Introduction
MicroRNAs (miRNAs) are a class of endogenous,
non-coding, single-stranded small RNAs of 19–24 nucleotides in
length. They inhibit target gene expression or translation at the
post-transcriptional level by binding target RNAs, thus affecting
cell proliferation, apoptosis or the cell cycle (1–3). miRNAs
also participate in tumorigenesis and development, acting as
oncogenes and tumor suppressor genes (4). miR-10b is involved in the invasion and
metastasis of tumor cells. It has a crucial role in tumor cell
proliferation, apoptosis, cell cycle or metastasis (5–7). miR-10b
also serves an important role in the regulation of breast cancer
metastasis (8). Therefore, it is
speculated that miR-10b may be involved in tumor invasion and
metastasis.
Esophageal cancer is one of the common tumors of the
digestive tract and its incidence and mortality varies globally
(9). China is a high-risk area for
esophageal cancer due to carcinogen exposure and nutritional
deficiency (10). It is reported
that abnormal miRNA expression is present in the serum of patients
with esophageal cancer (11).
The transforming growth factor-β (TGF-β) signaling
pathway is instrumental in mammalian development and in tumor
suppression via inhibiting proliferation and inducing apoptosis in
multiple cell types. However, TGF-β has a dual role in tumor
development as it can also promote tumor cell invasiveness and
metastasis via regulating the immune system and tumor
microenvironment (12,13). Phosphatase and tensin homolog (PTEN)
is a tumor suppressor gene, which regulates tumor cell invasion,
metastasis and proliferation (14).
PTEN may regulate PI3K signaling whilst a relationship between the
PI3K pathway and cancer has been established (15).
miR-10b is highly expressed in esophageal cancer
tissues and plasma, and is associated with poor prognosis for
patients with esophageal cancer (11,16).
However, the role and mechanism of miR-10b in esophageal cancer
cells remains unclear. In the present study, the role and mechanism
of miR-10b in esophageal cancer cells were investigated. Esophageal
cancer cell lines TE-1 and EC9706 were transfected with miR-10b
mimic and inhibitor to upregulate and downregulate miR-10b,
respectively. The effect of miR-10b on TGF-β and PTEN was also
analyzed. The present findings may provide a theoretical basis for
targeted therapy of esophageal cancer.
Materials and methods
Cell lines
Human esophageal cancer cell lines TE-1 (The Cell
Bank of Type Culture Collection of Chinese Academy of Sciences) and
EC9706 (The Cell Bank of Type Culture Collection of Chinese Academy
of Sciences) were cultured in RPMI-1640 complete medium (HyClone;
GE Healthcare Life Sciences) containing 10% fetal calf serum
(HyClone; GE Healthcare Life Sciences) and incubated in a 37°C, 5%
CO2 incubator.
Cell transfection and treatment
TE-1 cells and EC9706 cells were divided into the
control group, non-targeting negative control (NC) group, miR-10b
mimic group and miR-10b inhibitor group. The NC (sequence,
5′-TTCTCCGAACGTGTCACGT-3′; Beyotime Institute of Biotechnology),
miR-10b mimic (sequence, 5′-UACCCUGUAGAACCGAAUUUGUG-3′; 100 nmol/l;
Beyotime Institute of Biotechnology) and inhibitor (sequence,
5′-AUGGGACAUCUUGGCUUAAACAC-3′; 100 nmol/l; Beyotime Institute of
Biotechnology) were transfected with Lipofectamine® 2000
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C. Cells in the
control group were not transfected. The transfection time was
determined based on pilot experiments. At 24 h following
transfection, subsequent experiments were performed.
For growth factor experiments, TE-1 cells were
divided into the control group, TGF-β group, miR-10b mimic + TGF-β
group and miR-10b inhibitor + TGF-β group. Cells were transfected
with miR-10b mimic and miR-10b inhibitor as described above. At the
same time, cells were incubated with TGF-β (10 ng/ml; PeproTech EC,
Ltd.) for 48-h.
For analyzing the effect of TGF-β on cell
proliferation, TE-1 cells were treated with different
concentrations of TGF-β (0, 1.25, 2.5, 5 and 10 ng/ml) for 12, 24
and 48 h.
Flow cytometry
Following 48 h of transfection, cells were collected
by centrifugation at 800 × g and 4°C for 5 min. Cell apoptosis was
detected with an Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis kit (Sigma-Aldrich; Merck
KGaA). In brief, cells were stained with 5 µl Annexin V-FITC and 5
µl PI in the dark at 4°C for 30 min. After adding the Binding
Buffer Sigma-Aldrich (Merck KGaA), cells were analyzed on a flow
cytometer (Beckman Coulter, Inc.). The apoptosis rate was
calculated as the sum of the early apoptosis rate and late
apoptosis rate.
For cell cycle analysis, cells were incubated with
400 µl of PI (50 µg/ml) (Sigma-Aldrich; Merck KGaA) for 30–60 min
at 4°C. Flow cytometry was used to measure the proportion of cells
in each cell cycle.
