Introduction
Systemic lupus erythematosus (SLE) is a systemic
autoimmune disease characterized by the appearance of the
autoantibodies against several nuclear components. The deposition
of the formed immune complexes mediates the disease in a wide
variety of tissues and organs, including the kidneys, and multiple
organ systems. Lupus nephritis (LN) is one of the most common
complications in patients with SLE and influences the overall
outcome of these patients (1).
Estimates of lupus nephritis (LN) incidence among SLE patients
during the first 10 years ranges from 35 to 60% (2). It has been reported that two-thirds of
patients with SLE have renal disease at some stage, which is the
leading cause of mortality in these patients (3). Renal manifestations of lupus vary from
asymptomatic urinary abnormalities to rapidly progressive
crescentic glomerulonephritis, causing end-stage renal disease
(ESRD). However, the pathogenesis of LN remains unclear. Although
multiple factors are known to participate in LN development,
including genetic susceptibility (4), epigenetic regulation, sex and
environmental interactions (5), the
present study sought to identify the specific clinical diagnostic
markers and therapeutic targets.
Circular RNAs (circRNAs) are RNA molecules with
covalently joined 3′ and 5′ ends formed by back-splice events,
thereby presenting as covalently closed continuous loops. CircRNAs
have been demonstrated to cause loss of microRNA (miRNA/miR)
function accompanied by increased levels of endogenous targets,
acting as miRNA sponges (6). A
number of circRNAs that universally exist in a variety of
biological cells are abundant, stable (7) conserved, cell-type specific (8), tissue-specific (9) and potentially function as competing
endogenous (ce)-RNAs. Therefore, circRNAs are becoming important
biological molecules to understand the molecular mechanisms
underlying the disease and for investigating biomarkers for disease
diagnosis and targeting treatment. However, the role of circRNAs in
glomerular gene expression and their effects on LN remain
unknown.
Recent studies have reported and emphasized the
importance of circRNAs in regulating immunosenescence- associated
immunocytes (10–12). Thus far, little is known about the
expression and function of circRNAs in LN. Therefore, the present
study, to the best of our knowledge, investigated for the first
time the comprehensive expression profile of circRNAs in the
glomeruli of LN. In SLE-prone (NZB/W) F1 mice, the systemic
bioinformatics analysis was performed to identify circRNAs that are
essential for the biological processes of LN, which may provide
potential targets for the development of novel diagnostic and
therapeutic strategies for LN.
Materials and methods
Mice
Female 12-week-old NZB/W F1 mice (n=30) purchased
from the Jackson Laboratory Animal Center (Jackson, MS, USA) were
maintained in the Chinese Medical University Animal Laboratory
(Liaoning, China). The mice had free access to food and water
throughout the experimental period under controlled conditions
(humidity, 45±2%; temperature, 22±1°C), and a 12-h light/dark
artificial cycle in accordance with the guidelines of the Chinese
National Standard (GB 14925–2001). The present study complied with
the protocols approved by the Institutional Animal Care and Use
Committee at China Medical University. All animals were housed in
specific pathogen-free conditions until LN diagnosis, and after 16
weeks, mice were intraperitoneally anesthetized with 1% sodium
pentobarbital (40 mg/kg; Beijing Solarbio Science &Technology
Co, Ltd.) and sacrificed by exsanguination via the aorta
pectoralis.
Mouse groupings
The onset of renal disease was monitored by weekly
testing of fresh urine specimens using protein test strips to test
for proteinuria (±: 10 mg/dl; +: 30 mg/dl; 2+: 100 mg/dl; 3+, 300
mg/dl; 4+: >1,000 mg/dl). Mice with a proteinuria level ≥300
mg/dl in repeated tests were considered to have ‘severe’ LN, as
previously described (13,14). At the age of 28 weeks, the mice were
divided into two groups based on their degree of renal disease:
Mild LN (pro ±-1+) and severe LN (pro 3+-4+). The body weight of
the mice at 28 weeks of age were as follows: Mild LN, 36.433±2.332
g, n=5; and severe LN, 34.933±3.158 g; n=5. Data are expressed as
the mean ± standard error of the mean. The remaining 20 animals
were used for reverse transcription-quantitative PCR (RT-qPCR) to
verify the target circRNA of triple for each group [n=9 (pro±~
+1+); n=9 (pro3+~4+); experiments were repeated three times].
Euthanasia was intended via perfusion; however, 2 mice succumbed to
anesthetic due to accidental exsanguination.
Isolation of kidney glomeruli
Glomeruli were isolated through the abdominal aorta
as previously described (15),
alongside some improvements to achieve the experimental conditions
required. Glomeruli were isolated from kidneys perfused via the
aorta pectoralis with magnetic Dynabeads (Invitrogen; Thermo Fisher
Scientific, Inc.) at 28 weeks of age. First, kidneys were perfused
with ice-cold phosphate buffer saline (PBS) to remove any blood and
then Dynabeads (diameter, 4.5 µm) (4×106/ml PBS, 25
ml/mice) were perfused into kidneys at a constant rate of 7.4
ml/min/g per kidney. Following the removal, mincing, digestion and
filtration of tissue, the cell suspension was obtained and then
centrifuged at 200 × g for 5 min in 4°C. Once the supernatant was
discarded, the cell pellet was dissolved in 2 ml PBS. Finally, a
magnetic particle concentrator (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to collect the glomeruli containing
Dynabeads; the glomerular RNA was isolated using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). Following sacrifice,
other samples, such as urine, brain, liver, blood, heart, muscle
and lung, were obtained from the animals for future analysis in
other studies.
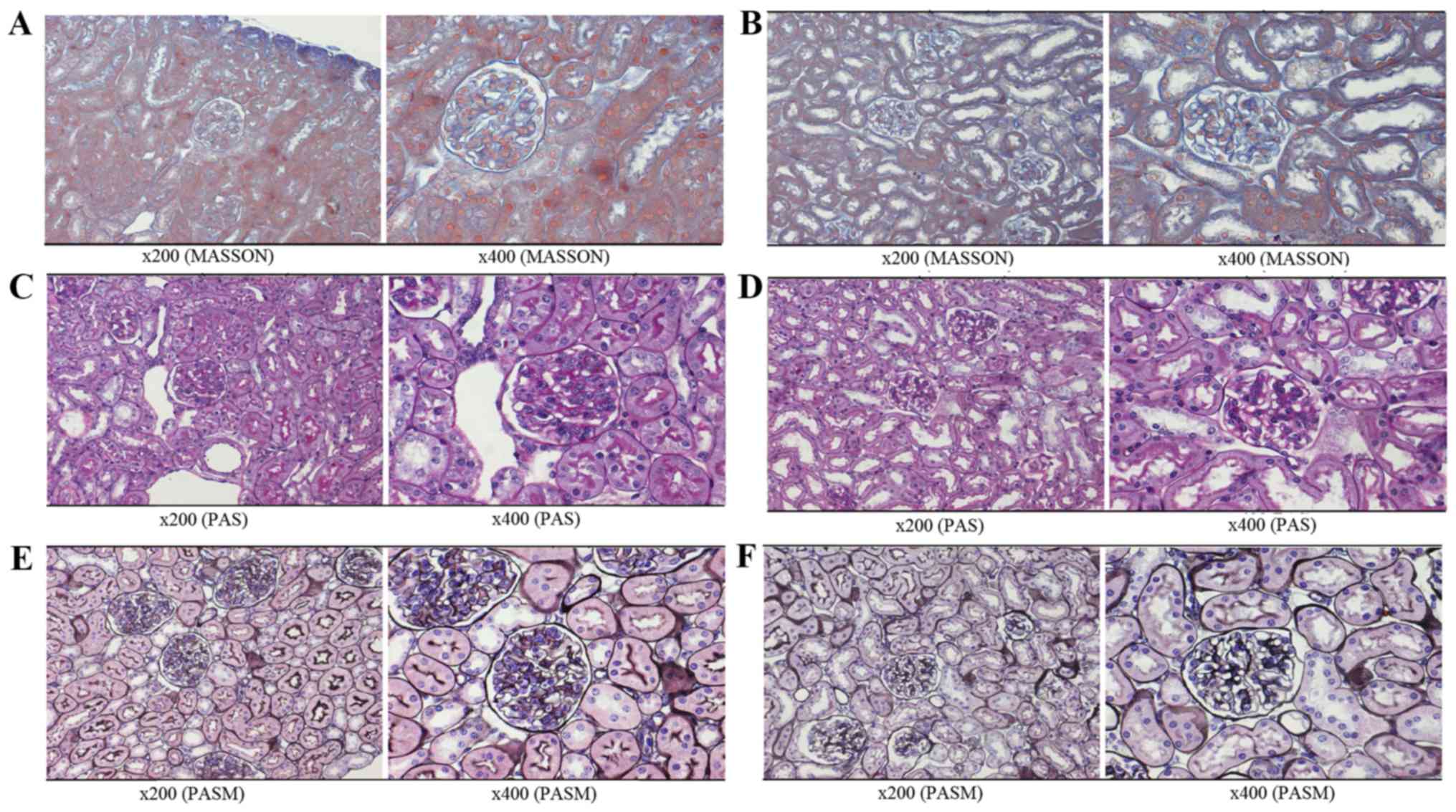
Histological determination of LN
Renal tissue from the severe and mild LN mice, and
Dynabead-perfused kidneys were fixed with Formalin-Aceto-Alcohol at
room temperature, dehydrated with alcohol and embedded in paraffin
for sectioning. Paraformaldehyde-fixed paraffin-embedded
histological kidney sections (4-µm-thick) were stained with
hematoxylin and eosin (H&E), Masson's trichrome, periodic
acid-Schiff and periodic Schiff-methenamine to evaluate disease
development by light microscopy. The severe and mild LN groups were
diagnosed according to kidney pathology.
