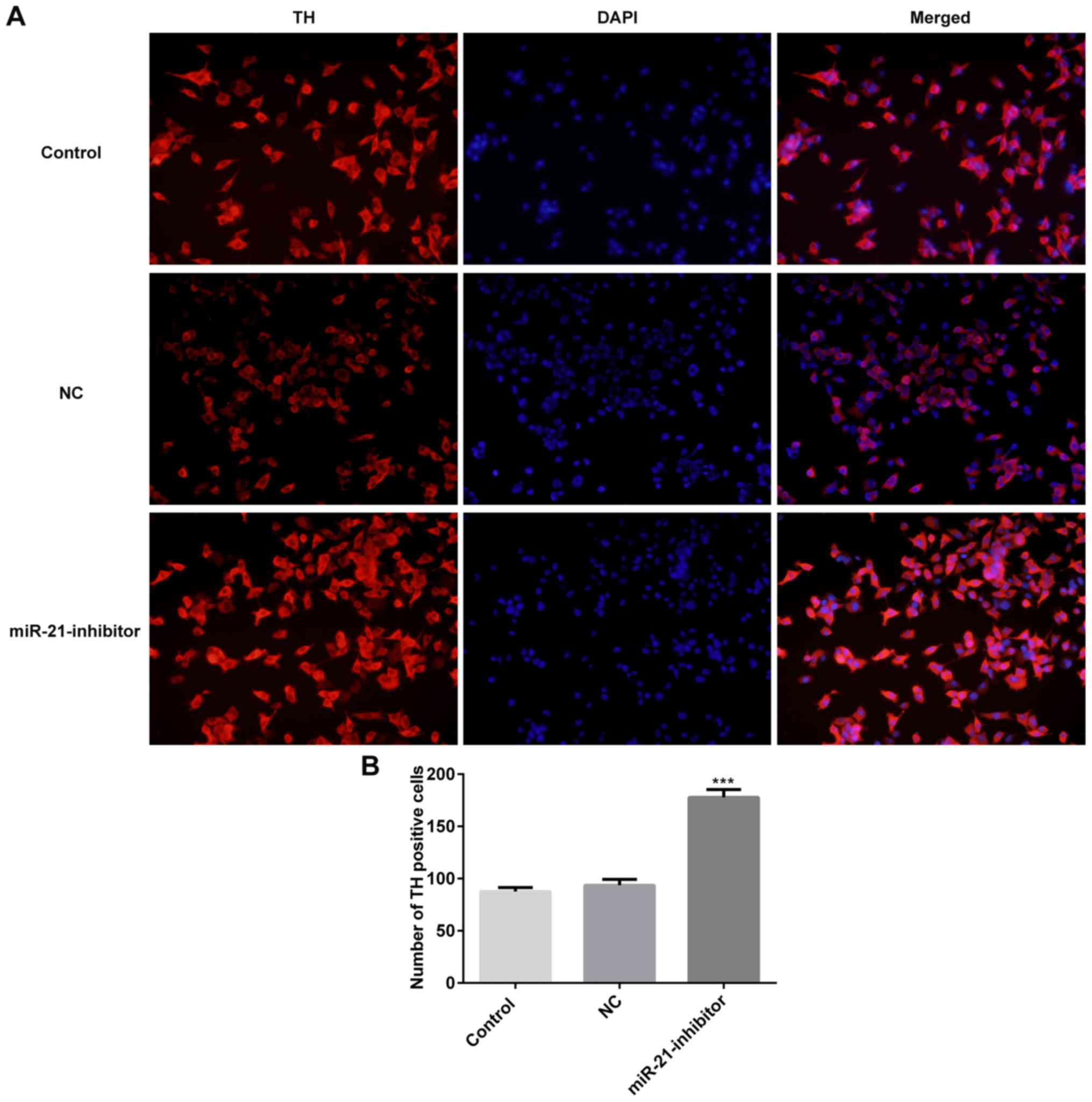
Moreover, the characteristics of active neurons were classified
according to the expression of TH (13). The results of the immunohistochemical
assay demonstrated that downregulation of miR-21 significantly
increased the number of TH positive cells (Fig. 2).
