Introduction
Over the past decades, the connective tissue has
been considered not only as a mechanical support structure for a
tissue but also as a biologically active component of the tissue
itself (1–3). The extracellular matrix (ECM) is a
dynamic, living structure, which is continuously remodeled and
involved in both physiological and pathological processes (1–3).
Accordingly, the analysis of ECM remodeling
represents an attractive topic for investigation. The loss of ECM
plasticity (i.e. the dysregulation of ECM turnover and barrier
effect) is a key factor for the onset and the progression of a
number of neoplastic, degenerative and inflammatory diseases
(4,5). In these diseases, the structure and
function of the ECM are affected by persistent low-grade chronic
systemic inflammation (LGCSI). LGCSI contributes to the development
of insulin resistance, dyslipidemia, atherogenesis, type II
diabetes and hypertension thus promoting cardiometabolic disease
(6). In addition, several cytokines
are produced in tissues affected by LGCSI that can affect the
homeostasis of ECM (6). These
alterations trigger the pathological process and promote both onset
and progression of a disease (7).
The loss of ECM homeostasis caused by LGCSI initially induces an
increase of matrix solubility and then the deposition of
disorganized collagens (types I, III and IV). The altered collagen
turnover leads to tissue fibrosis (8). Accordingly, the ECM shifts from
‘plasticity’ to ‘rigidity’ (9,10).
Within collagen types, collagen type III is directly responsible
for the elastic properties of the connective tissue and it is
involved in numerous fibrotic diseases (11,12).
Various studies corroborated the pathophysiological role of the
ECM, making it a potential therapeutic target (13–15). The
analysis of the effects of some natural substances on ECM
remodeling may lead to the development of a pharmacological
approach for the modulation of ECM turnover.
The aim of the current study was to analyze the
effect of a natural multi-component compound formulation named
Galium-Heel® on the growth, morphology and ECM
production of human skin fibroblasts. The present findings may
provide basis for the development of a new pharmacological approach
to counteract local abnormal ECM remodeling.
Materials and methods
Materials and reagents
Galium-Heel® is manufactured by
Biologische Heilmittel Heel GmbH. Sodium chloride 0.9% was obtained
from Eurospital. Galium-Heel® (from here on termed
Galium) contains: Acidum nitricum, Apis mellifica, argentum
metallicum, aurum metallicum, Betula pendula, calcium fluoratum,
Caltha palustris, Clematis recta, Echinacea, Galium mollugo, Galium
aparine, Hedera helix, Juniperus communis, Ononis spinosa,
phosphorus, Pyrogenium Nosode, Saponaria officinalis, Sedum
acre, Sempervivum tectorum ssp., Thuja occidentalis, Urtica
urens (Table I). Components were
dissolved in sodium chloride 0.9%.
 | Table I.Concentration of
Galium-Heel® components. |
Table I.
Concentration of
Galium-Heel® components.
|
Componenta | Concentration |
|---|
| Acidum
nitricum | 0.55 ng |
| Apis
mellifica | 0.0055 pg |
| Aurum
metallicum | 0.055 pg |
| Caltha
palustris | 1.1 µg |
| Clematis
recta | 0.165 µg |
|
Echinacea | 0.0165 µg |
| Galium
aparine | 0.88 µg |
| Galium
mollugo | 0.88 µg |
| Hedera
helix | 0.165 µg |
| Juniperus
communis | 0.165 µg |
| Ononis
spinosa | 0.165 µg |
| Phosphorus | 5.5 pg |
| Pyrogenium
Nosode | 5.5 ng |
| Saponaria
officinalis | 0.55 µg |
| Sedum
acre | 1.1 µg |
| Sempervivum
tectorum ssp. | 0.11 µg |
| Thuja
occidentalis | 1.65 µg |
| Urtica
urens | 4.4 µg |
| Argentum
metallicum | 5.5 pg |
| Calcium
fluoratum | 5.5 pg |
| Betula
pendula | 0.011 mg |
Sulforhodamine B (SRB), trypan blue and Amicon
centricon 100 and 30 micro concentrators were purchased from
Sigma-Aldrich (Merck KGaA). SimplyBlue SafeStain was purchased from
Invitrogen (Thermo Fisher Scientific, Inc.). Rabbit polyclonal
antibody against collagen type I (cat. no. NB600-408; 1:1,000) was
obtained from Novus Biologicals, LLC. Rabbit polyclonal antibody
against collagen type III (cat. no. AB747; 1:300) was purchased
from Chemicon International. Rabbit polyclonal antibodies against
matrix metalloproteinase (MMP)-2, −3, −7 (cat. no. SA-384; 1:500)
were obtained from Enzo Life Sciences. Rabbit monoclonal antibody
against AKT (C67E7; cat. no. 4691; 1:1,000), rabbit monoclonal
antibody against phosphorylated (p)-AKT (Ser473; D9E; cat. no.
4060; 1:1,000) were obtained from Cell Signaling Technology. Mouse
monoclonal antibody against JNK/mitogen-activated protein kinase 9
(SAPK) 1 (cat. no. BD 610627; 1:250), mouse monoclonal antibody
against JNK/SAPK (pT183/pY185; cat. no. BD 612540; 1:250), mouse
monoclonal antibody against p38α/SAPK2a (cat. no. BD 612168;
1:1,000) and mouse monoclonal antibody against p38 mitogen
activated kinase-like protein (MAPK; pT180/pY182; cat. no. BD
612280; 1:200) were obtained from BD Pharmingen (BD Biosciences).
Rabbit polyclonal antibody against extracellular signal-regulated
kinase (ERK)1/2 (C-14; cat. no. sc-154; 1:300), mouse monoclonal
antibody against p-ERK (E-4; cat. no. sc-7383; 1:300) and rabbit
polyclonal antibody against GLI family zinc finger 2 (GLI-2; H-300;
cat. no. sc-28674; 1:200) were obtained from Santa Cruz
Biotechnology, Inc. Rabbit polyclonal antibody against fibronectin
(cat. no. F3648; 1:1,000), rabbit polyclonal antibody against actin
(cat. no. A5060; 1:500) and peroxidase-conjugated goat anti-mouse
polyclonal (cat. no. A4416; 1:5,000) or anti-rabbit polyclonal
(cat. no. A6154; 1:10,000) immunoglobulin (Ig)G were obtained from
Sigma-Aldrich (Merck KGaA).
Cell lines and treatments
Human adult fibroblasts (HDF), purchased from Cell
Applications, Inc. (cat. no. 106-05a), and previously characterized
H-Meso-1 cells (16) were maintained
in DMEM high glucose (Aurogene s.r.l.) containing 10% fetal bovine
serum (FBS) (Aurogene s.r.l), 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were cultured at 37°C in a humidified incubator
with an atmosphere of 5% CO2.
For the treatments, cells were incubated for the
indicated times in the presence of Galium (dose range: 1:2 to 1:16)
or vehicle control (sodium chloride 0.9%; CTR; 1:2 to 1:16)
re-suspended in DMEM containing 0.2% bovine serum albumin (BSA)
(Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin and 100 µg/ml
streptomycin.
