Introduction
Kaempferol (KP; chemical name,
3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; also
known as kaempferol-3, kaempferide or kaempferol flavonol) is a
type of flavonoid. KP has a molecular weight of 286.23 and is a
pure yellow crystalline powder with a melting point of 276–278°C.
It is soluble in hot ethanol, ether alkaline, and slightly soluble
in water. KP has hydrophobic properties due to its diphenylpropane
structure.
The biosynthesis of KP is as follows, under
catalysis by chalcone synthase, KP is synthesized by
4-coumaroyl-CoA condensation with tripropionyl-CoA to produce
naringenin chalcone (1).
Subsequently, naringenin chalcone is transformed into a flavanone
called naringenin, which is then hydroxylated by flavanone
3-dioxygenase to produce dihydrokaempferol (2). Finally, by introduction of a double
bond at the C2-C3 position in the dihydrokaempferol skeleton, KP is
generated.
KP is a natural flavonol-type flavonoid and can be
be isolated from tea as well as numerous common vegetables and
fruits, including beans, broccoli, cabbage, gooseberries, grapes,
kale, strawberries, tomatoes, citrus fruits, brussel sprouts,
apples and grapefruit (3). It has
also been identified in different medicinal plants, including
Equisetum spp, Sophora japonica, Ginkgo bilobaEuphorbia
pekinensis (Rupr.) (3). The most
well-known properties of KP are its anti-inflammatory effects. KP
has been demonstrated to have beneficial effects on chronic
inflammatory diseases, including intervertebral disc (IVD)
degeneration, post-menopausal bone loss and colitis, and acute
inflammatory diseases, including acute lung injury (ALI). The
second most important feature of KP is the prevention of cancer.
Its anti-cancer role has been demonstrated in esophageal cancer,
breast cancer, cervical cancer, hepatocellular carcinoma (HCC),
ovarian cancer, gastric cancer (GC), non-small cell lung cancer
(NSCLC), leukemia, cholangiocarcinoma (CCA), pancreatic cancer,
bladder cancer and osteosarcoma, as well as benign conditions, e.g.
uterine fibroids. However, the detailed mechanisms of action of KP
against numerous cancer types, as well as its role in improving
liver injury, obesity, diabetes and symptoms of metabolic syndrome,
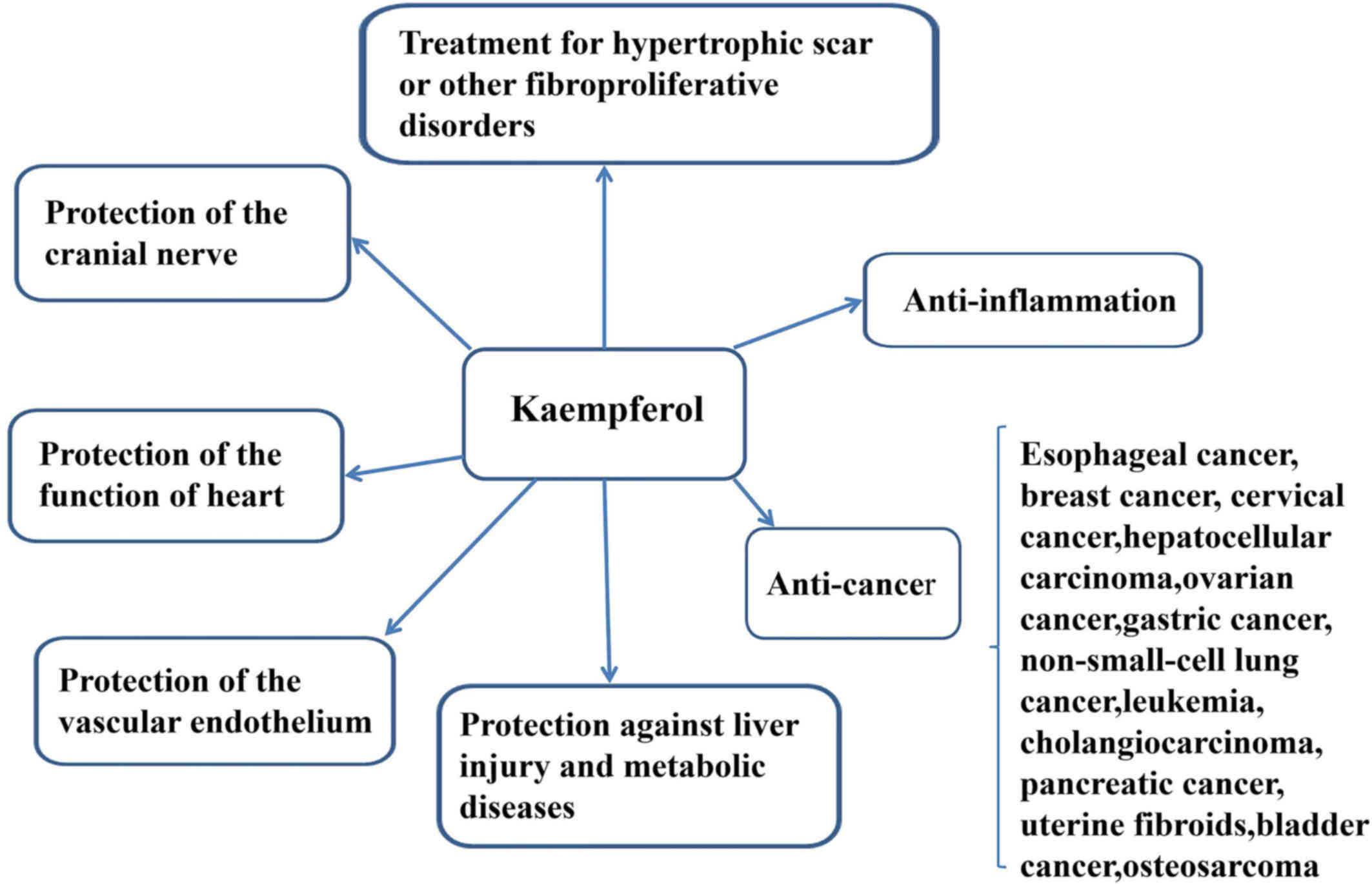
have remained elusive. KP may also inhibit vascular endothelial
inflammation, preserve the function of the heart, protect the
cranial nerve and treat fibroproliferative disorders, including
hypertrophic scar (HPS) (Fig.
1).
As a non-toxic, low price dietary ingredient, KP has
a very large economic value. As one of flavonoids, it can be
extracted from plants. An economic and low cost method for KP
preparation is enzymatic hydrolysis, using two KP glycosides in tea
seed (4). Additionally,
supercritical fluid extraction is also a method for rapidly
extracting natural biologically active compounds from plant
materials (5). Although poor
bioavailability epitomizes a major obstacle, nanotechnology has
emerged as a promising means to overcoming this problem (6).
The present review summarizes the role of KP in
disease and the current controversy surrounding KP treatment. The
aim of this review is to provide evidence for the application of KP
in human diseases in the future.
Anti-inflammatory effects
In vascular tissues, inflammation is an important
biological response, as it reflects tissue damage (7), which is caused by different pathogens,
irritants or cell damage (8). KP is
a common type of dietary flavonoid with anti-oxidative and
anti-inflammatory properties. Studies also indicated that KP
decreased lipopolysaccharide (LPS)-induced tumor necrosis factor-α
(TNF-α) and interleukin-1 (IL-1) expression by increasing the
number of activated macrophages; suppression of TNF-α mediates the
translocation of NF-κB p65 to the nucleus (9). As inflammation may be classified as
acute or chronic, the inflammatory diseases in different categories
were discussed separately to understand the role of KP in
inflammatory diseases (Fig. 1).
Chronic inflammation
KP slows IVD degeneration
IVDs are composed of the nucleus pulposus, the
annulus fibrosus and cartilage endplates (10), and IVD degeneration has been
considered an irreversible process when cell viability decreases,
type II collagen is synthesized and the nucleus pulposus is
dehydrated (11). Zhu et al
(11) demonstrated that KP inhibits
LPS-induced apoptosis by inhibiting LPS-induced decreases in the
levels of chondrogenic markers which means chondrogenic markers
SOX-9, Collagen II and Aggrecan, and reducing the level of
matrix-degrading enzymes. In addition, KP also inhibited the
expression of lipid anabolism-associated genes. The role of KP in
maximally reducing inflammation has also been experimentally
validated, as it reduced the levels of pro-inflammatory cytokines
and increased anti-inflammatory cytokines, including IL-10, by
inhibiting nuclear transcription factors. The above results
indicate that KP may be used as a novel treatment for IVD
degeneration.
KP may prevent osteoarthritis
(OA)
OA is the major cause of physical disability with
chronic pain (12), which is
characterized by the progressive degeneration of articular
cartilage along with the depletion of the cartilage matrix
(13). It is estimated that ~27
million American adults and 8.5 million British adults are
diagnosed with OA. The incidence increases with age, with 33.6% of
adults aged 65 being diagnosed with OA (14,15). The
progression of OA is closely linked to the overexpression of
pro-inflammatory mediators. Numerous studies have suggested that
the first-line treatment strategy for OA is the attenuation of
IL-1β-stimulated inflammatory mediators (15).
Increased nitric oxide (NO) formation and
prostaglandin E2 (PGE2) expression may be major contributors to the
development of OA (16) and various
studies have documented that stimulation of IL-1β may potentially
increase the expression of cyclooxygenase-2 (Cox-2) and inducible
NO synthase (iNOS) (17). The NF-κB
signaling pathway is thought to be associated with IL-1β-stimulated
OA.
In IL-1β-stimulated rat chondrocytes as a model of
OA, KP caused a reduction in the IL-1β-stimulated formation of PGE2
and NO in a KP concentration-dependent manner, and also upregulated
the expression of iNOS and Cox-2. The results suggest that KP has
significant anti-inflammatory and anti-arthritis effects by
inhibiting the NF-κB signaling pathway, suggesting that KP may be a
novel active therapeutic agent, which may prevent or retard the
progression of OA (17).
KP suppresses inflammation in
colitis
Ulcerative colitis (UC) is an intractable disease in
its chronic persistent form. Park et al (18) have demonstrated that the pathogenesis
of UC is associated with an imbalance between pro-inflammatory
cytokines and anti-inflammatory cytokines due to activation of the
phosphatidylinositol 3-kinase (PI3K)/Akt and NF-κB signaling
pathways that promote pro-inflammatory cytokine expression and
secretion.
KP ameliorates mastitis
Mastitis is inflammation of the breast and has the
following symptoms: Redness, swelling, pain and warmth, and is
classified as non-puerperal mastitis or puerperal mastitis. The
incidence rate in lactating women ranges from 10 to 20% (19). Previous studies have indicated that
angiopoietin-like protein 2 (ANGPTL2), which is expressed in
adipose tissue, the stomach, skeletal muscle, the heart, the
intestines and the uterus, is a decisive factor in mediating acute
and chronic inflammation of adipose tissue and in obesity.
Therefore, ANGPTL2 may be a beneficial pharmacological target for
the treatment of mastitis. Xiao et al (20) reported that treatment with KP may
prevent the development of mastitis and decrease myeloperoxidase
(MPO), IL-6, TNF-α and ANGPTL2 expression. These results suggest
that KP modulates the expression of ANGPTL2 to ameliorate
mastitis.
KP inhibits the migration and invasion
of fibroblast-like synoviocytes (FLS) in rheumatoid arthritis
(RA)
RA is a heterogeneous, chronic and systemic
autoimmune disease characterized by chronic inflammation (21). Elevated levels of matrix
metalloproteinases (MMPs) are important markers in patients with
RA, and MMP expression is associated with FLS migration and
invasion in RA.
Pan et al (22) demonstrated that KP further inhibited
TNF-α-induced mitogen-activated protein kinase (MAPK) activation
without affecting TNF-α receptor expression. They also demonstrated
that KP may reduce the severity of arthritis in mice with
collagen-induced arthritis. These results indicated that KP
inhibits the migration and invasion of FLS in RA by blocking MAPK
pathway activation without affecting the expression of TNF-α
receptors.
In addition, Lee et al (23) indicated that the basic fibroblast
growth factor (bFGF) concentration in the synovial fluid of
patients with RA was significantly higher than that in patients
with OA. bFGF stimulates the proliferation and migration of human
FLS by activating the bFGF-FGF receptor 3 (FGFR3)-ribosomal S6
kinase 2 signaling axis and the molecular docking study indicated
that KP inhibits FGFR3 activity by binding to the active pocket of
the FGFR3 kinase domain.
Acute inflammation
KP protects against ALI
Sepsis is a common and serious disease with high
morbidity and mortality rates, and is frequently associated with
multiple organ dysfunction, particularly acute lung injury (ALI)
and acute respiratory distress syndrome (ARDS) (24). In addition, severe hypotension,
abnormal blood coagulation and multiple organ dysfunction caused by
sepsis may be major risk factors of ALI development (25).
There are various therapeutic strategies for ALI,
including NO, surfactant and glucocorticoid administration, but
none of them reduce the mortality of patients with sepsis-induced
ALI/ARDS (11). The
pathophysiological mechanism of ALI/ARDS is thought to be
associated with the hyperinduction of cytokines (26), which together promote the migration
of polymorphonuclear neutrophils to the pulmonary interstitial and
alveolar spaces to cause inflammatory damage by producing reactive
oxygen species (ROS) (27). Rabha
et al (28) demonstrated that
KP reduces the plasma levels of the cytokines IL-6, IL-1β and
TNF-α, as well as the anti-oxidant enzyme superoxide dismutase
(SOD), but did not produce any reduction in the malondialdehyde
level and bacterial load.
In addition, the MAPK and NF-κB signaling pathways
have two pivotal roles that contribute to the development of
LPS-induced ALI (29). KP may
significantly reduce the upregulation of Toll-like receptor 4
(TLR4) and myeloid differentiation factor 88 (MyD88), the
phosphorylation level of inhibitor of NF-κB (IκBα) and NF-κB p65,
the DNA binding activity of NF-κB p65 and the phosphorylation level
of MAPKs (27). KP may also suppress
airway inflammation, e.g. in asthma, through disturbing NF-κB
signaling (30). Airway narrowing is
caused by smooth muscle contraction and mucus hypersecretion
(31), and the airway epithelium is
a target of inflammatory and physical insults, and an effector of
ongoing airway inflammation (32,33). The
interplay between airway epithelial cells and eosinophils is an
essential feature of allergic asthma (30).
Certain studies have indicated that in the
development of allergen-induced severe bronchial asthma,
endoplasmic-reticulum (ER) stress and the associated signaling
networks are important modulators (34,35). KP
was proven effective in ameliorating mucus hypersecretion through
disturbing the transforming growth factor β (TGF-β)-triggered ER
stress signaling of inositol-requiring enzyme 1α/TNF
receptor-associated factor 2/c-Jun N-terminal kinase (JNK) in
cellular or animal models of allergic asthma (36).
