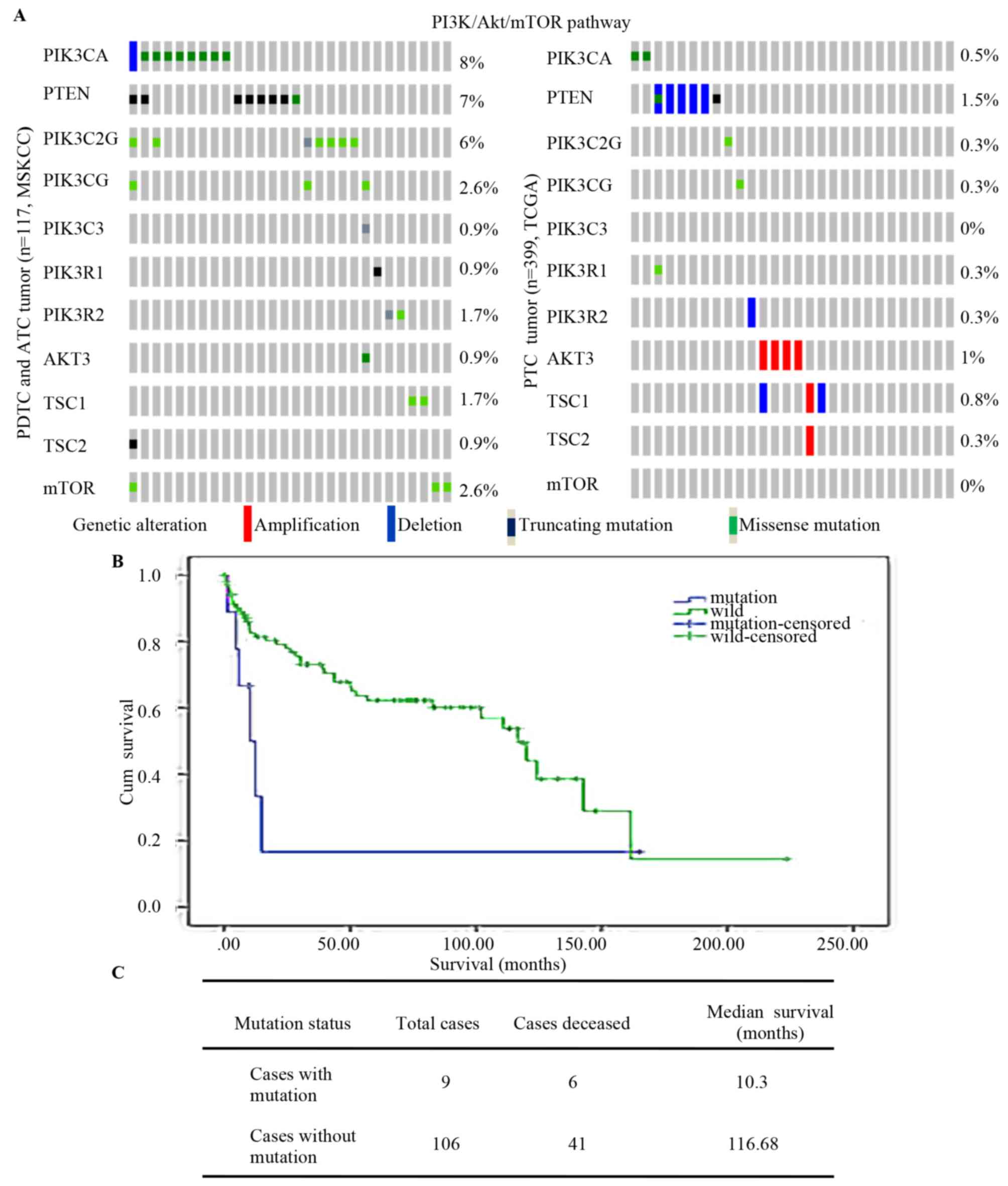
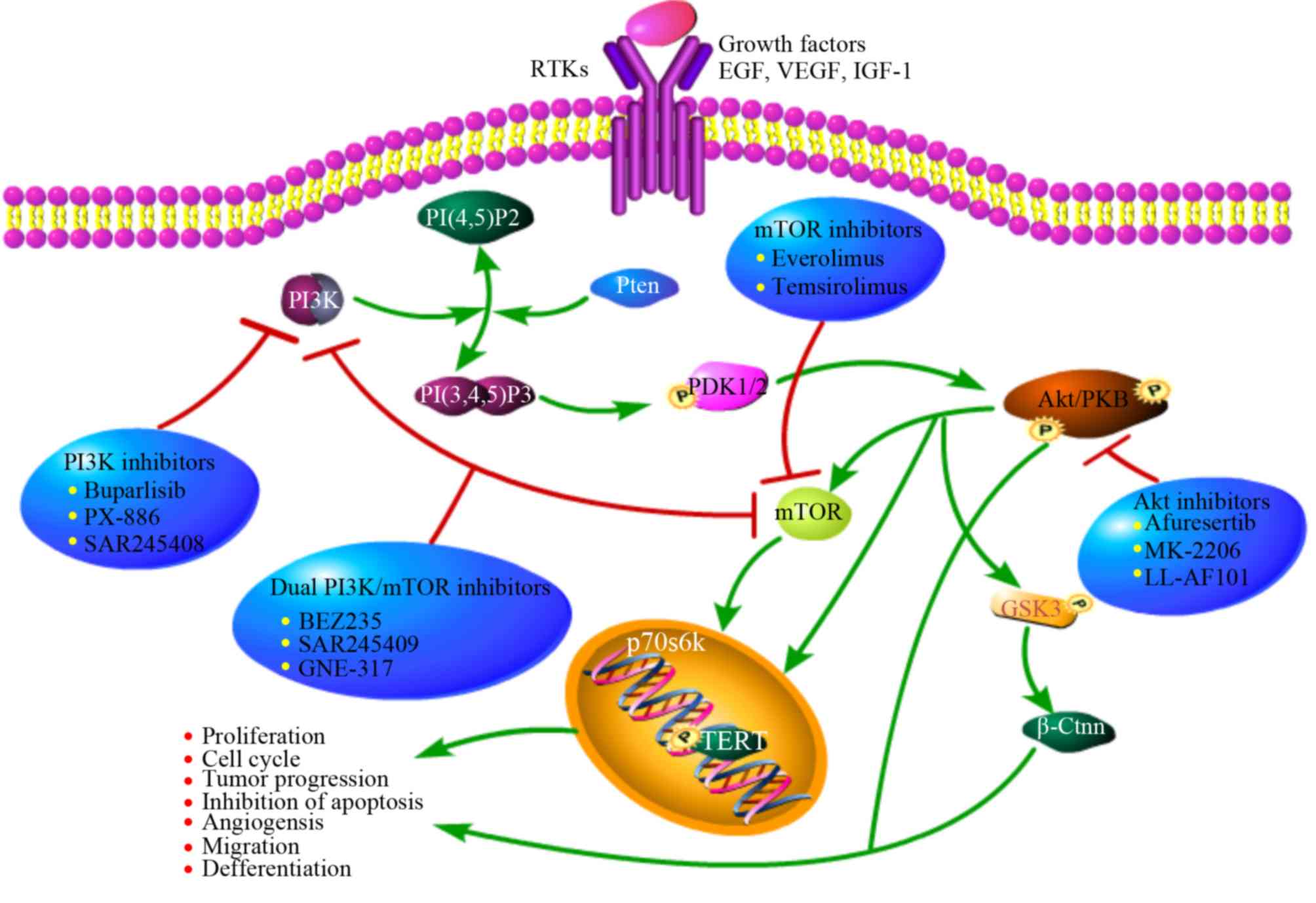
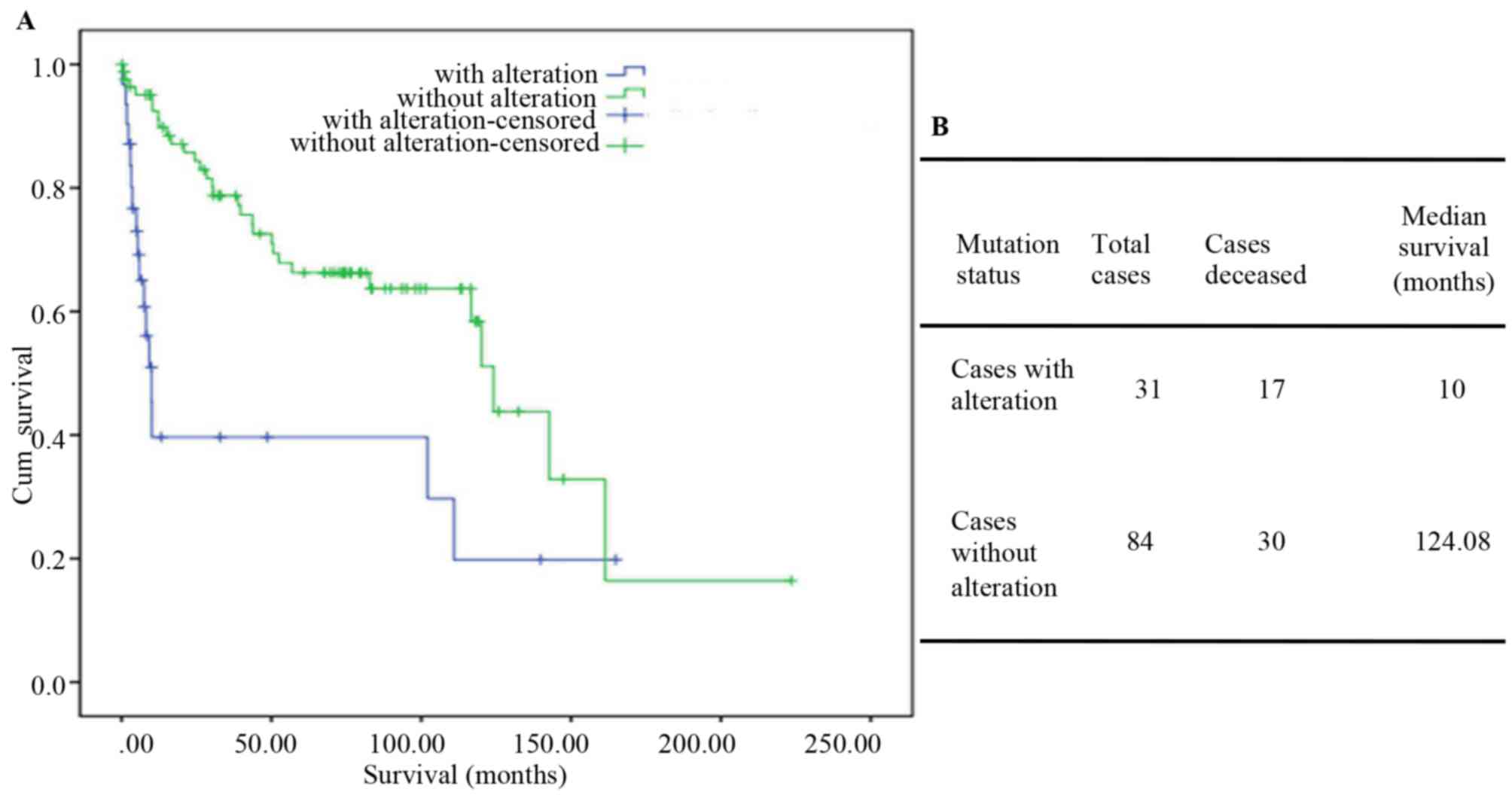
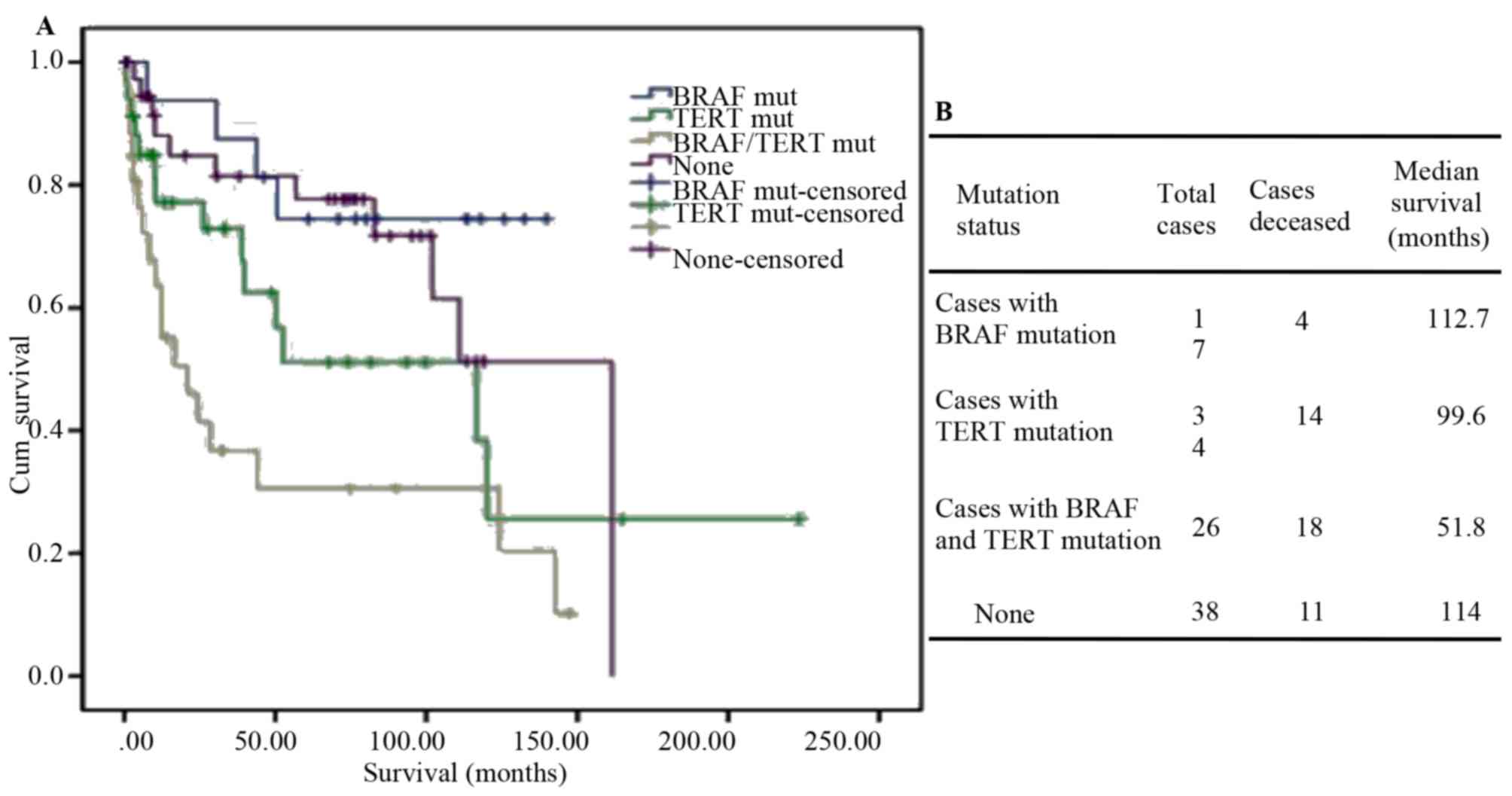
|
1
|
O'Neill JP and Shaha AR: Anaplastic
thyroid cancer. Oral Oncol. 49:702–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin S, Borkhuu O, Bao W and Yang YT:
Signaling pathways in thyroid cancer and their therapeutic
implications. J Clin Med Res. 8:284–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu KT, Yu XM, Audhya AW, Jaume JC, Lloyd
RV, Miyamoto S, Prolla TA and Chen H: Novel approaches in
anaplastic thyroid cancer therapy. Oncologist. 19:1148–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keutgen XM, Sadowski SM and Kebebew E:
Management of anaplastic thyroid cancer. Gland Surg. 4:44–51.