SRB assay
Cells were seeded at a density of
5×103/well in 96-well plates and incubated at 37°C to
allow cell attachment. After 24 h, the medium was changed and the
cells were treated with Galium or sodium chloride and incubated for
24, 48 or 72 h at concentrations of 1:2, 1:4, 1:8 or 1:16 in DMEM
0.2% BSA. The cells were then fixed with cold trichloroacetic acid
(final concentration 10%; Sigma-Aldrich; Merck KGaA) for 1 h at
4°C. After four washes with distilled water, the plates were
air-dried and stained for 30 min at room temperature with 0.4%
(wt/vol) SRB in 1% acetic acid (Sigma-Aldrich; Merck KGaA). After
four washes with 1% acetic acid to remove the unbound dye, the
plates were air-dried, and cell-bound SRB was dissolved with 100
µl/well of 10 mM unbuffered Tris base solution (Sigma-Aldrich;
Merck KGaA). The optical density (O.D.) of the samples was
determined at 540 nm with a spectrophotometric plate reader
(Multiskan FC; Thermo Fisher Scientific, Inc.). The percentage
survival of the cultures treated with Galium was calculated by
normalizing their O.D. values to each control culture treated with
sodium chloride at the same dilution (considered as 100%). The
experiments were performed in triplicate and repeated three times.
H-Meso-1 cells treated with 100 µM apigenin (Sigma-Aldrich; Merck
KGaA) were used as the positive control for the inhibition of cell
growth. The percentage of surviving cells treated with apigenin was
calculated by normalizing the O.D. value to that of the control
cultures treated with DMSO (≤0.1%), which is a solvent of apigenin
(17).
Trypan blue exclusion test
Cells were seeded at a density of
5×104/well in 24-well plates and incubated at 37°C to
allow cell attachment. After 24-h, the medium was changed and the
cells were treated with Galium or sodium chloride and incubated for
24, 48 or 72-h at concentrations of 1:2 and 1:4 in DMEM 0.2% BSA.
After 24, 48 and 72 h of adherence, cells from each well were
harvested and stained with trypan blue for 2 min at room
temperature, then counted with an Olympus IX50 inverted light
microscope (magnification, ×100) (18). Percentage of cell death was
determined by dividing dead cells by the total number of cells ×100
(19). Experiments were performed in
triplicate and repeated three times. H-Meso-1 cells treated with
apigenin (100 µM) were used as the positive control for the
inhibition of cells survival.
Fluorescent measurement of reactive
oxygen species (ROS)
To detect ROS production in HDF cells,
2′,7′-dichlorofluorescin diacetate (DCF-DA; Sigma-Aldrich; Merck
KGaA) was used. In brief, 2.5×105 cells were seeded into
six-well plates and incubated at 37°C to allow cell attachment
before treatment. After two PBS washes, cells were incubated with
10 µM DCF-DA in PBS at 37°C and 5% CO2 in the dark for
30 min (20). Following two washes,
cells were treated with Galium or sodium chloride at the dilution
of 1:2 in DMEM 0.2% BSA and incubated at 37°C and 5% CO2
in the dark for 30 min. Then, cells were harvested, centrifuged at
300 × g for 10 min at 4°C and seeded in 96-well plates
(2.5×104 cells in 100 µl per well). Fluorescence
intensity was measured after 30 min using a spectrophotometric
plate reader at an excitation wavelength of 495 nm and an emission
wavelength of 535 nm (Wallace 1420 Victor; Perkin Elmer Inc.).
Malignant mesothelioma H-Meso-1 cells treated with apigenin (100
µM) were used as the positive control for the intracellular ROS
production.
Fluorescence-activated cell sorting
(FACS) analysis
Log-phase growing HDF cells (60% confluent;
2.5×105/well in six-well plates) were treated with
Galium or sodium chloride at the dilution of 1:2 in DMEM 0.2% BSA.
Following a 48-h incubation, cells were harvested, centrifuged at
300 × g for 10 min at 4°C and washed twice with cold PBS. The cell
pellets were re-suspended in 70% ethanol and incubated for 1 h at
−20°C. The cells were then washed twice with cold PBS, centrifuged
at 300 × g for 10 min at 4°C, incubated for 1 h in the dark with
propidium iodide at a final concentration of 25 µg/ml in 0.1%
citrate and 0.1% Triton X-100 (all Sigma-Aldrich; Merck KGaA).
Samples were then analyzed by flow cytometry using a FACSCalibur™
cytometer with CellQuest Pro 5.2 software (both BD Biosciences)
(21).
Preparation of cell lysates and
western blotting
Cells were seeded at a density of 1×106
cells in 100-mm tissue culture dishes 24 h prior to the addition of
Galium or sodium chloride at the dilution of 1:2 in DMEM 0.2% BSA.
Following 48 h of incubation, the conditioned media was collected
and then centrifuged at 600 × g for 15 min at 4°C in 100 kDa or 30
kDa molecular weight cut-off Centricon tubes. Cells were harvested,
washed twice with cold PBS and lysed in RIPA lysis buffer (Triton
X-100 1%, SDS 0.1%, NaCl 200 mM, Tris HCl 50 mM pH 7.5, PMSF 1 mM,
and Na3VO4 1 mM). After 30 min at 4°C, the
mixtures were centrifuged at 12,000 × g for 15 min at 4°C, the
protein concentration of the lysates were quantified with Bradford
assay and then analyzed by western blotting. Handcast gels were
prepared from acrylamide and bisacrylamide monomer solutions (cat.
no. A3574; Sigma-Aldrich; Merck KGaA). SDS-PAGE and western blot
analysis were performed using Mini-PROTEAN Tetra Cell apparatus
(Bio-Rad Laboratories, Inc.) according to the manufacturer's
instructions. Electrophoresis (cat. no. 161-0732) and blot transfer
(cat. no. 161-0734) buffers were purchased from Bio-Rad
Laboratories, Inc. Hyper PAGE prestained markers (cat. no.
BIO-33066; Bioline; Meridian Bioscience) were used. For western
blot analysis, 50 µg of cell lysates per lane and 20 µl of
conditioned media per lane were resolved in 8–12% gels by SDS-PAGE
(150 V for 1 h) and then transferred to nitrocellulose membranes
(30 V for 90 min; GE Healthcare). After blocking with 5% skimmed
dry milk in PBS overnight at 4°C, the membranes were incubated
overnight at 4°C with the specific primary antibodies at the
dilution suggested by the manufacturers (Materials and reagents
section). Following washes, the membranes were incubated with
peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antibodies
for 1 h at room temperature. Protein bands were visualized by
enhanced chemiluminescence (LiteABlot Plus; Euroclone S.p.A.)