KP may treat gastric ulcer (GU)
One of the most common diseases among average and
particularly young individuals is GU in Japan, which has been
indicated to be as high as 80–82 people per 100,000 inhabitants.
However, the incidence rates in Kuwait, Los Angeles, California,
the United States and Mexico are very low (37). Acute GU may be caused by alcohol,
non-steroidal anti-inflammatory drugs and major stress events,
including shock, severe burns, trauma and surgery (38), resulting in severe upper
gastrointestinal bleeding (39). It
may also develop as a result of the presence of Helicobacter
pylori, decreased blood flow, increased acid secretion and
pepsin activity, imbalanced bile salt secretion, and reduced mucus
and bicarbonate secretion (40). The
development of GU arises from an imbalance between aggressive and
protective factors present in the gastric mucosa (41).
Several previous studies have demonstrated that
pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, have
important roles in the regulation of acute GU induced by ethanol
(42). In addition, ethanol markedly
reduces the level of NO required for physiological functions
(43) in the gastric mucosa and
decelerates the flow of gastric blood (44). Li et al (45) investigated the protective effects of
KP on acute ethanol-induced gastric mucosal injury in mice and the
underlying mechanisms. The results indicated that KP may protect
the stomach by inhibiting neutrophil accumulation and MPO activity,
regulating pro-inflammatory cytokine levels and improving NO
production to maintain gastric mucosal glycoprotein levels.
Anti-cancer efficacy
Cancer is primarily characterized by unrestricted
cell proliferation. Surgery, radiation therapy and chemotherapy
have been proven to be efficacious in the treatment of certain
cancer types, but none of these is a panacea. Since cancer cells
tend to have a stubborn inclination to mutate or metastasize, and
resistance to treatment is common, it is important to identify safe
and effective drugs (5).
KP may exert its anti-cancer effects via several
different mechanisms. KP is not only a potent promoter of apoptosis
(5), but may also activate the
host's immunity, inhibit tumor blood vessel growth and increase the
sensitivity of other anti-cancer drugs.
Arif et al (46) explained that another anti-cancer
mechanism of flavonoids is the increase in cellular DNA breakage;
furthermore, flavonoids act as pro-oxidants in the presence of
transition metal ions, including copper. Flavonoid treatment may
make cancer cells more susceptible to copper-induced oxidative DNA
breakage. This copper-dependent oxidative cytotoxic mechanism may
be a better explanation of the anti-cancer activity and
preferential cytotoxicity of dietary phytochemicals, which may be
beneficial in the treatment of cancer.
Esophageal cancer
Esophageal cancer is the eighth most common cancer
type in the world and the sixth leading cause of cancer-associated
death. From a histological perspective, it includes esophageal
squamous cell carcinoma and adenocarcinoma (47). However, difference are observed
between the two tumors. Esophageal cancer risk factors include
smoking and alcohol abuse, while adenocarcinoma is associated with
obesity and gastroesophageal reflux disease (48). It has been indicated that KP is able
to inhibit tumor cell proliferation and in vitro clonal
formation, and induces G0/G1 phase arrest of tumor cells (49). Furthermore, KP has a substantial
inhibitory effect against tumor glycolysis (49), and it may upregulate the expression
of B-cell lymphoma-2 (Bcl-2) associated X protein (Bax) and other
pro-apoptotic genes through the mitochondrial signaling pathway,
downregulate Bcl-2 and inhibit the expression of caspase-9.
Inhibition of caspase-9 expression further activates caspase-3,
triggering a cascade of caspases, which triggers apoptosis
(49). KP can also be applied to the
human oesophageal adenocarcinoma cell line. A previous study
demonstrated that with flavones treatment, G2/M arrest can be
caused through the upregulation of GADD45β and 14-3-3sρ and the
downregulation of cyclin B1 at the mRNA and protein levels, and can
induce p53-independent mitochondrial-mediated apoptosis through the
upregulation of PIG3 and the cleavage of caspase-9 and caspase-3
(50). These results indicate that
KP can be used for the treatment of esophageal cancer.
Breast cancer
Cancer is becoming one of the most malignant
diseases in the world, with >12.5 million new cases and 7.5
million deaths per year (51).
Breast cancer is an estrogen-associated cancer type, and its
development and progression are mainly linked to the multiple
effects of estrogen through the estrogen receptor (52). Previous studies have indicated that
flavonoids may inhibit the growth of breast cancer cells in
vitro and in vivo by competing for the estrogen binding
site of 17β-estradiol (E2) and the estrogen receptor (53).
Kim et al (52) reported that KP inhibits breast cancer
by suppressing cancer progression induced by triclosan (TCS) and E2
through acting as an antagonist for estrogen receptor and
insulin-like growth factor (IGF) 1 receptor signaling. It has been
suggested that KP-3-O-rhamnoside triggers cell death intrinsically
in MCF-7 cells via the mitochondrial caspase-9 signaling pathway
and activation of poly ADP-ribose polymerase (PARP) (54). These results demonstrate that KP has
an anti-cancer effect to antagonize the pro-cancer activity of E2
or TCS (52).
Li et al (55)
reported that KP inhibited the invasion of breast cancer cells by
blocking the protein kinase C (PKC)/MAPK/activator protein-1AP-1
cascade, and the subsequent expression and activity of MMPs; it
also inhibited TCS-induced MCF-7 breast cancer cells. Yi et
al (56) obtained similar
results, as incubation of MCF-7 cells with 40 and 80 µM KP for 24 h
resulted in numerous cells that possessed smaller nuclei with
chromatin condensation and perinuclear apoptotic bodies. It was
indicated that 40 and 80 µM KP may induce PARP cleavage in a
dose-dependent manner.
By analyzing the effects of KP on apoptosis and DNA
damage, Zhu et al (57)
revealed that it effectively inhibited the proliferation of the
triple-negative breast cancer cell line malondialdehyde
(MDA)-MB-231; this effect was stronger in MDA-MB-231 cells than in
the estrogen receptor-positive BT474 cell line. These results
indicated that KP may be a potential drug for the effective
treatment of breast cancer.
Cervical cancer
Cervical cancer ranks as the fourth most frequently
diagnosed cancer and the fourth leading cause of cancer death in
women, with an estimated 570,000 cases and 311,000 deaths in 2018,
worldwide. Cases are concentrated in Sub-Saharan Africa and
South-Eastern Asia (58).
Kashafi et al (59) indicated that KP decreased cell
viability in a concentration-and time-dependent manner. They
demonstrated that after incubation with KP, telomerase and the
PI3K/AKT signaling pathway were inhibited, and apoptosis was
induced via p53 and Bax/Bcl-2 in HeLa cervical cancer cells in a
time- and concentration-dependent manner, whereas this was not
observed in normal cells. There was no significant toxic effect,
which is a key advantage in the treatment of cervical cancer.
HCC
HCC is the most common type of primary liver cancer
(60). Early treatment for HCC is
transplantation, surgical resection or local ablation (61). An important cause of HCC development
is angiogenesis and the RAF kinase/MAPK kinase (MEK)/MAPK signaling
pathway that regulates cell proliferation (62). Other developmental factors in HCC are
increased cell proliferation and limited blood supply.
Hypoxia-inducible factor-1 is a key component of adaptation to
oxygen deprivation (63). At
present, due to its anti-tumor properties, KP has been proposed as
a potential agent for HCC treatment and activation of the ER
stress-CCAAT/enhancer-binding protein homologous protein (CHOP)
signaling pathway may be one of the molecular mechanisms of
KP-induced hepatocellular apoptosis (60). Mylonis et al (64) demonstrated that KP reduces the
survival of liver cancer cells more effectively under hypoxic
conditions. Seydi et al (65)
reported that KP induces selective cytotoxicity on hepatocytes in
HCC in a dose- and time-dependent manner, and also increases the
activation of caspase-3. Of note, KP is not toxic to healthy liver
cells and mitochondria. Therefore, KP is a good candidate for
supplemental anti-HCC treatment.
Ovarian cancer
It was estimated that in 2010, almost 850 females in
the US died from ovarian cancer, accounting for 5% of total
cancer-associated mortalities in females (66). The key approach to the prevention of
ovarian cancer is based on environmental factors rather than
genetic backgrounds (66).
Numerous studies have demonstrated that several
types of cancer cell, including ovarian cancer, pancreatic cancer,
gliomas, malignant melanoma and neuroblastoma, are TNF-related
apoptosis-inducing ligand (TRAIL) resistant (67). Increased expression of anti-apoptotic
proteins, including X-linked inhibitor of apoptosis protein, Bcl-2
and decoy receptors, have been associated with TRAIL resistance
(68). Furthermore, identifying
TRAIL sensitizers is crucial in cancer therapy, as the NF-κB,
PI3K/Akt and MAPK signaling pathways have all been implicated in
the resistance to TRAIL-induced apoptosis (67).
It was demonstrated that KP is able to regulate
pro-apoptotic and anti-apoptotic protein expression by inducing
apoptosis in A2780/CP70, A2780/WT and OVCAR-3 ovarian cancer cell
lines (66). This result was in line
with a study by Zhao et al (69), which indicated that KP was able to
enhance apoptosis and upregulate drought-repressed (DR)4, DR5,
CHOP, JNK, ERK1/2, p38 and apoptotic protein expression. RNA
silencing experiments indicated the involvement of CHOP in DR5
upregulation and also the contribution of DR5 in KP-enhanced
TRAIL-induced apoptosis.
In the cell cycle, checkpoint kinase 2 (Chk2) is a
stably expressed serine/threonine kinase and also a tumor
suppressor that regulates a variety of essential cellular functions
(70). Mutations and/or deletions in
Chk2 are associated with multiple cancer types (70). Gao et al (71) demonstrated that KP induces cell cycle
arrest in G2/M phase via the Chk2/cell division cycle (CDC)25C/cell
division control 2 signaling pathway. These results reflect the
anti-ovarian cancer properties of KP, which will be a potential
option for the treatment of ovarian cancer.
GC
GC is the fourth most common type of malignancy
worldwide and ranks second in terms of cancer-associated mortality
(72). Surgery is currently the
major treatment. Cytotoxic chemotherapy has proven to be an
effective treatment for advanced stages when surgery is not
feasible (73). However, long-term
chemotherapy leads to drug resistance and treatment-associated side
effects (74). Therefore, there is a
requirement for more effective anti-tumor drugs with fewer side
effects.
A previous study indicated that KP effectively
inhibits the proliferation of GC cells. They observed that GC cells
were arrested in G2/M phase, and the expression levels of
cyclin-dependent kinase 1 and CDC25C were decreased after KP
treatment. In addition, KP inhibited the ERK1/2 and PI3K/AKT
signaling pathways. Therefore, KP may be considered to have
potential therapeutic effects in GC.
NSCLC
NSCLC is the leading cause of cancer-associated
death worldwide each year and its prognosis is poor (75). In addition, the major cause of death
in patients with lung cancer is the metastasis of lung cancer
cells. TGF-β1 is a typical member of the TGF-β superfamily, the
function of which is regulated cell proliferation, differentiation,
apoptosis and migration (76).
TGF-β1 has been proved to inhibit tumor function, act as a
metastasis-inducing agent by inhibiting cell proliferation and
promoting apoptosis, and also promote endothelial-to-mesenchymal
transition (EMT) in advanced cancers (76). Jo et al (77) suggested that KP inhibits
TGF-β1-induced EMT and cell migration in human lung cancer cells by
targeting the phosphorylation of the 3 linker region of SMAD family
members, and this pharmacological inhibition may provide an
effective barrier to lung cancer progression.
Leukemia
Leukemia occurs in the blood and bone marrow. It
develops if blood-forming cells lack the ability to control their
growth (75). Leukemia has various
subtypes, including acute lymphocytic leukemia, acute myeloid
leukemia, acute promyelocytic leukemia (APL), chronic lymphocytic
leukemia and chronic myeloid leukemia (78). It has been indicated that KP inhibits
the growth of human leukemia mast cells by inhibiting DNA repair
protein expression in HL-60 human leukemic cells, including
p-ataxia telangiectasia mutated, p-ataxia telangiectasia Rad3,
BRCA-1, 14-3-3-like protein B, DNA-dependent protein kinase and
O-6-methylguanine-DNA methyltransferase (79).
In APL, fusion between retinoic acid receptor-α and
promyelocytic leukemia genes is common (80). Previous studies indicated that KP
increased apoptosis and inhibited telomerase expression (59). Another study suggested that KP
induced apoptosis by inhibiting multidrug resistance protein and
increasing the Bax/Bcl-2 ratio; the cytotoxic effect of KP was more
pronounced in leukemia cells than in normal human polymorphonuclear
leukocytes (81).
CCA
CCA is the most common biliary malignancy in the
world. It has been reported that KP induces apoptosis through
regulating Bcl-2 and caspase family proteins (82). Qin et al (83) confirmed that KP markedly reduced the
expression of Bcl-2, while it increased the expression of Bax, cell
surface death receptor, cleaved-caspase-3, cleaved-caspase-8,
cleaved-caspase-9 and cleaved-PARP. In addition, KP was indicated
to induce apoptosis via the fas cell surface death receptor death
receptor/caspase signaling pathway, and the pan-caspase inhibitor
Q-VD-OPH inhibited KP-mediated caspase-3 and PARP activation, as
well as KP-induced apoptosis.
Upregulation of MMP2 and MMP9 is also key to the
invasion of CCA cells into surrounding tissues. The study by Qin
et al (83) indicated that
the KP-treated groups had decreased MMP2 expression and increased
expression of tissue inhibitor of metalloproteinase 2 (TIMP2),
whereas there was no significant change in the expression of MMP9
and TIMP1.
In summary, KP is effective against CCA in
vitro and in vivo. Inactivation of the PI3K/AKT
signaling pathway and downstream proteins may be the mechanism by
which KP exerts its effects against CCA and prevents its
progression.
The 5-year survival rate of patients with pancreatic
cancer is <1% (84). Early local
tumor spread and progression are the major features of pancreatic
cancer (76).
Key factors in aggressive pancreatic cancer are
epidermal growth factor receptor (EGFR) and hepatocyte growth
factor receptor (85,86). It has been demonstrated that KP is
able to reduce EGFR and SRC proto-oncogene expression, as well as
the activation of the AKT and ERK1/2 signaling pathways to inhibit
the growth and migration of pancreatic cancer cells by blocking
EGFR-associated pathways in vitro (87).
Uterine fibroids
Uterine fibroids are most common in females between
30 and 50 years of age. Uterine fibroids are benign tumors and are
considered a hormone-dependent disease, but the exact cause remains
elusive (88). The most common
treatment of uterine fibroids is surgical resection, but this
affects the health of the patients. Flavonoids have been indicated
to have anti-inflammatory and anti-tumor effects, and may be used
in the treatment of uterine fibroids (89).
