Introduction
The effect of air pollution on the human body has
become an important problem in China and the focus of research.
According to epidemiological data, mortality due to respiratory
disease in north China significantly correlates with winter fog and
haze (1). Mixed pollutants,
including particulate matter (PM) and sulfide and nitrogen oxides,
have become the main causes of cardiovascular diseases in winter
(2). The deadliest form of air
pollution during haze is atmospheric particulate matter with a
diameter <2.5 µm (PM2.5) (3).
PM2.5 is associated with increased morbidity and mortality from
respiratory and cardiovascular diseases such as cardiac arrhythmia,
congestive heart failure, and ischemic heart disease (4,5).
Although most research on the links between
pathology and pollution have focused on cardiovascular and
respiratory effects, there are also indications that air pollutants
affect the human gastrointestinal tract (6). Epidemiological studies have revealed
associations between air pollution exposure and various
gastrointestinal diseases, including inflammatory bowel disease
(7), appendicitis (8), irritable bowel syndrome (9) and enteric infections in infants
(10). A rise in total measured air
pollutants was associated with an increase in hospitalizations for
inflammatory bowel disease (11) and
abdominal pain (3). Airborne PM2.5
in particular increases the permeability of the intestinal gut,
disrupting the epithelial barrier and thereby triggering
gastrointestinal disorders (11).
The gut microbiota is an important contributor to
human health (12). A dysfunctional
gut microbiome has been implicated in the development of many
disorders, such as diabetes (13),
obesity (14) and cardiovascular
disease (15). A study of pollutants
reported that smoking may exert pathological effects, at least in
part by regulating intestinal microbiota (11).
Little is known about how air pollution influences
the gut microbiome (16), but there
is evidence that the gastrointestinal tract can be affected by
respiratory tract exposure to PM2.5 (17). Human studies have shown that
mucociliary air pollutants are cleared quickly from the lungs
(18). The documented effect of air
pollutants on the respiratory and gastrointestinal systems, and the
effect of tobacco smoke on intestinal microbiota, supports the need
for more information regarding how PM2.5 may influence the gut
microbiota.
Spontaneously hypertensive rats (SHR) have been
deemed suitable for studying interactions of the digestive and
respiratory systems under air pollutant exposure (11). To gain greater understanding of such
interactions, in the present study we investigated alterations in
the intestinal flora microbiota of rats challenged by PM2.5
exposure, using 16S rDNA sequencing.
Materials and methods
SHR rats
Specific pathogen-free SHR male rats (n=10; aged
8–11 weeks; weight 200±10 g) were purchased from Beijing Weitong
Lihua Animal Technology (license number SCXK Beijing 2012-0001) and
housed at 22±2°C and 45–55% humidity, with natural day and night
hours and natural light. Food and water were provided ad
libitum. Adaptive feeding was allowed for one week prior to
experiments while their activity and eating was observed.
The present study was approved by the Ethics
Committee of the School of Basic Medical Sciences, Jilin University
(Changchun, China).
Source and treatment with PM2.5
The PM2.5 used in the present study was collected
from Shenyang city atmosphere with a 120 F flow dust sampler
(sampling flow, 1,000 l/min). A suspension of PM2.5 dust (4 mg/ml)
was prepared with sterile saline solution (19).
Preparation of mixed gases
Standard mixed gas was provided by Dalian Special
Gas Industry (SO2 2013.1×10−6; NO2
1187.0×10−6; CO 23199.6×10−6). The cylinder
filling pressure was 9.0 MPa and the volume was 40 l.
Animal handling
The Institutional Animal Care and Use Committee of
Shenyang Medical College approved the protocols for handling the
rats. The rats were anesthetized with an inhalation of nose and
mouth of chloral hydrate (0.7 mg/100 g) prior to each treatment
with PM2.5 dust-saline.
The rats were dosed with PM2.5 dust-saline (1 ml of
4 mg/ml) once per week for 12 weeks, using a non-exposed tracheal
perfusion method (19). Each rat was
treated individually to ensure the dosage. The rats breathed mixed
gases (SO2 2013.1 mg/m3; NO2,
1187.0 mg/m3; CO, 23199.6 mg/m3) by dynamic
inhalation (19). The flow rate of
the mixed gas was 1.2 l/min, each exposure time was 3 h.
The control group rats inhaled normal air in the
cage. During the experimental period, each rat was housed
individually in separate cages.
Sampling set up
Fecal samples were collected randomly from 3 rats at
0, 7, 15, 30, 60 and 90 days, <5 g fecal sample was collected
from each individual.
Stool DNA extraction
A Qiagen 51504 QIAamp DNA Stool Mini kit (Qiagen
GmbH) was used to extract the total DNA. The quantity of the
extracted DNA was determined with a NanoDrop 2000 (Thermo Fisher
Scientific, Inc.).
Amplicon generation and the quality
control of PCR products
The PCR primers were selected based on the sequences
of the V3 and V4 hyper-variable regions of the bacterial 16S rRNA
gene. The microbial V3-V4 region was amplified by PCR using the
primers F: 5′-ACTCCTACGGGAGGCAGCA and R: 3′-GGACTACHVGGGTWTCTAAT
(20). All PCR reactions were
performed with Phusion High-Fidelity PCR Master Mix (New England
Biolabs, Inc.). The quality of the PCR products was determined by
electrophoresis with 2% agarose gel for detection. Samples with
bright main strips between 400–450 bp were chosen for further
experiments. PCR products were mixed in equidensity ratios. The
mixed PCR products were purified with a Qiagen Gel Extraction kit
(Qiagen).