Transwell invasion and migration
assays
The invasion assay was performed using Transwell
chamber with 8 µm pores (Corning Inc.). Briefly, 50 µl of diluted
Matrigel (2 mg/ml, BD Biosciences) was placed on the inner surface
of the chamber and incubated at 37°C for 30 min. Cells were
transfected for 24 h and seeded in the top chamber. Culture medium
supplemented with 10% fetal bovine serum was then added to the
bottom chamber. Following 24-h incubation, non-invading cells were
removed from the top of the Matrigel with a cotton-tipped swab.
Invading cells at the bottom of the Matrigel were fixed using 70%
methanol at 37°C for 30 min and stained with 0.1% crystal violet at
room temperature for 10 min. The invasion efficiency was determined
by counting the penetrated cells under Olympus IX73 (Olympus
Corporation) at ×200 magnification in 5 random fields per well.
Each experiment was performed in triplicate.
The protocol of Transwell migration assay was
similar to the invasion assays except that no Matrigel was used on
the inner surface of the chamber.
MTT assay
Following TGF-β treatment for 12, 24 and 48 h, the
culture supernatant was removed and 90 µl of fresh medium was
added. Following this, 10 µl of MTT solution (Sigma-Aldrich; Merck
KGaA) was added and incubated at 37°C for 4 h. Next, 110 µl of DMSO
was added and then samples were shaken for 10 min at low speed to
fully dissolve the crystals. The absorbance was measured at 450 nm
on a microplate reader (Thermo Fisher Scientific, Inc.).
EdU assay
EdU assay was performed using an EdU assay kit from
Guangzhou RiboBio Co., Ltd. In brief, 24 h following transfection,
cells (4×103) were seeded into 96-well plates. Following
incubation at 37°C for 24 h, 50 µM EdU solution was added to each
well, which was then incubated at 37°C for 2 h. Cells were then
washed, fixed and stained with ApolloR Fluorescent dye
solution (Guangzhou RiboBio Co., Ltd). Three fields were randomly
selected and then imaged using a fluorescence microscope (×400)
(Olympus IX73; Olympus Corporation). Cells with green fluorescence
were considered positive. ImageJ software v1.8 (National Institutes
of Health) was used for cell counting. The relative EdU
incorporation rate was then calculated as number of cells with
green fluorescence/total cell number (100%).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells by TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) and subjected to
reverse transcription with a PrimeScript™ RT reagent kit (Takara
Bio, Inc.) at 42°C for 50 min. The prepared cDNA was subjected to
PCR amplification with SYBR Green PCR kit (KAPA Biosystems; Roche
Diagnostics). Primer sequences were as follows: miR-10b forward,
5′-GGGTACCCTGTAGAACCG-3′ and reverse, 5′-AACTGGTGTCGTGGAGTCGGC-3′
and U6 forward, 5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGT-3′. Thermocycling conditions were as
follows: 95°C for 3 min, 95°C for 5 sec, 56°C for 10 sec, 72°C for
25 sec, 39 cycles of 65°C for 5 sec and 95°C for 50 sec. The
2−ΔΔCq method was used to calculate the relative
expression (17).
Western blot analysis
Protein extraction followed standard methods using
radioimmunoprecipitation assay Lysis and Extraction Buffer (Thermo
Fisher Scientific). The protein was quantified using bicinchoninic
acid kit (Thermo Fisher Scientific, Inc.). Proteins (20 µg per
lane) were electrophoresis on 12% SDS-PAGE and then transferred
onto polyvinylidene difluoride membrane. After blocking with 5%
non-fat milk at room temperature for 1 h, membranes were incubated
with primary antibodies against TGF-β (Abcam; cat. no. ab31013;
1:1,000), PTEN (Abcam; cat. no. ab32199; 1:1,000), and β-actin
(Cell Signaling Technology, Inc.; cat. no. 4970L; 1:1,000)
overnight at room temperature or overnight at 4°C. Following
washing, membranes were incubated with horseradish
peroxidase-labeled secondary antibody (Thermo Fisher Scientific,
Inc.; cat. no. 31430; 1:5,000) for 1 h at room temperature. The
protein bands were visualized with enhanced chemiluminescence
(Bio-Rad Laboratories, Inc.). The ratio of each band was analyzed
with a fully automated chemiluminescence analyzer
(Chemiluminescence Imaging System; Clinx Science Instruments Co.
Ltd.).
Statistical analysis
Data were analyzed using statistical software SPSS
19.0 (IBM Corp). Data were expressed as mean ± standard deviation.
One-way analysis of variance was used to compare the differences
amongst multiple groups, followed by Student-Newman-Keuls test.
P<0.05 was considered to indicate statistical significance.