Li et al (89)
indicated that treatment with KP reduces the expression of the
estrogen receptor, IGF-1 and vascular endothelial growth factor
precursor (VEGF) at the mRNA and protein level by inhibiting the
proliferation of human uterine fibroid cells in vitro.
However, the effect of KP on the apoptosis of human uterine fibroid
cells remains elusive. In the above study, KP effectively reduced
the levels of myocardin in uterine fibroids compared to normal
uterine smooth muscle. Myocardin expression is the major auxiliary
factor of the transcription factor serum response factor, which
acts on the differentiation of smooth muscle cells (88).
Bladder cancer
Bladder cancer is a common type of malignant tumor
of the urinary system. Previous evidence has suggested that the
methylation of genomic DNA is closely linked to bladder cancer
(90). Therefore, modulating DNA
methylation with potent and low toxicity agents is a key strategy
to prevent and treat cancer (91,92).
DNA methyltransferases (DNMTs) are key enzymes in
the regulation of DNA methylation, which mostly relies on the
expression of DNMTs. Lee et al (93) indicated that KP promotes the
degradation of DNMT3B, which is closely linked to the Ub-proteasome
pathway in bladder cancer. A previous study suggested that KP
inhibits the activation of the PI3K/Akt signaling pathway through
increasing the tumor suppressor phosphatase and tensin homolog in
bladder cancer (88). These results
indicate that the downregulation of DNMT3B may be linked to the
PI3K/Akt-associated Ub-proteasome signaling pathway. Therefore, a
novel DNMT3B inhibitor, including KP, may be considered for bladder
cancer, which promotes DNA methylation and downregulates DNMT3B
levels by promoting the degradation of its Ub proteasome (90).
OS
OS is a malignant type of bone tumor and mainly
arises from the malignant transformation of mesenchymal cells
(94); it mostly affects children
and young adults (95). Huang et
al (96) demonstrated that KP
significantly reduced the viability of U-2 OS cells, human
osteoblasts and 143B cells, but exerted low cytotoxicity on human
fetal osteoblast progenitor and human fetal osteoblastic cells. KP
induced tumor cell apoptosis and inhibited tumor cell proliferation
through mitochondria-dependent and ER stress signaling pathways.
Chen et al (97) indicated
that KP decreased the expression of MMP-2, MMP-9 and urokinase
plasminogen activator at the protein levels, inhibited the invasion
and adhesion of OS cells and the migration of U-2 OS cells in a
concentration-dependent manner. These results demonstrated that
following KP treatment with 100 µM, the inhibition rate reached
>60%.
Application of KP in liver and metabolic
diseases
KP protects against
hepatotoxicity
As the first organ that metabolizes foreign
compounds, the liver is vulnerable to various diseases, including
hepatitis, cirrhosis or HCC (98).
One of the most common liver diseases caused by drugs is hepatitis
induced by anti-tuberculosis (TB) drugs (99). Drug-induced hepatotoxicity may be due
to oxidative stress caused by the production of toxic metabolites
or free radicals (100). The
primary pathway of inhibitor of isoniazid (INH) metabolism by
N-acetyltransferase 2 generates acetyl-INH. Acetyl-INH undergoes
hydrolysis to form acetyl-hydrazine and the nontoxic substance
isonicotinic acid, which is oxidized by cytochrome P450 family 2
subfamily E member 1 (CYP2E1) to form reactive acylating
hepatotoxins or its breakdown products (99).
Shih et al (101) demonstrated that KP significantly
reduced liver glutathione consumption and prevented an increase of
malondialdehyde formation in mice. In addition, KP did not affect
the anti-TB effect of INH/rifampicin.
Carbon tetrachloride (CCl4) is a
xenobiotic, which produces hepatotoxicity in humans and various
experimental animals (102).
CCI4 can be activated by cytochrome P450-dependent
monooxygenases to form highly reactive radicals and exhibit DNA
damage and lipid peroxidation (103). In a previous study, a hepatic
toxicity model was induced by carbon CCl4 in mice.
CCl4 induces lipid peroxidation, which causes membrane
breakdown of hepatocytes to subsequently release marker enzymes of
hepatotoxicity (104). It has
indicated that after KP 3-O-rutinoside and KP 3-O-glucoside
treatment, serum total protein levels were increased, induction of
serum aspartate aminotransferase serum alkaline phosphatase and
liver malondialdehyde levels by CCl4 was prevented
(105). In addition, glutathione
levels were significantly restored, and normal catalase and SOD
activities were observed. KP 3-O-rutinoside and KP 3-O-glucoside
have protective effects against acute CCl4-induced oxidative liver
damage.
KP protects against alcoholic liver
injury
Alcoholic liver injury is one of the major health
problems in the world, accounting for ~4% of the total global death
toll (106). High alcohol
consumption leads to hepatotoxicity associated with oxidative
stress due to the promotion of ROS production and reduction of
anti-oxidant effects (107).
Studies have indicated that CYP2E1 is an important factor in
alcohol-induced liver injury, as it is highly inducible and has a
high catalytic activity for alcohol (108).
Wang et al (107) indicated that KP attenuates the
activity and expression of CYP2E1, enhances the protective effect
of the anti-oxidant defense system and may thus protect the liver
from alcoholic liver injury. KP increased anti-oxidant effects and
also reduced ROS production through inhibiting CYP2E1. Although KP
was demonstrated to reduce lipid accumulation in non-alcoholic
fatty liver disease, its application in clinical treatment of
alcoholic liver disease remains to be evaluated (107). Zhou et al (109) indicated that the protein levels of
CYP2E1 in microsomes and mitochondria, heat shock protein 70
(Hsp70) in the cytosol and specificity protein 1 (SP1) in the
nucleus and cytosol were reduced in the KP-treated group, and KP
also increased the cell viability compared with that in the
ethanol-treated group. The study also suggested that the mechanism
of the protective effect of KP against ethanol-induced primary
hepatocyte injury involved mitochondrial and microsomal CYP2E1,
cytosolic Hsp70, and nuclear and cytosolic SP1 (109).
KP suppresses obesity
Obesity is a common chronic disease characterized by
excessive fat deposits in adipose tissue or other internal organs,
including the liver, heart, skeletal muscle and islets (110). Simple obesity may induce all
symptoms of metabolic syndrome, including insulin resistance,
non-alcoholic fatty liver, atherosclerosis and degenerative
diseases, e.g. dementia (111).
Experiments have suggested that KP inhibits lipid
accumulation and increases fatty acid oxidation signaling in
adipocytes. KP reduced cytoplasmic triglyceride accumulation during
adipocyte differentiation in a time- and dose-dependent manner. KP
broadly reduced the mRNA or protein levels of adipogenic
transcription factors and their lipid accumulation-associated
target genes, thereby inhibiting glucose uptake and the expression
of glucose transporter solute carrier family 4 mRNA in adipocytes
(112).
KP also reduces the accumulation of visceral fat by
increasing lipid metabolism through downregulation of sterol
regulatory element-binding proteins, and promoting the hepatic
expression of acyl-CoA oxidase and CYP450, family 4, subfamily a,
polypeptide 1 (113).
KP improves diabetes
Luo et al (114) reported that KP-mediated
downregulation of IκBα and inhibition of NF-κB pathway activation
may lead to a reduction in hepatic inflammatory lesions, which may
help to improve insulin signaling deficits in patients with
diabetes. Chen et al (115)
indicated that KP significantly inhibited inflammatory cytokine
expression and high glucose-induced ROS production. KP exerted
protective effects in diabetic cardiomyopathy by suppressing
nuclear translocation of NF-κB and activating NF-erythroid 2
p45-related factor-2.
In addition, KP may protect β-cells from glucose
toxicity and mediate anti-apoptotic effects by improving cyclic
adenosine monophosphate (cAMP)/protein kinase A and PI3K/Akt
signaling pathways. Zhang and Liu (116) indicated that KP treatment promoted
cell viability, inhibited apoptosis and reduced caspase-3 activity
in β-cells and human islets chronically exposed to hyperglycemic
conditions.
Furthermore, diabetes mellitus has been indicated to
increase the susceptibility of the myocardium to
ischemia-reperfusion (I/R) injury. It has been demonstrated that
advanced glycation end products (AGE) take part in the pathogenesis
of diabetic complications in various organs, including the heart
(117). Suchal et al
(118) indicated that KP is
involved in maintaining hemodynamic function, inhibiting
AGE-receptor axis activation, normalizing oxidative stress and
maintaining morphological changes. Their results suggested that
diabetic I/R group significantly reduced arterial pressure and
heart rate. Additionally, ventricular dysfunction was exhibited
through decreasing ventricular and relaxation and increasing
preload. The levels of inflammatory markers, including TNF-α, IL-6
and NF-κB, were decreased, and that the activity of JNK, p38 and
ERK1/2 was inhibited. For a period of 28 days, KP significantly
suppressed the activation of AGEs-RAGE, which is the receptor of
AGE. Furthermore, KP inhibited apoptosis by reducing the expression
of pro-apoptotic proteins, including Bax and caspase-3, decreasing
the expression of pro-apoptotic protein and terminal
deoxynucleotidyl transferase dUTP nick end labeling positive cells
and increasing the expression of Bcl-2. In conclusion, KP
significantly ameliorated IR-induced myocardial injury via the
normalization of hemodynamic parameters, maintaining oxidant
antioxidant status, reducing inflammation and apoptosis and by
inhibiting the MAPK/AGE-RAGE pathways.
Alkhalidy et al (119) reported that KP treatment increased
Akt and hexokinase activity, while decreasing pyruvate carboxylase
(PC) and glucose-6 phosphatase activity in the liver without
altering the protein expression. At the same time, KP reduced PC
activity and inhibited gluconeogenesis of HepG2 hepatoblastoma
cells and primary hepatocytes isolated from the liver of obese
mice.
Another noteworthy point is that KP is able to
alleviate diabetic neuropathic pain. Kishore et al (120) indicated that KP corrected
hyperglycemia in diabetic rats and partially reversed pain
responses by regulating oxidative and nitrosative stress, and
reducing AGE formation in diabetic rats. These results indicate
that KP may be a naturally occurring anti-diabetic compound and may
be applied to help treat diabetic neuropathic pain.
KP ameliorates symptoms of metabolic
syndrome
Metabolic syndrome is a complex disease, and the
risk of cardiovascular disease and type 2 diabetes is significantly
increased in patients with metabolic syndrome (99). It has been suggested that one
possible way to prevent and ameliorate these complex symptoms is to
activate the liver X receptor (LXR), which was identified as a
transcription factor that regulates hepatic gluconeogenesis
(121).
Chang et al (113) demonstrated that KP directly
interacts with LXR-α and LXR-β proteins. In obese apolipoprotein E
(ApoE)-deficient mice fed a high-fat diet, administration of KP
significantly reduced plasma glucose and triglyceride levels,
increased high-density lipoprotein cholesterol levels, and improved
insulin sensitivity and glucose tolerance. Furthermore, KP did not
induce hepatic steatosis, as it inhibited post-translational
activation of the precursor forms of sterol regulatory
element-binding protein-1 (pSREBP-1) to form the nuclear isoform of
SREBP-1. Selective activation of LXR-β, whose expression level is
low in the liver, may contribute to the avoidance of hepatic
lipogenesis.
In conclusion, KP independently regulates LXR-β and
peroxisome proliferator-activated receptor α, and improves the
overall metabolic status. These results may contribute to the
treatment of metabolic syndrome (122).
KP protects the vascular endothelium
KP inhibits vascular endothelial
inflammation
The vascular endothelium may be considered a
dynamic barrier that selectively inhibits the movement of plasma
and cells from the blood into adjacent tissues (123). Inhibition of vascular endothelial
inflammation is considered a key point in the treatment of numerous
vascular diseases, as excessively produced inflammatory mediators
may cause irreversible vascular damage and lead to excessive loss
of fluid from the circulation, resulting in insufficient tissue
perfusion, organ dysfunction and death (123).
Kim et al (124) reported that KP inhibited the
pro-inflammatory response, which was mediated by LPS or high
mobility group box 1 (HMGB1) by increasing barrier integrity and
inhibiting the expression of cell adhesion molecules. Therefore,
the anti-inflammatory properties of KP are considered to be based
on downregulation of the HMGB1 receptors TLR2 and TLR4, and this
may have a beneficial effect in the treatment of vascular
diseases.
KP inhibits vascular smooth muscle
cell (VSMC) migration
VSMCs retain the ability to switch between
differentiated and dedifferentiated phenotypes, in response to
environmental conditions including growth factor stimulation or
vascular injury (123). TGF-β and
bone morphogenetic protein (BMP)4 are important factors that
inhibit VSMC proliferation and migration, increase the expression
of contractile VSMC gene and promote the contractile phenotype
(125). This phenotype may be
switched to another phenotype via the platelet-derived growth
factor (PDGF) signaling pathway (126). PDGF may lead to resistance to TGF-β
and BMP signaling and promote the synthetic phenotype.
Kim et al (127) indicated that KP may activate the
BMP signaling pathway, induce microRNA (miR)-21 expression and
downregulate dedicator of cytokinesis 4 (DOCK4), DOCK5 and DOCK7,
thereby inhibiting cell migration and antagonizing PDGF-mediated
migration. KP may be considered a potential therapeutic for
cardiovascular diseases, as it inhibits VSMC migration by
modulating BMP-mediated miR-21 expression and miR regulation.
KP prevents atherosclerosis
Atherosclerotic cardiovascular disease is one of
the leading causes of mortality worldwide (128). Endothelial cells, macrophages and
smooth muscle cells are the major cell types involved in
atherosclerosis (129).
Osteopontin (OPN) together with its CD44 is
generally considered to be a key molecule in atherosclerosis
(130). Xiao et al (131) performed analysis of the aorta and
plasma from C57BL/6J control and ApoE-deficient mice treated with
or without KP. This aforementioned study reported that, prior to
the experiment, the expression of OPN and CD44, the production of
aortic ROS and the area of atherosclerotic lesions were higher
compared with the control group in the ApoE-deficient mice with KP.
For 4 weeks, KP significantly reduced atherosclerotic lesion
formation compared with mice treated with vehicle, restored
vasodilatation in response to acetylcholine, enhanced the Emax
value and reduced the EC50 value. The plasma level of OPN and the
expression of aortic OPN and CD44 were decreased. Therefore, KP
regulated the OPN-CD44 pathway to inhibit atherosclerosis in the
ApoE-deficient mouse aorta.