(22). Equal loading of conditioned
media on the gel was confirmed by Coomassie blue staining. Briefly,
after SDS-PAGE the gel was rinsed 3 times for 5 min with deionized
water, stained with SimplyBlue SafeStain for 1 h at room
temperature, and then washed 2 times for 1 h with water.
Densitometry was performed using Image J 1.42q software (National
Institutes of Health) after blot scanning (HP Scanjet 4890 Photo
Scanner; Hewlett-Packard).
Ultrastructural analysis
Ultrastructural analysis was performed on HDF cells
24 h following treatment with Galium or sodium chloride at the
dilution of 1:2 in DMEM 0.2% BSA. Cells were fixed with 2.5%
glutaraldehyde in PBS at 4°C for 48-h, and processed for
transmission electron microscopy (TEM), following routine
procedures (23,24). Briefly, cells were post-fixed with
1.33% osmium tetroxide at 4°C for 1 h, dehydrated in graded
alcohols (30%-50%-70%-95%-100% at 4°C), and then embedded in Epon
812 resin (Fisher Chemical Co.). The resin was allowed to
polymerize in a dry oven at 60°C overnight. Thin (1 µM) and
ultrathin (900Å) sections were cut on a Reichert ultra-microtome,
stained with uranyl acetate and lead citrate (3 min each at room
temperature), and observed under a Philips Morgagni 268D
transmission electron microscope at magnifications between ×7,000
and ×28,000.
Statistical analysis
Statistical analyses were performed with GraphPad
Prism 5.0 software (GraphPad Software, Inc.). Data were presented
as the mean ± SD. Data distribution of cell survival and trypan
blue exclusion test were preliminarily verified by
Kolmogorov-Smirnov test, and data sets were analyzed by one-way
analysis of variance followed by Newman-Keuls post-hoc test. Data
distribution of ROS production and FACS analysis, and differences
in the intensity of immunoreactive bands were analyzed by
two-tailed Student's t-test. P<0.05 was considered to indicate
statistical significance.
Results
Galium does not significantly affect
HDF cell growth or death
The survival of HDFs was evaluated by SRB assay
after treatment with increasing doses of Galium or sodium chloride
as control (1:2, 1:4, 1:8 and 1:16) for 24, 48 and 72 h. The
percentage of cell survival following treatment with Galium was
compared with cells treated with sodium chloride. No significant
differences between Galium and CTR were observed (P>0.05;
Fig. 1A). In addition, a Trypan blue
exclusion test was performed to test cell viability after treatment
with increasing doses of Galium or sodium chloride (1:2 and 1:4)
for 24, 48 and 72 h. No significant differences between treatments
were observed (P>0.05; Fig. 1B).
H-Meso-1 cells treated with apigenin (100 µM) were used as control.
Apigenin significantly inhibited H-Meso-1 cell survival (cell
growth: 65, 37, 22% for apigenin vs. 100% for control after 24, 48
and 72 h, respectively; P<0.01) and promoted cell death in a
time-dependent manner (cell death: 49, 67, 84% for apigenin vs. 2,
2, 3% for control after 24, 48 and 72 h, respectively; P<0.01;
data not shown).
Galium significantly decreases
intracellular ROS production in HDF cells
A DCF-DA assay was performed to determine the effect
of Galium on the intracellular ROS production in HDF. Galium
induced a significant decrease of intracellular ROS production when
compared with the control (P<0.01; Table II). H-Meso-1 cells treated with
apigenin (100 µM) were used as a positive control for monitoring
the intracellular ROS production. Apigenin significantly increased
the production of ROS in H-Meso-1 cells (3,353,636±272,041 for
apigenin vs. 184,118±3,530 for control; P<0.001; data not
shown).
 | Table II.Effects of Galium on the
intracellular ROS production in HDF. |
Table II.
Effects of Galium on the
intracellular ROS production in HDF.
| Treatment | Intracellular ROS
production |
|---|
| Sodium chloride 1:2
(CTR) | 320,564±2,251 |
| Galium 1:2 |
283,354±11,411a |
Galium does not significantly affect
cell cycle distribution
In order to evaluate the effect of Galium on cell
cycle distribution, FACS analysis of DNA content was performed on
HDF cells treated with Galium or sodium chloride at the dilution of
1:2. No significant changes were observed in any of the different
phases of the cell cycle following Galium treatment compared with
control cells treated with sodium chloride (P>0.05; Table III).
 | Table III.Effect of Galium on cell cycle
distribution. |
Table III.
Effect of Galium on cell cycle
distribution.
| Treatment | Sub-G1 | P-value | G0/G1 | P-value | S | P-value | G2/M | P-value |
|---|
| Sodium chloride 1:2
(CTR) | 1.70±0.71 |
| 89.70±1.44 |
| 3.05±0.11 |
| 5.65±0.86 |
|
| Galium 1:2 | 1.90±1.05 | >0.05 | 90.89±1.93 | >0.05 | 2.67±0.03 | >0.05 | 4.66±0.92 | >0.05 |
Effect of Galium on the expression of
extracellular matrix molecules and MMPs
The effect of Galium on the expression of
extracellular matrix molecules (collagen type I and III,
fibronectin) and metalloproteinases (MMP-2, −3, −7) in HDF was
evaluated by western blot analysis 48 h following Galium or sodium
chloride treatments. The analysis was performed both in HDF lysates
(Fig. 2A and C) and in the
conditioned medium (Fig. 2B and D).
Galium treatment significantly decreased the expression of collagen
type III both in the cell lysate (P<0.05) and in the conditioned
medium (P<0.01). Conversely, collagen type I was increased
following Galium treatment both at the intracellular level
(P<0.01) and in the extracellular space (P<0.001). Collagen
type III/I ratio was 0.81 for CTR vs. 0.43 for Galium in the cell
lysate and 1.0 for CTR vs. 0.59 for Galium in the extracellular
space. The expression of fibronectin was not affected by Galium
treatment. MMP-3 (P<0.01) and MMP-7 (P<0.01) significantly
decreased in the cell lysates following Galium treatment.
Conversely, MMP-3 (P<0.05) increased in the extracellular space
compared with CTR (Fig. 2). Galium
treatment did not affect the expression of MMP-2 (Fig. 2).