Library preparation and sequencing
process
Sequencing libraries were generated using a TruSeq
DNA PCR-free sample preparation kit (Illumina, Inc.) in accordance
with the manufacturers recommendations, and index codes were added.
The library quality was assessed on the Qubit 2.0 fluorometer
(Thermo Fisher Scientific, Inc.). Lastly, the library was sequenced
on an Illumina HiSeq 2500 platform (Illumina, Inc.) and 250-bp
paired-end reads were generated.
Data analysis
The reads were merged using the FLASH tool, which
merges paired-end reads from the original DNA fragments. Quality
filtering on the raw tags was performed under specific filtering
conditions in accordance with the QIIME (v1.9.1) quality-controlled
process. Sequences that overlapped by more than 10 bp were
assembled and junk reads were discarded.
Production sequences with ≥97% similarity were
assigned to the same operational taxonomic unit (OTU). The
representative sequence for each OTU was screened for further
annotation. Species annotation for each representative sequence was
picked from each OTU. The Green Gene Database was used based on the
RDP3 classifier phylogenetic tree. The relatedness of different
OTUs, and the differences in the dominant species in different
samples (groups), was conducted using MUSCLE software (version
3.8.31). OTU abundance information was normalized using a standard
sequence number corresponding to the sample with the least
sequences.
Alpha diversity and the community richness index
were calculated with QIIME (version 1.9.1) and displayed with R
software (version 2.15.3). Beta diversity of weighted and
unweighted UniFrac values were calculated using QIIME (version
1.9.1). Cluster analysis was preceded by principal component
analysis with the ‘ggplot-2’ package in the R software (version
2.15.3). Principal coordinates analysis (PCoA) was performed to
obtain the principal coordinates and visualize the complex
multidimensional data. UPGMA (unweighted pair group method with
arithmetic mean) clustering was performed for hierarchical
clustering to interpret the distance matrix using average linkage
and was conducted using QIIME (version 1.9.1).
PICRUSt (phylogenetic investigation of communities
by reconstruction of unobserved states) bioinformatics software was
applied for predicting the gene family abundance of bacterial
communities, based on the 16S rDNA gene data and a database of
reference genomes (21). PICRUSt
consisted of two steps, gene content inference and metagenome
inference, performed as previously described (22). The t-test (SPSS 19.0; Chicago, IL,
USA) was used to analyse the data with normal distribution.
P<0.05 was defined as the standard criterion for statistical
significance (23).
Results
Changes in the gut microbiota
associated with PM2.5 exposure
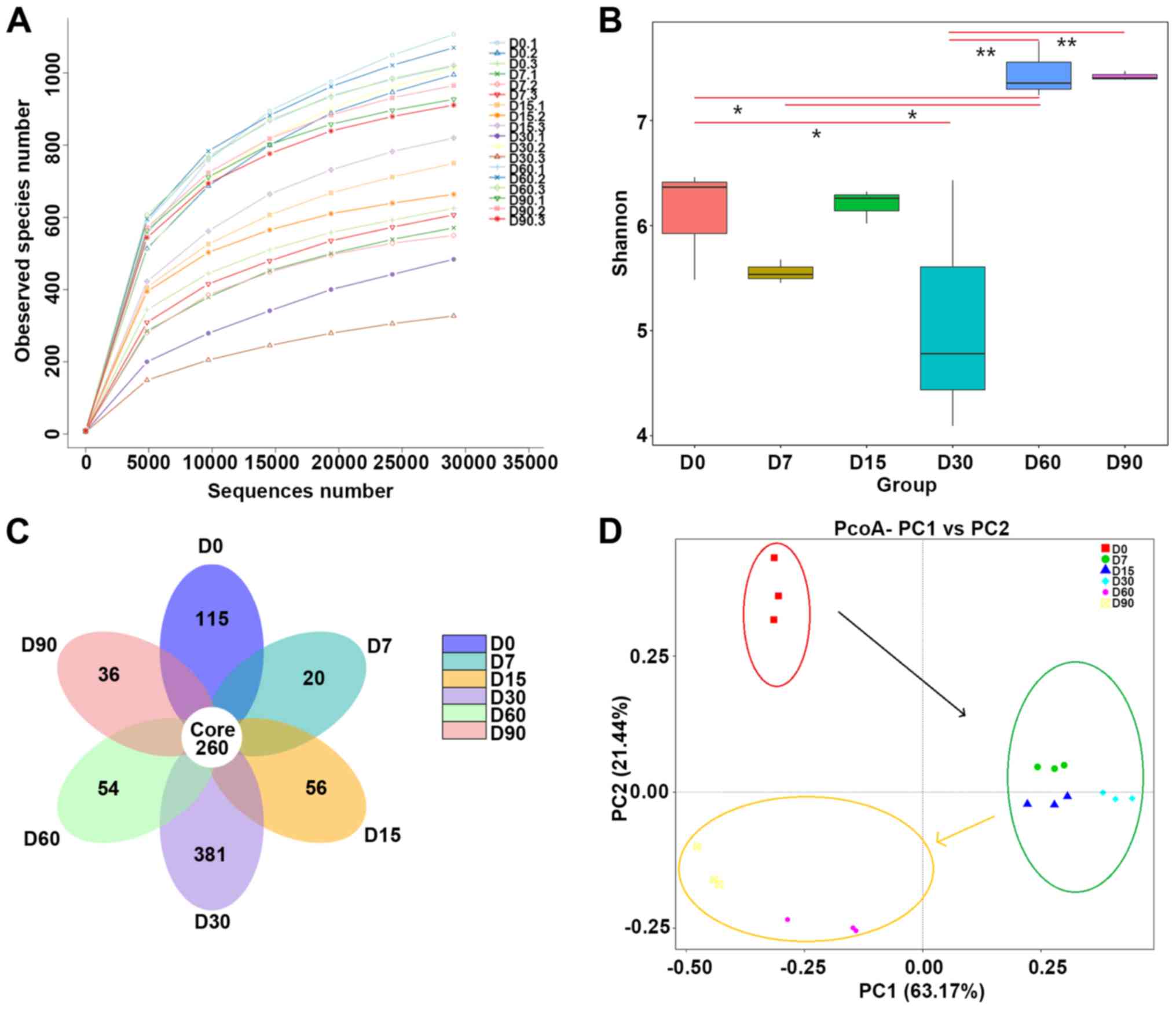
To investigate changes in the gut microbiota in
response to PM2.5, we performed amplicon sequencing of the fecal
samples from the exposed rats at 6 sampling timepoints (0, 7, 15,
30, 60 and 90 days). Rarefaction curves revealed no new observed
species after 20,000 reads, which meant that almost all bacterial
species were detected in all samples (Fig. 1A).