In addition, elevated plasma low-density
lipoprotein (LDL) cholesterol levels were identified to be a risk
factor for atherosclerosis and coronary heart disease (132). Ochiai et al (128) demonstrated that flavonoids
stimulate the activity of Sp1 by phosphorylating Thr-453 and
Thr-739 on ERK1/2, thereby increasing the DNA binding of Sp1 to the
promoter region of the low-density lipoprotein receptor gene.
Che et al (133) also reported that KP alleviated
oxidized (ox)-LDL-induced apoptosis. KP increased the ratio of
microtubule-associated protein 1 light chain 3α and beclin-1 levels
in ox-LDL-induced human umbilical vein endothelial cells (HUVECs).
Furthermore, the expression of phosphorylated (p-)Akt and
p-mammalian target of rapamycin (p-mTOR) was downregulated. The
effect of KP on cell viability and apoptosis was also reduced by
insulin in ox-LDL-induced HUVECs. From these results, it may be
concluded that KP attenuates ox-LDL-induced apoptosis by
upregulating autophagy through the PI3K/Akt/mTOR signaling pathway
in human endothelial cells.
KP inhibits thrombosis and platelet
activation
High-risk cerebrovascular disease is mainly caused
by abnormal blood vessel thrombosis after hemostasis, which may
include fibrinolytic system dysfunction caused by abnormal
fibrinolytic factors, e.g. plasmin and plasminogen activator, and
thrombosis induced by an imbalance of coagulation factors (134). A thrombus composed of an insoluble
fibrin polymer is formed by activating thrombin, which in turn
converts fibrinogen to fibrin and factor XIIIa (135).
KP has been demonstrated to inhibit procoagulant
activity and the interaction between fibrinogen and thrombin by
inhibiting ERK, serine protease p38, JNK and Akt activation. In
addition, platelet activation is a major event in acute vascular
thrombosis. Therefore, another treatment strategy is to prevent
enhanced platelet activation (136). ROS generated after collagen
stimulation is associated with various platelet activation events,
including phospholipase Cγ2 activation, cytosolic calcium
elevation, integrin-α and -β activation and granule release
(137). Wang et al (138) indicated that KP inhibits
collagen-induced triphosphopyridine nucleotide oxidase activation
and potently suppresses the tyrosine phosphorylation-based
glycoprotein VI signaling pathway by preventing oxidative
inactivation of SH2-containing phosphatase 2, and then attenuates
collagen-induced platelet aggregation and platelet-dependent
thrombosis.
KP suppresses angiogenesis of human
retinal endothelial cells (HRECs) under high-glucose
conditions
Diabetic retinopathy (DR) is a common microvascular
complication of diabetes, which is also the most likely cause of
blindness in developed countries (139). Retinal neovascularization is seen
as an essential factor in the pathogenesis of DR (140). VEGF may stimulate vasculogenesis
and angiogenesis. In the aqueous humor of patients with DR and in
endothelial cells exposed to high glucose, the levels of VEGF were
determined to be increased (141,142).
It has been demonstrated that the expression of thrombospondin 1
and metallopeptidases with TSP type 1 motif (ADAM), which are
endogenous inhibitors of angiogenesis, may have an important role
in diabetes-associated retinal vascular homeostasis and vascular
dysregulation (143).
Wu et al (144) reported that high glucose
significantly promoted the proliferation, migration and tube
formation of HRECs, which was antagonized by 10 and 30 mM KP in a
dose-dependent manner. They indicated that the expression levels of
TSP-1 and ADAM mRNA increased following treatment with 30 mM KP,
while TSP-1 and ADAM levels did not differ between high-glucose and
normal (5 mM) glucose conditions. Xu et al (145) also demonstrated that high glucose
increased the mRNA expression levels of VEGF and placental growth
factor (PGF), and that the concentration of secreted VEGF and PGF
from HRECs increased. Chin et al (146) also indicated that KP significantly
reduced VEGF-stimulated HUVEC proliferation. These results indicate
that in VEGF-stimulated ECs, KP may exert angiogenic inhibition
through regulation of VEGF/VEGF receptor 2 and its downstream
signaling cascades (PI3K/AKT, MEK and ERK).
KP protects heart function
KP protects against doxorubicin
(DOX)-induced cardiotoxicity
DOX is an anthracycline and is one of the most
widely used anti-tumor drugs with potent activity. However, it may
induce cardiotoxicity, which may cause cardiomyopathy or even
severe heart failure (147). The
mechanism of DOX-induced cardiomyocyte apoptosis has been
extensively studied (148) and
apoptosis is thought to start with two typical signaling pathways,
one intrinsic and one extrinsic (149). Xiao et al (149) suggested that KP may have a role in
inhibiting tumor suppressor proteins, protects against DNA damage
with p53-mediated mitochondria-dependent intrinsic apoptotic
signaling and participates in the ERK-dependent MAPK signaling
pathway.
KP protects against cardiac sinus node
dysfunction (SND)
The normal heartbeat begins with the sinus node
cells in the right atrium (150).
The electrical pulse in this conduction process is generated based
on the Ca2+ signal transduction pathway (151). Calmodulin kinase II (CaMKII) is a
key signaling molecule in pacing cardiomyocytes and regulates major
Ca2+ homeostasis proteins (152). In addition, after its oxidation,
overactivated CaMKII promotes cell death, which may lead to
arrhythmias, heart failure and sudden death (153).
An and Kim (15)
indicated that KP protects the sinus node from angiotensin II (Ang
II)-induced SND. Their results suggested that Ang II induced
caspase-3-activated apoptosis in sinus node cells, and reduced
CaMKII oxidation and death of sinoatrial node cells; KP maintained
normal impulse formation in the right atrium. These results suggest
that KP may hold value in the prevention of SND in high-risk
patients and protection of the sinoatrial node by inhibiting CaMKII
oxidation.
KP attenuates cardiac hypertrophy
Cardiac hypertrophy is a common disease that causes
heart dysfunction and various cardiovascular diseases as the size
and shape of the heart change (154). The MAPK signaling pathway is
frequently overactivated in pathological cardiac hypertrophy
(155). When the MAPK signaling
cascade is activated, its members phosphorylate various downstream
signal targets, thereby enhancing cardiac hypertrophy (155).
Feng et al (156) indicated that KP significantly
attenuated aortic banding-induced cardiac hypertrophy, which led to
a decreased myocardial cell area and interstitial fibrosis. KP
inhibited the activity of the apoptotic signal-regulating kinase 1
(ASK1)/JNK1/JNK2/p38 signaling pathway and the proliferation of
H9c2 cardiomyocytes. It may be concluded that KP is able to prevent
cardiac hypertrophy, and it may exert its role through the
regulation of the ASK1/MAPK signaling pathway and oxidative
stress.
Another factor that induces cardiac hypertrophy may
be Ang II (157) and the mechanism
that promotes Ang II-induced cardiac fibrosis is EMT. Zeisberg
et al (158) reported that
the endothelocytes were transformed into fibroblasts by EMT during
the development of myocardial fibrosis in mice. Liu et al
(159) indicated that, although KP
did not have a fundamental role, it reduced Ang II infusion or
TGF-β-induced EMT, and inhibited the proliferation and activation
of cardiac fibroblasts in vivo as well as in
vitro.
KP attenuates myocardial I/R
injury
Cardiovascular diseases are responsible for the
majority of mortalities in the elderly and the most important
presentation of cardiovascular disease is ischemia (160). Restoring the blood supply to the
ischemic myocardium may reduce myocardial damage, but may also
cause damage or even aggravate myocardial damage, known as I/R
injury (161).
Suchal et al (162) demonstrated that the major cause of
damage was the production of ROS and calcium overload (163). Recent evidence suggested that
mitochondrial protection has an important role in guiding apoptosis
(164). Khader et al
(165) demonstrated that sirtuin1
activation reduced oxidative stress and maintained mitochondrial
function in different tissue and cell types after I/R.
Suchal et al (162) also indicated that KP attenuated
myocardial I/R injury by reducing MAPK signaling-induced oxidative
stress and inflammation. Guo et al (164) obtained a similar result, namely
that KP protected cardiomyocytes by inhibiting the MAPK pathway,
with the effects including a decrease in the production of ROS,
maintenance of the mitochondrial membrane potential, inhibition of
mitochondrial membrane transition (mPTP) opening and reduction of
cytochrome C release.
In addition, Zhou et al (166) suggested that glycogen synthase
kinase-3 (GSK-3)β inhibition delays or suppresses mPTP opening and
inhibits the release of cytochrome C. They indicated that KP
decreased apoptosis induced by I/R injury via GSK-3β inhibition. KP
or thiadiazolidinone-8 increased the level of GSK-3β
phosphorylation and reduced the release of cytochrome C compared
with the control and I/R groups.
KP protects the cranial nerve
Protective effect of KP in
neurodegenerative diseases
The pathophysiology of neurodegenerative diseases
is complex and may be partially characterized by oxidative stress,
which is generally thought to have a key role in nerve damage
(167). Previous studies have
indicated that ROS is involved in neurodegenerative diseases and
that they are produced in high concentrations due to oxidative
metabolism in the brain (168).
Under normal conditions, the generation and elimination of ROS are
in a dynamic balance (169).
However, if the balance is destroyed, the level of ROS increases
due to the decreased activity of anti-oxidant enzymes, which
impairs the brain function, particularly in the hippocampus.
Lei et al (167) indicated that KP obviously reduces
cognitive impairment via upregulating the expression of proteins in
the ERK1/2-cAMP-responsive element-binding protein (CREB) signaling
pathway; KP may also enhance Na+/K+-ATPase
activity and reduce hippocampal oxidative stress. In addition, an
increase in the number of hydroxyl groups in the flavonol B ring
also contributes to the upregulation of the expression of proteins
from the ERK1/2-CREB signaling pathway in the hippocampus of mice
with D-gal-induced cognitive impairment.
KP has anti-depressant activities
Chronic stress is thought to be a risk factor for
psychosomatic and psychiatric illnesses, including anxiety and
depressive disorders (170).
Depression is the second most common chronic condition in clinical
medicine (171) and it is predicted
that by 2020, depression will become the second leading cause of
premature death or disability worldwide.
Park et al (172) used the tail suspension test (TST),
the forced swimming test (FST) and the rota-rod test in mice who
were stressed due to the chronic use of restraints or
immobilization to measure the anti-depressant activities of KP.
Mice in the experiment were orally administered KP or quercetin at
a dose of 30 mg/kg/day for 14 days while being tested. The final
experimental results suggested that KP or quercetin obviously
reduced the immobility time in the TST and FST, indicating that
flavonoids have potent anti-depressant effects.
KP has neuroprotective effects in
Parkinson's disease (PD)
PD is the second most common disorder of the
central nervous system (173). Its
pathological features are the loss of dopaminergic neurons and the
formation of cytoplasmic inclusions in the substantia nigra (SN).
When PD is present, ~80% of striatal dopamine is lost and damage to
the terminal region may preempt the loss of cell bodies in SN
(174).
Han et al (175) hypothesized that inflammasomes are
associated with immune homeostasis and their dysregulation leads to
neurodegenerative disorders. The NLR family pyrin domain containing
3 (NLRP3) inflammasome is involved in PD. KP inhibits NLRP3
inflammasome activation, leading to reduced NLRP3 protein
expression and deactivation of the NLRP3 inflammasome in order to
promote macroautophagy/autophagy in microglia.
Li and Pu (176)
indicated that pre-intake of KP markedly improved
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced
dopamine and dihydroxy-phenyl acetic acid (DOPAC) depletion in the
striatum, and also reduced the DOPAC/dopamine ratio and the
MPTP-induced loss of tyrosine hydroxylase-positive neurons in mouse
SN. KP has a neuroprotective effect in mice with MPTP-induced PD,
which may contribute to its anti-oxidant capacity to scavenge free
radicals and lead to the survival of more dopamine neurons.
In previous studies, KP 3-O-rutinoside and
anhydrosafflor yellow B, as the compounds isolated from SSFE, a
standardized safflower flavonoid extract, have been demonstrated to
reduce the levels of ROS, which are induced by hydrogen peroxide
and restore tyrosine hydroxylase activity in PC12 cells (177). Ren et al (178) indicated that KP 3-O-rutinoside and
dehydrin flavonoid B inhibited microtubule instability and reduced
the cell volume. In addition, SSFE, in the form of pills, may
inhibit astrocyte proliferation and improve the neurological
behavior in a 6-hydroxydopamine (6-OHDA)-induced PD rat model. In a
study using a magnetic resonance imaging-based, tracer-based
approach, 6-OHDA was indicated to alter diffusion parameters of
endothelial cells, including reduced bowing and clearance rate
constants and increased elimination half-life of the tracer in the
SN with 6-OHDA-induced lesions. These results indicated that
flavonoids may be potential anti-PD medicines.
KP prevents Alzheimer's disease
(AD)
Studies have indicated that the abnormal production
of various proteins and high concentrations of glutamate may lead
to excessive ROS production in cells, resulting in neurotoxicity
and neuronal death (179). Yang
et al (180) examined the
neuroprotective effects of KP in glutamate-treated HT22 hippocampal
neuronal cells. Lactate dehydrogenase assays and
fluorescein-5-isothiocyanate-connexin V/propidium iodide double
staining procedures were subsequently used to confirm the
protective effect of KP on HT22 cells. These results also indicated
that KP protects nerves by regulating the expression levels of
apoptosis-associated proteins, including Bcl-2, Bid,
apoptosis-inducing factor and MAPK.
AD is characterized by the accumulation and
deposition of β-amyloid peptides, leading to progressive neuronal
damage and cell loss. Among several hypotheses, oxidative stress
may be a mechanism that leads to neurodegeneration. To date, there
is no cure for AD, but the use of natural anti-oxidants may delay
the pathogenesis of the disease. These results suggest that KP may
serve as a useful drug for the prevention and treatment of
neurodegenerative diseases, including AD.
KP inhibits ischemic stroke
Ischemic stroke is a clinical syndrome
characterized by the rapid onset of neurological deficits, mostly
caused by arterial occlusion. The brain is particularly susceptible
to ischemic injury due to its high metabolic requirement (181).
It is reported that the process of ischemic brain
injury may include LPS binding to TLR4, causing microglia to be
activated by TLR2 and TLR4, followed by release of pro-inflammatory
factors from the TLR4/MyD88 signaling pathway and produce further
damage (182). The function of
blood-brain barrier (BBB) is to keep harmful substances in the
blood from being transported into the brain. One of the most
prominent causes of brain damage is BBB decomposition and
neuroinflammation, and therefore, the key to protecting the nerve
is the maintenance of anti-neuritis processes and BBB integrity
(183).
Cheng et al (184) indicated that KP reduces the
production of various pro-inflammatory and inflammatory proteins in
brain tissue, including IL-1, iNOS, COX-2, TNF-α, MCP-1 and IL-1β.