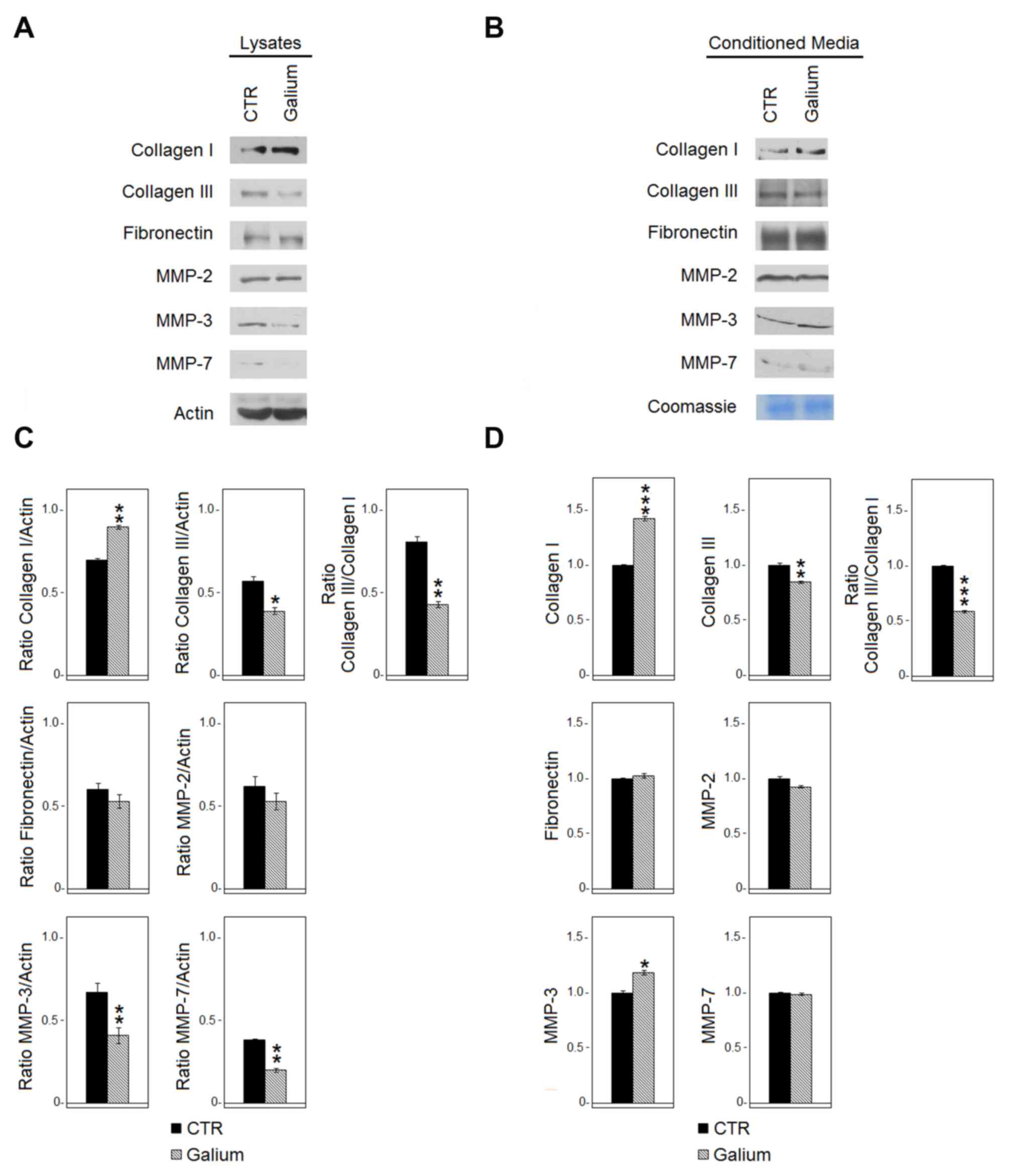 | Figure 2.Effects of Galium on the expression
of extracellular matrix molecules and MMPs. (A) Expression of
collagen type I and type III, fibronectin, MMP-2, MMP-3 and MMP-7
in cell lysates derived from HDF cells treated for 48 h with CTR or
Galium at the dilution of 1:2 in DMEM 0.2% BSA was assessed by
western blot analysis. (B) Expression of collagen type I and III,
fibronectin, MMP-2, MMP-3 and MMP-7 in the conditioned media
derived from HDF-treated cells was assessed by western blot
analysis. Equal loading of protein on the gel was confirmed by
Coomassie blue staining of the gel. Each protein was normalized to
total protein loading assessed by Coomassie blue staining. (C and
D) Densitometric ratios and statistical analysis of the respective
blots for sodium chloride- and Galium-treated cells. Data are
expressed as the mean ± SD of two experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. CTR. Galium,
Galium-Heel®; MMP, matrix metalloproteinase; HDF, human
dermal fibroblasts; CTR, sodium chloride. |
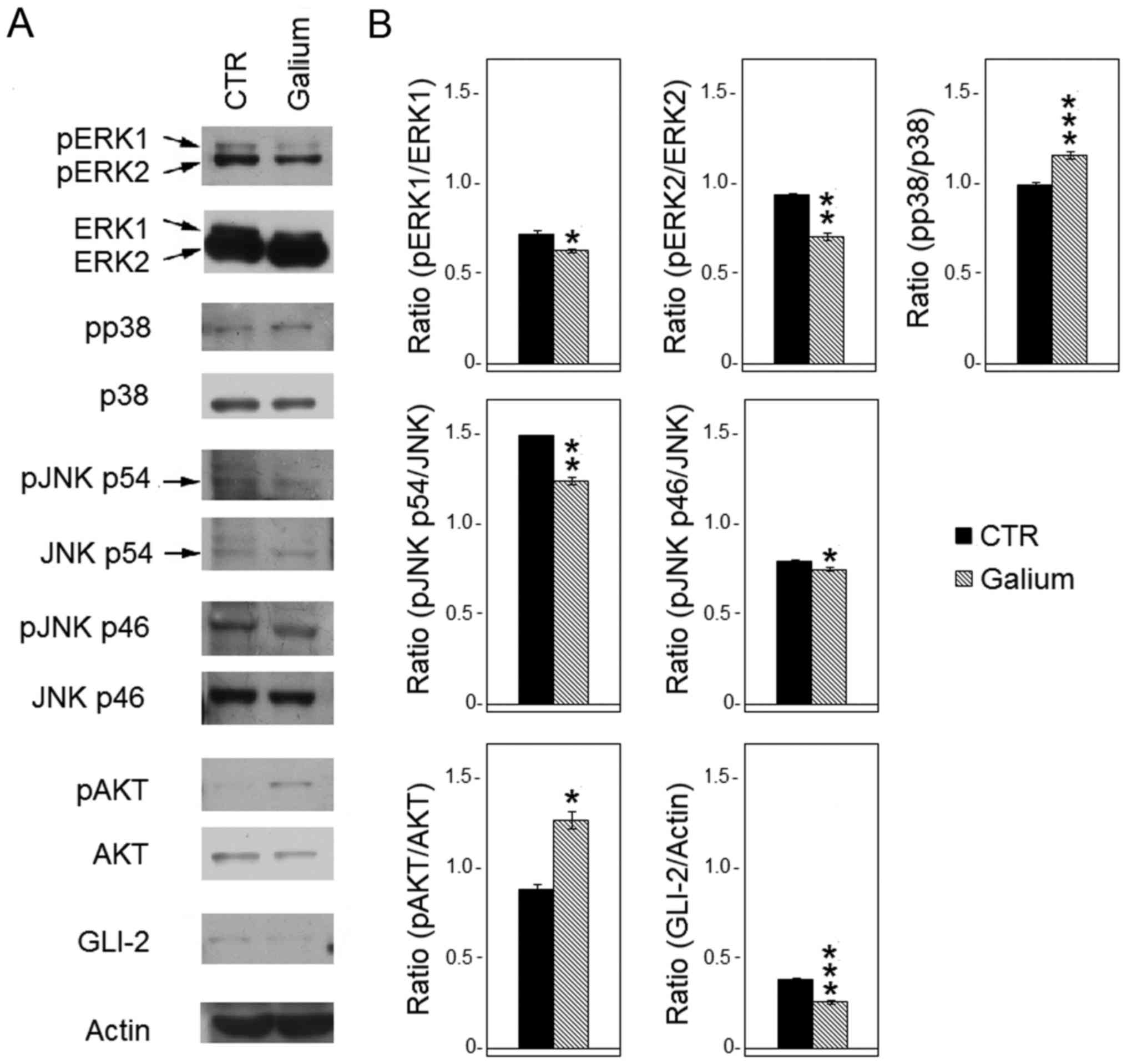
Galium upregulated expression and
activation of pro-survival signaling pathway molecules
To evaluate whether Galium affected the expression
and phosphorylation of MAPKs, western blot analysis was performed
after treating HDF with Galium or sodium chloride for 48 h.