Results
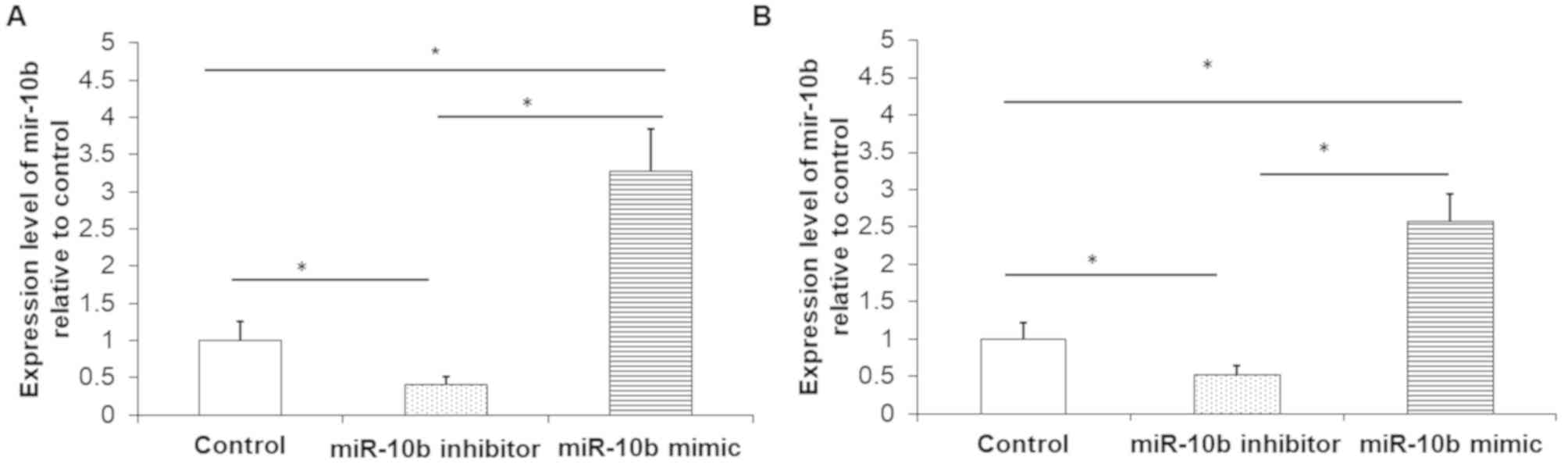
miR-10b mimic upregulates and miR-10b
inhibitor downregulates miR-10b expression, respectively
RT-qPCR was used to detect the relative expression
of miR-10b. Results demonstrated that, compared with the control
group, miR-10b inhibitor effectively silenced the expression of
miR-10b in TE-1 cells (Fig. 1A) and
EC9706 cells (Fig. 1B). Compared
with the control group, miR-10b mimic significantly increased the
relative expression of miR-10b in both TE-1 cells (Fig. 1A) and EC9706 cells (Fig. 1B). These experiments demonstrated
successful transfection efficiency.
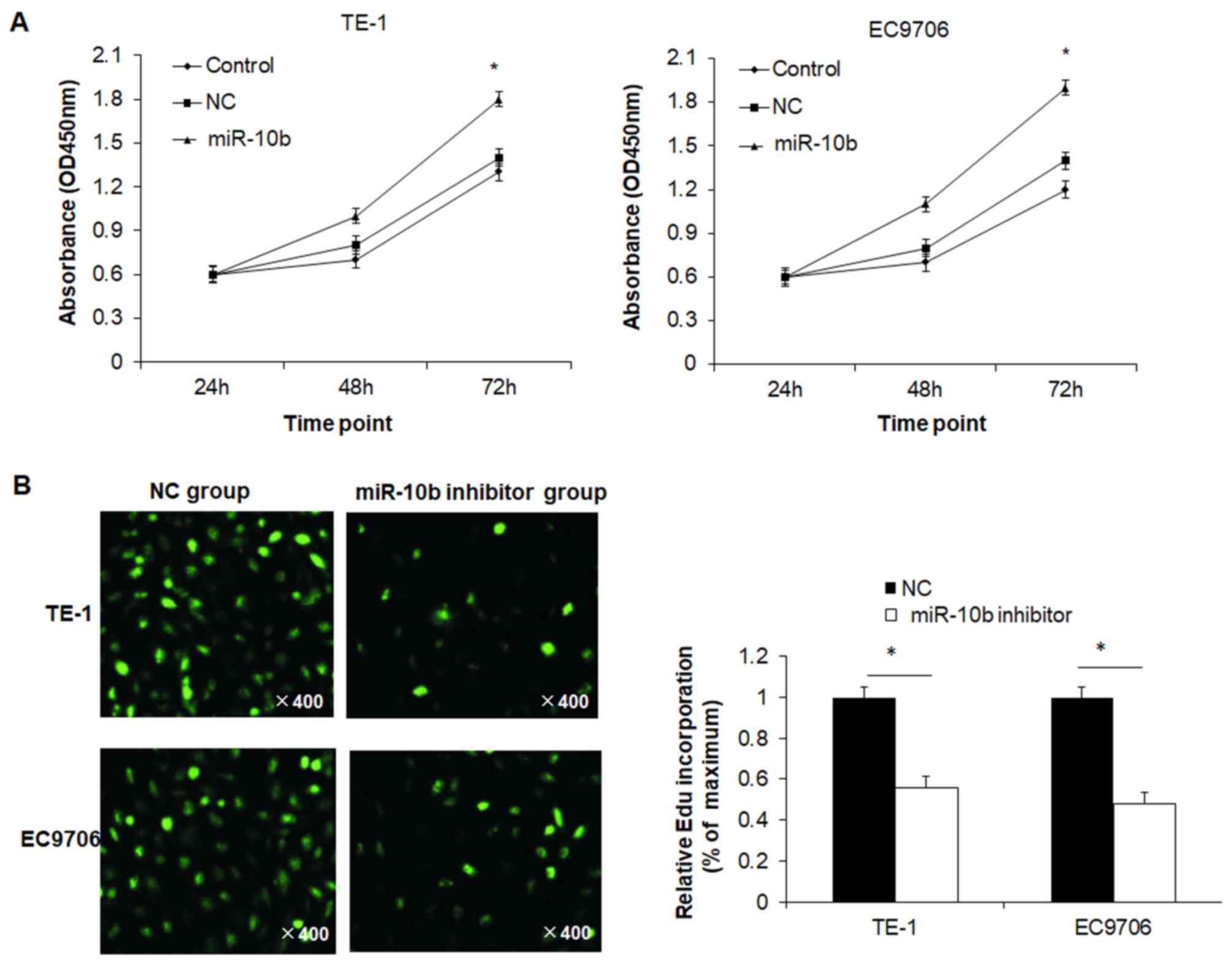
miR-10b increases proliferation of
esophageal cancer cells
To determine the effect of miR-10b on cell viability
of esophageal cancer cells, the MTT assay was performed. Cell
viability of TE-1 cells in the miR-10b mimic group at 72 h was
significantly higher than the NC group and control group (Fig. 2A). There was no significant
difference between the NC and control groups. Similar results were
also observed in EC9706 cells (Fig.