Based on 97% sequence similarity, all the sequences
of regions V3-V4 were clustered into 10,887 bacterial OTUs. The
diversity of the microbial communities was measured using Shannon
diversity indices (Fig. 1B). We
found that pollutant treatment was associated with these results;
there was a significant reduction in bacterial diversity at 7 days.
At day 60, the bacterial diversity was higher (Shannon index =
7.45) than at day 0 (Shannon index = 6.103). According to the flora
data (Fig. 1C), the fecal samples
shared 260 different OTUs. The day-30 fecal sample was the most
populous among the independent OTUs (381 independent OTUs), and the
next populous was the day 0 sample (115 independent OTUs).
The beta diversity of the bacterial communities
associated with rats was investigated through PCoA, which was
performed on the phylogenetic beta-diversity matrix obtained by
UniFrac (Fig. 1D). The samples
exhibited good repeatability. Moreover, ANOSIM of the weighted
UniFrac distances revealed significant differences in the bacterial
communities between groups (R=0.0689, P=0.033).
Intestinal bacteria at the phylum
level
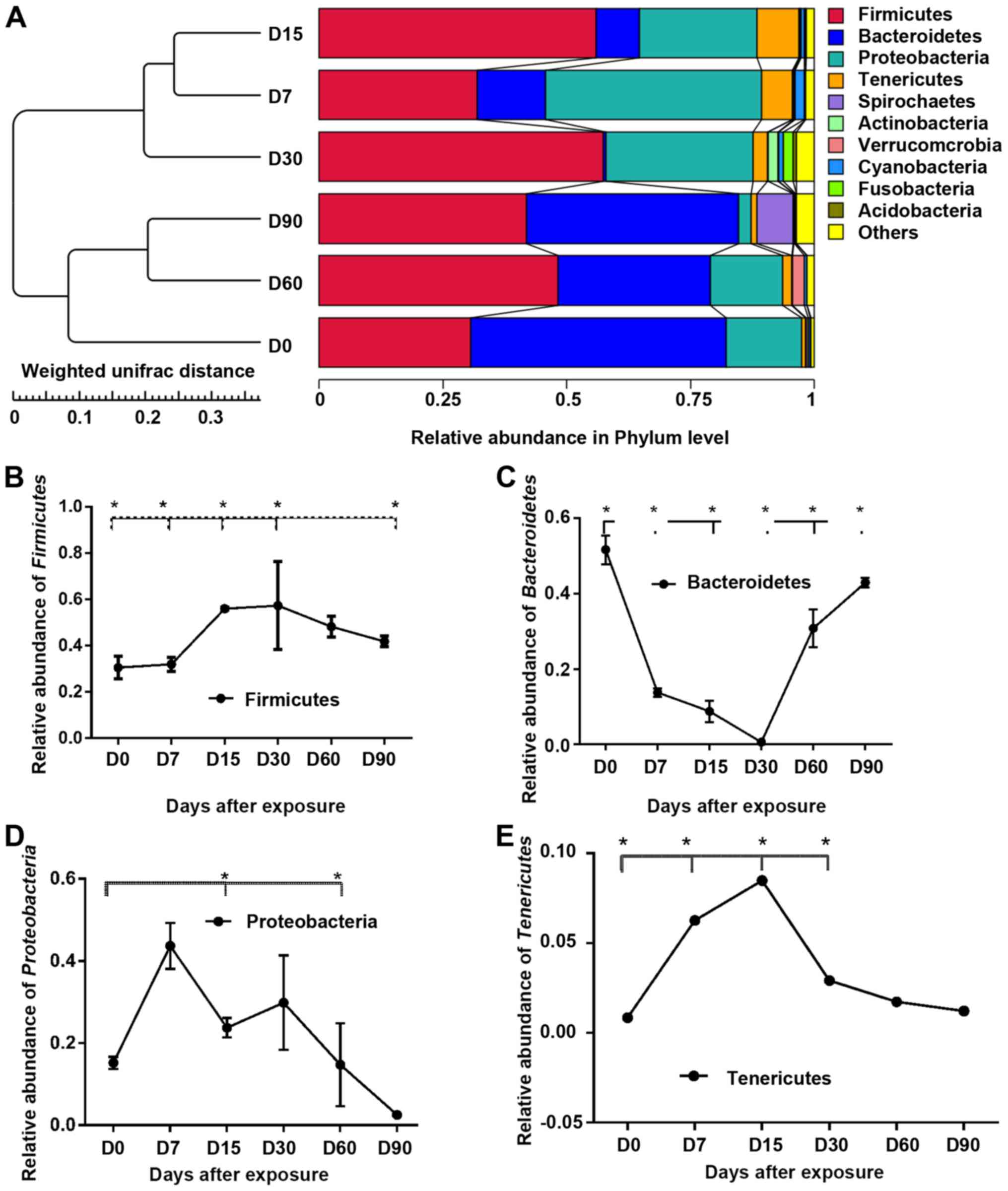
Taxonomic assignment analysis at the phylum level is
shown in Fig. 2A. After the
bacterial OTU representative sequences were taxonomically
classified, the results showed that the most abundant and common
phyla (abundance within the community ≥1%) in all samples were
Firmicutes (35%), Bacteroidetes (29%),
Proteobacteria (17%) and Tenericutes (1%).