In addition, KP protected BBB integrity in the mouse brain and
increased BBB-associated proteins (occludin-1, claudin-1 and
connexin43). KP also significantly reduced HMGB1 levels and
inhibited the expression of components of the TLR4/MyD88
inflammatory pathway at the transcriptional and translational
levels.
Mitochondrial dysfunction is a factor associated
with ischemic stroke. Wu et al (185) indicated that KP protected neurons
from ischemic injury by inhibiting mitochondrial fission and
maintaining mitochondrial hexokinase II (HK-II). KP promoted
mitochondrial binding of HK-II by inhibiting actin-related protein
1 (Drp1) activation, which was dependent on Akt. Furthermore, they
demonstrated that KP enhanced autophagy during oxygen and glucose
deprivation. In vivo, oral administration of KP resulted in
a reduced infarct volume in mice after I/R injury and exerted an
effect similar to mitochondrial protection in the infarcted area,
indicating an association between succinic acid accumulation and
I/R injury-induced nerve meta-mitochondrial dysfunction, and
suggesting that modulation of Drp1 phosphorylation is a potential
strategy for protecting neuronal mitochondrial integrity and
treating ischemic stroke.
KP for fibroproliferative disorders
HPS is a complex dermal fibroproliferative disorder
(185), which generally occurs
after burns, trauma or surgery, and is linked to excessive wound
healing (186). In HPS,
myofibroblasts cause scar contraction, which may cause dysfunction
of the affected body parts and even disability (187). Therefore, the major cause of HPS
formation is excessive development and hyperfunction of
scar-forming fibroblasts (188).
Li et al (189) discovered that KP obviously
inhibited HPS formation in a mechanically induced mouse model, and
the biological activity of KP was induced by selective binding of
KP to TGF-β1. Kinase binding assays indicated competitive binding
of KP to the ATP binding site of TGF-β1. Western blot analysis
further revealed that KP caused downregulation of SMAD2 and −3
phosphorylation in a dose-dependent manner. The bioactivity of KP,
which resulted from the inhibition of TGF-β1/SMADs signaling, was
induced by the selective binding of KP to TGF-β receptor type I.
These results suggested that KP may be a promising treatment for
fibro-proliferative diseases including HPS, and even liver
fibrosis, pulmonary fibrosis and myocardial fibrosis, which are
highly associated with the TGF-β1/SMADs signaling pathway.
Conclusion
Flavonoids have been widely recognized for their
effective anti-inflammatory and anti-oxidant properties. The major
signaling pathways via which KP exerts its effects in various
diseases are summarized in Fig.
2.
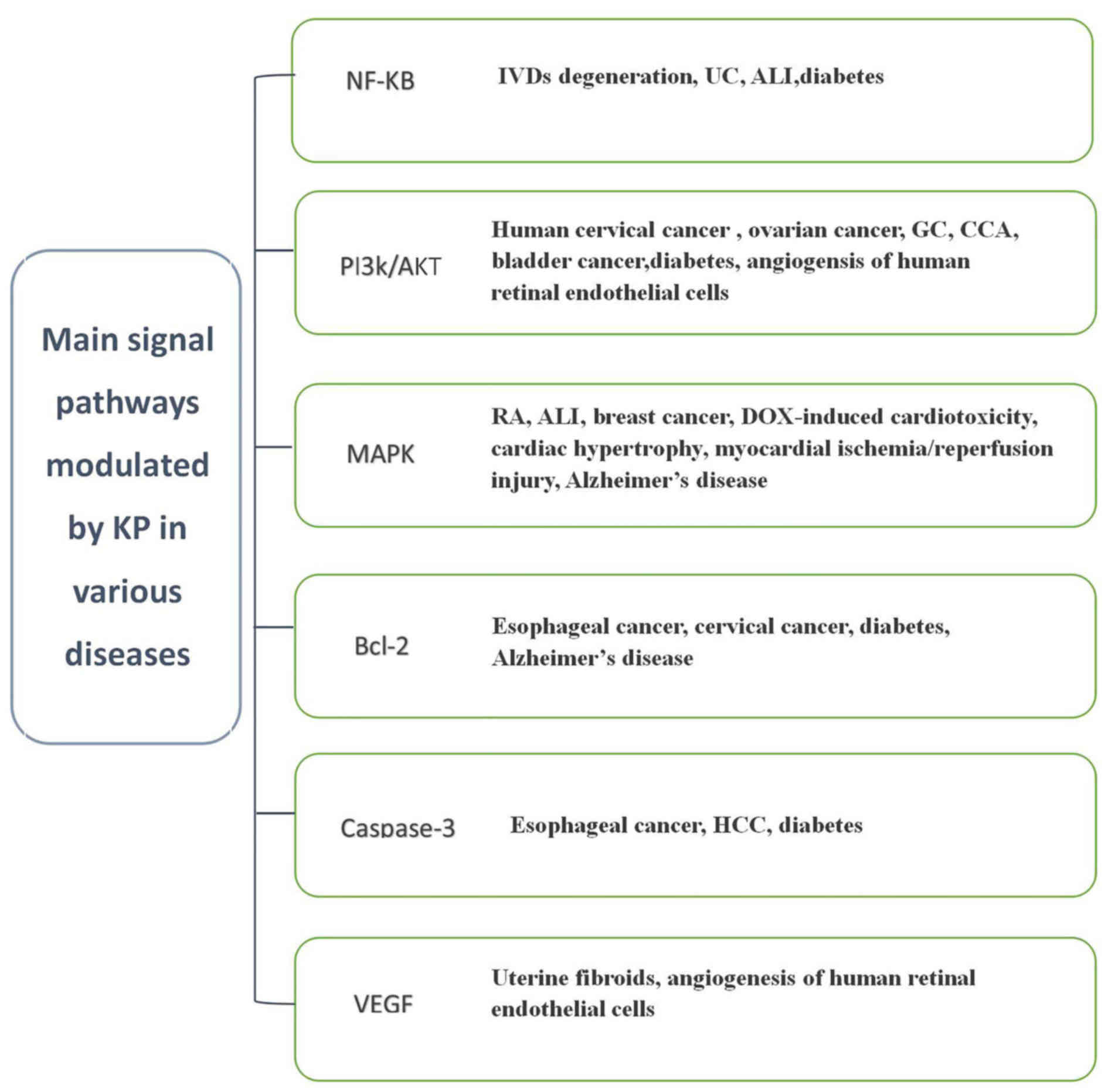 | Figure 2.Main signaling pathways modulated by
KP in various diseases. HCC, hepatocellular carcinoma; VEGF,
vascular endothelial growth factor; IVD, intervertebral disc; UC,
ulcerative colitis; ALI, acute lung injury; GC, gastric cancer;
CCA, cholangiocarcinoma; MAPK, mitogen-activated protein kinase;
RA, rheumatoid arthritis; DOX, doxorubicin; KP, kaempferol. |
The flavonoid ring structure is a structure
necessary for mutagenic activity, and this activity may depend on
characteristics including the degree of hydroxylation structural
features and the production of ROS (190). It may be hypothesized that the
major role of KP is to regulate the expression of genes involved in
inflammation, including the inhibition of transcription factors.
MAPK, PKC, PI3K, Janus kinase signaling and transcriptional
activator pathways are involved in the development of inflammation.
These kinases have roles in the regulation of the expression and
activity of transcription factors, including NF-κB and AP-1
(191). PI3K is an important
mediator in the inflammatory signaling cascade, and KP and
8-prenylkaempferol treatment have been indicated to inhibit PI3K
and Akt phosphorylation (192). In
conclusion, KP has therapeutic effects on inflammation-associated
diseases, including allergies, arthritis, diabetes, cardiovascular
diseases, cancers and neurological regression by inhibiting protein
kinases and transcription factors.
Although it has been reported that KP has the
highest anti-proliferative effect on the hepatoblastoma cell line,
the colon cancer cell line and the melanoma cell line (193), there is controversy regarding the
anti-cancer effect. Studies have indicated that KP represents a
double-edged sword in terms of exhibiting cytotoxic and
cytoprotective effects. Noroozi et al (194) reported that KP decreased oxidative
DNA damage in isolated human lymphocytes and increased cell
survival in HT-22 neuronal cells under oxidative stress. However,
in the study by Bestwick et al (195), KP was demonstrated to reduce ROS
levels in HL-60 cells at low doses of KP, but the occurrence of
single strand breaks was KP concentration-dependent. Thus, despite
low initial ROS levels, KP has an adverse effect on DNA integrity.
Furthermore, KP-induced DNA damage is highly localized and
specific. The cause of DNA damage may be highly localized changes
in ROS production. Sahu and Gray (196) hypothesized that HO.
formation close to DNA molecules may be the cause of chain breaks,
but this is also affected by the environment.
To date, flavonoids have been reported as typical
cytotoxic chemotherapeutic agents to treat acute myeloid leukemia
by causing DNA damage, which may make abnormal cells more
susceptible to therapeutic agents due to an environment with less
oxidative stress (197). Das et
al (198) suggested that the
flavonoid with the highest affinity for circulating tumor DNA
(ct-DNA) is quercetin, followed by myricetin, luteolin and KP; the
number and position of -OH moieties influences the binding
efficacies of flavonoids to ct-DNA. Therefore, the combination of
quercetin and myricetin may cause higher DNA damage, which can be
served as a potential anti-cancer agent.
The genotoxicity of KP is mainly due to its
pro-oxidative activity in vitro. Cos et al (199) introduced an anti-oxidant
selectivity index (ASI), which is the maximal non-toxic dose
divided by the IC50 value, used to assess the toxicity
of flavonoids. If the ASI of flavonoids is >100, it may be
considered safe and to possess a good anti-oxidant activity
profile, but the ASI requires to be further investigated.
Furthermore, KP has poor absorption, particularly
regarding oral bioavailability, which is likely due to the limited
solubility of flavonoids in water (200). However, the bioavailability of KP
may be increased if combined with different anti-cancer drugs
(5). Regarding its poor absorption,
considerations for improving the solubility and bioavailability of
KP may be the use of nanocarriers, which increase absorption and
enhance anti-free radical stability during consumption and storage
of food compounds (6). Qian et
al (201) developed a KP
nanosuspension, which is produced using high-pressure
homogenization technology, to improve pharmacokinetics and absolute
bioavailability. Resende et al (202) used poloxamer 407 to prepare a solid
dispersion of KP, which ultimately improved the problem of poor
water solubility. It has been demonstrated that mechanochemically
synthesized complexes of polysaccharides and oligosaccharides can
enhance bioavailability, and the supramolecular complexes are more
stable and soluble than the individual components (203). Xu et al (204) indicated that the combination of KP
with polysaccharide arabinogalactan and disodium glycyrrhizinate
improved the solubility and bioavailability of KP, which may be
used as a promising treatment for diabetes.
In addition, whether KP exerts toxicity in a
dietary environment remains elusive, and since KP is not the sole
active component, it is necessary to consider other active
polyphenols in the diet. Although there is little evidence that a
natural flavonoid-rich diet is harmful in the body, it may be
unwise to propose an enhanced supplemental intake. High doses of KP
may have side effects. For instance, studies have shown that
flavonoid compounds are markedly inhibited (3). H-folic acid uptake, means patients who
are deficient in iron and/or folic acid may exhibit abnormal side
effects after using KP, as the consumption of KP reduces the
bioavailability of iron and/or reduces the level of folic acid in
the cells (205). KP could be
incorporated into potential nanoparticles to achieve high targeting
efficacy against folate-overexpressing cancerous cells while
limiting the potential effects on normal cells (206).
In conclusion, KP inhibits protein kinases and
transcription factors due to its anti-inflammatory and antioxidant
properties. However, while protecting cells, cytotoxicity is also
exhibited, which is associated with ROS production by the
concentration of KP. Therefore, it is necessary to introduce a
standard for producing cytotoxicity. Additionally, the oral
bioavailability of KP is poor, and most of its current uses are as
nanocarriers. The combination of polysaccharides and
oligosaccharide complexes can improve bioavailability. In terms of
diet, whether KP is toxic requires further investigation as a high
intake of KP may cause adverse side effects. Patients with iron
and/or folic acid may exhibit abnormal side effects after using
KP.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81620108030 and
81873076), the Key project of Shanghai 3-year plan (grant no.
ZY2018-2020-CCCX-2002-01), the Shanghai Rising-Star Project (grant
no. 15QA1403500) and the Shanghai Talents development fund Project
(grant no. 2017090) and the Innovation Project for Undergraduates
of Shanghai University of Traditional Chinese Medicine (grant. no.
2017SHUTCM223).
Availability of data and materials
Not applicable.
Authors' contributions
TW and GJ designed the present study. JR and TW
prepared and wrote the manuscript. YL, YQ and BC performed a
literature search and selected the studies to be included. TW and
GJ revised the manuscript. All authors approved the final version
of the article.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Devi KP, Malar DS, Nabavi SF, Sureda A,
Xiao J, Nabavi SM and Daglia M: Kaempferol and inflammation: From
chemistry to medicine. Pharmacol Res. 99:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsao R: Chemistry and biochemistry of
dietary polyphenols. Nutrients. 2:1231–1246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calderón-Montaño JM, Burgos-Morón E,
Pérez-Guerrero C and López-Lázaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JS, Rho HS, Kim DH and Chang IS:
Enzymatic preparation of kaempferol from green tea seed and its
antioxidant activity. J Agric Food Chem. 54:2951–2956. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yadav KS and Sawant KK: Modified
nanoprecipitation method for preparation of cytarabine-loaded PLGA
nanoparticles. AAPS PharmSciTech. 11:1456–1465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hegde A and Bhatia M: Hydrogen sulfide in
inflammation: Friend or foe? Inflamm Allergy Drug Targets.
10:118–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkerts G, Kloek J, Muijsers RB and
Nijkamp FP: Reactive nitrogen and oxygen species in airway
inflammation. Eur J Pharmacol. 429:251–262. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin MK, Yu YL, Chen KC, Chang WT, Lee MS,
Yang MJ, Cheng HC, Liu CH, Chen DzC and Chu CL: Kaempferol from
Semen cuscutae attenuates the immune function of dendritic cells.
Immunobiology. 216:1103–1109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nosikova YS, Santerre JP, Grynpas M,
Gibson G and Kandel RA: Characterization of the annulus
fibrosus-vertebral body interface: Identification of new structural
features. J Anat. 221:577–589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu J, Tang H, Zhang Z, Zhang Y, Qiu C,
Zhang L, Huang P and Li F: Kaempferol slows intervertebral disc
degeneration by modifying LPS-induced osteogenesis/adipogenesis
imbalance and inflammation response in BMSCs. Int Immunopharmacol.