The levels of p-ERK1, p-ERK2, p-p38-MAPKα, p-JNK p46
and p-JNK p54 proteins were compared with their respective total
proteins. Results demonstrated that Galium treatment significantly
decreased the levels of phosphorylation of ERK1/2 (p-ERK1,
P<0.05; p-ERK2, P<0.01) compared with CTR (Fig. 3). In addition, Galium-treated cells
demonstrated significantly reduced phosphorylation of JNK p54
(P<0.01) and JNK p46 (P<0.05) compared with the CTR-treated
cells (Fig. 3). Conversely,
p38-MAPKα phosphorylation was increased following Galium treatment
(P<0.001) compared with sodium chloride treatment (Fig. 3). Furthermore, the effect of Galium
treatment on the expression of pro-survival kinase AKT was
evaluated. Results demonstrated that Galium significantly increased
the p-AKT protein levels compared with CTR (P<0.05; Fig. 3). Finally, GLI-2 expression was
significantly decreased after Galium treatment (P<0.001) when
compared with CTR treatment (Fig.
3).
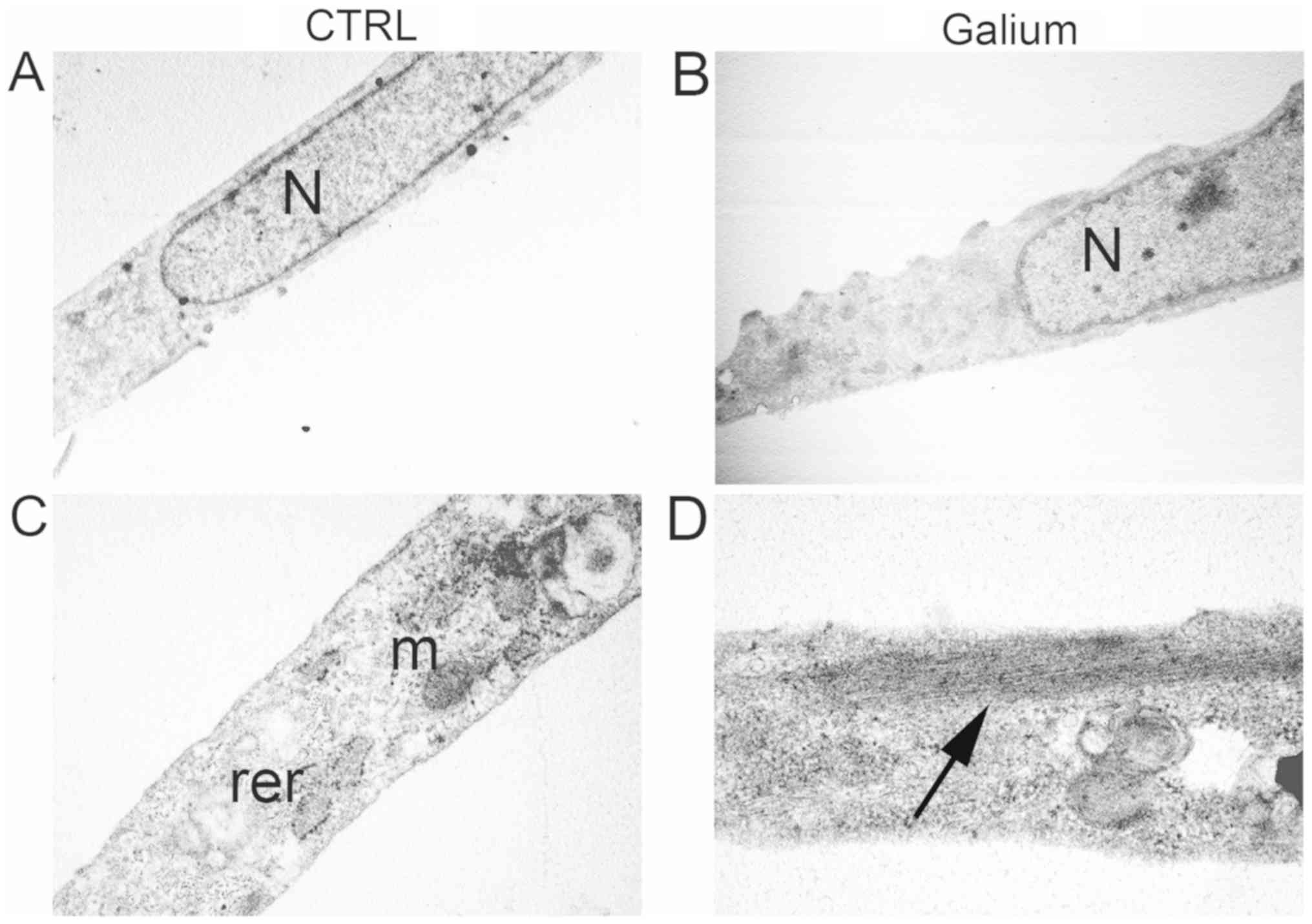
Ultrastructural analysis of HDF cells
treated with Galium
Ultrastructural analysis was performed on HDF cells
24-h following treatment with Galium or sodium chloride at the
dilution of 1:2 in DMEM 0.2% BSA. Sodium chloride-treated HDF cells
displayed an elongated morphology with elongated centrally located
nuclei, essentially formed by euchromatin with poor heterochromatin
and well organized nucleoli (Fig.
4A). Condensed mitochondria, dilated rough endoplasmic
reticulum, and primary and secondary lysosomes were also observed
(Fig. 4A). Intermediate filaments
were visible in the cytoplasm (Fig.
4C). Galium-treated cells had similar appearance to CTR-treated
cells (Fig. 4B). However, an
increase in the polymerization of intermediate filaments was
observed in Galium-treated cells (Fig.
4D).