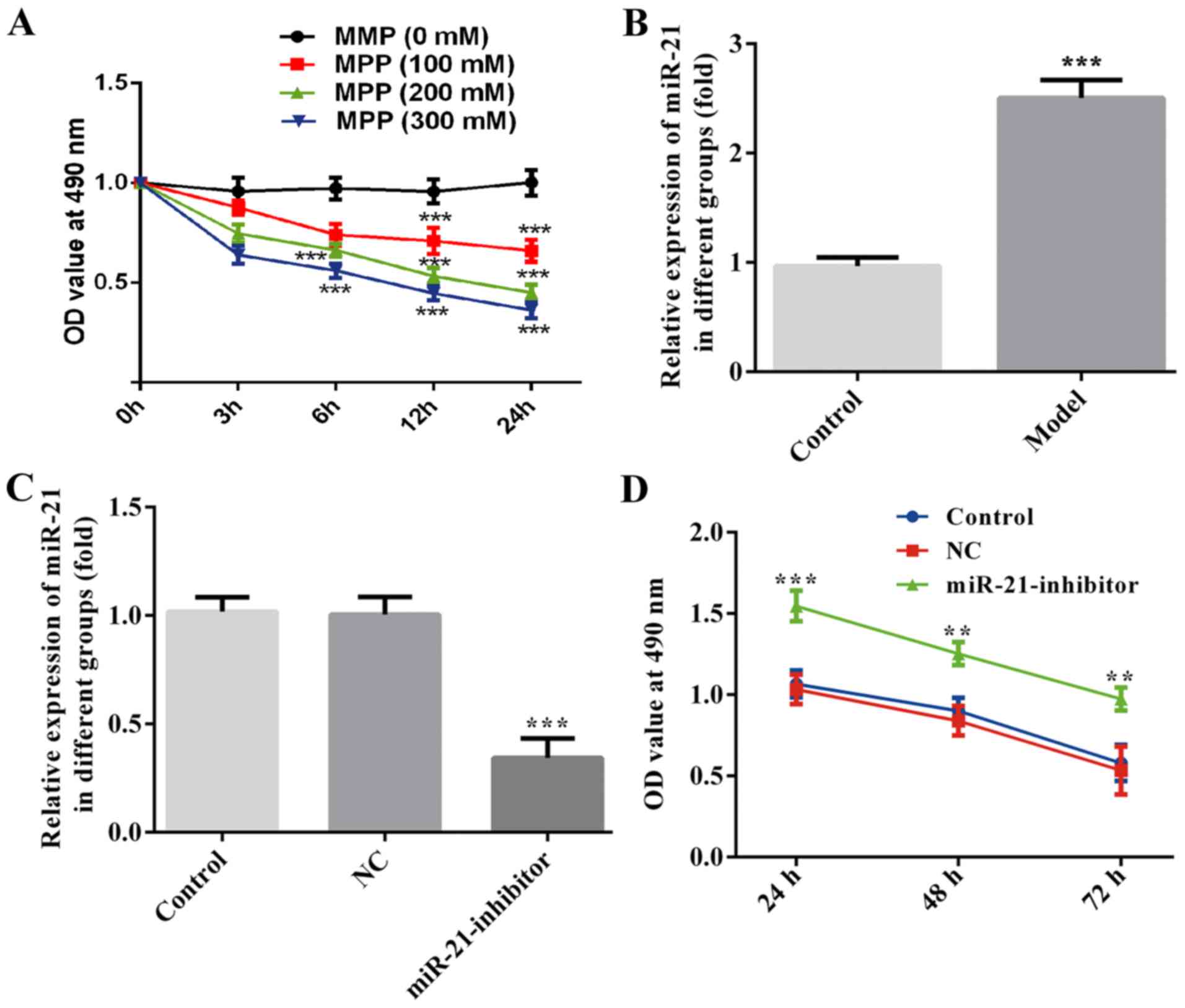 | Figure 1.Downregulation of miR-21 enhanced cell
survival in MPP+-treated MES23.5 cells. MES23.5 cells
were treated with MPP+ at the concentrations of 100, 200
and 300 µM. The CCK-8 assay was used to assess cell viability at 0,
3, 6, 12 or 24 h following MPP+ treatment. (A) Reverse
transcription-quantitative PCR was performed to measure miR-21
levels in MES23.5 cells with MPP+ treatment (B) and
miR-21inhibitior transfection. (C) CCK-8 assay was used to assess
cell viability at 24, 48 and 72 h following transfection of the
cells with the miR-21inhibitior (D) **P<0.01, ***P<0.001 vs.