43:236–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silverwood V, Blagojevic-Bucknall M, Jinks
C, Jordan JL, Protheroe J and Jordan KP: Current evidence on risk
factors for knee osteoarthritis in older adults: A systematic
review and meta-analysis. Osteoarthritis Cartilage. 23:507–515.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Asanbaeva A, Tam J, Schumacher BL, Klisch
SM, Masuda K and Sah RL: Articular cartilage tensile integrity:
Modulation by matrix depletion is maturation-dependent. Arch
Biochem Biophys. 474:175–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lawrence RC, Felson DT, Lawrence RC,
Gabriel S, Hirsch R, Kwoh CK, Liang MH, Kremers HM, Mayes MD,
Merkel PA, et al: Estimates of the prevalence of arthritis and
other rheumatic conditions in the United States. Part II. Arthritis
Rheum. 58:15–25. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu-Bryan R and Terkeltaub R: Emerging
regulators of the inflammatory process in osteoarthritis. Nat Rev
Rheumatol. 11:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abramson SB: Osteoarthritis and nitric
oxide. Osteoarthritis Cartilage. 16 (Suppl 2):S15–S20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Honghai Z, Zhang T, Xia C, Shi L, Wang S,
Zheng X, Hu T and Zhang B: Berberine ameliorates cartilage
degeneration in interleukin-1β-stimulated rat chondrocytes and in a
rat model of osteoarthritis via Akt signalling. J Cell Mol Med.
18:283–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park MY, Ji GE and Sung MK: Dietary
kaempferol suppresses inflammation of dextran sulfate
sodium-induced colitis in mice. Dig Dis Sci. 57:355–363. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sordillo LM and Streicher KL: Mammary
gland immunity and mastitis susceptibility. J Mammary Gland Biol
Neoplasia. 7:135–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao HB, Sui GG, Lu XY and Sun ZL:
Kaempferol modulates Angiopoietin-like protein 2 expression to
lessen the mastitis in mice. Pharmacol Rep. 70:439–445. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alam J, Jantan I and Bukhari SNA:
Rheumatoid arthritis: Recent advances on its etiology, role of
cytokines and pharmacotherapy. Biomed Pharmacother. 92:615–633.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan D, Li N, Liu Y, Xu Q, Liu Q, You Y,
Wei Z, Jiang Y, Liu M, Guo T, et al: Kaempferol inhibits the
migration and invasion of rheumatoid arthritis fibroblast-like
synoviocytes by blocking activation of the MAPK pathway. Int
Immunopharmacol. 55:174–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee CJ, Moon SJ, Jeong JH, Lee S, Lee MH,
Yoo SM, Lee HS, Kang HC, Lee JY, Lee WS, et al: Kaempferol
targeting on the fibroblast growth factor receptor 3-ribosomal S6
kinase 2 signaling axis prevents the development of rheumatoid
arthritis. Cell Death Dis. 9:4012018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gill SE, Rohan M and Mehta S: Role of
pulmonary microvascular endothelial cell apoptosis in murine
sepsis-induced lung injury in vivo. Respir Res. 16:1092015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Villar J, Blanco J, Añón JM, Santos-Bouza
A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa
S, et al: The ALIEN study: Incidence and outcome of acute
respiratory distress syndrome in the era of lung protective
ventilation. Intensive Care Med. 37:1932–1941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matthay MA and Zimmerman GA: Acute lung
injury and the acute respiratory distress syndrome: Four decades of
inquiry into pathogenesis and rational management. Am J Respir Cell
Mol Biol. 33:319–327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang R, Ai X, Duan Y, Xue M, He W, Wang
C, Xu T, Xu M, Liu B, Li C, et al: Kaempferol ameliorates H9N2
swine influenza virus-induced acute lung injury by inactivation of
TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed.
Pharmacother. 89:660–672. 2017. View Article : Google Scholar
|
|
28
|
Rabha DJ, Singh TU, Rungsung S, Kumar T,
Parida S, Lingaraju MC, Paul A, Sahoo M and Kumar D: Kaempferol
attenuates acute lung injury in caecal ligation and puncture model
of sepsis in mice. Exp Lung Res. 44:63–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Yang X, Liu T, Guan M, Feng X,
Dong W, Chu X, Liu J, Tian X, Ci X, et al: Kaempferol regulates
MAPKs and NF-κB signaling pathways to attenuate LPS-induced acute
lung injury in mice. Int Immunopharmacol. 14:209–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong JH, Shin D, Han SY, Kim JL and Kang
YH: Kaempferol suppresses eosionphil infiltration and airway
inflammation in airway epithelial cells and in mice with allergic
asthma. J Nutr. 142:47–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banerjee R and Puniyani RR: Exogenous
surfactant therapy and mucus rheology in chronic obstructive airway
diseases. J Biomater Appl. 14:243–272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davies DE: The role of the epithelium in
airway remodeling in asthma. Proc Am Thorac Soc. 6:678–682. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Erjefält JS: The airway epithelium as
regulator of inflammation patterns in asthma. Clin Respir J. 4
(Suppl 1):S9–S14. 2010. View Article : Google Scholar
|
|
34
|
Kim SR and Lee YC: Endoplasmic reticulum
stress and the related signaling networks in severe asthma. Allergy
Asthma Immunol Res. 7:106–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Makhija L, Krishnan V, Rehman R,
Chakraborty S, Maity S, Mabalirajan U, Chakraborty K, Ghosh B and
Agrawal A: Chemical chaperones mitigate experimental asthma by
attenuating endoplasmic reticulum stress. Am J Respir Cell Mol
Biol. 50:923–931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park SH, Gong JH, Choi YJ, Kang MK, Kim YH
and Kang YH: Kaempferol inhibits endoplasmic reticulum
stress-associated mucus hypersecretion in airway epithelial cells
and ovalbumin-sensitized mice. PLoS One. 10:e01435262015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lopez-Basave HN, Morales-Vásquez F,
Ruiz-Molina JM, Namendys-Silva SA, Vela-Sarmiento I, Ruan JM,
Rosciano AE, Calderillo-Ruiz G, Díaz-Romero C, Herrera-Gómez A and
Meneses-García AA: Gastric cancer in young people under 30 years of
age: Worse prognosis, or delay in diagnosis? Cancer Manag Res.
5:31–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li WF, Hao DJ, Fan T, Huang HM, Yao H and
Niu XF: Protective effect of chelerythrine against ethanol-induced
gastric ulcer in mice. Chem Biol Interact. 208:18–27. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bae S, Kim N, Kang JM, Kim DS, Kim KM, Cho
YK, Kim JH, Jung SW and Shim KN: Incidence and 30-day mortality of
peptic ulcer bleeding in Korea. Eur J Gastroenterol Hepatol.
24:675–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ribeiro AR, Diniz PB, Pinheiro MS,
Albuquerque-Júnior RL and Thomazzi SM: Gastroprotective effects of
thymol on acute and chronic ulcers in rats: The role of
prostaglandins, ATP-sensitive K(+) channels, and gastric mucus
secretion. Chem Biol Interact. 244:121–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ateufack G, Domgnim Mokam EC, Mbiantcha M,
Dongmo Feudjio RB, David N and Kamanyi A: Gastroprotective and
ulcer healing effects of Piptadeniastrum Africanum on
experimentally induced gastric ulcers in rats. BMC Complement
Altern Med. 15:2142015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Almasaudi SB, Abbas AT, Al-Hindi RR,
El-Shitany NA, Abdel-Dayem UA, Ali SS, Saleh RM, Al Jaouni SK,
Kamal MA and Harakeh SM: Manuka honey exerts antioxidant and
anti-inflammatory activities that promote healing of acetic
acid-induced gastric ulcer in rats. Evid Based Complement Alternat
Med. 2017:54139172017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Niv Y and Banić M: Gastric barrier
function and toxic damage. Dig Dis. 32:235–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hajrezaie M, Golbabapour S, Hassandarvish
P, Gwaram NS, A Hadi AH, Mohd Ali H, Majid N and Abdulla MA: Acute
toxicity and gastroprotection studies of a new schiff base derived
copper (II) complex against ethanol-induced acute gastric lesions
in rats. PLoS One. 7:e515372012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li Q, Hu X, Xuan Y, Ying J, Fei Y, Rong J,
Zhang Y, Zhang J, Liu C and Liu Z: Kaempferol protects
ethanol-induced gastric ulcers in mice via pro-inflammatory
cytokines and NO. Acta Biochim Biophys Sin (Shanghai). 50:246–253.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Arif H, Sohail A, Farhan M, Rehman AA,
Ahmad A and Hadi SM: Flavonoids-induced redox cycling of copper
ions leads to generation of reactive oxygen species: A potential
role in cancer chemoprevention. Int J Biol Macromol. 106:569–578.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bandla S, Pennathur A, Luketich JD, Beer
DG, Lin L, Bass AJ, Godfrey TE and Litle VR: Comparative genomics
of esophageal adenocarcinoma and squamous cell carcinoma. Ann
Thorac Surg. 93:1101–1106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pohl H and Welch HG: The role of
overdiagnosis and reclassification in the marked increase of
esophageal adenocarcinoma incidence. J Natl Cancer Inst.
97:142–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yao S, Wang X, Li C, Zhao T, Jin H and
Fang W: Kaempferol inhibits cell proliferation and glycolysis in
esophagus squamous cell carcinoma via targeting EGFR signaling
pathway. Tumour Biol. 37:10247–10256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Q, Zhao XH and Wang ZJ: Flavones and
flavonols exert cytotoxic effects on a human oesophageal
adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing
apoptosis. Food Chem Toxicol. 46:2042–2053. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Diantini A, Subarnas A, Lestari K, Halimah
E, Susilawati Y, Supriyatna, Julaeha E, Achmad TH, Suradji EW,
Yamazaki C, et al: Kaempferol-3-O-rhamnoside isolated from the
leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell
proliferation through activation of the caspase cascade pathway.
Oncol Lett. 3:1069–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim SH, Hwang KA and Choi KC: Treatment
with kaempferol suppresses breast cancer cell growth caused by
estrogen and triclosan in cellular and xenograft breast cancer
models. J Nutr Biochem. 28:70–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ren W, Qiao Z, Wang H, Zhu L and Zhang L:
Flavonoids: Promising anticancer agents. Med Res Rev. 23:519–534.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Konopleva M, Zhao S, Xie Z, Segall H,
Younes A, Claxton DF, Estrov Z, Kornblau SM and Andreeff M:
Apoptosis. Molecules and mechanisms. Adv Exp Med Biol. 457:217–236.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li S, Yan T, Deng R, Jiang X, Xiong H,
Wang Y, Yu Q, Wang X, Chen C and Zhu Y: Low dose of kaempferol
suppresses the migration and invasion of triple-negative breast
cancer cells by downregulating the activities of RhoA and Rac1.
Onco Targets Ther. 10:4809–4819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yi X, Zuo J, Tan C, Xian S, Luo C, Chen S,
Yu L and Luo Y: Kaempferol, a flavonoid compound from induced
apoptosis and growth inhibition in MCF-7 breast cancer cell. Afr J
Tradit Complement Altern Med. 13:210–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu L and Xue L: Kaempferol suppresses
proliferation and induces cell cycle arrest, apoptosis, and DNA
damage in breast cancer cells. Oncol Res. 27:629–634. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kashafi E, Moradzadeh M, Mohamadkhani A
and Erfanian S: Kaempferol increases apoptosis in human cervical
cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed
Pharmacother. 89:573–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Guo H, Ren F, Zhang L, Zhang X, Yang R,
Xie B, Li Z, Hu Z, Duan Z and Zhang J: Kaempferol induces apoptosis
in HepG2 cells via activation of the endoplasmic reticulum stress
pathway. Mol Med Rep. 13:2791–2800. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Calvisi DF, Ladu S, Gorden A, Farina M,
Conner EA, Lee JS, Factor VM and Thorgeirsson SS: Ubiquitous
activation of Ras and Jak/Stat pathways in human HCC.
Gastroenterology. 130:1117–1128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Bruix J and Llovet JM: Major achievements
in hepatocellular carcinoma. Lancet. 373:614–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mylonis I, Lakka A, Tsakalof A and Simos
G: The dietary flavonoid kaempferol effectively inhibits HIF-1
activity and hepatoma cancer cell viability under hypoxic
conditions. Biochem Biophys Res Commun. 398:74–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Seydi E, Salimi A, Rasekh HR, Mohsenifar Z
and Pourahmad J: Selective cytotoxicity of luteolin and kaempferol
on cancerous hepatocytes obtained from rat model of hepatocellular
carcinoma: Involvement of ROS-mediated mitochondrial targeting.
Nutr Cancer. 70:594–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Luo H, Rankin GO, Li Z, Depriest L and
Chen YC: Kaempferol induces apoptosis in ovarian cancer cells
through activating p53 in the intrinsic pathway. Food Chem.
128:513–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mellier G, Huang S, Shenoy K and Pervaiz
S: TRAILing death in cancer. Mol Aspects Med. 31:93–112. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Inoue T, Shiraki K, Fuke H, Yamanaka Y,
Miyashita K, Yamaguchi Y, Yamamoto N, Ito K, Sugimoto K and Nakano
T: Proteasome inhibition sensitizes hepatocellular carcinoma cells
to TRAIL by suppressing caspase inhibitors and AKT pathway.
Anticancer Drugs. 17:261–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhao Y, Tian B, Wang Y and Ding H:
Kaempferol sensitizes human ovarian cancer cells-OVCAR-3 and SKOV-3
to tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL)-induced apoptosis via JNK/ERK-CHOP pathway and
Up-regulation of death receptors 4 and 5. Med Sci Monit.
23:5096–5105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bartek J and Lukas J: Chk1 and Chk2
kinases in checkpoint control and cancer. Cancer Cell. 3:421–429.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gao Y, Yin J, Rankin GO and Chen YC:
Kaempferol induces G2/M cell cycle arrest via checkpoint Kinase 2
and promotes apoptosis via death receptors in human ovarian
carcinoma A2780/CP70 cells. Molecules. 23(pii): E10952018.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hundahl SA, Menck HR, Mansour EG and
Winchester DP: The national cancer data base report on gastric
carcinoma. Cancer. 80:2333–2341. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Alberts SR, Cervantes A and van de Velde
CJ: Gastric cancer: Epidemiology, pathology and treatment. Ann
Oncol. 14 (Suppl 2):ii31–ii36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ajani JA: Evolving chemotherapy for
advanced gastric cancer. Oncologist. 10 (Suppl 3):S49–S58. 2005.