2015.PubMed/NCBI
|
|
5
|
Saini S, Maker AV, Burman KD and Prabhakar
BS: Genetic aberrations and alterations in signaling cascades
implicated in the pathogenesis of anaplastic thyroid cancer.
Biochim Biophys Acta Rev Cancer. 2018.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guerra A, Di Crescenzo V, Garzi A, Cinelli
M, Carlomagno C, Tonacchera M, Zeppa P and Vitale M: Genetic
mutations in the treatment of anaplastic thyroid cancer: A
systematic review. BMC Surg. 13 (Suppl 2):S442013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu B and Ghossein R: Genomic landscape of
poorly differentiated and anaplastic thyroid carcinoma. Endocr
Pathol. 27:205–212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perri F, Pezzullo L, Chiofalo MG, Lastoria
S, Di Gennaro F, Scarpati GD and Caponigro F: Targeted therapy: A
new hope for thyroid carcinomas. Crit Rev Oncol Hematol. 94:55–63.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartholomeusz C and Gonzalez-Angulo AM:
Targeting the PI3K signaling pathway in cancer therapy. Expert Opin
Ther Targets. 16:121–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saji M and Ringel MD: The PI3K-Akt-mTOR
pathway in initiation and progression of thyroid tumors. Mol Cell
Endocrinol. 321:20–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Willems L, Tamburini J, Chapuis N, Lacombe
C, Mayeux P and Bouscary D: PI3K and mTOR signaling pathways in
cancer: New data on targeted therapies. Curr Oncol Rep. 14:129–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao F, Zhang C, Han W, Gao XJ, Ma J, Hu
YW, Gu X, Ding HZ, Zhu LX and Liu Q: p-Akt as a potential poor
prognostic factor for gastric cancer: A systematic review and
meta-analysis. Oncotarget. 8:59878–59888. 2017.PubMed/NCBI
|
|
13
|
Phyu SM and Smith TAD: Combination
treatment of cancer cells with pan-Akt and pan-mTOR inhibitors:
Effects on cell cycle distribution, p-Akt expression level and
radiolabelled-choline incorporation. Invest New Drugs. 37:424–430.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xing M: Genetic alterations in the
phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.
Thyroid. 20:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunstman JW, Juhlin CC, Goh G, Brown TC,
Stenman A, Healy JM, Rubinstein JC, Choi M, Kiss N, Nelson-Williams
C, et al: Characterization of the mutational landscape of
anaplastic thyroid cancer via whole-exome sequencing. Hum Mol
Genet. 24:2318–2329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landa I, Ibrahimpasic T, Boucai L, Sinha
R, Knauf JA, Shah RH, Dogan S, Ricarte-Filho JC, Krishnamoorthy GP,
Xu B, et al: Genomic and transcriptomic hallmarks of poorly
differentiated and anaplastic thyroid cancers. J Clin Invest.
126:1052–1066. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maira SM, Pecchi S, Huang A, Burger M,
Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, et al:
Identification and characterization of NVP-BKM120, an orally
available pan-class I PI3-kinase inhibitor. Mol Cancer Ther.
11:317–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sirohi B, Rastogi S and Dawood S:
Buparlisib in breast cancer. Future Oncol. 11:1463–1470. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Z, Xu X, Li Y, Zou K, Zhang Z, Xu X,
Liao Y, Zhao X, Jiang W, Yu W, et al: Synergistic antitumor effect
of BKM120 with prima-1met via inhibiting PI3K/AKT/mTOR and
CPSF4/hTERT signaling and reactivating mutant P53. Cell Physiol
Biochem. 45:1772–1786. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: PI3K/Akt/mTOR pathway inhibitors
enhance radiosensitivity in radioresistant prostate cancer cells
through inducing apoptosis, reducing autophagy, suppressing NHEJ
and HR repair pathways. Cell Death Dis. 5:e14372014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massacesi C, Di Tomaso E, Urban P, Germa
C, Quadt C, Trandafir L, Aimone P, Fretault N, Dharan B, Tavorath R
and Hirawat S: PI3K inhibitors as new cancer therapeutics:
Implications for clinical trial design. Onco Targets Ther.