Over time, the relative abundance of bacteria
changed to a great extent at the phylum level (Fig. 2B-E). Statistically significant
differences of the top phyla were found (compared with the day 0
sample), as follows: day 7, Firmicutes, Bacteroidetes and
Tenericutes; day 15, Firmicutes, Bacteroidetes,
Proteobacteria and Tenericutes; day 30,
Bacteroidetes; day 60, Proteobacteria and
Tenericutes; and day 90, Firmicutes and
Bacteroidetes. Compared with the day 0 sample, after 30 days
of exposure Firmicutes increased by 37% and
Tenericutes increased by 44%, and there were significant
decreases in Bacteroidetes (−17%) and Proteobacteria
(−83%).
Alterations in the gut bacterial
compositions
To investigate further the effects of air pollutants
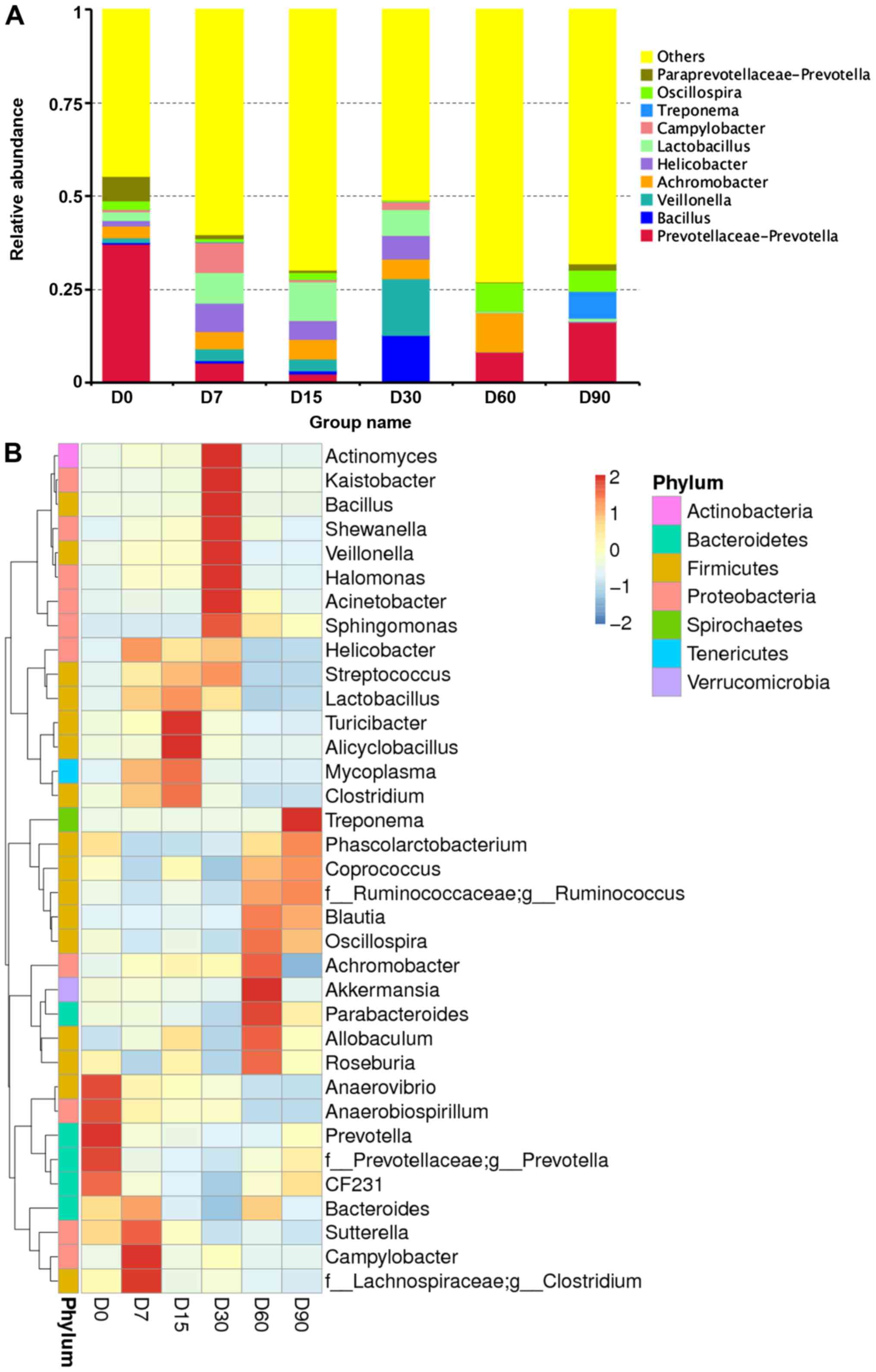
on the intestinal microflora of SHR rats, 209 genera were
identified from the gut bacterial communities of the samples. Among
these, 9 abundant genera constituted >0.1% of the total
sequences in at least one sample (Fig.
