Introduction
Pancreatic cancer (PaC), known as the king of
carcinoma, is a type of highly lethal malignant tumor with high
rate of incidence around the world (1). The 5-year relative survival rate of PaC
is <8%, due to its rapid progression, asymptomatic development,
poor prognosis and the relative lack of effects of treatment
(2). The etiology and pathogenesis
of PaC still remains unclear. However, regenerating gene protein
(Reg) 3A has been generally accepted to serve an important role
during pancreatic carcinogenesis and PaC progression, based on its
high expression in PaC and tumor-promoting activity (3–6). Reg
proteins are members of the calcium-dependent C-type lectin family,
which have been characterized as promoters of proliferation and
differentiation in a range of cell types (7). In humans, 5 Reg family proteins
including Reg1A, Reg1B, Reg3A, Reg3G and Reg4 have been identified
(4). Among them, Reg3A is
selectively expressed in the pancreas and small intestine (8). During pancreatic inflammation, Reg3A
expression is significantly increased and has thus also been named
pancreatitis-associated protein (5,6).
Elevated concentrations or expression of Reg3A in pancreatic
juice/serum (9,10) or tumor tissues (5,6) have
been reported in patients with PaC. Reg3A may promote the survival,
proliferation, growth of PaC cells and acinar-to-ductal metaplasia
formation, and overexpression of Reg3A in PaC is significantly
associated with the aggressiveness of the tumor (5,6).
Therefore, during pancreatic inflammation-mediated upregulation of
Reg3A, Reg3A may function as an oncogene to promote the development
and progression of PaC.
Although PaC therapies, including surgical
resection, chemotherapy and radiotherapy have improved over a
number of decades, the outcomes of patients with PaC have not
improved significantly (11).
Therefore, there is an need urgent to develop alternative
therapeutic approaches which may improve control of the
aggressiveness of PaC. Novel therapies based on natural products
with minimal toxicity have been demonstrated to benefit patients
with PaC (11,12).
Eckol, a phlorotannin component isolated from brown
algae, is a marine natural product with a variety of potent
biological activities (13). For
example, eckol has been demonstrated to exhibit antioxidant and
cytoprotective properties (14–17),
neuroprotective effect (18–20) and anti-adipogenic activities
(21). Additionally, eckol is also a
well-known anti-inflammatory agent. Jung et al (22) demonstrated that eckol could inhibit
lipopolysaccharide (LPS)-induced nitric oxide production in
RAW264.7 macrophages in a dose-dependent manner. In HepG2 cells,
eckol significantly inhibited LPS-stimulated inflammatory responses
without any cytotoxicity (23). A
few previous studies have demonstrated the cytotoxic effects of
eckol against tumors (24,25). As the source of eckol, brown algae
has long been considered to exhibit relatively higher anti-tumor
activities compared with other algae (24). Hyun et al (25) reported that eckol treatment decreased
the tumorigenic capacity of glioma cells, and sensitized these cell
to established anticancer treatments, suggesting a novel potential
adjuvant capacity of eckol for treating patients with cancer.
However, the effect of eckol on PaC has not been investigated, to
the best of our knowledge.
Based on the role of pancreatic
inflammation-upregulated Reg3A in PaC progression, together with
the existing evidence that eckol has potential anti-inflammatory
and anti-tumor effects, eckol may inhibit Reg3A-induced
proliferation of PaC cells, and thus protect against progression of
inflammation-associated PaC. To test this hypothesis, the effect of
eckol on the proliferation of Reg3A-stimulated human SW1990 PaC
cells was determined. Human pancreatic adenocarcinoma cell line
SW-1990 is a representative and common PaC cell line with
aggressive behaviors (5,6). A previous study studied the effect of
Reg3A on the proliferation of five different PaC cell lines;
AsPC-1, Mia Paca-2, BxPC-3, SW1990, PANC-1 (6). The results showed that the
proliferation-promoting effect of Reg3A was most marked in the
SW1990 cells, that is, SW1990 was the most sensitive cell line to
exogenous Reg3A stimulation amongst the studied cell lines. The aim
of the present study was to determine whether eckol may potentially
limit the malignant development of PaC driven by pancreatic
inflammatory mediators such as Reg3A, a pancreatic
inflammation-upregulated protein with pro-growth function.
Materials and methods
Cell culture and treatment
The human PaC cell line, SW1990 was obtained from
American Type Culture Collection (American Type Culture Collection)
was cultured in 5% CO2 atmosphere at 37°C in RPMI 1640
(Hyclone; GE Healthcare Life Sciences) supplemented with 10% fetal
calf serum and 100 units/ml penicillin/streptomycin (both
Invitrogen; Thermo Fisher Scientific, Inc.). SW1990 cells were
pre-treated with eckol (Rongbao Environmental Technology Co., Ltd)
for 48 h with 5, 10 and 20 µg/ml. Subsequently, Reg3A protein (Sino
Biological Inc.) was added to the culture media at a final
concentration of 50 ng/ml in the presence or absence of eckol for
24 h. The control cells were treated with the same volume of
vehicle (DMSO).
Cytotoxicity assay
The cytotoxic effects of eckol on SW1990 cells was
determined using an MTT assay as described previously (26). Briefly, 4×103 SW1990 cells
were plated per well in a 96 well microplates and pre-incubated for
24 h. Subsequently, the cells were treated with Reg3A and/or eckol
as described above, after which 20 µl 5 mg/ml MTT (Sigma-Aldrich;
Merck KGaA) was added to each well and the plates were incubated at
37°C for a further 4 h. The survival rate of SW1990 cells was
calculated as the A570 of treated cells/A570 of control cells.
There were a total of 8 wells per condition.
Cell cycle assay
Following treatment with Reg3A and/or eckol, the
SW1990 cells were stained with propidium iodide (PI; Sigma-Aldrich;
Merck KGaA) at 4°C for 30 min, and immediately analyzed using a
Flow Cytometer and CellQuest Pro software version 5.1 (both BD
Biosciences).