2A). To further confirm proliferation results, the EdU assay
was conducted. The relative EdU incorporation rate in the miR-10b
inhibitor group was significantly lower than in the control group
for both TE-1 and EC9706 cells (Fig.
2B). These results indicated that miR-10b promoted the
proliferation of esophageal cancer cells.
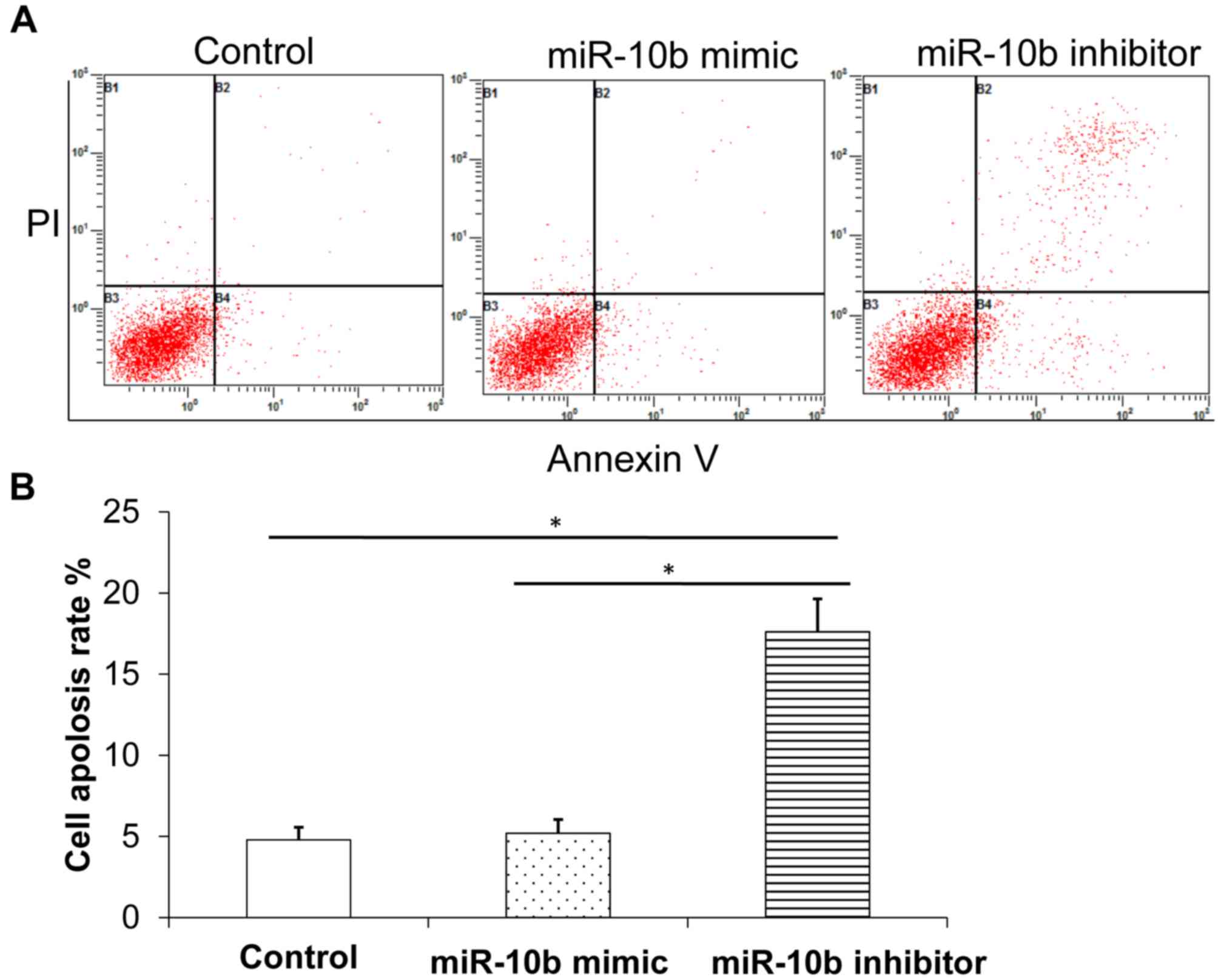
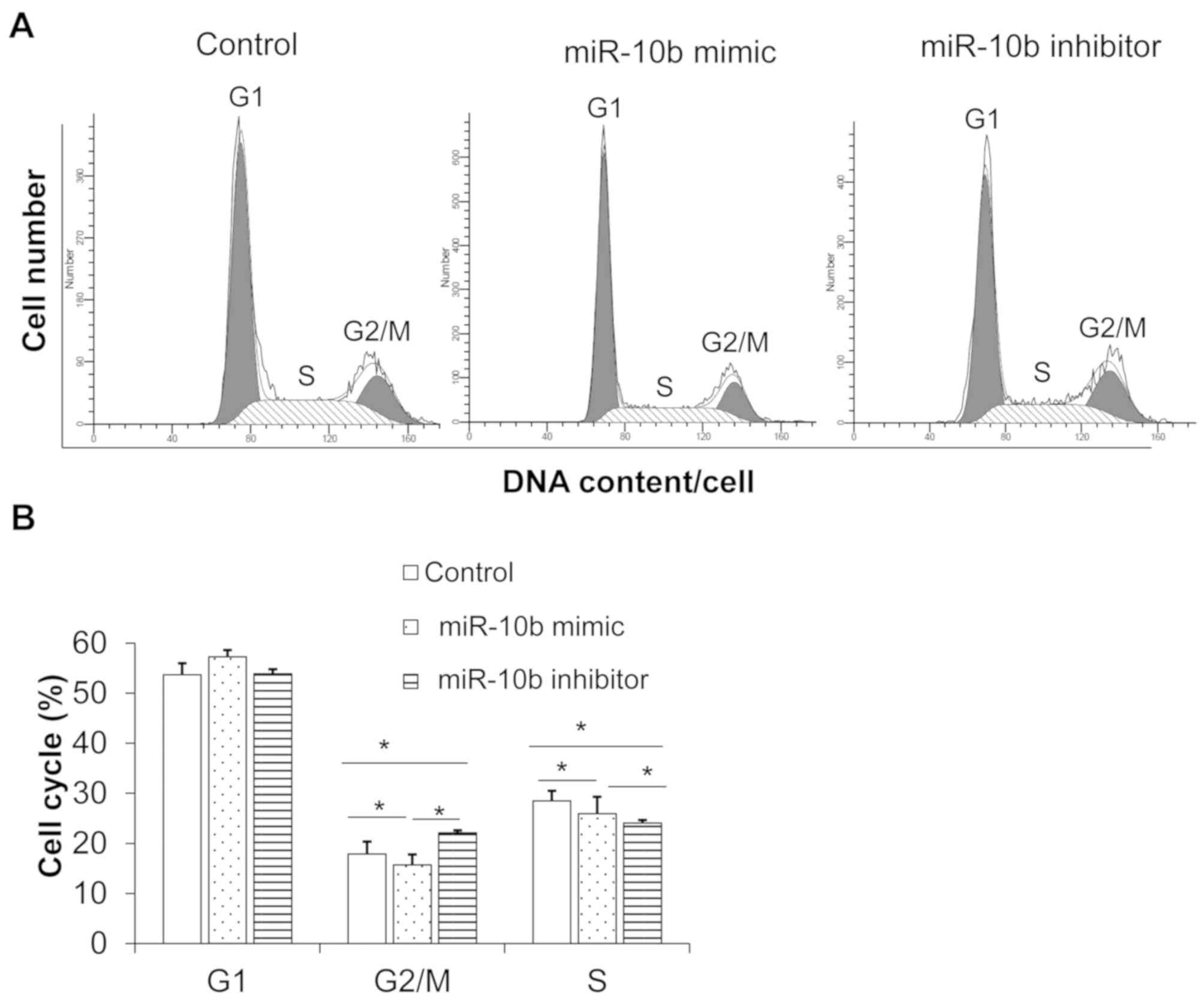
miR-10b mimic decreases esophageal
cancer cell apoptosis and promotes cell cycle progression whilst
miR-10b inhibitor has the reverse effect
Flow cytometry was used to detect the changes of
apoptosis in the three groups following transfection. Results
demonstrated that, compared with control group, miR-10b inhibitor
promoted cell apoptosis (Fig. 3A and
B) and arrested the cell cycle at the S phase and
G2/M phase (Fig. 4A and
B), indicating that miR-10b downregulation inhibited esophageal
cancer cell cycle progression and promoted apoptosis. By contrast,
miR-10b mimic inhibited apoptosis (Fig.
3A and B) and promoted cell cycle progression compared with
miR-10b inhibitor (Fig. 4A and
B).