RNA extraction
Total RNA was isolated using TRIzol reagent
according to the manufacturer's protocol. cDNA for RT-qPCR analysis
was synthesized from 1 µg of total RNA. Total RNA was quantified
using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher
Scientific, Inc.). The RNA integrity and genomic DNA contamination
of each sample was assessed by denaturing agarose gel
electrophoresis, as previously descried (16,17).
Labeling and hybridization
Sample labeling and array hybridization were
performed according to the manufacturers' protocols as described
below. To enrich circRNAs, linear RNAs were removed using Rnase R
(Epicentre; Illumina, Inc.) to digest total RNAs. Each sample of
enriched circRNAs was then amplified and transcribed into
fluorescent cRNA using the treating random primers method
(Arraystar Super RNA Labeling kit; Arraystar, Inc.). The labeled
cRNAs were purified using the RNeasy Mini kit (Qiagen, Inc.). The
concentration and specific activity of the labeled cRNAs (pmol
Cy3/µg cRNA) were measured using a NanoDrop ND-1000. A total of 1
µg of each labeled cRNA was fragmented by adding 5 µl 10X Blocking
Agent (Agilent Technologies, Inc.) and 1 µl of 25X Fragmentation
Buffer (Agilent Technologies, Inc.), and the mixture was incubated
at 60°C for 30 min. A total of 25 µl 2X Hybridization buffer
(Agilent Technologies, Inc.) was added to dilute the labeled cRNA,
50 µl of hybridization solution was added into the gasket slide and
assembled with the circRNA expression microarray slide. The slides
were then incubated for 17 h at 65°C in an Agilent Hybridization
Oven (G2545A; Agilent Technologies, Inc.). Finally, following
washing and fixing the slides, the hybridized arrays were scanned
using the Agilent Scanner G2505C (Agilent Technologies, Inc.).
Microarray and quality control
Agilent Feature Extraction software (version
11.0.1.1; Agilent Technologies, Inc.) was used to analyze the
acquired array images for raw data extraction. Quantile
normalization and subsequent data processing were performed using
the R software (version 3.1.2; http://www.r-project.org/) limma package. Following
quantile normalization of the raw data, low intensity filtering was
performed, and the circRNAs with at least one out of two samples
with flags in ‘P’ or ‘M’ (‘All Targets Value’) were retained for
further analyses. Differentially expressed circRNAs that were
statistically significant between the two groups were identified
via Volcano Plot filtering. Differentially expressed circRNAs
between two samples were identified via Fold Change filtering.
Hierarchical clustering was performed to reveal the distinguishable
circRNA expression pattern among samples. circRNAs with fold
changes ≥2 were selected as significantly differentially expressed.
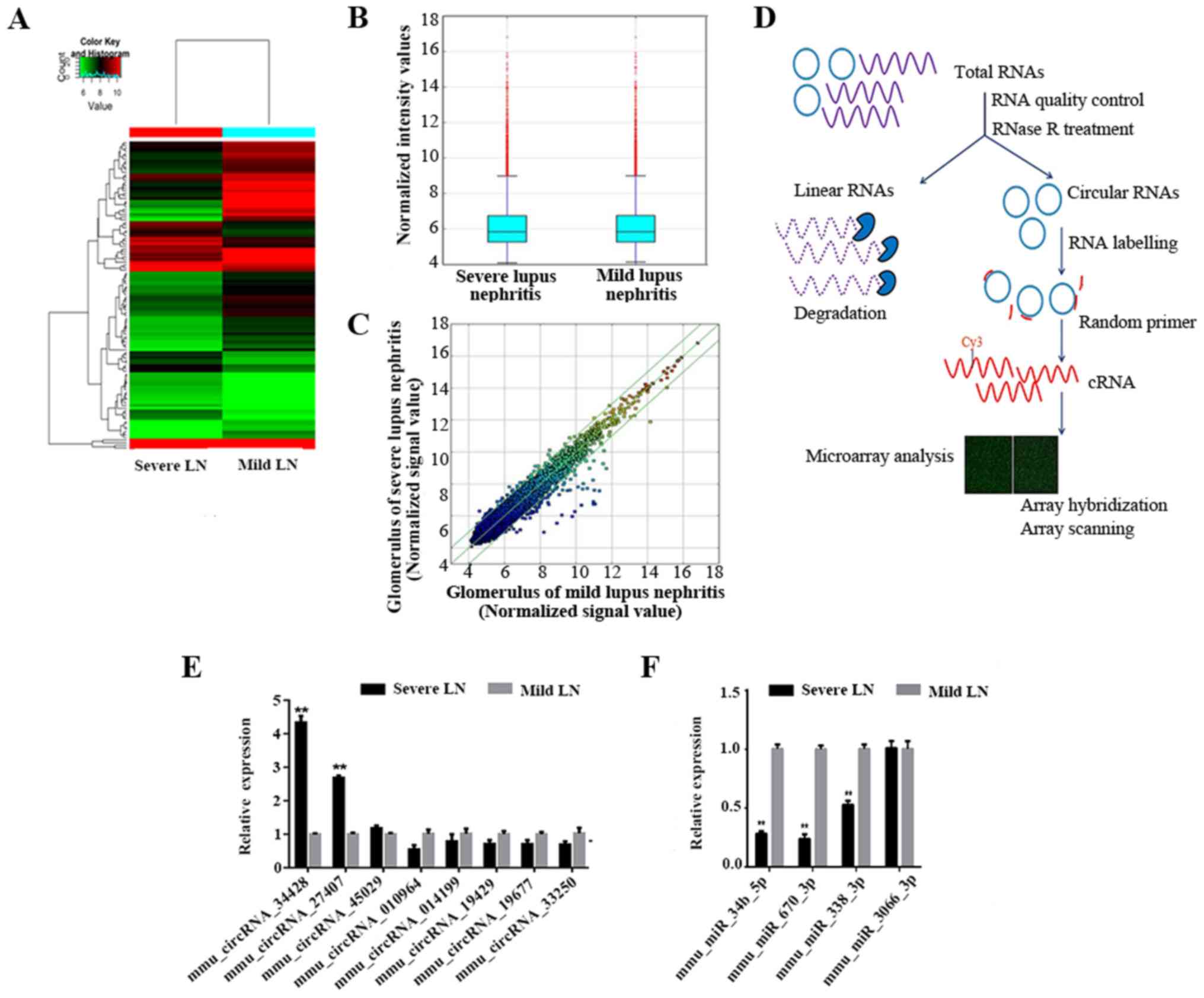
The experimental workflow is presented in Fig. 3D.
RT-qPCR analysis and statistical
analyses
Total RNA was isolated from glomeruli using TRIzol
reagent. A total of 1.0 µg of RNA was reverse transcribed to cDNA
using the SuperScript™ III Reverse Transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RT-qPCR was performed using the SYBR Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
fluorescence signal was detected by the ViiA 7 Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). cDNA samples
were prepared from the total RNA of glomeruli by RT-qPCR. In total,
5 upregulated circRNAs and 5 downregulated circRNAs were analyzed
by SYBR green I dye-based detection with specific primer sequences
(Table I). The following
thermocycling conditions were used: 95°C for 10 min; 40 cycles at
95°C for 10 sec and 60°C for 1 min. The relative expression of
circRNAs was calculated using the 2−∆∆Cq method
(18) with the housekeeping gene-β
actin expression to normalize the data. The quantitative data were
presented as the mean ± standard error of the mean. The statistical
data was calculated using a Student's t-test to analyze expression
levels. P<0.05 was considered to indicate a statistically
significant difference.
 | Table I.Specific circRNAs primers for
quantitative polymerase chain reaction analysis. |
Table I.