control. CCK-8, cell counting kit-8; MPP+,
1-methyl-4-phenylpyridinium; miR, microRNA; NC, negative
control. |
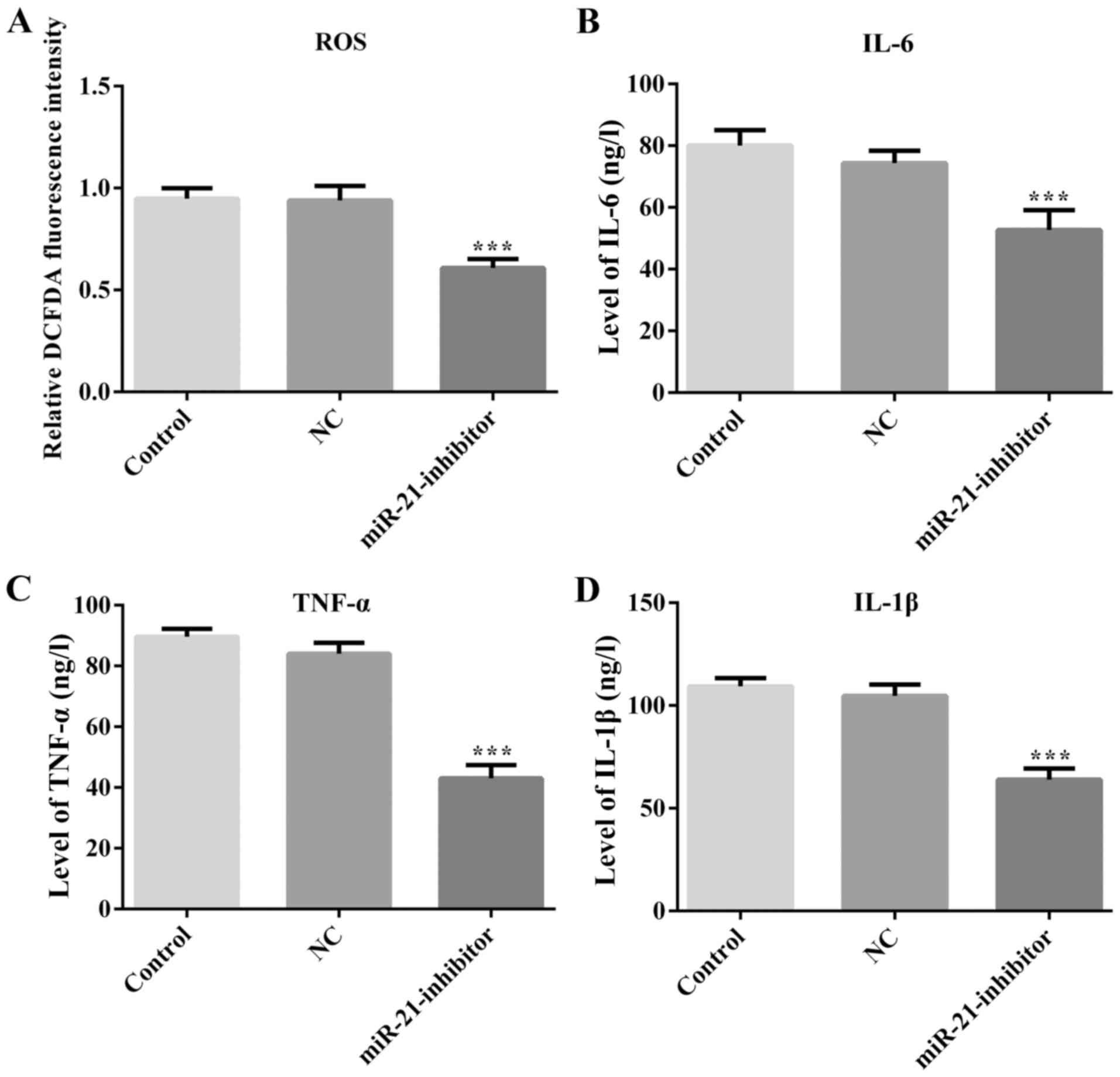
Downregulation of miR-21 causes
inhibition of the inflammatory response and ROS production in
MPP+-treated MES23.5 cells
To investigate the role of miR-21 in
MPP+-mediated cell damage, the production of ROS was
determined in MPP+-treated MES23.5 cells. The results
suggested that downregulation of miR-21 attenuated ROS production
compared with that of the NC group (Fig.