3A). These were: Prevotellaceae-Prevotella (37.25%),
Bacillus (0.45%), Veillonella (1.23%),
Achromobacter (3.11%), Helicobacter (2.31%),
Lactobacillus (1.42%), Campylobacter (0.59%),
Paraprevotellaceae-Prevotella (6.93%) and
Oscillospira (2.41%).
Compared with the day-0 sample, the most significant
differences in OTUs at the genus level were the following (Fig. 3B and C): Cetobacterium,
Mycoplasma, Treponema, Actinobacillus, Prevotella, Odoribacter,
Achromobacter, Spironema, Fusobacterium, Campylobacter,
Clostridium and Parvimonas. The result showed that two
genera (Actinobacillus and Fusobacterium)
significantly decreased after 30 days. However, the bacterial
community showed significant increases in relative abundance of
only one genus (Treponema). During 30 days of incubation,
other bacterial genera showed dramatic fluctuations in abundance
but, no changes were observed after 30 days.
Functional maturation of the gut
bacterial community and the shifts of disease-involved genes in gut
metagenomics
To investigate how pollutants affect the functional
profile of gut microbiota, PICRUSt analysis was used to analyse the
KEGG pathway compositions in bacterial populations (Fig. 4A). The result suggested that most
predicted genes were enriched in the following pathways: organismal
systems, cellular processes, environmental information processing,
human diseases, genetic information processing and metabolism.
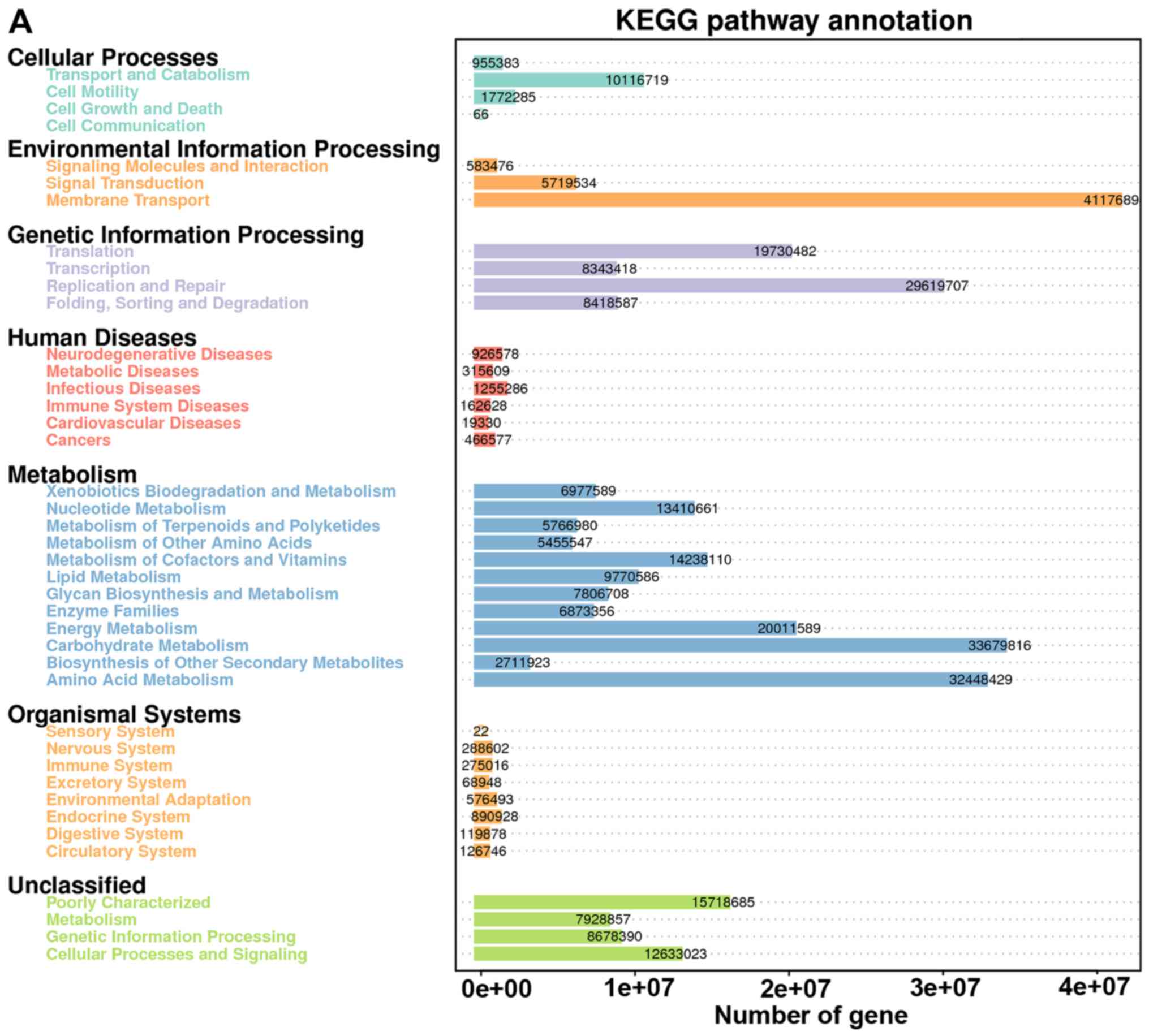 | Figure 4.KEGG pathway annotation and the
quantitative distribution of the gene enrichment. (A) Overview of
the predicted data. (B) Shifts in gut bacterial functional profiles
as the pollutant treated SHR rats. Heat map and hierarchical
clustering of differentially abundant KEGG pathways identified at 6
sampled time-points (0, 7, 15, 30, 60 and 90 days). The values of
color in the heat map represent the normalized relative abundance
of KEGG pathways (log 10). Heat map and hierarchical clustering of
differentially abundant KEGG pathways identified at 6 sampled
time-points (0, 7, 15, 30, 60 and 90 days). The values of color in
the heat map represent the normalized relative abundance of KEGG
path. (C-E) Analysis was performed to identify the significantly
differentially abundant of selected pathways (human diseases,
cardiovascular diseases, and environmental information processing)
among groups and day 0 sample. Asterisks indicate the significant
differences that were obtained between D0 sample and samples of
following observational days (*0.01<P<0.05, **P<0.01). |
The changes in most genes from treatment groups of
SHR rats did not correlate with the time-points (Fig. 4B). Changes in gut function or genes
in the SHR rats were not linear after prolonged exposure to the
pollutants. Genes related to human diseases increased significantly
(23%) prior to 30 days (Fig. 4C).
Surprisingly, the genes related to human diseases significantly
decreased after long-term pollutant exposure (15% lower at day 90
compared with that of day 0).
The percentage of genes linked to cardiovascular
diseases peaked at day 15 (Fig. 4D).
Moreover, the genes detected before day 15 were all significantly
higher than the baseline (day 0), and then decreased after 15 days.
At day 90, the percentage of genes related to cardiovascular
disease was significantly lower than that at day 0.
Most genes were annotated and enriched to the
environmental information-processing pathway (Fig. 4E). Compared with that of day 0, the
enrichment of related genes increased significantly at day 7, 15,
30, 60 and 90 (Fig. 4E).
Discussion
In the present study, we investigated shifts in gut