Specific circRNAs primers for
quantitative polymerase chain reaction analysis.
| Name | Sequence | PS, bp | Name | Sequence | PS, bp |
|---|
| mouse β-actin | F,
5′-GTACCACCATGTACCCAGGC-3′ | 247 |
mmu_circRNA_29625 | F,
5′-TCTGTCCTCGTCATGTTCCAC-3′ | 58 |
|
| R,
5′-AACGCAGCTCAGTAACAGTCC-3′ |
|
| R,
5′CTCTTCTCTCTTCAGGAGTCGTC 3′ |
|
|
mmu_circRNA_010964 | F,
5′-GGCGTGAAACCGTTAAGAGC-3′ | 86 |
mmu_circRNA_33250 | F,
5′-AAATGAGTTTGAGACCCTTCG-3′ | 145 |
|
| R,
5′-CATTCCCAAGCAACCCAACT-3′ |
|
| R,
5′-CACCTCGTTGTCCTTAAAATATGT-3′ |
|
|
mmu_circRNA_014199 | F,
5′-ACCTGCAACTTTGAGCAGGACT-3′ | 62 |
mmu_circRNA_34414 | F,
5′-TACTCATATCAAAACTTCGCCC-3′ | 103 |
|
| R,
5′-GAAACCTATGAGAGTGGGTTAGGG-3′ |
|
| R,
5′-GTGGGGTTGACTAGGATGATG-3′ |
|
|
mmu_circRNA_19429 | F,
5′-ACAACCCAGAAGAGCCAGGTA-3′ | 158 |
mmu_circRNA_34428 | F,
5′-CCAATGATGTGCCTTCTCCATA-3′ | 112 |
|
| R,
5′-TGAGTTATTCGCCCATACAGC-3′ |
|
| R,
5′-GCCTCTTGCAATGTCCACACTT-3′ |
|
|
mmu_circRNA_19677 | F,
5′-CTCTTGACCACGCCACCCTT-3′ | 78 |
mmu_circRNA_45029 | F,
5′-AAAAGTGGCTGTATTGGATGG-3′ | 123 |
|
| R,
5′-AGTGAAGCCAGATGCGAGGAA-3′ |
|
| R,
5′-ATGCAAGGACAAGTAACGAATAG-3′ |
|
|
mmu_circRNA_27407 | F, 5′
CTGAGCCTGACGCCATTTCT 3′ | 88 |
|
|
|
|
| R,
5′-′TGTACCTGGCTGCCGTCTC-3′ |
|
|
|
|
Construction of the competing
endogenous RNA (ceRNA) network for candidate circRNAs
It was hypothesized that the RNA transcripts can
crosstalk by competing for common miRNAs, with miRNA response
elements (MREs) as the foundation of this interaction (19). These RNA transcripts have been termed
as ceRNAs (20). Any RNA transcript
with MREs that may function as ceRNAs, and ceRNAs containing
pseudogene transcripts, long non-coding RNAs, circRNAs and mRNAs,
can compete for the same MREs. To identify the potential target of
miRNAs, the target miRNAs were predicted with miRNA target
prediction software TargetScan 7.2 (http://www.targetscan.or) and miRanda (http://www.microrna.org/microrna/home.do) (21–28).
Validated candidate circRNAs were used as seeds to enrich the
circRNA/miRNA/gene network according to the analysis.
Statistical analysis
Statistical analyses were performed using GraphPad
software (version 6.01; GraphPad Software, Inc.). The Student's
t-test was applied for comparison of two groups. P<0.05 were
considered to indicate a statistically significant difference. Gene
Ontology (GO; www.geneontology.org) and Kyoto Encyclopedia of Genes
and Genomes (KEGG) pathway (www.genome.jp/kegg) analysis was utilized based on the
predicted gene from the online TargetScan and miRanda analysis. A
network map of circRNA-miRNA-mRNA interactions was constructed with
Cytoscape 3.5.0 (www.cytoscape.org).
Results
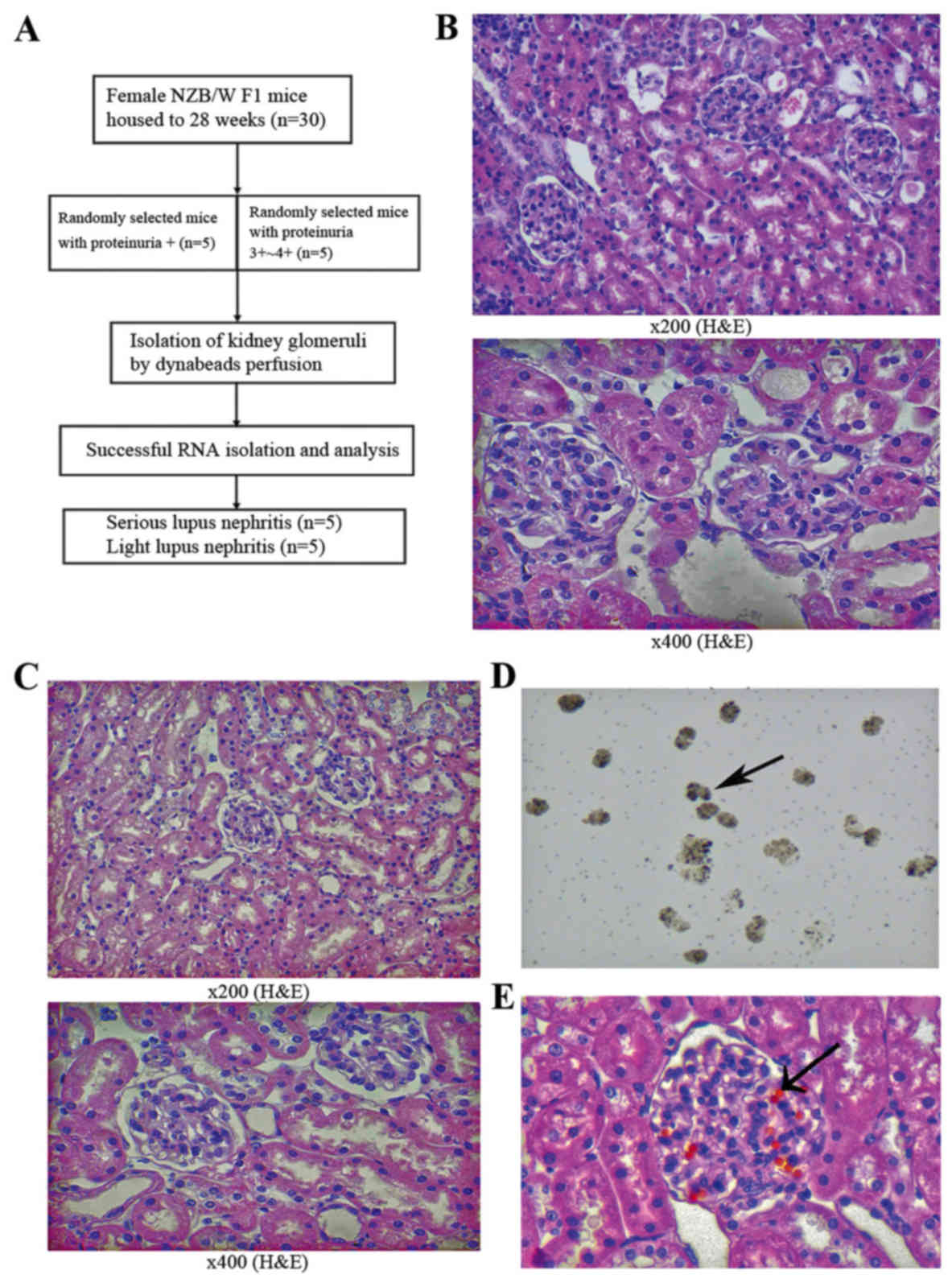
Pathology of kidneys and efficiency of
glomeruli isolation
With the development of renal disease, NZB/W F1 mice
present with severe proteinuria at only a few weeks of age.
According to the different groups (as presented in Fig. 1A), the predominant pathological
manifestations observed in severe LN included glomerular diffuse
mesangial cell proliferation, matrix expansion and
segmental-spherical endothelial cell proliferation (Figs. 1B and 2A,
C and E). The pathology of the kidneys in the mild LN group was
decreased when compared with the severe group (Figs. 1C and 2B,
D and F).
The mouse glomeruli were isolated by perfusion with
Dynabeads (diameter, 4.5 µm) through the aorta pectoralis. The
glomerular structure was only slightly affected by the collagenase
digestion of the kidney (15).