Colony formation in soft agarose
Following treatment with Reg3A and/or eckol, the
SW1990 cells were seeded in 6-well plates at a density of 500 cells
per well with five replicates, and incubated for 2 weeks for the
colony formation assay in soft agarose culture as described
previously (5,6). RPMI 1640 medium (X2 concentration) was
mixed with an equal volume of 1.2% melted agarose (Invitrogen;
Thermo Fisher Scientific, Inc.) to prepare the agar. The number of
colonies formed per 100 seeded SW1990 cells was assessed by
counting under a compound light microscope at a magnification of
×100.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was isolated from the cells using
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The mRNAs were reverse-transcribed to cDNAs using a
PrimeScript® RT Master mix (Perfect Real Time; Takara
Bio, Inc.) under the reaction conditions of 37°C for 15 min and
85°C for 5 sec. RT-qPCR was performed using an Applied Biosystems
StepOnePlus™ system using a SYBR Green Premix kit (Takara Bio Inc.)
as the fluorophore. Primers were as follows: Cyclin D1 forward,
5′-TCTACACCGACAACTCCATCCG-3 and reverse,
5′-TCTGGCATTTTGGAGAGGAAGTG-3; STAT3 forward,
5′-CCCATCCAGGCTGAGTATGT-3 and reverse, 5′-GATCCAGTCCGTGGAACCAT-3;
JAK2 forward, 5′-CCTTGTACTTCACGATGTTGTC-3 and reverse,
5′-GTGGAGATGTGCCGCTATG-3; NF-κB p65 forward,
5′-CTTCAGAATGGCAGAAGATGA-3 and reverse,
5′-CACATACATAACGGAAACGAAA-3; β-actin forward,
5′-TCACCCACACTGTGCCCATCTACGA-3 and reverse,
5′-CAGCGGAACCGCTCATTGCCAATGG-3. The thermocycling conditions were:
i) 95°C For 10 min; and ii) 40 cycles of 95°C for 30 sec, 58°C for
30 sec and 72°C for 30 sec. The mRNA levels of cyclin D1, STAT3,
JAK2, and NF-κB p65 were normalized to those of β-actin. The
results were analyzed using the 2−∆∆Cq method (27).
Western blotting
The protein expression levels of Cyclin D1, STAT3,
JAK2 and NF-κB p65 were detected by western blotting. The total
protein in the SW1990 cells was extracted using RIPA lysis and
extraction buffer containing protease inhibitor cocktail (Pierce;
Thermo Fisher Scientific, Inc.). A bicinchoninic acid protein assay
kit (Pierce; Thermo Fisher Scientific, Inc.) was used to determine
the protein concentration of each sample. Equal quantities of
protein (50 µg per lane) were resolved by SDS-PAGE on a 12% gel and
transferred onto a nitrocellulose membrane by electroblotting. The
membrane was blocked overnight at 4°C with 5% skimmed milk in TBST
(20 mM Tris-HCl, 0.1% Tween-20 and 150 mM NaCl) and subsequently
incubated at 37°C for 2 h with a 1:1,000 dilution of rabbit
monoclonal anti-human phosphorylated (p)STAT3 (cat. no. 9145),
pJAK2 (cat. no. 4406), STAT3 (cat. no. 4904), JAK2 antibody (cat.
no. 3230; all form CST Biological Reagents Co., Ltd.), rabbit
monoclonal anti-human CyclinD1 antibody (cat. no. ab16663; Abcam),
rabbit monoclonal NF-κB p65 antibody (cat. no. AP3749a; Abgent,
Inc.) or mouse monoclonal anti-β-actin antibody (cat. no. sc-47778;
Santa Cruz Biotechnology Inc.). Horseradish peroxidase-conjugated
sheep anti-rabbit or anti-mouse immunoglobulin G (cat. nos. LK2001L
or LK2003L; Tianjin Sungene Biotech Co., Ltd.) was used as the
secondary antibody. After incubation with the corresponding
secondary antibody at a dilution of 1:2,000 for 2 h at room
temperature, signals were visualized using an enhanced
chemiluminescence detection kit (Advansta, Inc.) and scanned using
an EPSON Perfection V370 Photo Scanner (Seiko Epson Corporation).
Densitometric analysis of the image was performed using ImageJ
(version 1.8.0; National Institutes of Health). Protein expression
was normalized to the amount of β-actin in the same sample.