microbial populations in SHR rats within 90 days of continuous
exposure to PM2.5 air pollutant. The results revealed changes in
gut microbiota composition and functional adaptation of the gut
bacterial community associated with long-term pollutant
exposure.
A recent study showed that Bacteroidaceae,
Oscillospira, and Ruminococcus are the most abundant
genera in the guts of Wistar rats reared under normal conditions
(24). By contrast, the present
study found that Prevotellaceae-Prevotella,
Paraprevotellaceae-Prevotella and Achromobacter were the
most abundant. The gut microbiota structure of the SHR rat is
different from those of Wistar rats (1). In previous studies, PM2.5 was thought
to affect the microbial community in the gut after going through
the digestive tract (25). In this
study, we found a significant decrease in alpha diversity in the
gut bacterial community associated with exposure to air pollutants.
To some extent, damaging effects of air pollutants on gut microbial
diversity were found in animal experiments.
In the present study, after long-term exposure the
restoration of intestinal microbial diversity indicated that the
intestinal system of rats has a certain ability to maintain
microbial diversity. Whether humans have such rapid recovery is
unknown. The decrease in diversity during the early days (0–15
days) of exposure indicated gut diseases and simplification of
metabolic types. This matches previous epidemiological
investigation results, in which PM could alter the gut microbiome
and result in gut disease (6). How
this process was triggered and whether it is related to the
influence of PM2.5 on microbiota colonization in the
gastrointestinal tract (26) remains
unknown. In particular, in the present study the change in gut
microbes in the SHR rats was characterized by a major transition
from Bacteroidetes to Firmicutes under pollutant
exposure (Fig. 2A). Our results
showed significant decline in the relative abundances of 2 phyla
(Bacteroidetes and Spirochaetea) after 90 days of
long-term exposure, and the number of phyla did not significantly
increase. In the present study, pollutant exposure did not affect
the structure of the phyla in the SHR rats, but the quantitative
changes were significant and responded rapidly to pollutant
exposure.
At the genus level, Cetobacterium, Mycoplasma,
Treponema, Actinobacillus, Prevotella and Odoribacter
were the genera that declined most significantly after 90 days
(>5% decline). Mycoplasma and Treponema are specific pathogens
that are associated with chronic respiratory infections (28). These pathogens that are common in the
respiratory tract, which changed dramatically in the intestinal
tract, and the mechanism of this change is unclear. Moreover, the
flora of the lungs and intestines may interchange and colonize via
the lymphatic system (25). Our
experiments seem to confirm this finding.
Prevotella is a newly discovered strain in a
recently isolated genus that includes 20 species (16), with the most common species being a
black pigmented strain (P. melaninogenica). This genus is
mainly concentrated in the healthy human oral cavity, female
genital tract (29). It is a common
opportunistic pathogen in the clinic and can cause endogenous
infections, especially of the female genital tract and oral cavity
(29). Thus, Actinobacillus
may be a candidate pathogen of the host upper respiratory tract,
digestive tract, and urogenital tract; it belongs to thousands of
normal flora. In the present study, the change in the abundance of
Prevotella may be related to the oral perfusion of the
pollutants.