3A). Subsequently, the levels of neuroinflammation were
assessed by ELISA analysis. The expression levels of the
inflammatory markers IL-6, IL-1β and TNF-α were significantly
reduced following transfection of the cells with the miR-21
inhibitor (Fig. 3).
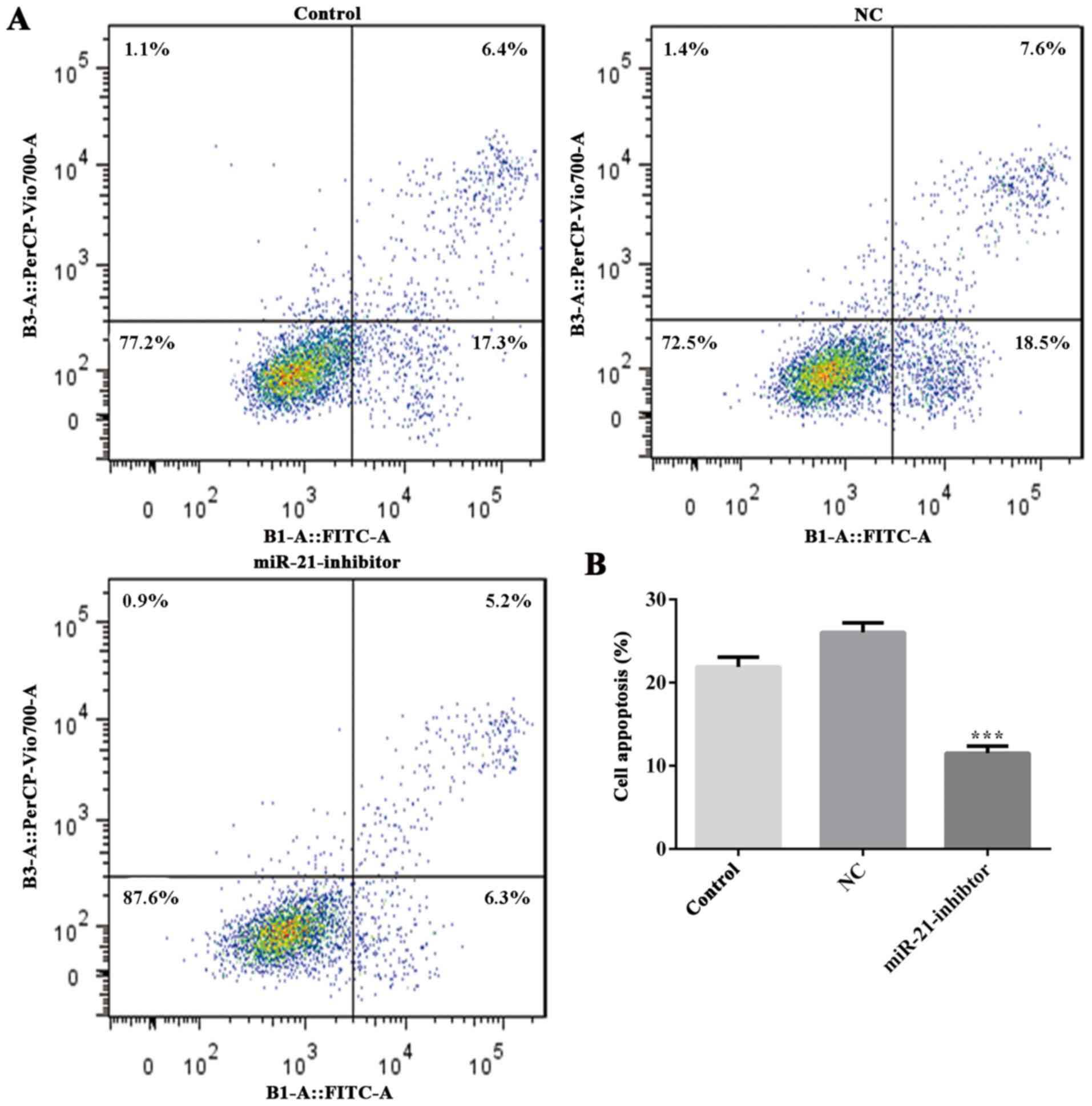
Downregulation of miR-21 suppresses
cell apoptosis and Bcl-2 is its direct target
The effects of miR-21 in the induction of apoptosis
were further evaluated. The data demonstrated that downregulation