View Article : Google Scholar
|
|
75
|
Roberts AB and Wakefield LM: The two faces
of transforming growth factor beta in carcinogenesis. Proc Natl
Acad Sci USA. 100:8621–8623. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
DiMagno EP, Reber HA and Tempero MA: AGA
technical review on the epidemiology, diagnosis, and treatment of
pancreatic ductal adenocarcinoma. American Gastroenterological
Association. Gastroenterology. 117:1464–1484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jo E, Park SJ, Choi YS, Jeon WK and Kim
BC: Kaempferol suppresses transforming growth factor-β1-induced
Epithelial-to-Mesenchymal transition and migration of A549 lung
cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3
at threonine-179. Neoplasia. 17:525–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
McCoyd M, Gruener G and Foy P: Neurologic
aspects of lymphoma and leukemias. Handb Clin Neurol.
120:1027–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu LY, Lu HF, Chou YC, Shih YL, Bau DT,
Chen JC, Hsu SC and Chung JG: Kaempferol induces DNA damage and
inhibits DNA repair associated protein expressions in human
promyelocytic leukemia HL-60 cells. Am J Chin Med. 43:365–382.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Adams J and Nassiri M: Acute Promyelocytic
leukemia: A review and discussion of variant translocations. Arch
Pathol Lab Med. 139:1308–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Moradzadeh M, Tabarraei A, Sadeghnia HR,
Ghorbani A, Mohamadkhani A, Erfanian S and Sahebkar A: Kaempferol
increases apoptosis in human acute promyelocytic leukemia cells and
inhibits multidrug resistance genes. J Cell Biochem. 119:2288–2297.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Song W, Dang Q, Xu D, Chen Y, Zhu G, Wu K,
Zeng J, Long Q, Wang X, He D and Li L: Kaempferol induces cell
cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38
signaling. Oncol Rep. 31:1350–1356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Qin Y, Cui W, Yang X and Tong B:
Kaempferol inhibits the growth and metastasis of cholangiocarcinoma
in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai).
48:238–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Neoptolemos JP, Dunn JA, Stocken DD,
Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P,
Dervenis C, et al: Adjuvant chemoradiotherapy and chemotherapy in
resectable pancreatic cancer: A randomised controlled trial.
Lancet. 358:1576–1585. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Brandes F, Schmidt K, Wagner C, Redekopf
J, Schlitt HJ, Geissler EK and Lang SA: Targeting cMET with INC280
impairs tumour growth and improves efficacy of gemcitabine in a
pancreatic cancer model. BMC Cancer. 15:712015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Di Renzo MF, Poulsom R, Olivero M,
Comoglio PM and Lemoine NR: Expression of the Met/hepatocyte growth
factor receptor in human pancreatic cancer. Cancer Res.
55:1129–1138. 1995.PubMed/NCBI
|
|
87
|
Lee J and Kim JH: Kaempferol inhibits
pancreatic cancer cell growth and migration through the blockade of
EGFR-related pathway in vitro. PLoS One. 11:e01552642016.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Liang J, Wu L, Xiao H, Li N, Wang H, Cheng
C, Bai R, Zhao Y and Zheng H: Use of myocardin in the
classification of mesenchymal tumors of the uterus. Int J Gynecol
Pathol. 29:55–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li Y, Ding Z and Wu C: Mechanistic study
of the inhibitory effect of kaempferol on uterine fibroids in
vitro. Med Sci Monit. 22:4803–4808. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Qiu W, Lin J, Zhu Y, Zhang J, Zeng L, Su M
and Tian Y: Kaempferol modulates DNA methylation and downregulates
DNMT3B in bladder cancer. Cell Physiol Biochem. 41:1325–1335. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Azad N, Zahnow CA, Rudin CM and Baylin SB:
The future of epigenetic therapy in solid tumours-lessons from the
past. Nat Rev Clin Oncol. 10:256–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Maio M, Covre A, Fratta E, Di Giacomo AM,
Taverna P, Natali PG, Coral S and Sigalotti L: Molecular pathways:
At the crossroads of cancer epigenetics and immunotherapy. Clin
Cancer Res. 21:4040–4047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lee SJ, Park SS, Kim WJ and Moon SK:
Gleditsia sinensis thorn extract inhibits proliferation and
TNF-α-induced MMP-9 expression in vascular smooth muscle cells. Am
J Chin Med. 40:373–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen HJ, Lin CM, Lee CY, Shih NC, Peng SF,
Tsuzuki M, Amagaya S, Huang WW and Yang JS: Kaempferol suppresses
cell metastasis via inhibition of the ERK-p38-JNK and AP-1
signaling pathways in U-2 OS human osteosarcoma cells. Oncol Rep.
30:925–932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Alter MJ: Epidemiology of viral hepatitis
and HIV co-infection. J Hepatol. 44 (1 Suppl):S6–S9. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lee SS, Buters JT, Pineau T,
Fernandez-Salguero P and Gonzalez FJ: Role of CYP2E1 in the
hepatotoxicity of acetaminophen. J Biol Chem. 271:12063–12067.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Nicod L, Viollon C, Regnier A, Jacqueson A
and Richert L: Rifampicin and isoniazid increase acetaminophen and
isoniazid cytotoxicity in human HepG2 hepatoma cells. Hum Exp
Toxicol. 16:28–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Shih TY, Young TH, Lee HS, Hsieh CB and Hu
OY: Protective effects of kaempferol on isoniazid- and
rifampicin-induced hepatotoxicity. AAPS J. 15:753–762. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Rudnicki M, Silveira MM, Pereira TV,
Oliveira MR, Reginatto FH, Dal-Pizzol F and Moreira JC: Protective
effects of Passiflora alata extract pretreatment on carbon
tetrachloride induced oxidative damage in rats. Food Chem Toxicol.
45:656–661. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sun F, Hamagawa E, Tsutsui C, Ono Y, Ogiri
Y and Kojo S: Evaluation of oxidative stress during apoptosis and
necrosis caused by carbon tetrachloride in rat liver. Biochim
Biophys Acta. 1535:186–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Szymonik-Lesiuk S, Czechowska G,
Stryjecka-Zimmer M, Słomka M, Madro A, Celiński K and Wielosz M:
Catalase, superoxide dismutase, and glutathione peroxidase
activities in various rat tissues after carbon tetrachloride
intoxication. J Hepatobiliary Pancreat Surg. 10:309–315. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang Y, Tang C and Zhang H:
Hepatoprotective effects of kaempferol 3-O-rutinoside and
kaempferol 3-O-glucoside from Carthamus tinctorius L. on
CCl4-induced oxidative liver injury in mice. J Food Drug
Anal. 23:310–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
O'Shea RS, Dasarathy S and McCullough AJ;
Practice Guideline Committee of the American Association for the
Study of Liver Diseases; Practice Parameters Committee of the
American College of Gastroenterology, : Alcoholic liver disease.
Hepatology. 51:307–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang M, Sun J, Jiang Z, Xie W and Zhang X:
Hepatoprotective effect of kaempferol against alcoholic liver
injury in mice. Am J Chin Med. 43:241–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
French SW: The importance of CYP2E1 in the
pathogenesis of alcoholic liver disease and drug toxicity and the
role of the proteasome. Subcell Biochem. 67:145–164. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhou B, Jiang Z, Li X and Zhang X:
Kaempferol's protective effect on ethanol-induced mouse primary
hepatocytes injury involved in the synchronous inhibition of SP1,
Hsp70 and CYP2E1. Am J Chin Med. 46:1093–1110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
McCarthy MI: Genomics, type 2 diabetes,
and obesity. N Engl J Med. 363:2339–2350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tilg H and Moschen AR: Adipocytokines:
Mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–873. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lee B, Kwon M, Choi JS, Jeong HO, Chung HY
and Kim HR: Kaempferol isolated from nelumbo nucifera inhibits
lipid accumulation and increases fatty acid oxidation signaling in
adipocytes. J Med Food. 18:1363–1370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chang CJ, Tzeng TF, Liou SS, Chang YS and
Liu IM: Kaempferol regulates the lipid-profile in high-fat diet-fed
rats through an increase in hepatic PPARα levels. Planta Med.
77:1876–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Luo C, Yang H, Tang C, Yao G, Kong L, He H
and Zhou Y: Kaempferol alleviates insulin resistance via hepatic
IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol.
28:744–750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen X, Qian J, Wang L, Li J, Zhao Y, Han
J, Khan Z, Chen X, Wang J and Liang G: Kaempferol attenuates
hyperglycemia-induced cardiac injuries by inhibiting inflammatory
responses and oxidative stress. Endocrine. 60:83–94. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhang Y and Liu D: Flavonol kaempferol
improves chronic hyperglycemia-impaired pancreatic beta-cell
viability and insulin secretory function. Eur J Pharmacol.
670:325–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kass DA: Getting better without AGE: New
insights into the diabetic heart. Circ Res. 92:704–706. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Suchal K, Malik S, Khan SI, Malhotra RK,
Goyal SN, Bhatia J, Ojha S and Arya DS: Molecular pathways involved
in the amelioration of myocardial injury in diabetic rats by
kaempferol. Int J Mol Sci. 18(pii): E10012017. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Alkhalidy H, Moore W, Wang A, Luo J,
McMillan RP, Wang Y, Zhen W, Hulver MW and Liu D: Kaempferol
ameliorates hyperglycemia through suppressing hepatic
gluconeogenesis and enhancing hepatic insulin sensitivity in
diet-induced obese mice. J Nutr Biochem. 58:90–101. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Kishore L, Kaur N and Singh R: Effect of
Kaempferol isolated from seeds of Eruca sativa on changes of pain
sensitivity in Streptozotocin-induced diabetic neuropathy.
Inflammopharmacology. 26:993–1003. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Chawla A, Repa JJ, Evans RM and
Mangelsdorf DJ: Nuclear receptors and lipid physiology: Opening the
X-files. Science. 294:1866–1870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hoang MH, Jia Y, Mok B, Jun HJ, Hwang KY
and Lee SJ: Kaempferol ameliorates symptoms of metabolic syndrome
by regulating activities of liver X receptor-β. J Nutr Biochem.
26:868–875. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Fisher M: Injuries to the vascular
endothelium: Vascular wall and endothelial dysfunction. Rev Neurol
Dis. 5 (Suppl 1):S4–S11. 2008.PubMed/NCBI
|
|
124
|
Kim TH, Ku SK and Bae JS: Inhibitory
effects of kaempferol-3-O-sophoroside on HMGB1-mediated
proinflammatory responses. Food Chem Toxicol. 50:1118–1123. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
ten Dijke P and Arthur HM: Extracellular
control of TGFbeta signalling in vascular development and disease.
Nat Rev Mol Cell Biol. 8:857–869. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Chan MC, Hilyard AC, Wu C, Davis BN, Hill
NS, Lal A, Lieberman J, Lagna G and Hata A: Molecular basis for
antagonism between PDGF and the TGFbeta family of signalling
pathways by control of miR-24 expression. EMBO J. 29:559–573. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Kim K, Kim S, Moh SH and Kang H:
Kaempferol inhibits vascular smooth muscle cell migration by
modulating BMP-mediated miR-21 expression. Mol Cell Biochem.
407:143–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ochiai A, Miyata S, Iwase M, Shimizu M,
Inoue J and Sato R: Kaempferol stimulates gene expression of
low-density lipoprotein receptor through activation of Sp1 in
cultured hepatocytes. Sci Rep. 6:249402016. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Tabas I, García-Cardeña G and Owens GK:
Recent insights into the cellular biology of atherosclerosis. J
Cell Biol. 209:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Singh M, Ananthula S, Milhorn DM,
Krishnaswamy G and Singh K: Osteopontin: A novel inflammatory
mediator of cardiovascular disease. Front Biosci. 12:214–221. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Xiao HB, Lu XY, Sun ZL and Zhang HB:
Kaempferol regulates OPN-CD44 pathway to inhibit the atherogenesis
of apolipoprotein E deficient mice. Toxicol Appl Pharmacol.
257:405–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Keys A, Taylor HL, Blackburn H, Brozek J,
Anderson JT and Simonson E: Mortality and coronary heart disease
among men studied for 23 years. Arch Intern Med. 128:201–214. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Che J, Liang B, Zhang Y, Wang Y, Tang J
and Shi G: Kaempferol alleviates ox-LDL-induced apoptosis by
up-regulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in
human endothelial cells. Cardiovasc Pathol. 31:57–62. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Weisel JW, Stauffacher CV, Bullitt E and
Cohen C: A model for fibrinogen: Domains and sequence. Science.
230:1388–1391. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Choi JH, Park SE, Kim SJ and Kim S:
Kaempferol inhibits thrombosis and platelet activation. Biochimie.
115:177–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Mackman N: Triggers, targets and
treatments for thrombosis. Nature. 451:914–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Begonja AJ, Gambaryan S, Geiger J, Aktas
B, Pozgajova M, Nieswandt B and Walter U: Platelet
NAD(P)H-oxidase-generated ROS production regulates
alphaIIbbeta3-integrin activation independent of the NO/cGMP
pathway. Blood. 106:2757–2760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wang SB, Jang JY, Chae YH, Min JH, Baek
JY, Kim M, Park Y, Hwang GS, Ryu JS and Chang TS: Kaempferol
suppresses collagen-induced platelet activation by inhibiting NADPH
oxidase and protecting SHP-2 from oxidative inactivation. Free
Radic Biol Med. 83:41–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hampton BM, Schwartz SG, Brantley MA Jr
and Flynn HW Jr: Update on genetics and diabetic retinopathy. Clin
Ophthalmol. 9:2175–2193. 2015.PubMed/NCBI
|
|
140
|
Behl T and Kotwani A: Exploring the
various aspects of the pathological role of vascular endothelial
growth factor (VEGF) in diabetic retinopathy. Pharmacol Res.
99:137–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Jenkins AJ, Joglekar MV, Hardikar AA,
Keech AC, O'Neal DN and Januszewski AS: Biomarkers in diabetic
retinopathy. Rev Diabet Stud. 12:159–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li JK, Wei F, Jin XH, Dai YM, Cui HS and
Li YM: Changes in vitreous VEGF, bFGF and fibrosis in proliferative
diabetic retinopathy after intravitreal bevacizumab. Int J
Ophthalmol. 8:1202–1206. 2015.PubMed/NCBI
|
|
143
|
Sorenson CM, Wang S, Gendron R, Paradis H
and Sheibani N: Thrombospondin-1 deficiency exacerbates the
pathogenesis of diabetic retinopathy. J Diabetes Metab. (Suppl
12):2013.doi: 10.4172/2155-6156.S12-005. PubMed/NCBI
|
|
144
|
Wu Y, Zhang Q and Zhang R: Kaempferol
targets estrogen-related receptor a and suppresses the angiogenesis
of human retinal endothelial cells under high glucose conditions.