Statistical analysis
The statistical analyses were performed using SPSS
version 12.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation of at least five repeats. The
statistical significance of difference between groups was
determined using a one-way ANOVA followed by a post-hoc Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Eckol decreases Reg3A-induced SW1990
cell survival
The direct effect of eckol on the survival of human
SW1990 PaC cells was determined by treating cells with 5, 10 or 20
µg/ml eckol for 72 h. The treatment of SW1990 cells with 5–20 µg/ml
eckol did not result in a significant change in cell viability as
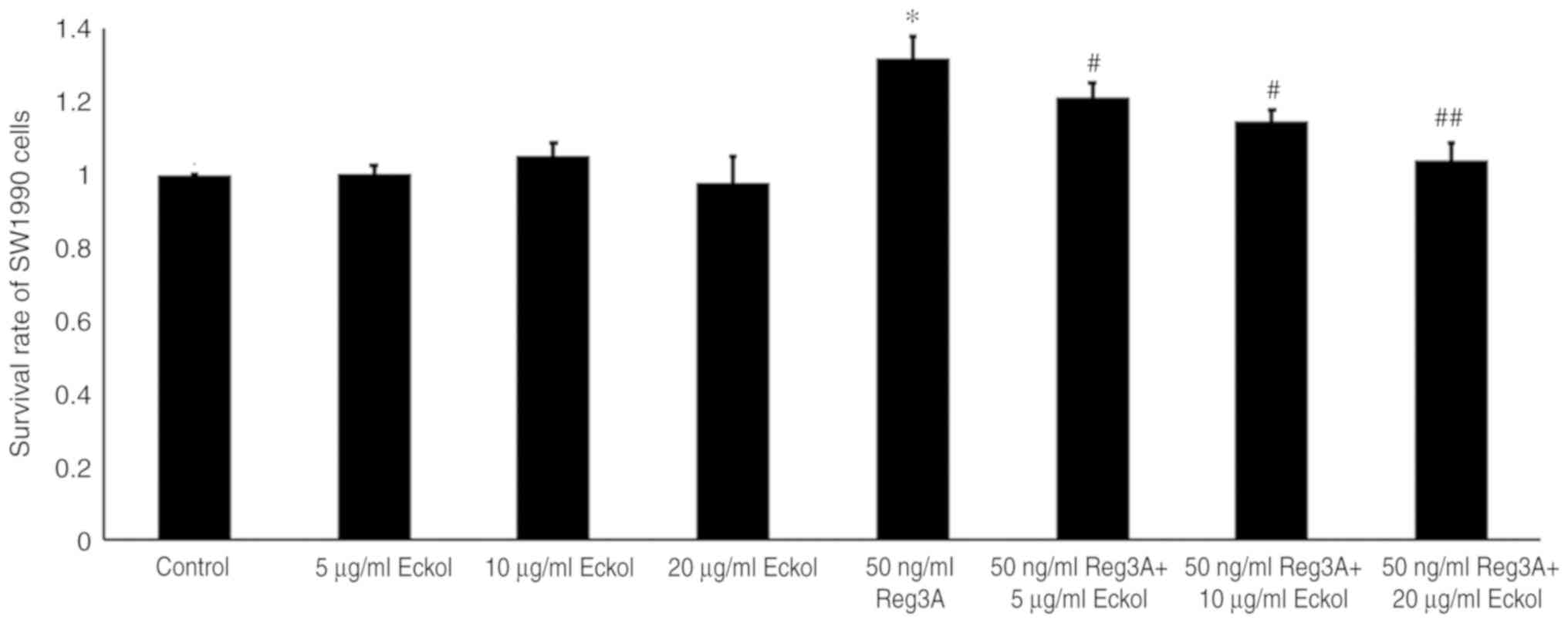
determined by an MTT assay (Fig. 1).
Cells treated with 50 ng/ml exogenous Reg3A protein demonstrated a
31.8% increase in survival compared with the control (P<0.05;
Fig. 1). Cell survival was
significantly decreased in SW1990 cells treated with 50 ng/ml Reg3A
and 5, 10 or 20 µg/ml eckol by 10.2, 16.5, and 22.9%, respectively,
when compared with cells treated with Reg3A alone (5 µg/ml,
P<0.05; 10 µg/ml, P<0.05; 20 µg/ml, P<0.01). Therefore,
these data suggest that 5–10 µg/ml eckol did not have a significant
direct cytotoxic effect on human SW1990 PaC cells, however,
attenuated the Reg3A-mediated increase in SW1990 cell survival.
Eckol attenuates Reg3A-induced cell
cycle progression in human SW1990 PaC cells
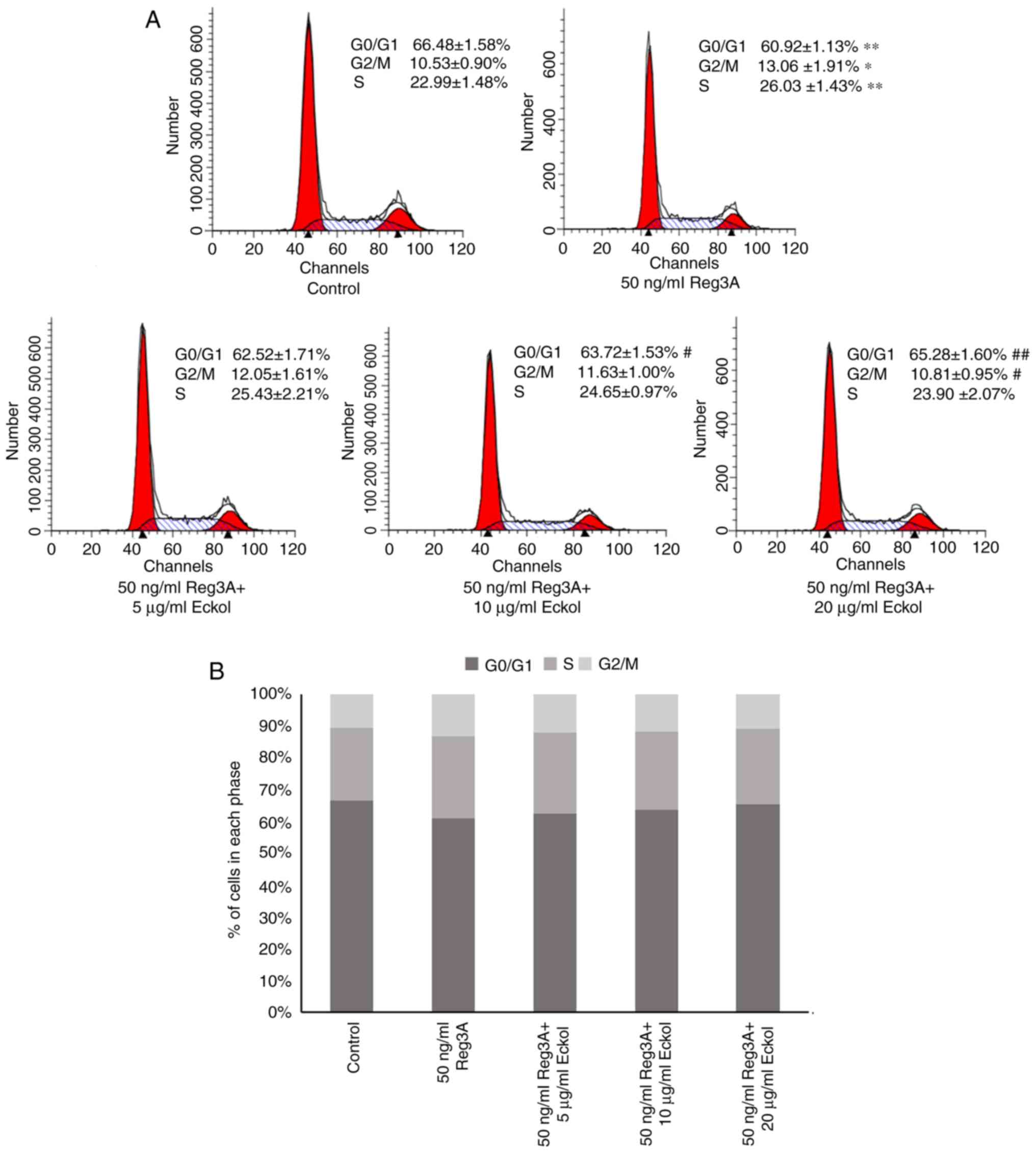
Based on the above preliminary assays, the effect of
eckol on the cell cycle progression in cells treated with and
without Reg3A was determined using flow cytometry analysis. As
shown in Fig. 2, incubation with
exogenous Reg3A for 24 h caused a significantly lower number of
cells in the G0/G1 phase and the accumulation of cells in the G2/M
and S phases, compared with the control group (P<0.01). However,
treatment of cells with Reg3A and eckol resulted in the reduction
in the number of cells in the G2/M and S phases, and the increase
in the proportion of cells in the G0/G1 phase, particularly at with
10 and 20 µg/ml (P<0.05, P<0.01, respectively) when compared
with cells treated with Reg3A only. The data in Fig. 2 suggest that eckol may attenuate the
proliferation-promoting effect of Reg3A on human SW1990 PaC
cells.