9:203–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saran U, Foti M and Dufour JF: Cellular
and molecular effects of the mTOR inhibitor everolimus. Clin Sci
(Lond). 129:895–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneider TC, de Wit D, Links TP, van Erp
NP, van der Hoeven JJ, Gelderblom H, Roozen IC, Bos M, Corver WE,
van Wezel T, et al: Everolimus in patients with advanced
follicular-derived thyroid cancer: Results of a phase II clinical
trial. J Clin Endocrinol Metab. 102:698–707. 2017.PubMed/NCBI
|
|
25
|
Onoda N, Nakamura M, Aomatsu N, Noda S,
Kashiwagi S, Kurata K, Uchino S and Hirakawa K: Significant
cytostatic effect of everolimus on a gefitinib-resistant anaplastic
thyroid cancer cell line harboring PI3KCA gene mutation. Mol Clin
Oncol. 3:522–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yi H, Ye X, Long B, Ye T, Zhang L, Yan F,
Yang Y and Li L: Inhibition of the AKT/mTOR pathway augments the
anticancer effects of sorafenib in thyroid cancer. Cancer Biother
Radiopharm. 32:176–183. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin SF, Huang YY, Lin JD, Chou TC, Hsueh C
and Wong RJ: Utility of a PI3K/mTOR inhibitor (NVP-BEZ235) for
thyroid cancer therapy. PLoS One. 7:e467262012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Petrulea MS, Plantinga TS, Smit JW,
Georgescu CE and Netea-Maier RT: PI3K/Akt/mTOR: A promising
therapeutic target for non-medullary thyroid carcinoma. Cancer
Treat Rev. 41:707–713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nitulescu GM, Margina D, Juzenas P, Peng
Q, Olaru OT, Saloustros E, Fenga C, Spandidos DΑ, Libra M and
Tsatsakis AM: Akt inhibitors in cancer treatment: The long journey
from drug discovery to clinical use (Review). Int J Oncol.
48:869–885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu R, Liu D, Trink E, Bojdani E, Ning G
and Xing M: The akt-specific inhibitor MK2206 selectively inhibits
thyroid cancer cells harboring mutations that can activate the
PI3K/Akt pathway. J Clin Endocrinol Metab. 96:E577–E585. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pflaum J, Schlosser S and Muller M: P53
family and cellular stress responses in cancer. Front Oncol.
4:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parrales A and Iwakuma T: Targeting
oncogenic mutant p53 for cancer therapy. Front Oncol. 5:2882015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maslon MM and Hupp TR: Drug discovery and
mutant p53. Trends Cell Biol. 20:542–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bullock AN and Fersht AR: Rescuing the
function of mutant p53. Nat Rev Cancer. 1:68–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wiman KG: Strategies for therapeutic
targeting of the p53 pathway in cancer. Cell Death Differ.
13:921–926. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Larson C, Oronsky B, Scicinski J, Fanger
GR, Stirn M, Oronsky A and Reid TR: Going viral: A review of
replication-selective oncolytic adenoviruses. Oncotarget.