of miR-21 resulted in a significant inhibition of apoptosis in
MPP+-treated MES23.5 cells compared with that of the NC
group (Fig. 4). In addition, the
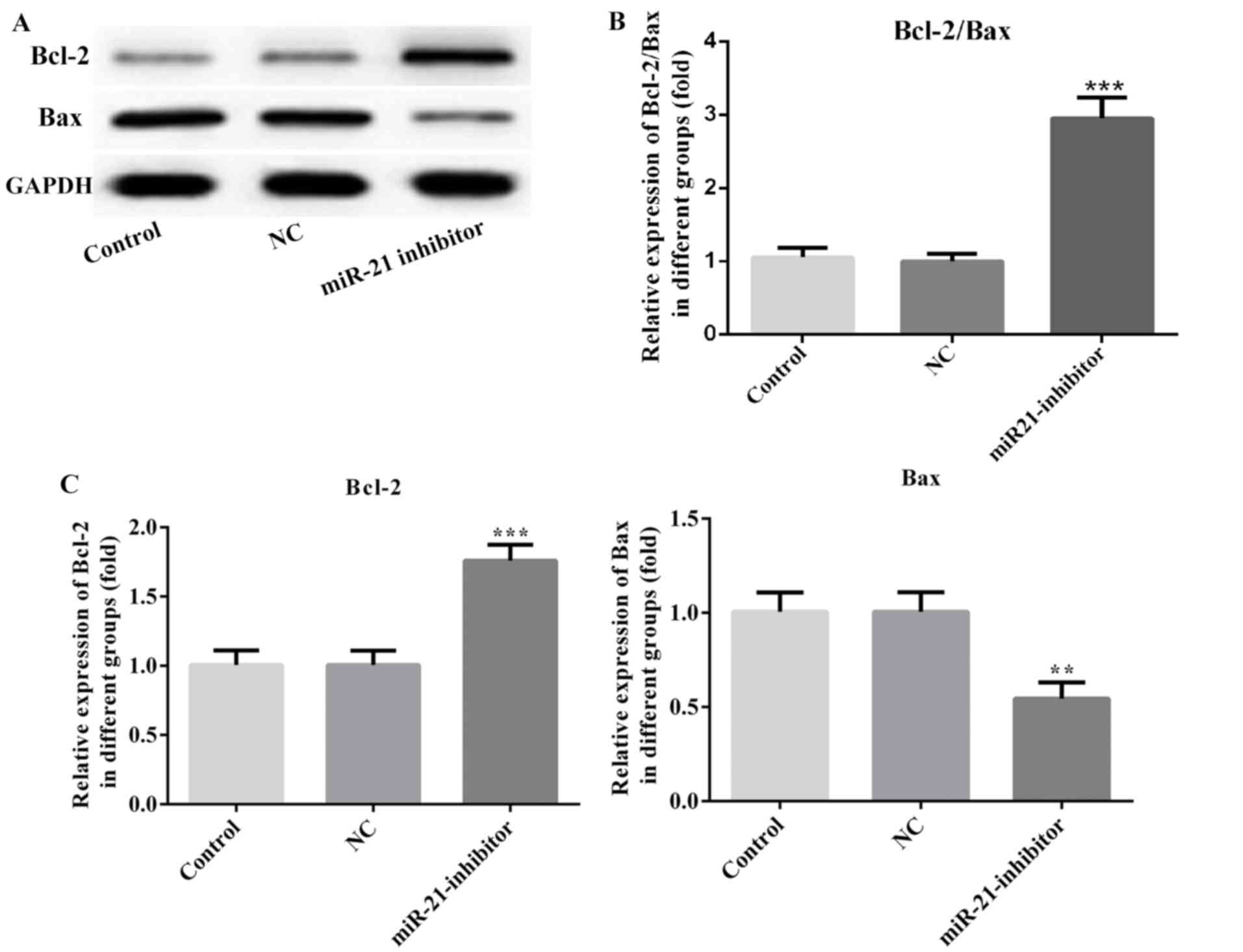
effects of miR-21 on the expression levels of the apoptotic
proteins were also investigated. Inhibition of miR-21 resulted in
increased Bcl-2 and decreased Bax levels compared with those of the
NC group (Fig. 5). Moreover, MES23.5
cells were transfected with miR-NC and miR-21 mimic, and the miR-21
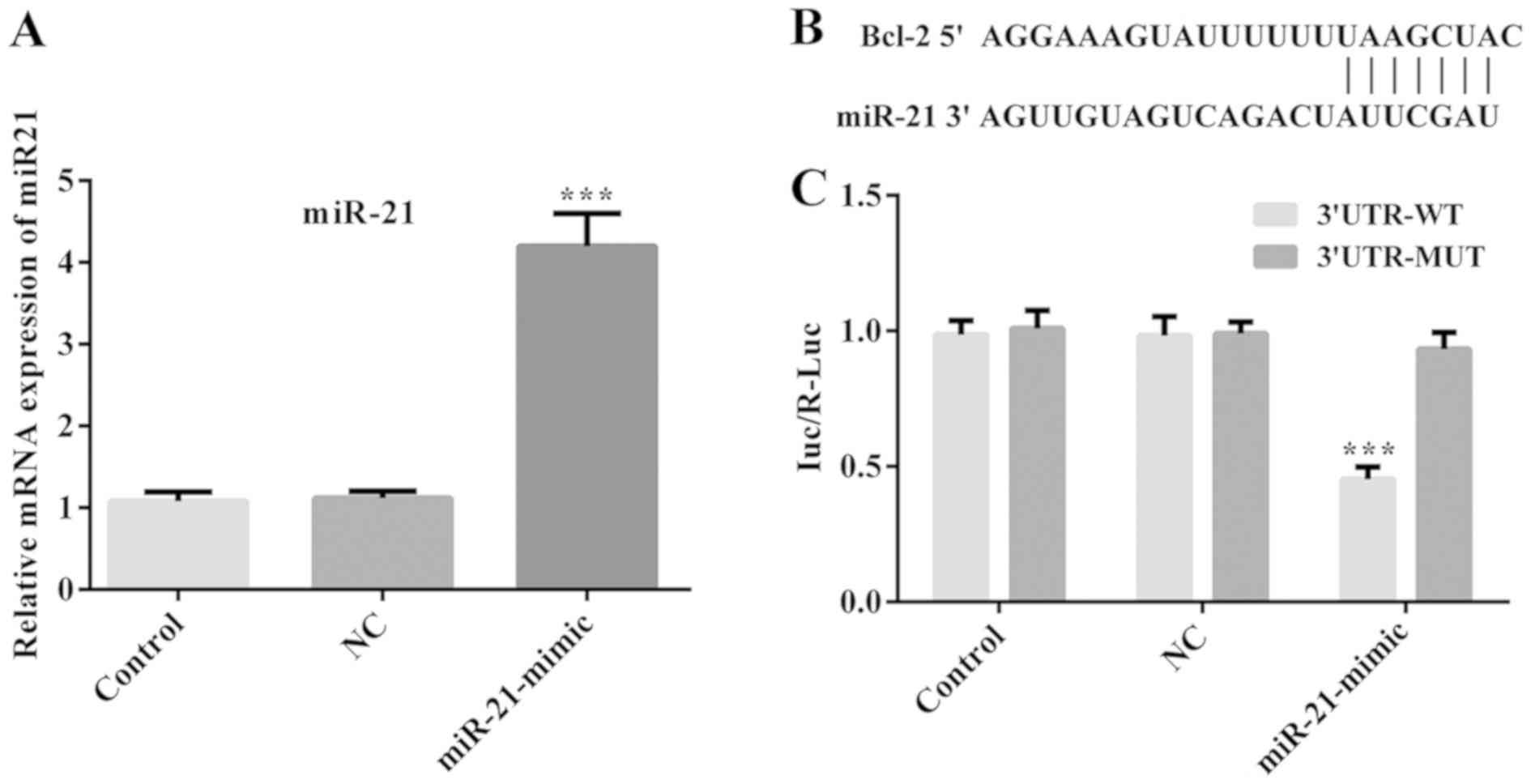
mimic significantly increased the levels of miR-21 (Fig. 6A). Furthermore, the results of
Dual-luciferase reporter assay showed that cells co-transfected
with miR-21 mimics and Bcl-2 3′UTR-WT, exhibited lower luciferase
activity compared with those co-transfected with miR-21 mimics and
Bcl-2 3′MUT (Fig. 6C). However, no
significant difference was noted in the luciferase activity of the
NC groups (Fig. 6C). These results
confirmed that miR-21 could directly bind to the 3′UTR of the Bcl-2
protein.