Eckol attenuates Reg3A-mediated SW1990
PaC cell colony formation in soft agar
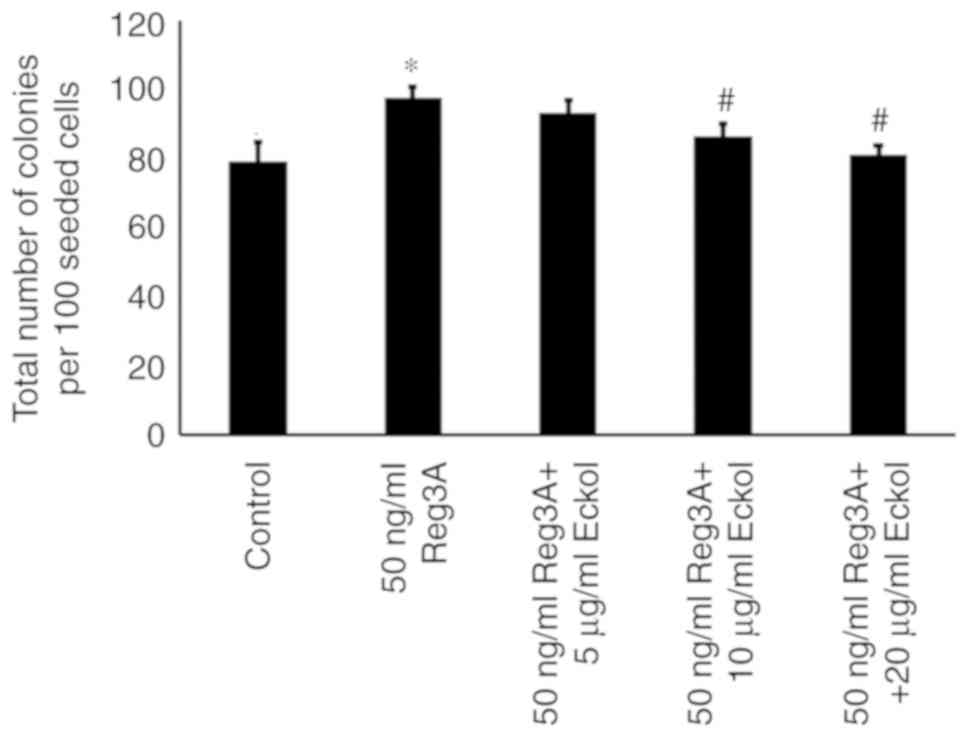
SW1990 cells were seed in soft agar to determine the
effect of eckol on the anchorage-independent growth and
colony-forming capacity of Reg3A-treated PaC cells. As shown in
Fig. 3, SW1990 cells formed colonies
in soft agar, and Reg3A incubation for 24 h significantly enhanced
the colony growth (P<0.01 compared with control). Compared with
the cells incubated with exogenous Reg3A alone, fewer colonies were
observed in cells treated with Reg3A in combination with 10 or 20
µg/ml eckol (P<0.05), suggesting that eckol may decrease
Reg3A-mediated anchorage-independent SW1990 PaC cell growth.