6:19976–19989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galanis E, Okuno SH, Nascimento AG, Lewis
BD, Lee RA, Oliveira AM, Sloan JA, Atherton P, Edmonson JH,
Erlichman C, et al: Phase I–II trial of ONYX-015 in combination
with MAP chemotherapy in patients with advanced sarcomas. Gene
Ther. 12:437–445. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Makower D, Rozenblit A, Kaufman H, Edelman
M, Lane ME, Zwiebel J, Haynes H and Wadler S: Phase II clinical
trial of intralesional administration of the oncolytic adenovirus
ONYX-015 in patients with hepatobiliary tumors with correlative p53
studies. Clin Cancer Res. 9:693–702. 2003.PubMed/NCBI
|
|
39
|
Chen GX, Zhang S, He XH, Liu SY, Ma C and
Zou XP: Clinical utility of recombinant adenoviral human p53 gene
therapy: Current perspectives. Onco Targets Ther. 7:1901–1909.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Pan J, Zhu X, Su Y, Bao L, Qiu S,
Zou C, Cai Y, Wu J and Tham IW: Recombinant adenovirus-p53
(Gendicine) sensitizes a pancreatic carcinoma cell line to
radiation. Chin J Cancer Res. 25:715–721. 2013.PubMed/NCBI
|
|
41
|
Wade M, Li YC and Wahl GM: MDM2, MDMX and
p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 13:83–96.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oren M, Tal P and Rotter V: Targeting
mutant p53 for cancer therapy. Aging (Albany NY). 8:1159–1160.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Selivanova G: Wild type p53 reactivation:
From lab bench to clinic. FEBS Lett. 588:2628–2638. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Urso L, Calabrese F, Favaretto A, Conte P
and Pasello G: Critical review about MDM2 in cancer: Possible role
in malignant mesothelioma and implications for treatment. Crit Rev
Oncol Hematol. 97:220–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Puca R, Nardinocchi L, Porru M, Simon AJ,
Rechavi G, Leonetti C, Givol D and D'Orazi G: Restoring p53 active
conformation by zinc increases the response of mutant p53 tumor
cells to anticancer drugs. Cell Cycle. 10:1679–1689. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bykov VJ and Wiman KG: Mutant p53
reactivation by small molecules makes its way to the clinic. FEBS
Lett. 588:2622–2627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Messina RL, Sanfilippo M, Vella V, Pandini
G, Vigneri P, Nicolosi ML, Gianì F, Vigneri R and Frasca F:
Reactivation of p53 mutants by prima-1 [corrected] in thyroid
cancer cells. Int J Cancer. 130:2259–2270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr-Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Melo M, da Rocha AG, Vinagre J, Batista R,
Peixoto J, Tavares C, Celestino R, Almeida A, Salgado C, Eloy C, et
al: TERT promoter mutations are a major indicator of poor outcome
in differentiated thyroid carcinomas. J Clin Endocrinol Metab.
99:E754–E765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu R and Xing M: TERT promoter mutations
in thyroid cancer. Endocr-Relat Cancer. 23:R143–R155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin A, Xu J and Wang Y: The role of TERT
promoter mutations in postoperative and preoperative diagnosis and
prognosis in thyroid cancer. Medicine (Baltimore). 97:e115482018.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dong H, Shen WZ, Yan YJ, Yi JL and Zhang
L: Effects of BRAF(V600E) mutation on Na(+)/I(−) symporter
expression in papillary thyroid carcinoma. J Huazhong Uni Sci
Technolog. Med Sci. 36:77–81. 2016.
|
|
53
|
Shi X, Liu R, Qu S, Zhu G, Bishop J, Liu
X, Sun H, Shan Z, Wang E, Luo Y, et al: Association of TERT
promoter mutation 1,295,228 C>T with BRAF V600E mutation, older
patient age, and distant metastasis in anaplastic thyroid cancer. J
Clin Endocrinol Metab. 100:E632–E637. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Charles RP, Silva J, Iezza G, Phillips WA
and McMahon M: Activating BRAF and PIK3CA mutations cooperate to
promote anaplastic thyroid carcinogenesis. Mol Cancer Res.
12:979–986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang W: BRAF inhibitors: The current and
the future. Curr Opin Pharmacol. 23:68–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim KB, Cabanillas ME, Lazar AJ, Williams
MD, Sanders DL, Ilagan JL, Nolop K, Lee RJ and Sherman SI: Clinical
responses to vemurafenib in patients with metastatic papillary
thyroid cancer harboring BRAF(V600E) mutation. Thyroid.
23:1277–1283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Brose MS, Cabanillas ME, Cohen EE, Wirth
LJ, Riehl T, Yue H, Sherman SI and Sherman EJ: Vemurafenib in
patients with BRAF(V600E)-positive metastatic or unresectable
papillary thyroid cancer refractory to radioactive iodine: A
non-randomised, multicentre, open-label, phase 2 trial. Lancet
Oncol. 17:1272–1282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Marten KA and Gudena VK: Use of
vemurafenib in anaplastic thyroid carcinoma: A case report. Cancer
Biol Ther. 16:1430–1433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cabanillas ME, Patel A, Danysh BP, Dadu R,
Kopetz S and Falchook G: BRAF inhibitors: Experience in thyroid
cancer and general review of toxicity. Horm Cancer. 6:21–36. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lim AM, Taylor GR, Fellowes A, Cameron L,
Lee B, Hicks RJ, McArthur GA, Angel C, Solomon B and Rischin D:
BRAF Inhibition in BRAFV600E-Positive Anaplastic Thyroid Carcinoma.