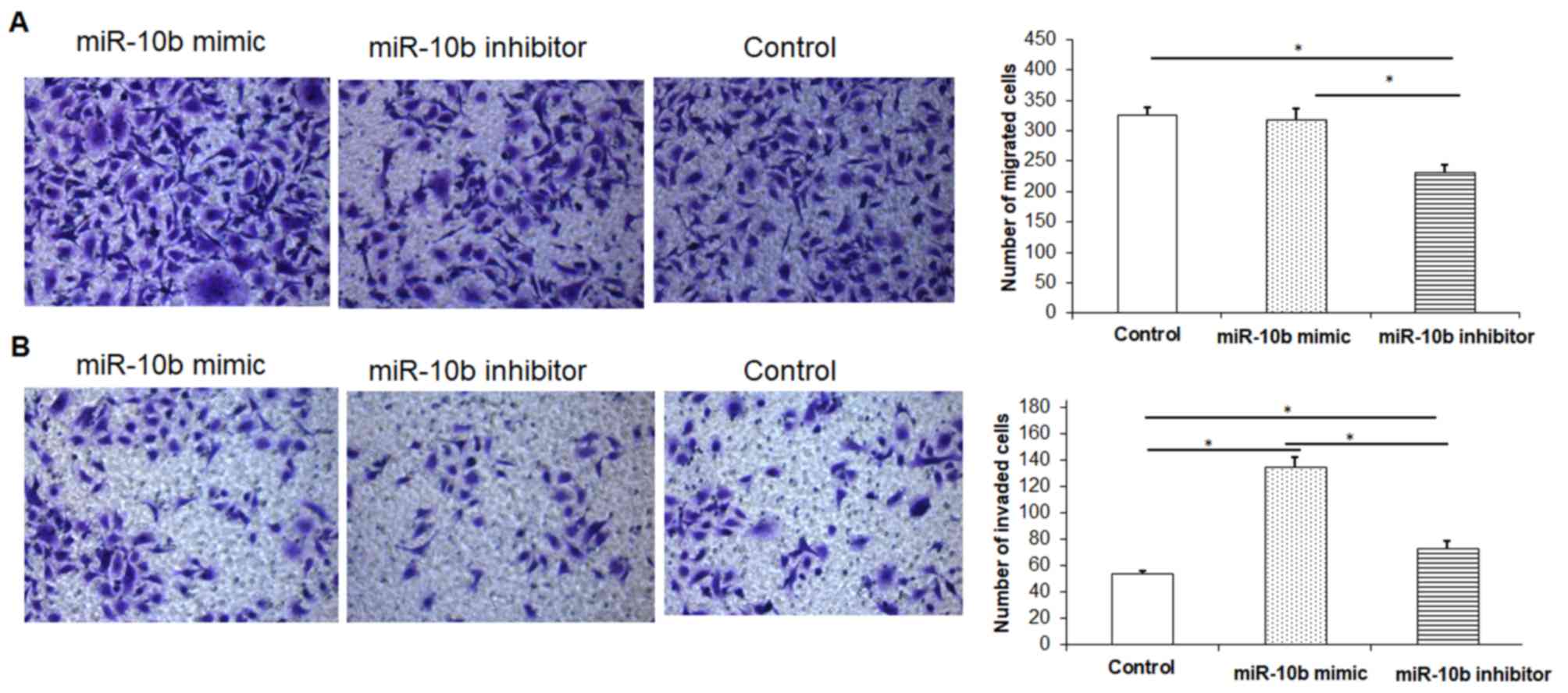
miR-10b inhibitor decreases migration
and invasion of esophageal cancer cells whilst miR10b mimic has the
reverse effect
In order to investigate the effect of miR-10b mimic
or miR-10b inhibitor on the migration and invasion ability of
esophageal cancer cells, the Transwell assay was performed.
Compared with the control group, esophageal cancer cells
transfected with miR-10b mimic significantly promoted the migration
and invasion of esophageal cancer cells (Fig. 5A and B). Esophageal cancer cells
transfected with miR-10b inhibitor significantly inhibited
esophageal cancer cell migration and invasion compared with the
miR-10b mimic group and the control group (Fig. 5A and B).
TGF-β treatment increases the
proliferation of the human esophageal cancer cell line TE-1
The proliferation of esophageal cancer cells under
different concentrations of TGF-β was detected with MTT assay. As
demonstrated in Table I, within
12-h, the proliferation of esophageal cancer cells increased in a
TGF-β dose dependent manner. The proliferation of esophageal cancer
cells increased significantly with the increase of TGF-β
concentration. Within 48-h of culture, the proliferation of
esophageal cancer cells was significantly increased with the
increase in TGF-β concentration. Thus, for subsequent experiments,
10 ng/ml concentration of TGF-β was selected.
 | Table I.Effects of different doses of TGF-β on
the proliferation of TE-1 cells at 450 nm. |
Table I.