Odoribacter was reported as enriched in mice with
colorectal cancer and may be related to tumor development (30). Genera of the bacterial phyla
Cetobacterium may be related to digestive tract function.
Cetobacterium is associated with the biosynthesis of acetic
acid (31).
It is reported that intestinal microbiota metabolism
of choline/phosphatidylcholine produces trimethylamine (TMA), which
is further metabolized to a proatherogenic species,
trimethylamine-N-oxide (TMAO) (15). TMAO is closely related to the
increased occurrence of major adverse events of cardiovascular
diseases. The bacterial community and its composition provide
different TMAOs (32). Increased
TMAO levels will significantly affect systemic cholesterol
accumulation, leading to increased generation of atherosclerotic
plaque (33).
The gut microbiota have also been proven to be
involved in the anabolism of TMAO as the producer of the precursor
(TMA) (34). Microecology studies
have shown that higher TMAO plasma concentrations were associated
with the Prevotella enterotype, as opposed to the
Bacteroides enterotype (34).
In the present study, from investigation at the genera level we
found that Prevotellaceae-Prevotella (relative abundance
37.25%) is the most enriched genus in all samples. Moreover,
pollutant exposure reduced the abundance of this population within
30 days (Fig. 3C). This may be
related to the intestinal microbial background of SHR rats
(24), the relative abundance of
Bacteroides was decreased after treatment with pollutant (Fig. 3A). This may suggest that a metabolic
disorder of TMAO leads to an increased risk of cardiovascular
disease at the microbial level.
PICRUSt is a closed-access analysis based on an
established database. It is not comprehensive, but can provide
accurate directional guidance. In this study, the rat intestinal
metagenome responded to pollutant exposure within 30 days (Fig. 4). After 30 days, the intestinal
metagenome showed an ability to repair damage. The accumulation of
cardiovascular disease-related genes in the gut showed a
statistically significant association with air pollutants. This is
consistent with other reports (33).
Previous studies have shown that, in response to changes in
environmental factors, the changes in microbial functional
diversity were greater than changes in system diversity (35). The evidence from metagenomic studies
may be more useful for clinical studies than that of microbial
systematic investigations.
In conclusion, herein the effects of air pollutants
on the gut microbiota of SHR rats are reported. Moreover,
statistically significant changes in the microbiota were
investigated. Changes in phylum levels indicate that the intake of
air pollutants highly affects the lower taxa, and abnormalities in
metabolism and nutrient absorption may be triggered by the intake
of air contaminants. The pathological features of these changes
require further investigation. The results of this study support
that the consequences and destruction of air pollutants on the
microbial structure of the intestinal tract are no less than that
of the respiratory system.
Acknowledgements
We would like to thank the undergraduate students of
SYMC: Gang Li, Yuting Deng and Guotong Zhao for taking care of the
rats, and Jiamiao Wang, Xin Chen and Sun Rui for the pollutant
exposure experiment.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 30872083), the Shenyang
Science and Technology Public Welfare Research Project (4190070101)
and the Shenyang Science and Technology Project (grant nos.
F13-221-9-36 and F14-181-1-00) by the Science and Technology Bureau
of Shenyang.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors contributions
DC wrote the manuscript. CX and HJ helped with the
animal handling. DC, BY and JN contributed to DNA extraction and
PCR. SY and YS were responsible for the data collection and
analysis. YZ and XW were in charge of the library preparation and
sequencing process. The final version was read and adopted by all
the authors.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the School of Basic Medical Sciences, Jilin University
(Changchun, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma M, Li S, Jin H, Zhang Y, Xu J, Chen D,
Kuimin C, Yuan Z and Xiao C: Characteristics and oxidative stress
on rats and traffic policemen of ambient fine particulate matter
from Shenyang. Sci Total Environ. 526:110–115. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stockfelt L, Andersson EM, Molnár P,
Gidhagen L, Segersson D, Rosengren A, Barregard L and Sallsten G:
Long-term effects of total and source-specific particulate air
pollution on incident cardiovascular disease in Gothenburg, Sweden.
Environ Res. 158:61–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen H, Goldberg MS and Villeneuve PJ: A
systematic review of the relation between long-term exposure to
ambient air pollution and chronic diseases. Rev Environ Health.
23:243–297. 2008.PubMed/NCBI
|
|
4
|
Chen YC, Weng YH, Chiu YW and Yang CY:
Short-term effects of coarse particulate matter on hospital
admissions for cardiovascular diseases: A case-crossover study in a
tropical city. J Toxicol Environ Health A. 78:1241–1253. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhalla DK: Ozone-induced lung inflammation
and mucosal barrier disruption: Toxicology, mechanisms, and
implications. J Toxicol Environ Health B Crit Rev. 2:31–86. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salim SY, Kaplan GG and Madsen KL: Air
pollution effects on the gut microbiota: A link between exposure
and inflammatory disease. Gut Microbes. 5:215–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuo T, Kamm MA, Colombel JF and Ng SC:
Urbanization and the gut microbiota in health and inflammatory
bowel disease. Nat Rev Gastroenterol Hepatol. 15:440–452. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sekirov I, Russell SL, Antunes LC and
Finlay BB: Gut microbiota in health and disease. Physiol Rev.