Discussion
PD is characterized by progressive and irreversible
loss of dopaminergic neurons in the substantia nigra. This disease
is the second most common neurodegenerative disorder following
Alzheimer disease (1). Current
evidence suggests that miRs are involved in diverse biological
processes including pathogenesis of neurodegenerative disorders and
abnormal brain function (8,14). With regard to PD, several miRs have
been reported to participate and play important roles in PD
progression, via the regulation of various processes, such as
apoptosis, autophagy, inflammation, mitochondrial dysfunction, and
ROS activity (15,16). These molecules can be targeted for
the development of future diagnostic tools and treatment
strategies. For example, patients with PD and
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced animals
exhibit a significant decrease in the levels of miR-7 in brain
tissue areas associated with dopaminergic neurodegeneration
(17). Moreover, miR-7 has recently
been shown to target α-synuclein, a protein involved in the
pathological process of PD, and can therefore be used for the
treatment of this disease (18).
It is important to note that the miR-21 levels of a
SH-SY5Y cell PD model were significantly higher than those noted in
normal cells as reported by recent studies (9,10). Su
et al (10) demonstrated that
miR-21 upregulated the expression levels of α-synuclein by directly
targeting lysosome-associated membrane protein 2 in SH-SY5Y cells
pretreated with MPP+. However, the effects and the
underlying molecular mechanism of miR-21 in neural cells are still
unclear. In the present study, the role and underlying molecular
mechanism of miR-21 in PD was investigated using the neurotoxin
MPP+, a well-established compound in PD cell models
(13). It was demonstrated that
treatment of MPP+ resulted in a dose- and time-dependent
cytotoxicity in MES23.5 cells. The present study demonstrated that
miR-21 levels were considerably increased in
MPP+-treated dopaminergic neuronal MES23.5 cells, which
is consistent with a previous study (10). To further investigate the role of
miR-21, MES23.5 cells were transfected with miR-21 inhibitor
sequences. The results suggested that downregulation of miR-21
considerably enhanced cell survival and inhibited cell apoptosis
induced by MPP+ treatment. Moreover, inhibition of
miR-21 significantly increased the TH positive cell number in
MPP+-treated MES23.5 cells. The levels of the
anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax were
also examined. These two proteins are considered important
regulators of apoptosis and the disruption of the balance of the
Bcl-2/Bax ratio could lead to the release of pro-apoptotic proteins
from the mitochondria to the cytoplasm (19). Western blot analysis was performed to
detect the expression levels of the aforementioned proteins, and
the results indicated that downregulation of miR-21 increased the
Bcl-2/Bax ratio in the MPP+-treated MES23.5 cells.
Moreover, dual-luciferase reporter assay demonstrated that Bcl-2
was a direct target gene of miR-21, which may indicate that miR-21
participated in the induction of cell apoptosis in MES23.5 cells
via the regulation of Bcl-2. In addition, MPP+ has been
shown to cause neurotoxicity by the induction of ROS and the
secretion of inflammatory factors (20). Devathasan et al (21) demonstrated that patients with PD
exhibited a significant increase in the production of ROS in
specific brain regions. The overproduction of ROS and the induction
of oxidative stress have been shown to be involved in PD and to
lead to dopaminergic neuronal cell death and apoptosis (22). The data reported in the present study
demonstrated that downregulation of miR-21 resulted in a
significant inhibition of ROS production in MPP+-induced
MES23.5 cells. The results indicated that downregulation of miR-21
may exert a neuroprotective effect in MPP+-treated
MES23.5 cells by the inhibition of cell apoptosis and the induction
of inflammation and ROS production.