J Natl Compr Canc Netw. 14:249–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Maggisano V, Celano M, Lombardo GE, Lepore
SM, Sponziello M, Rosignolo F, Verrienti A, Baldan F, Puxeddu E,
Durante C, et al: Silencing of hTERT blocks growth and migration of
anaplastic thyroid cancer cells. Mol Cell Endocrinol. 448:34–40.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bu R, Siraj AK, Divya SP, Kong Y,
Parvathareddy SK, Al-Rasheed M, Al-Obaisi KAS, Victoria IG,
Al-Sobhi SS, Al-Dawish M, et al: Telomerase reverse transcriptase
mutations are independent predictor of disease-free survival in
middle eastern papillary thyroid cancer. Int J Cancer.
142:2028–2039. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Namba H, Saenko V and Yamashita S: Nuclear
factor-kB in thyroid carcinogenesis and progression: A novel
therapeutic target for advanced thyroid cancer. Arq Bras Endocrinol
Metabol. 51:843–851. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhu W, He S, Li Y, Qiu P, Shu M, Ou Y,
Zhou Y, Leng T, Xie J, Zheng X, et al: Anti-angiogenic activity of
triptolide in anaplastic thyroid carcinoma is mediated by targeting
vascular endothelial and tumor cells. Vascul Pharmacol. 52:46–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sastre-Perona A and Santisteban P:
Wnt-independent role of β-catenin in thyroid cell proliferation and
differentiation. Mol Endocrinol. 28:681–695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang L, Shao YY and Ballock RT: Thyroid
hormone interacts with the Wnt/beta-catenin signaling pathway in
the terminal differentiation of growth plate chondrocytes. J Bone
Miner Res. 22:1988–1995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ito Y, Onoda N, Ito KI, Sugitani I,
Takahashi S, Yamaguchi I, Kabu K and Tsukada K: Sorafenib in
japanese patients with locally advanced or metastatic medullary
thyroid carcinoma and anaplastic thyroid carcinoma. Thyroid.
27:1142–1148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gupta-Abramson V, Troxel AB, Nellore A,
Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT,
Loevner LA, O'Dwyer PJ and Brose MS: Phase II trial of sorafenib in
advanced thyroid cancer. J Clin Oncol. 26:4714–4719. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kloos RT, Ringel MD, Knopp MV, Hall NC,
King M, Stevens R, Liang J, Wakely PE Jr, Vasko VV, Saji M, et al:
Phase II trial of sorafenib in metastatic thyroid cancer. J Clin
Oncol. 27:1675–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bible KC, Suman VJ, Menefee ME, Smallridge
RC, Molina JR, Maples WJ, Karlin NJ, Traynor AM, Kumar P, Goh BC,
et al: A multiinstitutional phase 2 trial of pazopanib monotherapy
in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab.
97:3179–3184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Takahashi S, Kiyota N, Yamazaki T,
Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H,
Imamura Y, et al: A Phase II study of the safety and efficacy of
lenvatinib in patients with advanced thyroid cancer. Future Oncol.
15:717–726. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sasanakietkul T, Murtha TD, Javid M, Korah
R and Carling T: Epigenetic modifications in poorly differentiated
and anaplastic thyroid cancer. Mol Cell Endocrinol. 469:23–37.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Celano M, Mio C, Sponziello M, Verrienti
A, Bulotta S, Durante C, Damante G and Russo D: Targeting
post-translational histone modifications for the treatment of
non-medullary thyroid cancer. Mol Cell Endocrinol. 469:38–47. 2018.
View Article : Google Scholar : PubMed/NCBI
|