Dynabeads accumulated in the glomerular vessels, making the
glomeruli easy to isolate using a magnet with a low degree of
contaminating tissues. The isolated glomeruli with Dynabeads were
distributed across the slide when observed under a light microscope
(Fig. 1D). H& E staining of the
kidneys from mice perfused by Dynabeads revealed that the beads
were primarily distributed in the glomeruli, and that only a few
beads could be detected in the surrounding renal tissues and were
mainly concentrated in the afferent and efferent arterioles
(Fig. 1E).
Analysis of differentially expressed
circRNAs
In total, 13,538 circRNAs were detected using the
Arraystar mouse circRNA Microarray. Through the circRNA array
profiles, differentially expressed circRNAs were identified in each
pairwise comparison. Hierarchical clustering was performed to
present the circRNAs based on the expression levels of two samples
(Fig. 3A). Box plots revealed that
the distribution of the circRNAs for the compared samples were
nearly the same following normalization (Fig. 3B). The present study set a threshold
of fold-change ≥2.0.
Among the differentially-expressed circRNAs, 41 were
significantly upregulated in severe LN when compared with mild LN
according to the pre-defined fold-change of ≥2.0 (the top 25 are
presented in Table II). In
addition, 75 circRNAs that were significantly downregulated in
severe LN when compared with mild LN were also identified (the top
25 are presented in Table
III).
 | Table II.the top up-regulated 25 circRNAs
between serious LN and light LN. |
Table II.
the top up-regulated 25 circRNAs
between serious LN and light LN.
| circRNA | FC | circRNA_type | Gene symbol | MRE1 | MRE2 | MRE3 | MRE4 | MRE5 |
|---|
|
mmu_circRNA_30815 | 3.25 | Exonic | L3mbtl4 |
mmu-miR-6986-3p |
mmu-miR-216c-3p |
mmu-miR-9768-5p | mmu-miR-873b |
mmu-miR-219b-5p |
|
mmu_circRNA_36259 | 3.02 | Exonic | Lmo4 | mmu-miR-6337 | mmu-miR-143-3p |
mmu-miR-5621-3p | mmu-miR-547-5p | mmu-miR-433-3p |
|
mmu_circRNA_30673 | 2.93 | Exonic | Adgre1 | mmu-miR-207 |
mmu-miR-7007-5p |
mmu-miR-7054-5p |
mmu-miR-6925-5p |
mmu-miR-6965-5p |
|
mmu_circRNA_41054 | 2.61 | Exonic | Bcat1 | mmu-miR-298-5p |
mmu-miR-7080-5p | mmu-miR-544-5p |
mmu-miR-6931-5p | mmu-miR-5113 |
|
mmu_circRNA_45030 | 2.6 | Exonic | Wdr82 | mmu-miR-3471 | mmu-miR-493-3p |
mmu-miR-7230-3p |
mmu-miR-3069-5p | mmu-miR-6354 |
|
mmu_circRNA_35594 | 2.46 | Intronic |
|
mmu-miR-7092-3p | mmu-miR-1187 | mmu-miR-185-5p |
mmu-miR-297c-5p |
mmu-miR-6925-5p |
|
mmu_circRNA_45029 | 2.38 | Exonic | Wdr82 |
mmu-miR-7232-5p | mmu-miR-345-3p |
mmu-miR-3097-5p |
mmu-miR-466c-5p | mmu-miR-221-5p |
|
mmu_circRNA_39054 | 2.37 | Exonic | Wdfy3 |
mmu-miR-6919-3p |
mmu-miR-3544-3p | mmu-miR-138-5p | mmu-miR-1902 |
mmu-miR-7072-3p |
|
mmu_circRNA_27407 | 2.36 | Exonic | Sh3bp5 | mmu-miR-22-5p | mmu-miR-207 |
mmu-miR-6938-3p | mmu-miR-877-3p | mmu-miR-484 |
|
mmu_circRNA_27357 | 2.32 | Exonic | Tkt | mmu-miR-6403 |
mmu-miR-7074-3p | mmu-miR-1668 | mmu-miR-182-5p |
mmu-miR-5134-3p |
|
mmu_circRNA_42341 | 2.28 | Exonic | Itgam | mmu-miR-136-5p |
mmu-miR-3084-5p |
mmu-miR-29b-1-5p |
mmu-miR-1198-5p | mmu-miR-107-3p |
|
mmu_circRNA_31618 | 2.28 | Exonic | Hars2 | mmu-miR-361-3p | mmu-miR-6370 |
mmu-miR-1968-3p |
mmu-miR-3087-3p |
mmu-miR-6926-3p |
|
mmu_circRNA_19193 | 2.25 |
Senseoverlapping | Adgre1 |
mmu-miR-6957-5p | mmu-miR-207 |
mmu-miR-7007-5p |
mmu-miR-6925-5p | mmu-miR-877-3p |
|
mmu_circRNA_38229 | 2.24 | Exonic | Orc5 | mmu-miR-34c-5p | mmu-miR-34b-5p |
mmu-miR-195a-3p |
mmu-miR-299a-3p |
mmu-miR-6916-5p |
|
mmu_circRNA_43151 | 2.24 | Exonic | Smad1 |
mmu-miR-7085-5p | mmu-miR-667-5p |
mmu-miR-92a-2-5p |
mmu-miR-3094-5p |
mmu-miR-3100-5p |
|
mmu_circRNA_42194 | 2.22 | Exonic | Arl6ip1 |
mmu-miR-3100-5p | mmu-miR-761 | mmu-miR-214-3p |
mmu-miR-6901-5p | mmu-miR-6370 |
|
mmu_circRNA_38710 | 2.21 | Exonic | Limch1 |
mmu-miR-7034-3p | mmu-miR-1903 |
mmu-miR-103-1-5p |
mmu-miR-103-2-5p | mmu-miR-107-5p |
|
mmu_circRNA_24654 | 2.2 |
Senseoverlapping | AK135963 | mmu-miR-5110 |
mmu-miR-7665-5p |
mmu-miR-6976-5p |
mmu-miR-7012-5p | mmu-miR-5113 |
|
mmu_circRNA_24113 | 2.2 | Exonic | Kat7 | mmu-miR-1904 | mmu-miR-6344 | mmu-miR-137-5p |
mmu-miR-7671-3p | mmu-miR-8120 |
|
mmu_circRNA_32604 | 2.19 | Exonic | Tm9sf3 |
mmu-miR-7011-3p | mmu-miR-421-5p |
mmu-miR-7016-5p | mmu-miR-6384 | mmu-miR-107-5p |
|
mmu_circRNA_30668 | 2.18 | Exonic | Adgre1 |
mmu-miR-181d-5p | mmu-miR-191-3p |
mmu-miR-181b-5p |
mmu-miR-7668-3p | mmu-miR-6360 |
|
mmu_circRNA_41078 | 2.18 | Exonic | Tm7sf3 |
mmu-miR-7012-5p |
mmu-miR-7057-5p | mmu-miR-8113 | mmu-miR-504-3p |
mmu-miR-7686-5p |
|
mmu_circRNA_34428 | 2.15 | Exonic | Pdia3 | mmu-miR-1903 |
mmu-miR-7092-3p | mmu-miR-495-5p |
mmu-miR-7649-3p | mmu-miR-338-3p |
|
mmu_circRNA_29625 | 2.14 | Exonic | L3mbtl4 |
mmu-miR-6986-3p |
mmu-miR-216c-3p |
mmu-miR-9768-5p | mmu-miR-873b |
mmu-miR-219b-5p |
|
mmu_circRNA_19115 | 2.12 |
Senseoverlapping | Tkt |
mmu-miR-6973a-5p | mmu-miR-880-3p | mmu-miR-298-5p |
mmu-miR-6974-3p |
mmu-miR-3099-5p |
 | Table III.Top 25 downregulated circRNAs between
severe LN and light LN. |
Table III.