90:859–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shouval DS and Rufo PA: The role of
environmental factors in the pathogenesis of inflammatory bowel
diseases: A Review. JAMA Pediatr. 171:999–1005. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calkins BM: A meta-analysis of the role of
smoking in inflammatory bowel disease. Dig Dis Sci. 34:1841–1854.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mutlu EA, Engen PA, Soberanes S, Urich D,
Forsyth CB, Nigdelioglu R, Chiarella SE, Radigan KA, Gonzalez A,
Jakate S, et al: Particulate matter air pollution causes
oxidant-mediated increase in gut permeability in mice. Part Fibre
Toxicol. 8:192011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kinross JM, Darzi AW and Nicholson JK: Gut
microbiome-host interactions in health and disease. Genome Med.
3:142011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barton W, Penney NC, Cronin O,
Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD and
OSullivan O: The microbiome of professional athletes differs from
that of more sedentary subjects in composition and particularly at
the functional metabolic level. Gut. 67:625–633. 2018.PubMed/NCBI
|
|
14
|
Kowalski TJ, Liu SM, Leibel RL and Chua SC
Jr: Transgenic complementation of leptin-receptor deficiency. I.
Rescue of the obesity/diabetes phenotype of LEPR-null mice
expressing a LEPR-B transgene. Diabetes. 50:425–435. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang WH and Hazen SL: The gut microbiome
and its role in cardiovascular diseases. Circulation.
135:1008–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Man WH, de Steenhuijsen Piters WA and
Bogaert D: The microbiota of the respiratory tract: Gatekeeper to
respiratory health. Nat Rev Microbiol. 15:259–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hernández AF and Tsatsakis AM: Human
exposure to chemical mixtures: Challenges for the integration of
toxicology with epidemiology data in risk assessment. Food Chem
Toxicol. 103:188–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moller W, Haussinger K, Winkler-Heil R,
Stahlhofen W, Meyer T, Hofmann W and Heyder J: Mucociliary and
long-term particle clearance in the airways of healthy nonsmoker
subjects. J Appl Physiol (1985). 97:2200–2206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiao C, Li S, Zhou W, Shang D, Zhao S, Zhu
X, Chen K and Wang R: The effect of air pollutants on the
microecology of the respiratory tract of rats. Environ Toxicol
Pharmacol. 36:588–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma Y, Ding S, Liu G, Fang J, Yan W,
Duraipandiyan V, Al-Dhabi NA, Esmail GA and Jiang H: Egg protein
transferrin-derived peptides IRW and IQW regulate citrobacter
rodentium-induced, inflammation-related microbial and metabolomic
profiles. Front Microbiol. 10:6432019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Catry E, Bindels LB, Tailleux A, Lestavel
S, Neyrinck AM, Goossens JF, Lobysheva I, Plovier H, Essaghir A,
Demoulin JB, et al: Targeting the gut microbiota with inulin-type
fructans: Preclinical demonstration of a novel approach in the
management of endothelial dysfunction. Gut. 67:271–283. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Henning SM, Yang J, Shao P, Lee RP, Huang
J, Ly A, Hsu M, Lu QY, Thames G, Heber D, et al: Health benefit of
vegetable/fruit juice-based diet: Role of microbiome. Sci Rep.
7:21672017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Deng Y and Cao L: Characterising
the interspecific variations and convergence of gut microbiota in
Anseriformes herbivores at wintering areas. Sci Rep.
6:326552016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shatzkes K, Tang C, Singleton E, Shukla S,
Zuena M, Gupta S, Dharani S, Rinaggio J, Connell ND and Kadouri DE:
Effect of predatory bacteria on the gut bacterial microbiota in
rats. Sci Rep. 7:434832017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakradhar S: A curious connection:
Teasing apart the link between gut microbes and lung disease. Nat
Med. 23:402–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barrett KE and Wu GD: Influence of the
microbiota on host physiology - moving beyond the gut. J Physiol.
595:433–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arrieta MC, Bistritz L and Meddings JB:
Alterations in intestinal permeability. Gut. 55:1512–1520. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mortaz E, Adcock IM, Folkerts G, Barnes
PJ, Paul Vos A and Garssen J: Probiotics in the management of lung
diseases. Mediators Inflamm. 2013:7510682013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Gao Y and Zhao F: Phage-bacteria
interaction network in human oral microbiome. Environ Microbiol.
18:2143–2158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Viaud S, Saccheri F, Mignot G, Yamazaki T,
Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ,
et al: The intestinal microbiota modulates the anticancer immune
effects of cyclophosphamide. Science. 342:971–976. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
De Vadder F, Kovatcheva-Datchary P,
Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F and Mithieux
G: Microbiota-generated metabolites promote metabolic benefits via
gut-brain neural circuits. Cell. 156:84–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang WH, Wang Z, Levison BS, Koeth RA,
Britt EB, Fu X, Wu Y and Hazen SL: Intestinal microbial metabolism
of phosphatidylcholine and cardiovascular risk. N Engl J Med.
368:1575–1584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koeth RA, Wang Z, Levison BS, Buffa JA,
Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al: Intestinal
microbiota metabolism of L-carnitine, a nutrient in red meat,
promotes atherosclerosis. Nat Med. 19:576–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang WH, Kitai T and Hazen SL: Gut
microbiota in cardiovascular health and disease. Circ Res.
120:1183–1196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kostovcik M, Bateman CC, Kolarik M,
Stelinski LL, Jordal BH and Hulcr J: The ambrosia symbiosis is
specific in some species and promiscuous in others: Evidence from
community pyrosequencing. ISME J. 9:126–138. 2015. View Article : Google Scholar : PubMed/NCBI
|