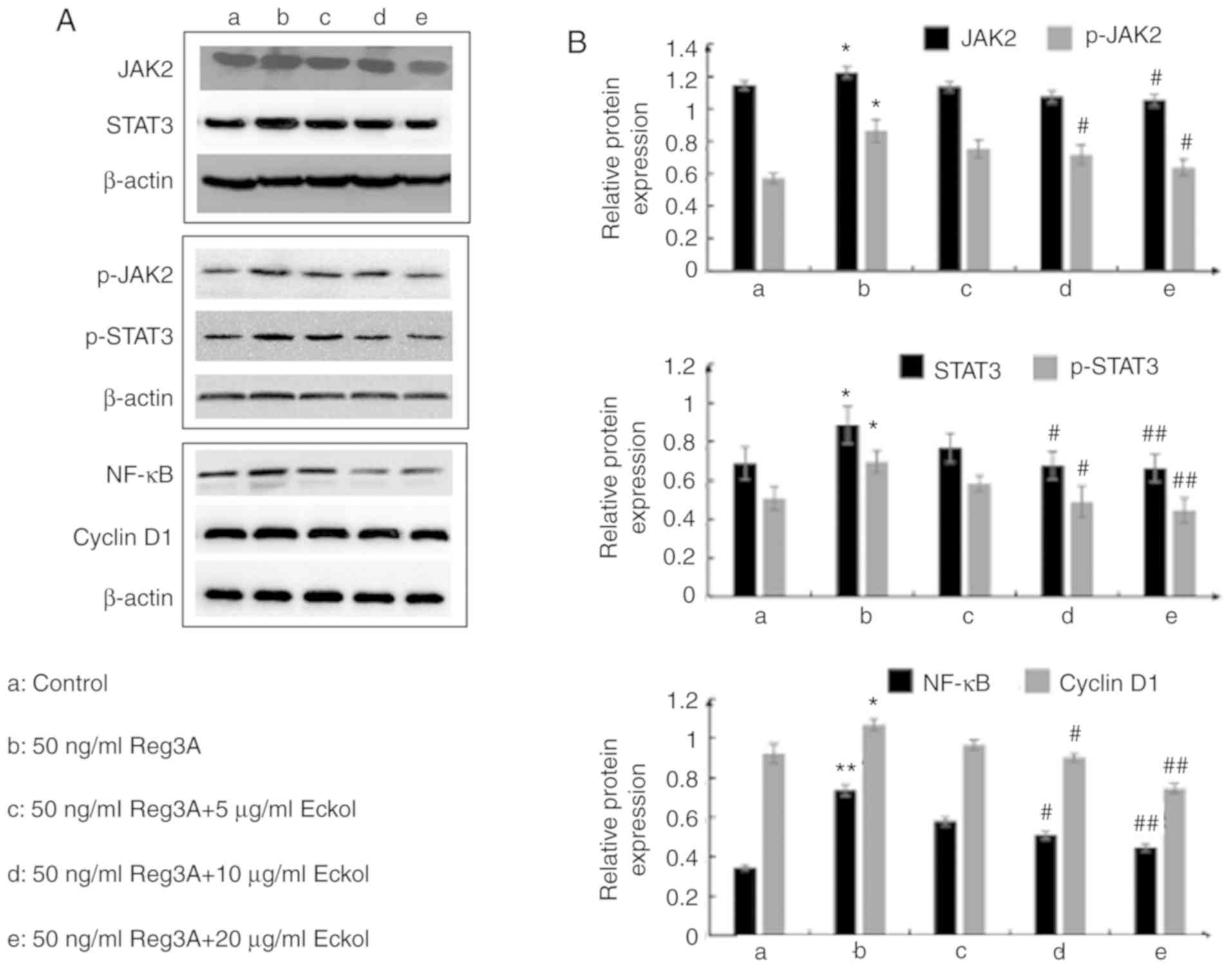
JAK/STAT3 and NF-κB/cyclin D1 pathways
may contribute to the protective effect of eckol on Reg3A-induced
over-proliferation of SW1990 PaC cells
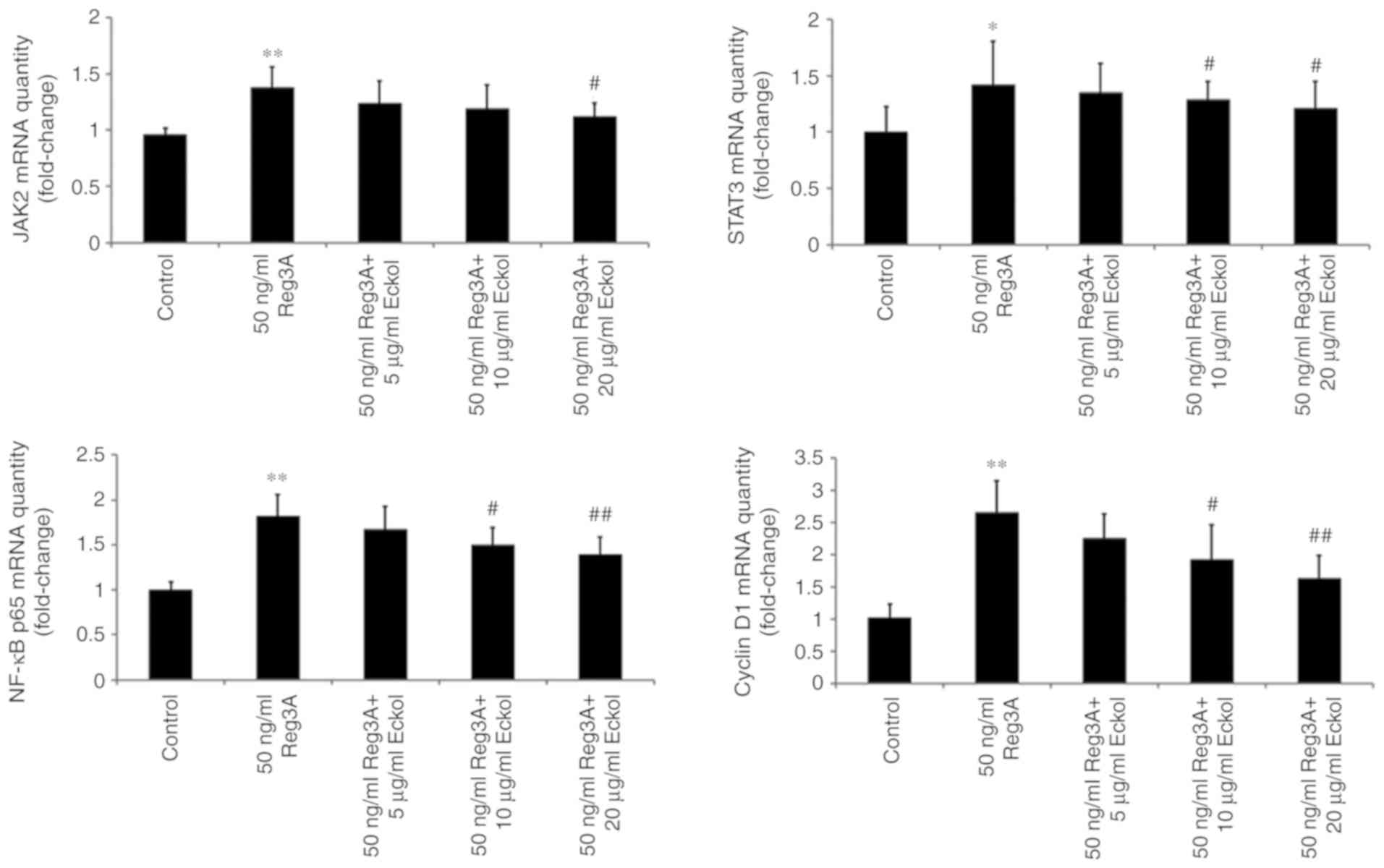
Based on the above results, the possible underlying
molecular mechanism involved in the protective effects of eckol on
Reg3A-induced over-proliferation of SW1990 PaC cells were
determined using RT-qPCR and western blot analysis of Reg3A
signaling-associated molecules, including JAK2, STAT3, NF-κB and
cyclin D1. As shown in Fig. 4,
administration of 50 ng/ml exogenous Reg3A for 24 h significantly
upregulated the expression of JAK2 (P<0.01), STAT3 (P<0.05),
NF-κB (P<0.01) and cyclin D1 (P<0.01) in SW1990 cells. The
addition of varying concentrations of eckol reduced the
Reg3a-induced increases in JAK2, STAT3, NF-κB and cyclin D1 mRNA
expression levels. Compared with the cells incubated with Reg3A
alone, eckol treatment decreased the activation of JAK2 (20 µg/ml
eckol; P<0.05) and STAT3 (10 and 20 µg/ml eckol; both P<0.05)
in SW1990 cells. Additionally, 10 µg/ml (P<0.05) and 20 µg/ml
(P<0.01) eckol reduced the mRNA expression levels of both NF-κB
and Cyclin D1. Compared with cells incubated with Reg3A alone,
>10 µg/ml eckol treatment decreased the phosphorylation of
JAK2/STAT3 and protein expression levels of NF-κB/cyclin D1 in
SW1990 cells treated with Reg3A. The data in Figs. 4 and 5
suggest that the protective effect of eckol on Reg3A-induced
over-proliferation may be associated with the JAK/STAT3 and
NF-κB/cyclin D1 signaling pathways in human PaC.
Discussion
The association between inflammation and cancer is
well-known and researched (28).
Inflammation is considered a hallmark and a dominant feature of
cancer (28). Evidence has shown
that almost all tumors possess an inflammatory tumor
microenvironment (28). In PaC,
there is an indispensable contribution of pre-existing inflammation
to pancreatic carcinogenesis, which frequently manifests as a
chronic inflammatory response to pancreatic diseases such as
chronic pancreatitis, hereditary pancreatitis, and is a
contributing risk factors in patients with PaC (29). Furthermore, PaC is characterized by a
highly inflammatory tumor microenvironment, and inflammatory
mediators are implicated in the progression of this tumor (30). Sustained inflammation within PaC
tissues aggravates the behavior of cancer cells (such as cell
proliferation and survival), affects the tumor microenvironment,
and is conducive to the invasive growth of PaC (29,31).
During the progression of PaC, additional inflammatory cells are
recruited into the cancer microenvironment and the interaction