Effects of different doses of TGF-β on
the proliferation of TE-1 cells at 450 nm.
|
| TGF-β (ng/ml) |
|---|
|
|
|
|---|
| Group | 0 | 1.25 | 2.5 | 5 | 10 |
|---|
| 12-h | 0.362±0.003 |
0.398±0.002a |
0.409±0.002a |
0.435±0.007a,b |
0.451±0.003a–c |
| 24-h |
0.435±0.006e |
0.546±0.007a,e |
0.614±0.006a,b,e |
0.755±0.007a–c,e |
0.816±0.006a–e |
| 48-h |
0.513±0.006e,f |
0.670±0.005a,e,f |
0.788±0.002a–b,e,f |
0.925±0.007a–c,e,f |
1.010±0.029a–d,f |
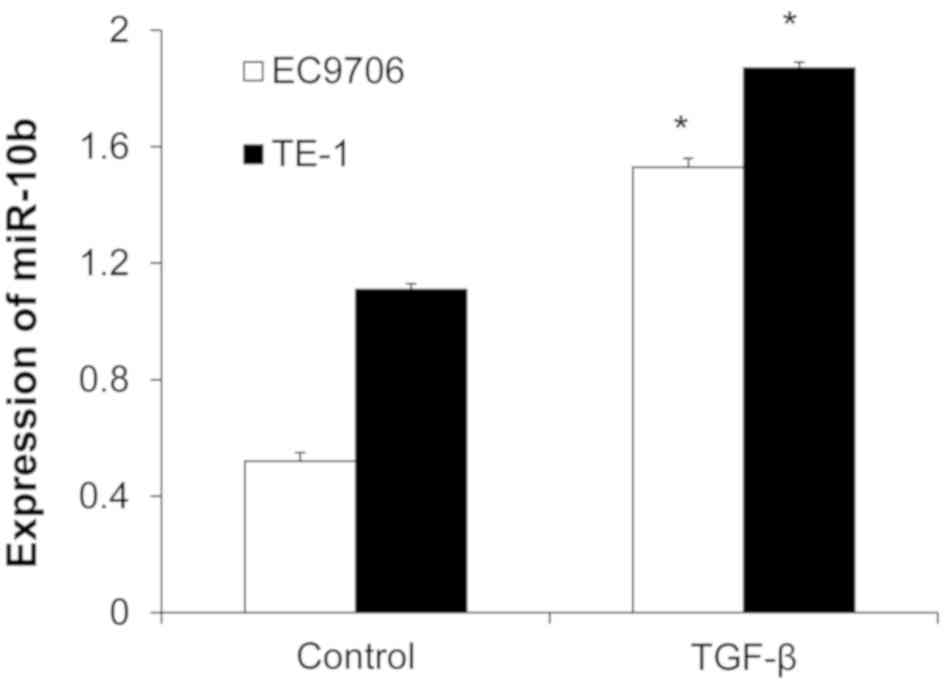
TGF-β increases miR-10b
expression
TE-1 and EC9706 cells were treated with 10 ng/ml
TGF-β for 48 h and miR-10b expression was detected with RT-qPCR.
miR-10b expression was significantly increased in the TGF-β group
compared with the control group (Fig.
6), indicating that TGF-β induced the expression of
miR-10b.
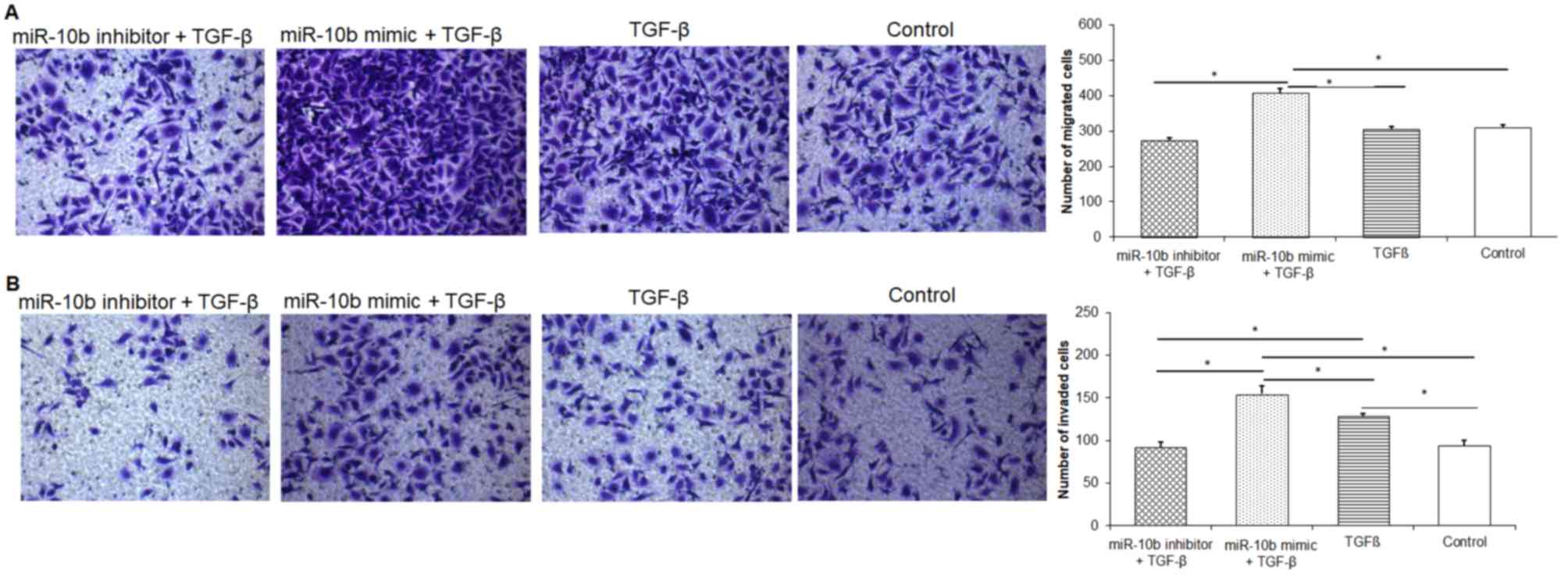
TGF-β promotes migration and invasion
of esophageal cancer cells
Results demonstrated that TGF-β alone could promote
the migration and invasion of esophageal cancer cells compared with
the control group (Fig. 7A and B).