Discussion
The ECM produces a tissue microenvironment able to
affect and regulate cellular signalling (1–3). It is
fundamental for optimal cell crosstalk to maintain ECM homeostasis
(1–3). The loss of ECM plasticity, which is
dependent on the anabolic and catabolic phases, is associated with
the onset of several pathologies, especially those involving
chronic inflammation. Persistent LGCSI causes the ECM to lose its
plasticity, making it become rigid with consequent fibrotic
degeneration (4–6). Accordingly, it is important to
counteract the vicious cycle of inflammation and ECM turnover
alterations by primary therapeutic intervention. However, the
therapy needs to affect several different aspects involving both
the inflammatory processes leading to reactive oxygen species
production and ECM turnover mechanisms.
The present study aimed to investigate the activity
of a natural multi-component compound formulation named
Galium-Heel® on HDF growth, morphology and ECM
production. The results demonstrated that Galium-Heel®
had no significant effect on the cell cycle, cell growth, cell
survival and cell morphology, which highlighted the
biocompatibility of Galium-Heel®. The observed
antioxidant activity of Galium-Heel® on HDF might be due
to its components Galium aparine, Sedum acre, Sempervivum
tectorum or Urtica urens, which are able to regulate
redox processes (25–27). The modifications of collagen type I
and type III and MMP-3, observed after the treatment of HDF with
Galium-Heel® suggested that the multi-component compound
formulation had a role in the modulation of ECM anabolic and
catabolic phases. Galium-Heel® treatment significantly
decreased the expression of collagen type III and MMP-3, whilst
collagen type I expression increased at the intracellular level. In
addition, Galium-Heel® significantly decreased the
expression of collagen type III and increased collagen I and MMP-3
expression in the extracellular space. Increased MMP-3 expression
might support subsequent ECM remodelling (28). Tumour necrosis factor-α (TNF-α) is
able to increase the expression of MMP-3 and subsequent ECM
remodelling with the process dependent on p38 phosphorylation
(29). This effect can be reversed
by TGF-β via activation of MAPKs namely ERK1/2 (30). The present results demonstrated that
Galium-Heel® may have a similar effect to TNF-α acting
as a TGF-β inhibitor, inducing activation of p38 and increasing the
expression of MMP-3 whilst inhibiting ERK1/2 activity. Increased
expression of MMP-3 could be responsible for the decrease in
collagen type III/I ratio observed in the cell lysate and
extracellular space following Galium-Heel® treatment.
TGF-β is able to induce fibrosis by upregulating the expression of
collagen type III and increasing the collagen type III/I ratio
(31). Collagen type III is
considered a marker of lung fibrosis (32). In addition, it has been demonstrated
that collagen type III expression is increased in liver cirrhosis
and this increased expression is related to the development of
portal hypertension (33).
GLI proteins are the effectors of the Hedgehog
signalling pathway (34). GLI-2 is
an early gene target of the TGF-β/SMAD cascade independent of
Hedgehog signalling (34). Hedgehog
and TGF-β signalling pathways overlap in GLI-2, which integrates
those signals to promote tissue fibrosis (35). The present study observed that
Galium-Heel® decreased the expression of GLI-2
therefore, it could potentially prevent the early stages of
fibrosis by maintaining homeostasis of the ECM. The effects of
Galium-Heel® on ECM modulation might be due to certain
components contained in the formulation including: i) Acidum
nitricum, which participates as a nitric oxide (NO) donor
through the nitrate/nitrite/NO pathway, and is involved in the
collagen synthesis mechanism (36–39); ii)
argentum metallicum and aurum metallicum are donors
of the trace elements silver and gold respectively, and exert an
antioxidant effect (40); iii)
aurum metallicum regulates MMP expression (41); iv) phosphorus contributes to the
tissue inhibitor of metalloproteinases synthetic pathways (42); v) calcium fluoratum is
involved in the synthesis of MMP-2 and −9 (43); vi) Caltha palustris promotes
collagen synthesis (44); vii)
Juniperus communis controls the synthesis of collagen type I
and III (45); viii) Betula
pendula reduces the expression of MMP-3 (46,47); and
ix) Pyrogenium and Thuja occidentalis exert an
immunomodulating effect through stimulation of T helper type I
immune response and the synthesis of MMPs (48–50). In
addition, it should be considered that the various components
contained in the formulation could have an additive or a
synergistic effect. Previous studies by our group demonstrated that
the simultaneous administration of polyphenols exerts additive or
synergistic antitumoral effects (51–53).
Finally, an increase in the polymerization of intermediate
filaments was observed in Galium-treated cells by ultrastructural
analysis.
It has been established that fibroblasts within the
connective tissue are able to remodel the ECM (54). Upon ECM remodelling, fibroblasts
adapt their cytoskeleton to a different extracellular
microenvironment (54), which can
affect the viscoelastic and stretching properties of the connective
tissue (55). Therefore, the present
results suggested that polymerization of the cytoskeleton in
Galium-treated HDF might be a consequence of ECM remodelling or
that ECM remodelling might be a consequence of the fibroblast
cytoskeleton polymerization.
Based on the fundamental role of the tissue
microenvironment in the onset and maintenance of many pathological
conditions, the importance of ECM turnover is crucial, and the
recovery of ECM homeostasis can be considered as a potential
therapeutic goal. The present results demonstrating the beneficial
effects of Galium-Heel® on ECM plasticity highlight that
it may be possible to pharmacologically control some mechanisms of
ECM metabolism. The present findings may provide basis for the
development of novel pharmacological approaches to counteract local
abnormal ECM remodeling.
Acknowledgements
The authors would like to thank Mrs. Barbara
Bulgarini (Department of Clinical Sciences and Translational
Medicine, University of Rome ‘Tor Vergata’) for editorial
assistance in the preparation of the manuscript.
Funding
This study was supported by a grant from GUNA S.p.a,
(UPB: BeiRCtGUNA) which provided the Galium-Heel®.
However, this did not influence the authors' scientific
neutrality.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MB performed cell proliferation, western blot
analysis, FACS, statistical analysis and analyzed the results. RM
performed cell death experiments, reaction oxygen species
production, western blot analysis and analyzed the results. MTM
performed statistical analysis and analyzed the results. MGG, IT,
LA, VM and AM analyzed the results and critically revised the
manuscript. LM performed ultrastructural analysis and analyzed the
results. RB supervised the project, analyzed the results and wrote
the manuscript. All the authors have critically revised the
manuscript and gave the final approval of the version to be
published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The present study was partially funded by GUNA S.p.a
which provided Galium-Heel®. However, this did not
influence the authors' scientific neutrality. No products, patents
or other commercial interests relate to this study.
References
|
1
|
Nyström A and Bruckner-Tuderman L: Matrix
molecules and skin biology. Semin Cell Dev Biol. 89:136–146. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bei R, Masuelli L, Palumbo C, Tresoldi I,
Scardino A and Modesti A: Long-lasting tissue inflammatory
processes trigger autoimmune responses to extracellular matrix
molecules. Int Rev Immunol. 27:137–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iozzo RV and Gubbiotti MA: Extracellular
matrix: The driving force of mammalian diseases. Matrix Biol.