between cancer-associated inflammation and tumor cells promotes the
malignant progression of PaC (32).
The molecular events involved in
inflammation-associated cancer have been gradually uncovered
(3–6,28–31).
IL-6 has long been demonstrated as a well-known mediator involved
in inflammation-associated pancreatic carcinogenesis (5,6). There
is a synergistic effect of Reg3A and IL-6 on PaC development
(5). As a mediator induced by
pancreatic inflammation and a promoter of cancer cell growth in
PaC, Reg3A serves an important role in the progression of PaC
accompanied by pancreatic inflammation (3–6). Reg3A
has been demonstrated to be overexpressed in 79% (30 out of 38) of
pancreatic tissues from individuals with PaC (10). In a cohort of 36 patients who
underwent resection for PaC (6),
Reg3A mRNA expression levels in tumor tissues were significantly
higher in the patients with inflammation history, in tumors >3
cm, in relatively undifferentiated tumors and in patients in
Tumor-Node-Metastasis stage III–IV, indicating the association
between the inflammatory mediator Reg3A and PaC malignancy.
In line with previous studies (5,6), 50
ng/ml exogenous Reg3A promoted cell growth, proliferation and tumor
formation in human SW1990 PaC cells. In the present study, despite
the fact that cell viability as assessed by MTT assay was not
significantly influenced by eckol, there was a reduction in
Reg3A-induced cell survival, the proportion of cells in the G2/M
and S phases and the colony-forming ability of cells in
eckol-treated SW1990 cells. The protective role of eckol in normal
tissue cells has been well documented (25). Eckol was previously found to exhibit
cytoprotective activity in hepatocytes (17), neurocytes (18,19),
keratinocytes (33) and lung
fibroblast cells (34). In addition,
eckol showed hepatoprotective activity in tacrine-treated in HepG2
cells (15). Hyun et al
(25) reported that treatment of
glioma cells cultured in sphere forming condition, induced with
epidermal growth factor and basic fibroblast growth factor, with 50
µM eckol significantly decreased cell proliferation and the
formation of spheres and there was no significant increase in cell
death when treated with 10–80 µM eckol. These previous studies
suggest that eckol appears to exert dual regulatory effects on cell
proliferation and viability. In the present study, eckol treatment
inhibited the Reg3A-induced increase in proliferation without
decreasing the viability of SW1990 cells. Therefore, eckol may be a
safe compound for controlling the progression of PaC accompanied by
pancreatic inflammation, which possesses activity against
inflammation-driven PaC malignancies without significantly
affecting the cell viability.