Top 25 downregulated circRNAs between
severe LN and light LN.
| circRNA | FC | circRNA_type | Gene symbol | MRE1 | MRE2 | MRE3 | MRE4 | MRE5 |
|---|
|
mmu_circRNA_26644 | −20.03 | Exonic | Edil3 | mmu-miR-215-3p |
mmu-miR-3075-3p | mmu-miR-8118 | mmu-miR-129-5p | mmu-miR-8092 |
|
mmu_circRNA_35752 | −18.88 | Exonic | Dap3 |
mmu-miR-3104-3p |
mmu-miR-7116-3p |
mmu-miR-7051-5p | mmu-miR-337-3p |
mmu-miR-181d-3p |
|
mmu_circRNA_003795 | −16.94 |
Senseoverlapping | Cep350 |
mmu-miR-1249-5p | mmu-miR-504-3p | mmu-miR-6399 |
mmu-miR-7054-5p | mmu-miR-667-5p |
|
mmu_circRNA_013216 | −14.06 |
Senseoverlapping | Sulf1 |
mmu-miR-7012-3p |
mmu-miR-486b-5p |
mmu-miR-486a-5p |
mmu-miR-181a-1-3p |
|
|
mmu_circRNA_34193 | −13.39 | Exonic | Lgr4 |
mmu-miR-146a-3p |
mmu-miR-450a-2-3p | mmu-miR-6359 |
mmu-miR-7087-5p |
mmu-miR-7647-3p |
|
mmu_circRNA_26765 | −12.68 |
Senseoverlapping | Wdr41 |
mmu-miR-3082-5p | mmu-miR-466f |
mmu-miR-466i-5p | mmu-miR-1187 |
mmu-miR-466a-5p |
|
mmu_circRNA_23904 | −12.36 | Exonic | Acaca | mmu-miR-320-5p | mmu-miR-1903 | mmu-miR-677-3p |
mmu-miR-7090-5p | mmu-miR-20b-3p |
|
mmu_circRNA_006620 | −10.69 | Exonic | Mllt10 | mmu-miR-5098 |
mmu-miR-7059-3p |
mmu-miR-7092-3p |
mmu-miR-7210-5p | mmu-miR-136-5p |
|
mmu_circRNA_25774 | −10.41 | Exonic | Ppp4r4 |
mmu-miR-3097-5p |
mmu-miR-7235-3p |
mmu-miR-7033-5p |
mmu-miR-7661-5p |
mmu-miR-7214-5p |
|
mmu_circRNA_45713 | −9.33 |
Senseoverlapping | Tex16 |
mmu-miR-7092-3p |
mmu-miR-297a-5p |
mmu-miR-297c-5p | mmu-miR-1187 |
mmu-miR-466c-5p |
|
mmu_circRNA_41925 | −8.9 | Exonic | Ints4 | mmu-miR-708-3p | mmu-miR-6342 | mmu-miR-670-3p |
mmu-miR-6938-5p |
mmu-miR-3075-3p |
|
mmu_circRNA_38586 | −7.49 | Exonic | Adgra2 |
mmu-miR-3058-5p |
mmu-miR-3059-5p | mmu-miR-677-3p | mmu-miR-1946a |
mmu-miR-7219-3p |
|
mmu_circRNA_014199 | −6.64 |
Senseoverlapping | Mamdc4 |
mmu-miR-125a-3p |
mmu-miR-6930-5p |
mmu-miR-6932-5p |
mmu-miR-7068-5p | mmu-miR-5710 |
|
mmu_circRNA_33250 | −6.55 | Exonic | Tbc1d13 |
mmu-miR-7089-3p |
mmu-miR-7018-5p |
mmu-miR-3113-5p |
mmu-miR-6899-3p | mmu-miR-1956 |
|
mmu_circRNA_20247 | −6.44 | Intronic | Ino80d |
mmu-miR-7661-5p |
mmu-miR-3089-5p | mmu-miR-339-5p |
mmu-miR-7222-3p |
mmu-miR-7222-5p |
|
mmu_circRNA_010498 | −5.25 | Antisense | Dstn |
mmu-miR-7661-5p | mmu-miR-152-3p | mmu-miR-93-5p | mmu-miR-350-5p | mmu-miR-20a-5p |
|
mmu_circRNA_29589 | −4.93 | Exonic | Heg1 |
mmu-miR-7075-5p |
mmu-miR-7029-3p |
mmu-miR-7081-5p | mmu-miR-149-3p |
mmu-miR-6935-5p |
|
mmu_circRNA_010964 | −4.85 | Intergenic |
| mmu-miR-20a-3p |
mmu-miR-7019-5p | mmu-miR-1969 |
mmu-miR-7092-3p |
mmu-miR-7684-5p |
|
mmu_circRNA_19429 | −4.77 |
Senseoverlapping | Plxnd1 |
mmu-miR-6953-5p |
mmu-miR-7054-5p |
mmu-miR-6925-5p |
mmu-miR-7047-5p | mmu-miR-665-5p |
|
mmu_circRNA_016016 | −4.15 | Exonic | Nhlrc2 | mmu-miR-320-5p |
mmu-miR-7233-3p |
mmu-miR-7668-5p |
mmu-miR-6976-3p | mmu-miR-130c |
|
mmu_circRNA_24437 | 3.77 | Exonic | Rnf157 |
mmu-miR-3087-5p |
mmu-miR-5107-5p |
mmu-miR-7663-5p | mmu-miR-877-3p | mmu-miR-1946b |
|
mmu_circRNA_45841 | 3.44 | Exonic | Kdm5c |
mmu-miR-148b-5p |
mmu-miR-7667-5p |
mmu-miR-7659-3p | mmu-miR-320-5p |
mmu-miR-6959-5p |
|
mmu_circRNA_20692 | 3.4 | Exonic | Epb4.1l5 |
mmu-miR-7078-3p | mmu-miR-206-5p |
mmu-miR-129b-5p | mmu-miR-1187 |
mmu-miR-7079-5p |
|
mmu_circRNA_35336 | 3.35 | Exonic | Slc7a11 | mmu-miR-509-5p | mmu-miR-541-5p |
mmu-miR-7226-5p | mmu-miR-6539 | mmu-miR-1946b |
|
mmu_circRNA_19322 | 3.32 |
Senseoverlapping | Reck | mmu-miR-298-5p |
mmu-miR-7012-5p |
mmu-miR-7067-5p |
mmu-miR-1966-5p |
mmu-miR-6953-5p |
RT-qPCR validation of differential
circRNAs
Based on the raw intensity, the gene length and fold
change of the circRNA profile, the present study selected 10
dysregulated circRNAs, including five upregulated (34414, 29625,
27407, 34428 and 45029) and five downregulated circRNAs (010964,
014199, 19429, 19677 and 33250) for RT-qPCR validation in the
samples. The RT-qPCR results revealed that, except for two
upregulated circRNAs (34414 and 29625), three upregulated circRNAs
and all five downregulated circRNAs were expressed consistently
with microarray results (Fig. 3E).
The relative fold changes in circRNA expression were calculated
using the ΔΔCq method, and the values were expressed as
2−ΔΔCq are presented as the expression level relative to
the control group with the standard deviation of the mean of
triplicate measures for each group. mmu_circRNA_34428 was the most
significantly differentially expressed when compared between the
two groups (P<0.001).
Construction of the circRNA-miRNA-mRNA
interaction network and predicting the circRNAs that act as
ceRNAs
In order to identify more targeted miRNAs, the
present study constructed a ceRNA network. Based on the results of
the RT-qPCR, the key circRNAs (27407, 34428, 45029, 010964, 014199
and 19429) were selected to predict ceRNAs, and a network map of
circRNA-miRNA-mRNA interactions was constructed with Cytoscape
(Fig. 4A). Through specific base
pairing, the genetic crosstalk between the selected circRNAs and
the predicted miRNA targets were detected using an miRNA target
prediction software created based on TargetScan & miRanda. The
target miRNA of mmu_circRNA_34428 was investigated further. Based
on the ceRNA analysis, four miRNAs were predicted to have an
interaction with mmu_circRNA_34428, which was consistent with the
results of RT-qPCR of these four miRNAs (Fig. 3F). A total of four miRNAs and eight
mRNAs were demonstrated to interact with mmu_circRNA_34428, as
presented in Fig. 4A. The ceRNA
analysis of the interaction network of mmu_circRNA_34428 indicated
that miR-34b-5p exhibited the greatest number of interactions,
followed by mmu-miR-670-3p and mmu-miR-338-3p (Fig. 4A).