In conclusion, the present study demonstrated that
miR-21 expression was increased in MPP+-treated MES23.5
cells, whereas inhibition of miR-21 alleviated
MPP+-induced MES23.5 cell damage by suppressing
intracellular ROS and inflammatory factor production, and by
inhibiting apoptosis. Moreover, the present study reported that
Bcl-2 was a direct target gene of miR-21 and that the
downregulation of miR-21 could significantly increase TH expression
in MPP+-treated MES23.5 cells. Although further studies
should be conducted, miR-21 may be considered a potential target
for PD diagnosis and treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HWM and LDD participated in experiment design, data
collection and paper writing.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kalia LV and Lang AE: Parkinson's disease.
Lancet. 386:896–912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andican G, Konukoglu D, Bozluolcay M,
Bayulkem K, Firtiina S and Burcak G: Plasma oxidative and
inflammatory markers in patients with idiopathic Parkinson's
disease. Acta Neurol Belg. 112:155–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mullin S and Schapira AH: Pathogenic
mechanisms of neurodegeneration in Parkinson disease. Neurol Clin.
33:1–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maiti P, Manna J and Dunbar GL: Current
understanding of the molecular mechanisms in Parkinson's disease:
Targets for potential treatments. Transl Neurodegener. 6:282017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moss EG: MicroRNAs: Hidden in the genome.
Curr Biol. 12:R138–R140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma L, Wei L, Wu F, Hu Z, Liu Z and Yuan W:
Advances with microRNAs in Parkinson's disease research. Drug Des
Devel Ther. 7:1103–1113. 2013.PubMed/NCBI
|
|
8
|
Quinlan S, Kenny A, Medina M, Engel T and
Jimenez-Mateos EM: MicroRNAs in neurodegenerative diseases. Int Rev
Cell Mol Biol. 334:309–343. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu Y, Zhen J and Lu Z: Synergetic
neuroprotective effect of docosahexaenoic acid and aspirin in
SH-Y5Y by inhibiting miR-21 and activating RXRalpha and PPARalpha.
DNA Cell Biol. 36:482–489. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su C, Yang X and Lou J: Geniposide reduces
alpha-synuclein by blocking microRNA-21/lysosome-associated
membrane protein 2A interaction in Parkinson disease models. Brain
Res 1644. 98–106. 2016. View Article : Google Scholar
|
|
11
|
Singer TP, Ramsay RR, McKeown K, Trevor A
and Castagnoli NE Jr: Mechanism of the neurotoxicity of
1-methyl-4-phenylpyridinium (MPP+), the toxic bioactivation product
of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicology.
49:17–23. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng J, Liu Q, Rao MS and Zeng X: Using
human pluripotent stem cell-derived dopaminergic neurons to
evaluate candidate Parkinson's disease therapeutic agents in MPP+
and rotenone models. J Biomol Screen. 18:522–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge X, Li W, Huang S, Yin Z, Yang M, Han Z,
Han Z, Chen F, Wang H, Lei P and Zhang J: Increased miR-21-3p in
injured brain microvascular endothelial cells following traumatic
brain injury aggravates blood-brain barrier damage by promoting
cellular apoptosis and inflammation through targeting MAT2B. J
Neurotrauma. 36:1291–1305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oh SE, Park HJ, He L, Skibiel C, Junn E
and Mouradian MM: The Parkinson's disease gene product DJ-1
modulates miR-221 to promote neuronal survival against oxidative
stress. Redox Biol. 19:62–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Sun Y and Chen J: Identification of
critical genes and miRNAs associated with the development of
parkinson's disease. J Mol Neurosci. 65:527–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong G and Nass R: miRNAs and their
putative roles in the development and progression of Parkinson's
disease. Front Genet. 3:3152013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Titze-de-Almeida R and Titze-de-Almeida
SS: miR-7 replacement therapy in parkinson's disease. Curr Gene
Ther. 18:143–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lotharius J, Dugan LL and O'Malley KL:
Distinct mechanisms underlie neurotoxin-mediated cell death in
cultured dopaminergic neurons. J Neurosci. 19:1284–1293. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devathasan G, Chong PN, Puvanendran K, Lun
KC and Wong PK: Low-dose bromocriptine therapy in severe
Parkinson's disease. Clin Neuropharmacol. 7:231–237. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yadava N and Nicholls DG: Spare
respiratory capacity rather than oxidative stress regulates
glutamate excitotoxicity after partial respiratory inhibition of
mitochondrial complex I with rotenone. J Neurosci. 27:7310–7317.
2007. View Article : Google Scholar : PubMed/NCBI
|