The JAK2/STAT3 signal transduction pathway is
closely associated with Reg3A-mediated PaC cell growth (5,6). The
JAK2/STAT3 signaling pathway is an important pathway in a wide
variety of malignant diseases including PaC (35) and is required for expression of Reg3A
in response to the stimulation of proinflammatory factors (such as
IL-6) (4,6). In the present study it was demonstrated
that, Reg3A regulated the activation of JAK2 and STAT3, which is in
agreement with Wang et al (6)
and Liu et al (5). Therefore,
there may be crosstalk between Reg3A and JAK2/STAT3 signaling,
forming a positive feedback loop, ultimately promoting PaC
development (5). In addition, STAT3
co-operates with NF-κB, another key molecule associated with
malignant conversion and progression (36) by promoting cell proliferation,
transformation and tumor development (37). Cyclin D1 is one of important
transcriptional targets of NF-κB which contributes to cell cycle
progression (38). Acceleration of
the G1/S transition via regulation of the cell-cycle-associated
protein cyclin D1 represents a key mechanism by which NF-κB
promotes cell proliferation and functions as a cancer promoter
(38). Previous studies found that
NF-κB and its gene product cyclin D1 are also involved in the
proliferation-promoting effect of Reg3A on PaC cells (5,6).
Therefore, Reg3A upregulates the NF-κB/cyclin D1 signaling pathway
and interacts with the JAK2/STAT3 pathway to create a positive
feedback loop to promote PaC development and progression. Treatment
with eckol resulted in a significant suppression of
Reg3A-upregulated the expression of JAK2, STAT3, NF-κB and cyclin
D1 in SW1990 PaC cells. We therefore hypothesized that eckol may
exert its protective activity against Reg3A-incduced SW1990 cell
proliferation, at least partly, by inhibition of JAK/STAT3 and
NF-κB/cyclin D1 signaling pathways.
In summary, in the SW1990 PaC cells, eckol exerted a
protective effect on PaC cells from Reg3A-induced proliferation in
a concentration-dependent manner, which was observed by the
reduction of Reg3A-promoted cell survival and attenuation of
Reg3A-induced cell cycle progression in the Reg3A-stimulated cells
co-treated with eckol. In addition, colony growth of SW1990 PaC
cells in soft agar was significantly increased in the presence of
exogenous Reg3A, which was reversed by the addition of eckol and
the expression levels of JAK2, STAT3, NF-κB and cyclin D1 were
upregulated following treatment with Reg3A, and these were reversed
when co-treated with eckol. Therefore, the Reg3A associated
JAK/STAT3 and NF-κB/cyclin D1 signaling pathways may be involved in
the protective effect of eckol on Reg3A-induced proliferation of
SW1990 PaC cells. As Reg3A is as a protein upregulated during
pancreatic inflammation with pro-growth functions, it serves an
important role during inflammation-driven PaC malignancies, and the
above results support the notion that eckol may be used as a
potential protective agent against progression of PaC accompanied
by pancreatic inflammation. However, the protective effects of
eckol on the proliferation of PaC cells induced by other
pro-proliferative factors other than Reg3A still require further
study, to determine whether eckol effects are limited to the
activity of Reg3A or not.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Science Foundation of China (grant no. 81602108).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW designed the study, conducted the experiments and
wrote the manuscript. MZ and WZ collected the data and performed
the experiments. SZ, SL and DY performed data analysis. MZ and WZ
revised the manuscript. All authors collaborated to develop the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma Y, Wu Q, Li X, Gu X, Xu J and Yang J:
Pancreatic cancer: From bench to bedside. Ann Transl Med.
4:4582016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Q, Fu R, Yin G, Liu X, Liu Y and Xiang
M: Microarray-based gene expression profiling reveals genes and
pathways involved in the oncogenic function of REG3A on pancreatic
cancer cells. Gene. 578:263–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Q, Wang H, Zogopoulos G, Shao Q, Dong
K, Lv F, Nwilati K, Gui XY, Cuggia A, Liu JL and Gao ZH: Reg
proteins promote acinar-to-ductal metaplasia and act as novel
diagnostic and prognostic markers in pancreatic ductal
adenocarcinoma. Oncotarget. 7:77838–77853. 2016.PubMed/NCBI
|
|
5
|
Liu X, Wang J, Wang H, Yin G, Liu Y, Lei X
and Xiang M: REG3A accelerates pancreatic cancer cell growth under
IL-6-associated inflammatory condition: Involvement of a
REG3A-JAK2/STAT3 positive feedback loop. Cancer Lett. 362:45–60.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Zhou H, Han Y, Liu X, Wang M, Wang
X, Yin G, Li X and Xiang M: SOCS3 methylation in synergy with Reg3A
overexpression promotes cell growth in pancreatic cancer. J Mol Med
(Berl). 92:1257–1269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen ZF, Huang ZM, Xue HB, Lin XQ, Chen
RP, Chen MJ and Jin RF: REG3A promotes the proliferation,
migration, and invasion of gastric cancer cells. Onco Targets Ther.
10:2017–2023. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye Y, Xiao L, Wang SJ, Yue W, Yin QS, Sun
MY, Xia W, Shao ZY and Zhang H: Up-regulation of REG3A in
colorectal cancer cells confers proliferation and correlates with
colorectal cancer risk. Oncotarget. 7:3921–3933. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukushima N, Koopmann J, Sato N, Prasad N,
Carvalho R, Leach SD, Hruban RH and Goggins M: Gene expression
alterations in the non-neoplastic parenchyma adjacent to
infiltrating pancreatic ductal adenocarcinoma. Mod Pathol.