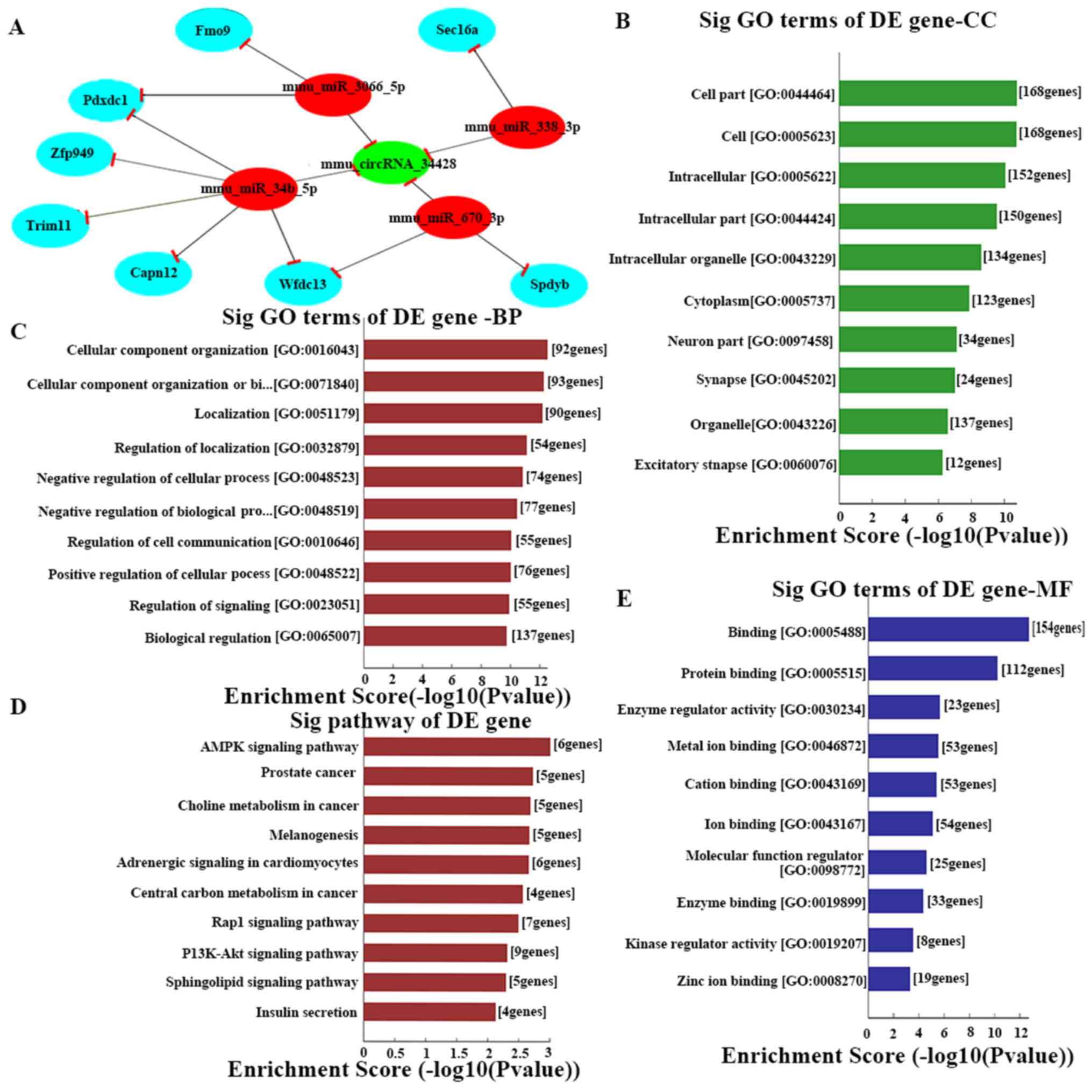 | Figure 4.(A) The predicted mmu_circRNA_34428
targeted gene network (circRNA-miRNA-mRNA) according to
sequence-pairing prediction. The interactions predicted by ceRNA.
Nodes colored red are miRNAs, light blue nodes are protein coding
RNAs and green nodes are circRNAs. Edges with T-shape arrows
represent the direction of associations and edges without arrows
represent the general association. GO and Kyoto Encyclopedia of
Genes and Genomes pathway analysis of mRNAs based on
mmu_circRNA_34428. The top 10 enrichment scores of (B) CC, (C) BP,
(D) signaling pathway and (E) MF. BP, biological process; CC,
cellular component; MF, molecular function; circRNA, circular RNA;
miRNA, microRNA; ceRNA, competing endogenous RNA; GO, Gene
Ontology; DE, differentially expressed. |
However, these eight mRNAs were not enough to
predict the function of the four miRNAs as well as
mmu_circRNA_34428. A total of four miRNAs were conserved between
the online TargetScan and miRanda prediction software, including,
mmu-miR-34b-5p, mmu-miR-670-3p, mmu-miR-338-3p and-mmu-miR-3066-3p.
These four miRNAs were validated by RT-qPCR, and mmu-miR-34b-5p,
mmu-miR-670-3p and mmu-miR-338-3p were significantly differentially
expressed between the two groups (P<0.001; Fig. 3F).
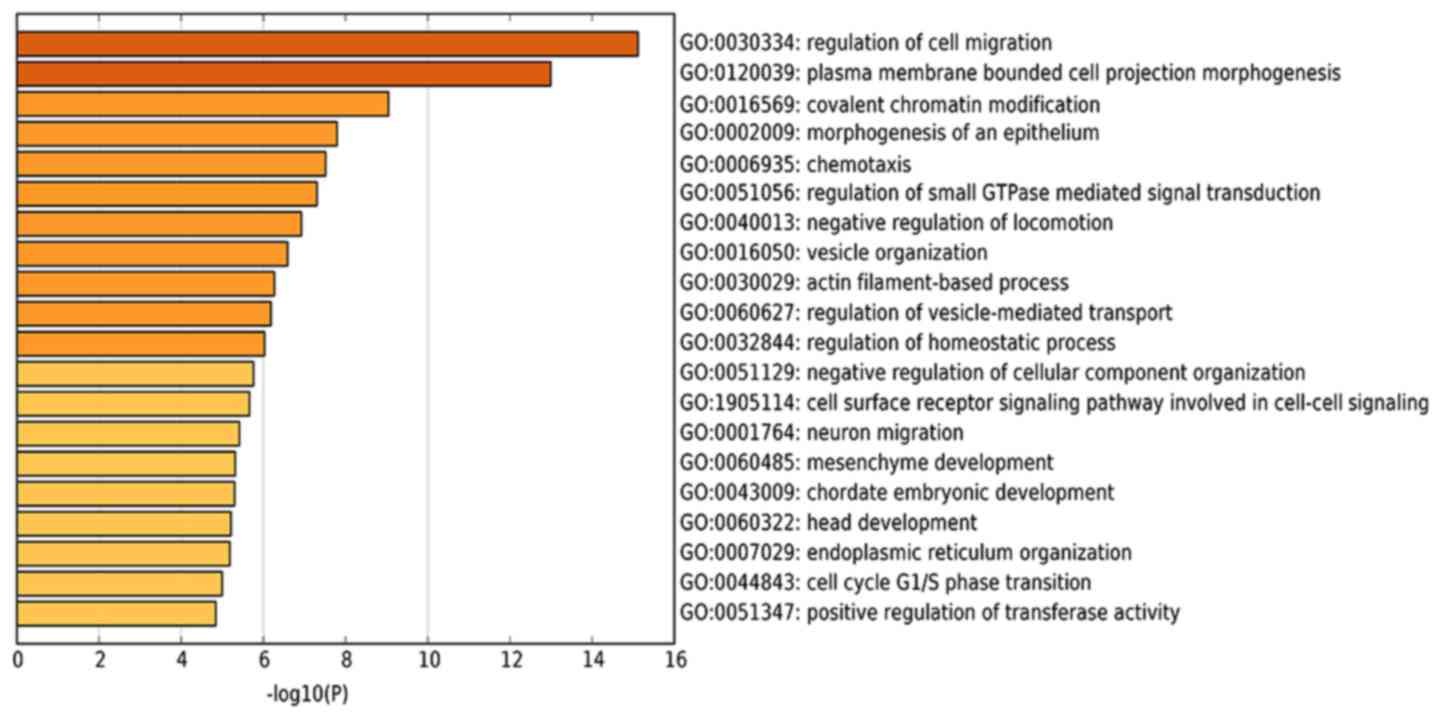
To expand the current understanding of the genetic
functions of mmu_circRNA_34428, GO and KEGG pathway analysis was
utilized based on the predicted results from the online TargetScan
and miRanda analysis (Figs. 4A and
S1). mmu_circRNA_34428 had the
greatest number of interactions with mmu_miR_34b_5p, mmu_miR_670_3p
and mmu_mirR_338_3p within the biological process of cell migration
(Figs. 4B-E and 5).
Discussion
With the growing amount of attention being paid to
circRNAs in the field, increasing evidence has demonstrated their
roles and mechanisms underlying the pathogenesis of many diseases.
Xia et al (29) reported that
the overexpressed circRNA cia-cGAS is the effective inhibitor of
cGAS-mediated autoimmune disease. The most predominant function of
circRNAs is acting as miRNA sponges, and therefore, they could be
ideal biomarkers for diagnosing disease. However, little is known
about the role of circRNAs in LN, particularly in the glomeruli. In
the present study, high-throughput circRNA microarray was utilized
to detect differentially expressed circRNAs that were in the
glomeruli of those with serious LN when compared with corresponding
mild LN glomeruli. Following validation of the expression of 10
circRNAs among the expressed profile, mmu_circRNA_34428 and
mmu_circRNA_27407 were significantly upregulated in severe LN
(P<0.001). Therefore, the most significantly upregulated circRNA
(mmu_circRNA_34428) was selected for investigating the multiple
biological processes of LN development.