71-72:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walker C, Mojares E and Del Río Hernández
A: Role of extracellular matrix in development and cancer
progression. Int J Mol Sci. 19(pii): E30282018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Zhao Q and Kong W: Extracellular
matrix remodeling and cardiac fibrosis. Matrix Biol. 68-69:490–506.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minihane AM, Vinoy S, Russell WR, Baka A,
Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, et
al: Low-grade inflammation, diet composition and health: Current
research evidence and its translation. Br J Nutr. 114:999–1012.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Theocharis AD, Skandalis SS, Gialeli C and
Karamanos NK: Extracellular matrix structure. Adv Drug Deliv Rev.
97:4–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soylemezoglu O, Wild G, Dalley AJ, MacNeil
S, Milford-Ward A, Brown CB and el Nahas AM: Urinary and serum type
III collagen: Markers of renal fibrosis. Nephrol Dial Transplant.
12:1883–1889. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arpino V, Brock M and Gill SE: The role of
TIMPs in regulation of extracellular matrix proteolysis. Matrix
Biol. 44-46:247–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Randelli F, Menon A, Giai Via A, Mazzoleni
MG, Sciancalepore F, Brioschi M and Gagliano N: Effect of a
collagen-based compound on morpho-functional properties of cultured
human tenocytes. Cells. 7(pii): E2462018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karsdal MA, Nielsen MJ, Sand JM, Henriksen
K, Genovese F, Bay-Jensen AC, Smith V, Adamkewicz JI, Christiansen
C and Leeming DJ: Extracellular matrix remodeling: The common
denominator in connective tissue diseases. Possibilities for
evaluation and current understanding of the matrix as more than a
passive architecture, but a key player in tissue failure. Assay
Drug Dev Technol. 11:70–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barascuk N, Vassiliadis E, Larsen L, Wang
J, Zheng Q, Xing R, Cao Y, Crespo C, Lapret I, Sabatini M, et al:
Development and validation of an enzyme-linked immunosorbent assay
for the quantification of a specific MMP-9 mediated degradation
fragment of type III collagen-A novel biomarker of atherosclerotic
plaque remodeling. Clin Biochem. 44:900–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang Z, Li T, Jiang S, Xu J, Di W, Yang
Z, Hu W and Yang Y: AMPK: A novel target for treating hepatic
fibrosis. Oncotarget. 8:62780–62792. 2017.PubMed/NCBI
|
|
14
|
Zhang N, Wei WY, Li LL, Hu C and Tang QZ:
Therapeutic potential of polyphenols in cardiac fibrosis. Front
Pharmacol. 9:1222018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katoh M: Multi-layered prevention and
treatment of chronic inflammation, organ fibrosis and cancer
associated with canonical WNT/β catenin signaling activation
(Review). Int J Mol Med. 42:713–725. 2018.PubMed/NCBI
|
|
16
|
Reale FR, GriYn TW, Compton JM, Graham S,
Townes PL and Bogden A: Characterization of a human malignant
mesothelioma cell line (H-MESO-1): A biphasic solid and ascitic
tumor model. Cancer Res. 47:3199–3205. 1987.PubMed/NCBI
|
|
17
|
Masuelli L, Benvenuto M, Mattera R, Di
Stefano E, Zago E, Taffera G, Tresoldi I, Giganti MG, Frajese GV,
Berardi G, et al: In vitro and in vivo anti-tumoral effects of the
flavonoid apigenin in malignant mesotelioma. Front Pharmacol.
8:3732017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol. 111:A3.B.1–3. 2015. View Article : Google Scholar
|
|
19
|
Benvenuto M, Mattera R, Sticca JI, Rossi
P, Cipriani C, Giganti MG, Volpi A, Modesti A, Masuelli L and Bei
R: Effect of the BH3 mimetic polyphenol (−)-Gossypol (AT-101) on
the in vitro and in vivo growth of malignant mesothelioma. Front
Pharmacol. 9:12692018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masuelli L, Pantanella F, La Regina G,
Benvenuto M, Fantini M, Mattera R, Di Stefano E, Mattei M,
Silvestri R, Schippa S, et al: Violacein, an indole-derived
purple-colored natural pigment produced by Janthinobacterium
lividum, inhibits the growth of head and neck carcinoma cell lines
both in vitro and in vivo. Tumour Biol. 37:3705–3717. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benvenuto M, Mattera R, Masuelli L,
Taffera G, Andracchio O, Tresoldi I, Lido P, Giganti MG, Godos J,
Modesti A and Bei R: (±)-Gossypol induces apoptosis and autophagy
in head and neck carcinoma cell lines and inhibits the growth of
transplanted salivary gland cancer cells in BALB/c mice. Int J Food
Sci Nutr. 68:298–312. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masuelli L, Benvenuto M, Di Stefano E,
Mattera R, Fantini M, De Feudis G, De Smaele E, Tresoldi I, Giganti
MG, Modesti A and Bei R: Curcumin blocks autophagy and activates
apoptosis of malignant mesothelioma cell lines and increases the
survival of mice intraperitoneally transplanted with a malignant
mesothelioma cell line. Oncotarget. 8:34405–34422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masuelli L, Granato M, Benvenuto M,
Mattera R, Bernardini R, Mattei M, d'Amati G, D'Orazi G, Faggioni
A, Bei R and Cirone M: Chloroquine supplementation increases the
cytotoxic effect of curcumin against Her2/neu overexpressing breast
cancer cells in vitro and in vivo in nude mice while counteracts it
in immune competent mice. OncoImmunology. 6:e13561512017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Angelucci C, D'Alessio A, Lama G, Binda E,
Mangiola A, Vescovi AL, Proietti G, Masuelli L, Bei R, Fazi B, et
al: Cancer stem cells from peritumoral tissue of glioblastoma
multiforme: The possible missing link between tumor development and
progression. Oncotarget. 9:28116–28130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stanković M, Radojević I, Ćurčić M, Vasić
S, Topuzović M, Čomić L and Marković S: Evaluation of biological
activities of goldmoss stonecrop (Sedum acre L.). Turk J
Biol. 36:580–588. 2012.