18:779–787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie MJ, Motoo Y, Iovanna JL, Su SB,
Ohtsubo K, Matsubara F and Sawabu N: Overexpression of
pancreatitis-associated protein (PAP) in human pancreatic ductal
adenocarcinoma. Digest Dis Sci. 48:459–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yue Q, Gao G, Zou G, Yu H and Zheng X:
Natural products as adjunctive treatment for pancreatic cancer:
Recent trends and advancements. Biomed Res Int. 2017:84125082017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marasini B and Sahu RP: Natural
anti-cancer agents: Implications in gemcitabine-resistant
pancreatic cancer treatment. Mini Rev Med Chem. 17:920–927. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Fu X, Duan D, Liu X, Xu J and Gao X:
Extraction and identification of phlorotannins from the brown alga,
sargassum fusiforme (Harvey) setchell. Mar Drugs. 15(pii): E492017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang MC, Cha SH, Wijesinghe WA, Kang SM,
Lee SH, Kim EA, Song CB and Jeon YJ: Protective effect of marine
algae phlorotannins against AAPH- induced oxidative stress in
zebrafish embryo. Food Chem. 138:950–955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee MS, Shin T, Utsuki T, Choi JS, Byun DS
and Kim HR: Isolation and identification of phlorotannins from
Ecklonia stolonifera with antioxidant and hepatoprotective
properties in tacrine-treated HepG2 cells. J Agric Food Chem.
60:5340–5349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim AD, Kang KA, Piao MJ, Kim KC, Zheng J,
Yao CW, Cha JW, Hyun CL, Kang HK, Lee NH and Hyun JW:
Cytoprotective effect of eckol against oxidative stress-induced
mitochondrial dysfunction: Involvement of the FoxO3a/AMPK pathway.
J Cell Biochem. 115:1403–1411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung HA, Kim JI, Choung SY and Choi JS:
Protective effect of the edible brown alga Ecklonia stolonifera on
doxorubicin-induced hepatotoxicity in primary rat hepatocytes. J
Pharm Pharmacol. 66:1180–1188. 2014.PubMed/NCBI
|
|
18
|
Ahn BR, Moon HE, Kim HR, Jung HA and Choi
JS: Neuroprotective effect of edible brown alga Eisenia bicyclis on
amyloid beta peptide-induced toxicity in PC12 cells. Arch Pharm
Res. 35:1989–1998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang SM, Cha SH, Ko JY, Kang MC, Kim D,
Heo SJ, Kim JS, Heu MS, Kim YT, Jung WK and Jeon YJ:
Neuroprotective effects of phlorotannins isolated from a brown
alga, Ecklonia cava, against H2O2-induced oxidative stress in
murine hippocampal HT22 cells. Environ Toxicol Pharmacol.
34:96–105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung HA, Roy A, Jung JH and Choi JS:
Evaluation of the inhibitory effects of eckol and dieckol isolated
from edible brown alga Eisenia bicyclis on human monoamine oxidases
A and B. Arch Pharm Res. 40:480–491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jung HA, Jung HJ, Jeong HY, Kwon HJ, Ali
MY and Choi JS: Phlorotannins isolated from the edible brown alga
Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1
adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia.
92:260–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jung HA, Jin SE, Ahn BR, Lee CM and Choi
JS: Anti- inflammatory activity of edible brown alga Eisenia
bicyclis and its constituents fucosterol and phlorotannins in
LPS-stimulated RAW264. 7 macrophages. Food Chem Toxicol.
59:199–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang YM, Eom SH and Kim YM: Protective
effect of phlorotannins from Eisenia bicyclis against
lipopolysaccharide-stimulated inflammation in HepG2 cells. Environ
Toxicol Pharmacol. 35:395–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noda H, Amano H, Arashima K, Hashimoto S
and Nisizawa K: Studies on the antitumor activity of marine algae.
Nippon Suisan Gakk. 55:1259–1264. 1989. View Article : Google Scholar
|
|
25
|
Hyun KH, Yoon CH, Kim RK, Lim EJ, An S,
Park MJ, Hyun JW, Suh Y, Kim MJ and Lee SJ: Eckol suppresses
maintenance of stemness and malignancies in glioma stem-like cells.
Toxicol Appl Pharmacol. 254:32–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ochoa JJ, Farquharson AJ, Grant I, Moffat
LE, Heys SD and Wahle KW: Conjugated linoleic acids (CLAs) decrease
prostate cancer cell proliferation: Different molecularmechanisms
for cis-9, trans-11 and trans-10, cis-12 isomers. Carcinogenesis.
25:1185–1191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pesic M and Greten FR: Inflammation and
cancer: Tissue regeneration gone awry. Curr Opin Cell Biol.
43:55–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamada S, Masamune A and Shimosegawa T:
Inflammation and pancreatic cancer: Disease promoter and new
therapeutic target. J Gastroenterol. 49:605–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gukovsky I, Li N, Todoric J, Gukovskaya A
and Karin M: Inflammation, autophagy, and obesity: Common features
in the pathogenesis of pancreatitis and pancreatic cancer.
Gastroenterology. 144:1199–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zambirinis CP, Pushalkar S, Saxena D and
Miller G: Pancreatic cancer, inflammation, and microbiome. Cancer
J. 20:195–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Fan W, Xu Z, Chen H, He Y, Yang G,
Hu H, Tang S, Wang P, Zhang Z, et al: Inhibiting tumor necrosis
factor-alpha diminishes desmoplasia and inflammation to overcome
chemoresistance in pancreatic ductal adenocarcinoma. Oncotarget.
7:81110–81122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Piao MJ, Lee NH, Chae S and Hyun JW: Eckol
inhibits ultraviolet B-induced cell damage in human keratinocytes
via a decrease in oxidative stress. Biol Pharm Bull. 35:873–880.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang KA, Lee KH, Chae S, Zhang R, Jung MS,
Lee Y, Kim SY, Kim HS, Joo HG, Park JW, et al: Eckol isolated from
Ecklonia cava attenuates oxidative stress induced cell damage in
lung fibroblast cells. FEBS Lett. 579:6295–6304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harada D, Takigawa N and Kiura K: The Role
of STAT3 in non-small cell lung cancer. Cancers (Basel). 6:708–722.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ledoux AC and Perkins ND: NF-κB and the
cell cycle. Biochem Soc Trans. 42:76–81. 2014. View Article : Google Scholar : PubMed/NCBI
|