An increasing body of evidence has reported that
circRNAs may act as ceRNAs and serve roles in biological functions,
including acting as miRNA sponges to regulate mRNA transcription
and protein production (6,30). In addition, a previous study reported
that circRNAs have more miRNA binding sites and may be more
effective in silencing miRNAs when compared with liner RNAs
(31–33). Therefore, the immune-inflammatory
response mechanisms of circRNAs may occur in LN by miRNA-mediated
effects. The present study demonstrated that mmu_circRNA_34428 was
the most likely to interact (i.e., contain complementary base-pair
sites) with the following four miRNAs: mmu-miR-34b-5p,
mmu-miR-670-3p, mmu-miR-338-3p and mmu-miR-3066-3p. In addition,
the present study validated via RT-qPCR that three miRNAs
(mmu-miR-34b-5p, mmu-miR-670-3p and mmu-miR-338-3p) had
significantly different expression levels between the two groups
(P<0.001).
A previous study demonstrated that miR-34b and
miR-34c are targets of p53 and cooperate in the control of cell
proliferation and adhesion-independent growth (34); miR-34 serves a redundant function in
the p53 pathway, suggesting that there may be additional
p53-independent functions for this family of miRNAs, which was
identified in miR-34-deficient mice (35). In addition, miR-449 and miR-34b/c
function was redundant in murine testes by targeting the E2F
transcription factor-retinoblastoma protein (E2F-pRb) pathway
(36). Research on the p53 pathway
in SLE has discovered the pathway participating in the activation
of inflammatory factors and B-cells (37,38). In
the present ceRNA analysis, mmu-miR-34b-5p was revealed to be one
of the binding miRNAs that strongly associates with
mmu_circRNA_34428. Finally, in line with all the aforementioned
studies that focus on mmu-miR-34b-5p in immune disease, it was
hypothesized that mmu_circRNA_34428 acts as an miRNA sponge to
inhibit the expression and function of mmu-miR-34b-5p.
Similarly, a previous study demonstrated that
miR-338-3p expression inhibits cell proliferation following
expression profile analysis in LO2/HBx-d382 cells (39), which indicates that downregulation of
miR-338-3p may promote cell proliferation. Furthermore, miR-338-3p
was reportedly downregulated in patients with celiac disease with
more severe histological lesions when compared with the controls,
and this affected the expression of innate and adaptive immunity
proteins (40). Zhang et al
(41) demonstrated that miR-338-3p
serves a role as a novel tumor suppressor to prevent the invasion
of renal cell carcinoma by affecting ALK5 (activin receptor-like
kinase 5, ALK5) expression. Thus, the low expression level and
inhibited function of miR-338-3p and miR-670-3p in cell
proliferation as well as in the immune system support the
hypothesis that mmu_circRNA_34428 acts as a miRNA sponge to
accelerate LN development.
In addition, the results from the GO and KEGG
pathway analysis support and identify the important mRNAs in
mmu-circRNA-34428 including biological progresses, cellular
components, molecular function and meaningful biological signaling
pathways. In the KEGG pathway analysis, the AMP kinase signaling
pathway serves a key role in controlling cell growth, cell
proliferation and stability; it also participates in SLE (42,43). The
phosphoinositide-3 kinase/protein kinase B signaling pathway is
also closely associated with SLE (44,45).
mmu_circRNA_34428 was predicted to serve an important role in cell
migration and plasma membrane bounded cell projection
morphogenesis, which is involved in immune cell proliferation and
immune inflammatory responses in SLE (46–48). It
was also assumed that mmu_circRNA_34428 functions as an miRNA
sequestering factor for its predicted miRNA binding partners, as
aforementioned. It has also been reported that cell migration and
morphogenesis were involved in immune pathogenesis and disease
activities in SLE and LN (47). It
is therefore possible to hypothesize that mmu_circRNA_34428
functions in the regulation of chemokine-mediated cell migration,
causing immune factors and cell proliferation to affect LN
development; however, further studies are required in order to
elucidate its mechanism.
There is evidence to suggest that mesangial cell
proliferation is involved in the pathogenesis of LN (49), which can eventually lead to renal
failure. Notably, the present study identified that
mmu_circRNA_34428 was positively associated with LN disease
deterioration in mice depending on the degree of mouse glomerular
lesion, which indicates that a high expression of mmu_circRNA_34428
in LN glomeruli may promote mesangial cell and matrix
proliferation, and glomerular sclerosis progression. Therefore, the
results of the present study indicate that mmu_circRNA_34428 may be
an ideal potential diagnostic biomarker for LN with a high degree
of accuracy, specificity and sensitivity. In the future, further
studies focusing on the circRNA function as miRNA sequestering
factors in the regulation of LN occurrence and development will be
completed. The small sample size in the present study is one
limitation and thus, future experiments using a bigger sample size
are underway. To identify the candidate circRNA for the development
of albuminuria in LN in vivo and in vitro, future
studies will be pursued, including gene sequencing, molecular
biological function analysis, intervention experiment and an
investigation of functional linkage and crosstalk between these
differentially expressed circRNAs and relative proteins of LN.
In conclusion, the present study provided a unique
circRNA profile of LN in NZB/W F1 mice, based on which a
substantial circRNA signature was demonstrated to indicate possible
involvement in LN development. Furthermore, the present study
characterized and functionally evaluated one abundant circRNA,
mmu_circRNA_34428, thereby offering a potential and credible
pathogenicity link and treatment target for LN in the future.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professors XIAOLI
LI, ZILONG LI and LINING WANG affiliated with the Department of
Nephrology, The First Hospital of China Medical University
(Shenyang, China) for acquisition of funding.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81273297), the
Science and Technology Plan of Liaoning Provincial Technology
Department (grant no. 2012225021), the National Science and
Technology Support Program during the 12th five-year plant period
(grant no. 2011BAI10B04) and the Natural Science Foundation of
Liaoning Province (grant no. 201202254).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST and YL designed the study, curated the data and
performed the analysis. YL and LY acquired the funding. ST and XL
performed the experiments. YL performed the project administration
and supervised the experiments. ST, YL, QF, JM and LY assisted in
analysing, integrating and checking the data. ST assisted with the
application of the software and wrote the manuscript. YL wrote and
reviewed the manuscript.
Ethics approval and consent to
participate
The present study complied with the protocols
approved by the Institutional Animal Care and Use Committee at
China Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Plantinga L, Lim SS, Patzer R, McClellan
W, Kramer M, Klein M, Pastan S, Gordon C, Helmick C and Drenkard C:
Incidence of end-stage renal disease among newly diagnosed systemic
lupus erythematosus patients: The georgia lupus registry. Arthritis
Care Res (Hoboken). 68:357–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hahn BH, McMahon MA, Wilkinson A, Wallace
WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ,
Yazdany J, et al: American College of Rheumatology guidelines for
screening, treatment, and management of lupus nephritis. Arthritis
Care Res (Hoboken). 64:797–808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cameron JS: Lupus nephritis. J Am Soc
Nephrol. 10:413–424. 1999.PubMed/NCBI
|
|
4
|
Tsao BP: Genetic susceptibility to lupus
nephritis. Lupus. 7:585–590. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frangou EA, Bertsias GK and Boumpas DT:
Gene expression and regulation in systemic lupus erythematosus. Eur
J Clin Invest. 43:1084–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: Circular RNAs are
abundant, conserved, and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szabo L, Morey R, Palpant NJ, Wang PL,
Afari N, Jiang C, Parast MM, Murry CE, Laurent LC and Salzman J:
Statistically based splicing detection reveals neural enrichment
and tissue-specific induction of circular RNA during human fetal
development. Genome Biol. 16:1262015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YH, Yu XH, Luo SS and Han H:
Comprehensive circular RNA profiling reveals that circular
RNA100783 is involved in chronic CD28-associated CD8(+)T cell
ageing. Immun Ageing. 12:172015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lal N, White BS, Goussous G, Pickles O,
Mason MJ, Beggs AD, Taniere P, Willcox BE, Guinney J and Middleton
GW: KRAS mutation and consensus molecular subtypes 2 and 3 are
independently associated with reduced immune infiltration and
reactivity in colorectal cancer. Clin Cancer Res. 24:224–233. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouyang Q, Wu J, Jiang Z, Zhao J, Wang R,
Lou A, Zhu D, Shi GP and Yang M: Microarray expression profile of
circular RNAs in peripheral blood mononuclear cells from rheumatoid
arthritis patients. Cell Physiol Biochem. 42:651–659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang D, Fujio K, Jiang Y, Zhao J, Tada N,
Sudo K, Tsurui H, Nakamura K, Yamamoto K, Nishimura H, et al:
Dissection of the role of MHC class II A and E genes in autoimmune
susceptibility in murine lupus models with intragenic
recombination. Proc Natl Acad Sci USA. 101:13838–13843. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sitrin J, Suto E, Wuster A,
Eastham-Anderson J, Kim JM, Austin CD, Lee WP and Behrens TW: The
Ox40/Ox40 ligand pathway promotes pathogenic Th cell responses,
plasmablast accumulation, and lupus nephritis in NZB/W F1 mice. J
Immunol. 199:1238–1249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takemoto M, Asker N, Gerhardt H, Lundkvist
A, Johansson BR, Saito Y and Betsholtz C: A new method for large
scale isolation of kidney glomeruli from mice. Am J Pathol.