|
|
26
|
Sentjurc M, Nemec M, Connor HD and Abram
V: Antioxidant activity of Sempervivum tectorum and its
components. J Agric Food Chem. 51:2766–2771. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marrassini C, Acevedo C, Miño J, Ferraro G
and Gorzalczany S: Evaluation of antinociceptive, antinflammatory
activities and phytochemical analysis of aerial parts of Urtica
urens L. Phytother Res. 24:1807–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3(pii):
a0050582011.PubMed/NCBI
|
|
29
|
Sanchavanakit N, Saengtong W,
Manokawinchoke J and Pavasant P: TNF-α stimulates MMP-3 production
via PGE2 signalling through the NF-kB and p38 MAPK pathway in a
murine cementoblast cell line. Arch Oral Biol. 60:1066–1074. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang H, Gao F, Li X, Wang J, Liu H and
Zheng Z: TGF-β1 antagonizes TNF-α induced up-regulation of matrix
metalloproteinase 3 in nucleus pulposus cells: Role of the ERK1/2
pathway. Connect Tissue Res. 56:461–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saed GM, Zhang W, Chegini N, Holmdahl L
and Diamond MP: Alteration of type I and III collagen expression in
human peritoneal mesothelial cells in response to hypoxia and
transforming growth factor-beta1. Wound Repair Regen. 7:504–510.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Forel JM, Guervilly C, Farnarier C, Donati
SY, Hraiech S, Persico N, Allardet-Servent J, Coiffard B, Gainnier
M, Loundou A, et al: Transforming growth factor-β1 in predicting
early lung fibroproliferation in patients with acute respiratory
distress syndrome. PLoS One. 13:e02061052018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rojkind M, Giambrone MA and Biempica L:
Collagen types in normal and cirrhotic liver. Gastroenterology.
76:710–719. 1979.PubMed/NCBI
|
|
34
|
Javelaud D, Pierrat MJ and Mauviel A:
Crosstalk between TGF-β and hedgehog signaling in cancer. FEBS
Lett. 586:2016–2025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang R, Šumová B, Cordazzo C, Mallano T,
Zhang Y, Wohlfahrt T, Dees C, Ramming A, Krasowska D,
Michalska-Jakubus M, et al: The transcription factor GLI2 as a
downstream mediator of transforming growth factor-β-induced
fibroblast activation in SSc. Ann Rheum Dis. 76:756–764. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moncada S and Higgs A: The
L-arginine-nitric oxide pathway. N Engl J Med. 329:2002–2012. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia W, Szomor Z, Wang Y and Murrell GA:
Nitric oxide enhances collagen synthesis in cultured human tendon
cells. J Orthop Res. 24:159–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lundberg JO, Weitzberg E and Gladwin MT:
The nitrate-nitrite-nitric oxide pathway in physiology and
therapeutics. Nat Rev Drug Discov. 7:156–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gilkes DM, Semenza GL and Wirtz D: Hypoxia
and the extracellular matrix: Drivers of tumour metastasis. Nat Rev
Cancer. 14:430–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Negahdary M, Chelongar R, Zadeh SK and
Ajdary M: The antioxidant effects of silver, gold, and zinc oxide
nanoparticles on male mice in in vivo condition. Adv Biomed Res.
4:692015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hashimoto M, Sasaki JI, Yamaguchi S, Kawai
K, Kawakami H, Iwasaki Y and Imazato S: Gold nanoparticles inhibit
matrix metalloproteases without cytotoxicity. J Dent Res.
94:1085–1091. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Veerendhar A, Reich R and Breuer E:
Phosphorus based inhibitors of matrix metalloproteinases. C R Chim.
13:1191–1202. 2010. View Article : Google Scholar
|
|
43
|
Slompo C, Buzalaf CP, Damante CA, Martins
GM, Hannas AR, Buzalaf MA and Oliveira RC: Fluoride modulates
preosteoblasts viability and matrix metalloproteinases-2 and −9
activities. Braz Dent J. 23:629–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Suszko A and Obmińska-Mrukowicz B: Effects
of polysaccharide fractions isolated from Caltha palustris
L. on the activity of phagocytic cells & humoral immune
response in mice with collagen-induced arthritis: A comparison with
methotrexate. Indian J Med Res. 145:229–236. 2017.PubMed/NCBI
|
|
45
|
Han X and Parker TL: Anti-inflammatory
activity of Juniper (Juniperus communis) berry essential oil
in human dermal fibroblasts. Cogent Med. 4:13062002017. View Article : Google Scholar
|
|
46
|
Rastogi S, Pandey MM and Kumar Singh Rawat
A: Medicinal plants of the genus Betula-traditional uses and a
phytochemical-pharmacological review. J Ethnopharmacol. 159:62–83.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ra HJ, Lee HJ, Jo HS, Nam DC, Lee YB, Kang
BH, Moon DK, Kim DH, Lee CJ and Hwang SC: Betulin suppressed
interleukin-1β-induced gene expression, secretion and proteolytic
activity of matrix metalloproteinase in cultured articular
chondrocytes and production of matrix metalloproteinase in the knee
joint of rat. Korean J Physiol Pharmacol. 21:19–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Silacci P, Dayer JM, Desgeorges A, Peter
R, Manueddu C and Guerne PA: Interleukin (IL)-6 and its soluble
receptor induce TIMP-1 expression in synoviocytes and chondrocytes,
and block IL-1-induced collagenolytic activity. J Biol Chem.
273:13625–13629. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Offergeld R, Reinecker C, Gumz E, Schrum
S, Treiber R, Neth RD and Gohla SH: Mitogenic activity of high
molecular polysaccharide fractions isolated from the cuppressaceae
Thuja occidentalis L. enhanced cytokine-production by
thyapolysaccharide, g-fraction (TPSg). Leukemia. 6 (Suppl
3):189S–191S. 1992.PubMed/NCBI
|
|
50
|
Sunila ES, Hamsa TP and Kuttan G: Effect
of Thuja occidentalis and its polysaccharide on
cell-mediated immune responses and cytokine levels of metastatic
tumor-bearing animals. Pharm Biol. 49:1065–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Masuelli L, Marzocchella L, Focaccetti C,
Tresoldi I, Palumbo C, Izzi V, Benvenuto M, Fantini M, Lista F,
Tarantino U, et al: Resveratrol and diallyl disulfide enhance
curcumin-induced sarcoma cell apoptosis. Front Biosci (Landmark
Ed). 17:498–508. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Masuelli L, Di Stefano E, Fantini M,
Mattera R, Benvenuto M, Marzocchella L, Sacchetti P, Focaccetti C,
Bernardini R, Tresoldi I, et al: Resveratrol potentiates the in
vitro and in vivo anti-tumoral effects of curcumin in head and neck
carcinomas. Oncotarget. 5:10745–10762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fantini M, Benvenuto M, Masuelli L,
Frajese GV, Tresoldi I, Modesti A and Bei R: In vitro and in vivo
antitumoral effects of combinations of polyphenols, or polyphenols
and anticancer drugs: Perspectives on cancer treatment. Int J Mol
Sci. 16:9236–9282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rhee S: Fibroblasts in three dimensional
matrices: Cell migration and matrix remodelling. Exp Mol Med.
41:858–865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Langevin HM, Bouffard NA, Fox JR, Palmer
BM, Wu J, Iatridis JC, Barnes WD, Badger GJ and Howe AK: Fibroblast
cytoskeletal remodeling contributes to connective tissue tension. J
Cell Physiol. 226:1166–1175. 2011. View Article : Google Scholar : PubMed/NCBI
|