Exp Ther Med. 14:5576–5582. 2017.PubMed/NCBI
|
|
145
|
Xu XH, Zhao C, Peng Q, Xie P and Liu QH:
Kaempferol inhibited VEGF and PGF expression and in vitro
angiogenesis of HRECs under diabetic-like environment. Braz J Med
Biol Res. 50:e53962017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chin HK, Horng CT, Liu YS, Lu CC, Su CY,
Chen PS, Chiu HY, Tsai FJ, Shieh PC and Yang JS: Kaempferol
inhibits angiogenic ability by targeting VEGF receptor-2 and
downregulating the PI3K/AKT, MEK and ERK pathways in
VEGF-stimulated human umbilical vein endothelial cells. Oncol Rep.
39:2351–2357. 2018.PubMed/NCBI
|
|
147
|
Zhang YW, Shi J, Li YJ and Wei L:
Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch
Immunol Ther Exp (Warsz). 57:435–445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Kawamura T, Hasegawa K, Morimoto T,
Iwai-Kanai E, Miyamoto S, Kawase Y, Ono K, Wada H, Akao M and Kita
T: Expression of p300 protects cardiac myocytes from apoptosis in
vivo. Biochem Biophys Res Commun. 315:733–738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Xiao J, Sun GB, Sun B, Wu Y, He L, Wang X,
Chen RC, Cao L, Ren XY and Sun XB: Kaempferol protects against
doxorubicin-induced cardiotoxicity in vivo and in vitro.
Toxicology. 292:53–62. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Liu J, Xin L, Benson VL, Allen DG and Ju
YK: Store-operated calcium entry and the localization of STIM1 and
Orai1 proteins in isolated mouse sinoatrial node cells. Front
Physiol. 6:692015. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Swulius MT and Waxham MN:
Ca(2+)/calmodulin-dependent protein kinases. Cell Mol Life Sci.
65:2637–2657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Wang Y, Cui H, Wang W, Zhao B and Lai J:
The region-specific activation of Ca2+/calmodulin dependent protein
kinase II and extracellular signal-regulated kinases in hippocampus
following chronic alcohol exposure. Brain Res Bull. 89:191–196.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Swaminathan PD, Purohit A, Soni S, Voigt
N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, et al:
Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J
Clin Invest. 121:3277–3288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Schiattarella GG and Hill JA: Inhibition
of hypertrophy is a good therapeutic strategy in ventricular
pressure overload. Circulation. 131:1435–1447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Rose BA, Force T and Wang Y:
Mitogen-activated protein kinase signaling in the heart: Angels
versus demons in a heart-breaking tale. Physiol Rev. 90:1507–1546.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Feng H, Cao J, Zhang G and Wang Y:
Kaempferol attenuates cardiac hypertrophy via regulation of
ASK1/MAPK signaling pathway and oxidative stress. Planta Med.
83:837–845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Weng X, Yu L, Liang P, Chen D, Cheng X,
Yang Y, Li L, Zhang T, Zhou B, Wu X, et al: Endothelial MRTF-A
mediates angiotensin II induced cardiac hypertrophy. J Mol Cell
Cardiol. 80:23–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zeisberg EM, Tarnavski O, Zeisberg M,
Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT,
Roberts AB, et al: Endothelial-to-mesenchymal transition
contributes to cardiac fibrosis. Nat Med. 13:952–961. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Liu Y, Gao L, Guo S, Liu Y, Zhao X, Li R,
Yan X, Li Y, Wang S, Niu X, et al: Kaempferol alleviates
angiotensin II-induced cardiac dysfunction and interstitial
fibrosis in mice. Cell Physiol Biochem. 43:2253–2263. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Fan Q, Chen L, Cheng S, Li F, Lau WB, Wang
le F and Liu JH: Aging aggravates nitrate-mediated ROS/RNS changes.
Oxid Med Cell Longev. 2014:3765152014. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Juhaszova M, Zorov DB, Yaniv Y, Nuss HB,
Wang S and Sollott SJ: Role of glycogen synthase kinase-3beta in
cardioprotection. Circ. Res. 104:1240–1252. 2009.
|
|
162
|
Suchal K, Malik S, Gamad N, Malhotra RK,
Goyal SN, Chaudhary U, Bhatia J, Ojha S and Arya DS: Kaempferol
attenuates myocardial ischemic injury via inhibition of MAPK
signaling pathway in experimental model of myocardial
ischemia-reperfusion injury. Oxid Med Cell Longev.
2016:75807312016. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Shen AC and Jennings RB: Kinetics of
calcium accumulation in acute myocardial ischemic injury. Am J
Pathol. 67:441–452. 1972.PubMed/NCBI
|
|
164
|
Guo Z, Liao Z, Huang L, Liu D, Yin D and
He M: Kaempferol protects cardiomyocytes against
anoxia/reoxygenation injury via mitochondrial pathway mediated by
SIRT1. Eur J Pharmacol. 761:245–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Khader A, Yang WL, Kuncewitch M, Jacob A,
Prince JM, Asirvatham JR, Nicastro J, Coppa GF and Wang P: Sirtuin
1 activation stimulates mitochondrial biogenesis and attenuates
renal injury after ischemia-reperfusion. Transplantation.
98:148–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhou M, Ren H, Han J, Wang W, Zheng Q and
Wang D: Protective effects of kaempferol against myocardial
Ischemia/Reperfusion injury in isolated rat heart via antioxidant
activity and inhibition of glycogen synthase kinase-3β. Oxid Med
Cell Longev. 2015:4814052015. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Lei Y, Chen J, Zhang W, Fu W, Wu G, Wei H,
Wang Q and Ruan J: In vivo investigation on the potential of
galangin, kaempferol and myricetin for protection of
D-galactose-induced cognitive impairment. Food Chem. 135:2702–2707.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Zhong SZ, Ge QH, Qu R, Li Q and Ma SP:
Paeonol attenuates neurotoxicity and ameliorates cognitive
impairment induced by d-galactose in ICR mice. J Neurol Sci.
277:58–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Palsamy P, Sivakumar S and Subramanian S:
Resveratrol attenuates hyperglycemia-mediated oxidative stress,
proinflammatory cytokines and protects hepatocytes ultrastructure
in streptozotocin-nicotinamide-induced experimental diabetic rats.
Chem Biol Interact. 186:200–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
McEwen BS and Stellar E: Stress and the
individual. Mechanisms leading to disease. Arch Intern Med.
153:2093–2101. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Whooley MA and Simon GE: Managing
depression in medical outpatients. N Engl J Med. 343:1942–1950.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Park SH, Sim YB, Han PL, Lee JK and Suh
HW: Antidepressant-like effect of kaempferol and quercitirin,
isolated from opuntia ficus-indica var. saboten. Exp Neurobiol.
19:30–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Reeve A, Simcox E and Turnbull D: Ageing
and Parkinson's disease: Why is advancing age the biggest risk
factor? Ageing Res Rev. 14:19–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Tian L, Karimi M, Loftin SK, Brown CA, Xia
H, Xu J, Mach RH and Perlmutter JS: No differential regulation of
dopamine transporter (DAT) and vesicular monoamine transporter 2
(VMAT2) binding in a primate model of Parkinson disease. PLoS One.
7:e314392012. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Han X, Sun S, Sun Y, Song Q, Zhu J, Song
N, Chen M, Sun T, Xia M, Ding J, et al: Small molecule-driven NLRP3
inflammation inhibition via interplay between ubiquitination and
autophagy: Implications for Parkinson disease. Autophagy. 1–22.
2019.10.1080/15548627.2019.1596481 (Epub ahead of print).
View Article : Google Scholar
|
|
176
|
Li S and Pu XP: Neuroprotective effect of
kaempferol against a
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of
Parkinson's disease. Biol Pharm Bull. 34:1291–1296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ma Z, Li C, Qiao Y, Lu C, Li J, Song W,
Sun J, Zhai X, Niu J, Ren Q and Wen A: Safflower yellow B
suppresses HepG2 cell injury induced by oxidative stress through
the AKT/Nrf2 pathway. Int J Mol Med. 37:603–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Ren R, Shi C, Cao J, Sun Y, Zhao X, Guo Y,
Wang C, Lei H, Jiang H, Ablat N, et al: Neuroprotective effects of
A standardized flavonoid extract of safflower against
neurotoxin-induced cellular and animal models of Parkinson's
disease. Sci Rep. 6:221352016. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Jang JY, Kim HN, Kim YR, Choi YW, Choi YH,
Lee JH, Shin HK and Choi BT: Hexane extract from Polygonum
multiflorum attenuates glutamate-induced apoptosis in primary
cultured cortical neurons. J Ethnopharmacol. 145:261–268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Yang EJ, Kim GS, Jun M and Song KS:
Kaempferol attenuates the glutamate-induced oxidative stress in
mouse-derived hippocampal neuronal HT22 cells. Food Funct.
5:1395–1402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Lai TW, Zhang S and Wang YT:
Excitotoxicity and stroke: Identifying novel targets for
neuroprotection. Prog Neurobiol. 115:157–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Zhou X, Sun X, Gong X, Yang Y, Chen C,
Shan G and Yao Q: Astragaloside IV from Astragalus membranaceus
ameliorates renal interstitial fibrosis by inhibiting inflammation
via TLR4/NF-кB in vivo and in vitro. Int Immunopharmacol. 42:18–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Zhang H, Park JH, Maharjan S, Park JA,
Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, et al: Sac-1004,
a vascular leakage blocker, reduces cerebral ischemia-reperfusion
injury by suppressing blood-brain barrier disruption and
inflammation. J Neuroinflammation. 14:1222017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Cheng X, Yang YL, Yang H, Wang YH and Du
GH: Kaempferol alleviates LPS-induced neuroinflammation and BBB
dysfunction in mice via inhibiting HMGB1 release and
down-regulating TLR4/MyD88 pathway. Int Immunopharmacol. 56:29–35.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Wu B, Luo H, Zhou X, Cheng CY, Lin L, Liu
BL, Liu K, Li P and Yang H: Succinate-induced neuronal
mitochondrial fission and hexokinase II malfunction in ischemic
stroke: Therapeutical effects of kaempferol. Biochim Biophys Acta.
1863:2307–2318. 2017. View Article : Google Scholar
|
|
186
|
Zhao J, Shu B, Chen L, Tang J, Zhang L,
Xie J, Liu X, Xu Y and Qi S: Prostaglandin E2 inhibits collagen
synthesis in dermal fibroblasts and prevents hypertrophic scar
formation in vivo. Exp Dermatol. 25:604–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Ledon JA, Savas J, Franca K, Chacon A and
Nouri K: Intralesional treatment for keloids and hypertrophic
scars: A review. Dermatol Surg. 39:1745–1757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Sarrazy V, Billet F, Micallef L, Coulomb B
and Desmoulière A: Mechanisms of pathological scarring: Role of
myofibroblasts and current developments. Wound Repair Regen. 19
(Suppl 1):S10–S15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Li H, Yang L, Zhang Y and Gao Z:
Kaempferol inhibits fibroblast collagen synthesis, proliferation
and activation in hypertrophic scar via targeting TGF-β receptor
type I. Biomed Pharmacother. 83:967–974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Rietjens IM, Boersma MG, van der Woude H,
Jeurissen SM, Schutte ME and Alink GM: Flavonoids and
alkenylbenzenes: Mechanisms of mutagenic action and carcinogenic
risk. Mutat Res. 574:124–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Sheng WY, Chen YR and Wang TC: A major
role of PKC theta and NFkappaB in the regulation of hTERT in human
T lymphocytes. FEBS Lett. 580:6819–6824. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Park SE, Sapkota K, Kim S, Kim H and Kim
SJ: Kaempferol acts through mitogen-activated protein kinases and
protein kinase B/AKT to elicit protection in a model of
neuroinflammation in BV2 microglial cells. Br J Pharmacol.
164:1008–1025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wang J, Fang X, Ge L, Cao F, Zhao L, Wang
Z and Xiao W: Antitumor, antioxidant and anti-inflammatory
activities of kaempferol and its corresponding glycosides and the
enzymatic preparation of kaempferol. PLoS One. 13:e01975632018.
View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Noroozi M, Angerson WJ and Lean ME:
Effects of flavonoids and vitamin C on oxidative DNA damage to
human lymphocytes. Am J Clin Nutr. 67:1210–1218. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Bestwick CS, Milne L, Pirie L and Duthie
SJ: The effect of short-term kaempferol exposure on reactive oxygen
levels and integrity of human (HL-60) leukaemic cells. Biochim
Biophys Acta. 1740:340–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Sahu SC and Gray GC: Kaempferol-induced
nuclear DNA damage and lipid peroxidation. Cancer Lett. 85:159–164.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Liesveld JL, Abboud CN, Lu C, McNair C,
Menon A, Smith A, Rosell K and Rapoport AP: Flavonoid effects on
normal and leukemic cells. Leuk Res. 27:517–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Das A, Majumder D and Saha C: Correlation
of binding efficacies of DNA to flavonoids and their induced
cellular damage. J Photochem Photobiol B. 170:256–262. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Cos P, Calomme M, Sindambiwe JB, De Bruyne
T, Cimanga K, Pieters L, Vlietinck AJ and Vanden Berghe D:
Cytotoxicity and lipid peroxidation-inhibiting activity of
flavonoids. Planta Med. 67:515–519. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Gupta A, Kaur CD and Saraf S and Saraf S:
Formulation, characterization, and evaluation of ligand-conjugated
biodegradable quercetin nanoparticles for active targeting. Artif
Cells Nanomed Biotechnol. 44:960–970. 2016.PubMed/NCBI
|
|
201
|
Qian YS, Ramamurthy S, Candasamy M, Shadab
M, Kumar RH and Meka VS: Production, characterization and
evaluation of kaempferol nanosuspension for improving oral
bioavailability. Curr Pharm Biotechnol. 17:549–555. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Resende FA, Vilegas W, Dos Santos LC and
Varanda EA: Mutagenicity of flavonoids assayed by bacterial reverse
mutation (Ames) test. Molecules. 17:5255–5268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Polyakov NE and Kispert LD: Water soluble
biocompatible vesicles based on polysaccharides and
oligosaccharides inclusion complexes for carotenoid delivery.
Carbohydr Polym. 128:207–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Xu W, Wen M, Yu J, Zhang Q, Polyakov NE,
Dushkin AV and Su W: Mechanochemical preparation of kaempferol
intermolecular complexes for enhancing the solubility and
bioavailability. Drug Dev Ind Pharm. 44:1924–1932. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Lemos C, Peters GJ, Jansen G, Martel F and
Calhau C: Modulation of folate uptake in cultured human colon
adenocarcinoma Caco-2 cells by dietary compounds. Eur J Nutr.
46:329–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Sunoqrot S, Bae JW, Pearson RM, Shyu K,
Liu Y, Kim DH and Hong S: Temporal control over cellular targeting
through hybridization of folate-targeted dendrimers and PEG-PLA
nanoparticles. Biomacromolecules. 13:1223–1230. 2012. View Article : Google Scholar : PubMed/NCBI
|