161:799–805. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adachi H and Yu YT: Purification of
radiolabeled RNA products using denaturing gel electrophoresis.
Curr Protoc Mol Biol. 105:Unit 4.20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aranda PS, LaJoie DM and Jorcyk CL: Bleach
gel: A simple agarose gel for analyzing RNA quality.
Electrophoresis. 33:366–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Phelps M, Coss C, Wang H and Cook M;
Reproducibility Project, : Cancer Biology; Reproducibility Project
Cancer Biology: Registered report: Coding-independent regulation of
the tumor suppressor PTEN by competing endogenous mRNAs. Elife.
5:2016. View Article : Google Scholar
|
|
21
|
Jin X, Feng CY, Xiang Z, Chen YP and Li
YM: CircRNA expression pattern and circRNA-miRNA-mRNA network in
the pathogenesis of nonalcoholic steatohepatitis. Oncotarget.
7:66455–66467. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Zhang S, Wu J, Cui J, Zhong L,
Zeng L and Ge S: circRNA_100290 plays a role in oral cancer by
functioning as a sponge of the miR-29 family. Oncogene.
36:4551–4561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang M, Zhong Z, Lv M, Shu J, Tian Q and
Chen J: Comprehensive analysis of differentially expressed profiles
of lncRNAs and circRNAs with associated co-expression and ceRNA
networks in bladder carcinoma. Oncotarget. 7:47186–47200.
2016.PubMed/NCBI
|
|
24
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcia DM, Baek D, Shin C, Bell GW,
Grimson A and Bartel DP: Weak seed-pairing stability and high
target-site abundance decrease the proficiency of lsy-6 and other
microRNAs. Nat Struct Mol Biol. 18:1139–1146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Enright AJ, John B, Gaul U, Tuschl T,
Sander C and Marks DS: MicroRNA targets in Drosophila. Genome Biol.
5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia P, Wang S, Ye B, Du Y, Li C, Xiong Z,
Qu Y and Fan Z: A circular RNA protects dormant hematopoietic stem
cells from DNA Sensor cGAS-mediated exhaustion. Immunity.
48:688.e7–701.e7. 2018. View Article : Google Scholar
|
|
30
|
Chen I, Chen CY and Chuang TJ: Biogenesis,
identification, and function of exonic circular RNAs. Wiley
Interdiscip Rev RNA. 6:563–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wilusz JE and Sharp PA: Molecular biology.
A circuitous route to noncoding RNA. Science. 340:440–441. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomas LF and Sætrom P: Circular RNAs are
depleted of polymorphisms at microRNA binding sites.
Bioinformatics. 30:2243–2246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dudekula DB, Panda AC, Grammatikakis I, De
S, Abdelmohsen K and Gorospe M: CircInteractome: A web tool for
exploring circular RNAs and their interacting proteins and
microRNAs. RNA Biol. 13:34–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang TC, Wentzel EA, Kent OA,
Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M,
Ferlito M, Lowenstein CJ, et al: Transactivation of miR-34a by p53
broadly influences gene expression and promotes apoptosis. Mol
Cell. 26:745–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Concepcion CP, Han YC, Mu P, Bonetti C,
Yao E, D'Andrea A, Vidigal JA, Maughan WP, Ogrodowski P and Ventura
A: Intact p53-dependent responses in miR-34-deficient mice. PLoS
Genet. 8:e10027972012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao J, Li D, Wang L, Wu J, Hu Y, Wang Z,
Chen Y, Cao X, Jiang C, Yan W and Xu C: MicroRNA-449 and
microRNA-34b/c function redundantly in murine testes by targeting
E2F transcription factor-retinoblastoma protein (E2F-pRb) pathway.
J Biol Chem. 287:21686–21698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miret C, Molina R, Filella X,
García-Carrasco M, Claver G, Ingelmo M, Ballesta A and Font J:
Relationship of p53 with other oncogenes, cytokines and systemic
lupus erythematosus activity. Tumour Biol. 24:185–188. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luo S, Liu Y, Liang G, Zhao M, Wu H, Liang
Y, Qiu X, Tan Y, Dai Y, Yung S, et al: The role of microRNA-1246 in
the regulation of B cell activation and the pathogenesis of
systemic lupus erythematosus. Clin Epigenetics. 7:242015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu X, Tan D, Hou Z, Hu Z, Liu G, Ouyang Y
and Liu F: The effect of miR-338-3p on HBx deletion-mutant
(HBx-d382) mediated liver-cell proliferation through CyclinD1
regulation. PLoS One. 7:e432042012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Magni S, Buoli Comani G, Elli L, Vanessi
S, Ballarini E, Nicolini G, Rusconi M, Castoldi M, Meneveri R,
Muckenthaler MU, et al: miRNAs affect the expression of innate and
adaptive immunity proteins in celiac disease. Am J Gastroenterol.
109:1662–1674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang X, Wang C, Li H, Niu X, Liu X, Pei
D, Guo X, Xu X and Li Y: miR-338-3p inhibits the invasion of renal
cell carcinoma by downregulation of ALK5. Oncotarget.
8:64106–64113. 2017.PubMed/NCBI
|
|
42
|
Benatti FB, Miyake CNH, Dantas WS,
Zambelli VO, Shinjo SK, Pereira RMR, Silva MER, Sá-Pinto AL, Borba
E, Bonfá E and Gualano B: Exercise increases insulin sensitivity
and skeletal muscle AMPK expression in systemic lupus
erythematosus: A randomized controlled trial. Front Immunol.
9:9062018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schuiveling M, Vazirpanah N, Radstake
TRDJ, Zimmermann M and Broen JCA: Metformin, a new era for an old
drug in the treatment of immune mediated disease? Curr Drug
Targets. 19:945–959. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Badr G, Sayed A, Abdel-Maksoud MA, Mohamed
AO, El-Amir A, Abdel-Ghaffar FA, Al-Quraishy S and Mahmoud MH:
Infection of female BWF1 lupus mice with malaria parasite
attenuates B cell autoreactivity by modulating the CXCL12/CXCR4
axis and its downstream signals PI3K/AKT, NFκB and ERK. PLoS One.
10:e01253402015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Beşliu AN, Pistol G, Marica CM, Bănică LM,
Chiţonu C, Ionescu R, Tănăseanu C, Tamsulea I, Matache C and
Stefănescu M: PI3K/Akt signaling in peripheral T lymphocytes from
systemic lupus erythematosus patients. Roum Arch Microbiol Immunol.
68:69–79. 2009.PubMed/NCBI
|
|
46
|
Qingjuan L, Xiaojuan F, Wei Z, Chao W,
Pengpeng K, Hongbo L, Sanbing Z, Jun H, Min Y and Shuxia L:
miR-148a-3p overexpression contributes to glomerular cell
proliferation by targeting PTEN in lupus nephritis. Am J Physiol
Cell Physiol. 310:C470–C478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Adalid-Peralta L, Mathian A, Tran T,
Delbos L, Durand-Gasselin I, Berrebi D, Peuchmaur M, Couderc J,
Emilie D and Koutouzov S: Leukocytes and the kidney contribute to
interstitial inflammation in lupus nephritis. Kidney Int.
73:172–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schiffer L, Bethunaickan R, Ramanujam M,
Huang W, Schiffer M, Tao H, Madaio MP, Bottinger EP and Davidson A:
Activated renal macrophages are markers of disease onset and
disease remission in lupus nephritis. J Immunol. 180:1938–1947.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ichinose K, Rauen T, Juang YT, Kis-Toth K,
Mizui M, Koga T and Tsokos GC: Cutting edge:
Calcium/Calmodulin-dependent protein kinase type IV is essential
for mesangial cell proliferation and lupus nephritis. J Immunol.
187:5500–5504. 2011. View Article : Google Scholar : PubMed/NCBI
|