The miR-10b mimic + TGF-β group exhibited increased esophageal
cancer cell migration and invasion ability compared with the
miR-10b inhibitor + TGF-β group. Results indicated that
upregulation of miR-10b expression worked synergistically with
TGF-β to enhance the migration and invasion ability of esophageal
cancer cells.
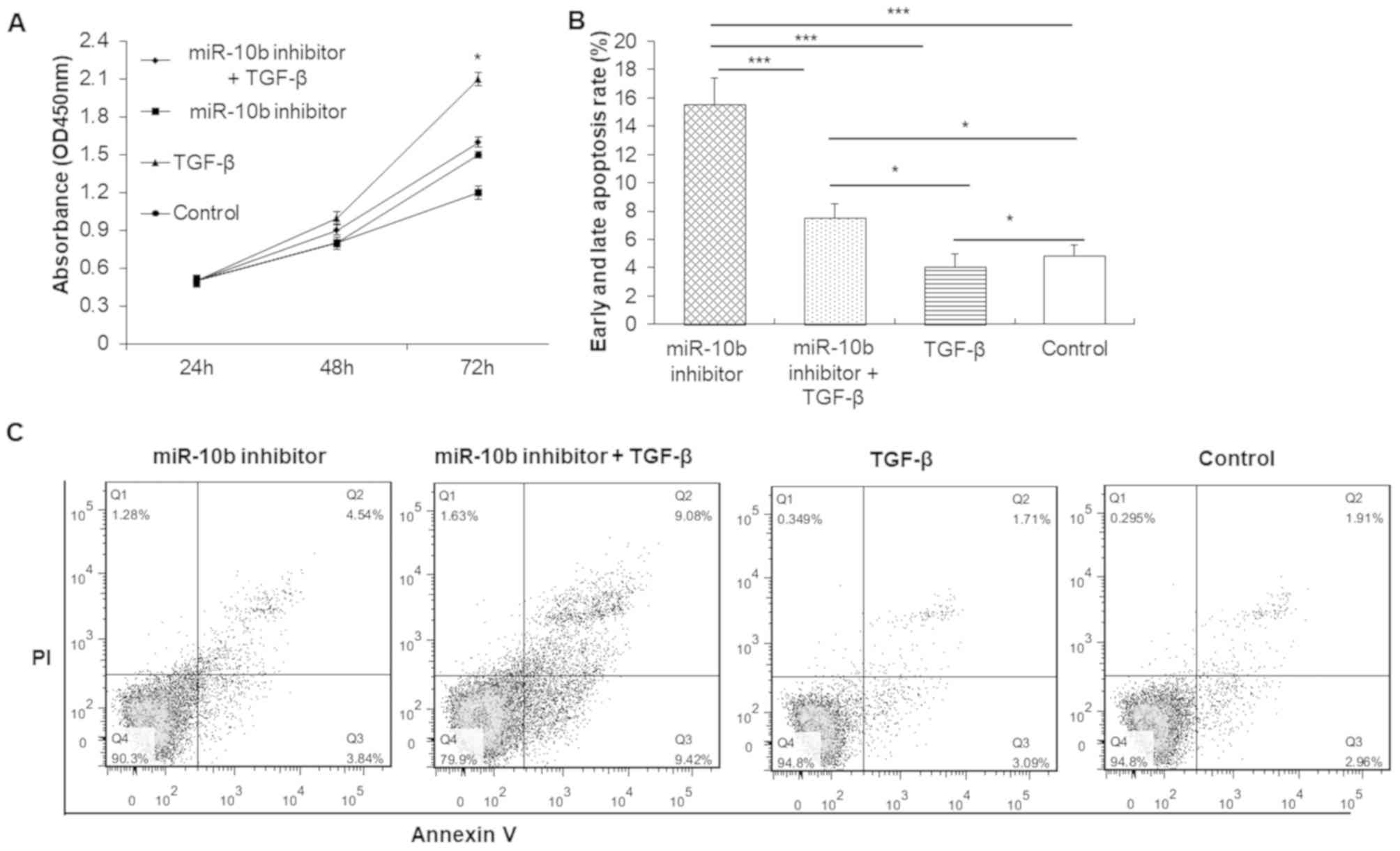
TGF-β promotes cell proliferation and
reduces apoptosis of esophageal cancer cells
MTT results demonstrated that TGF-β alone promoted
the proliferation of esophageal cancer cells compared with the
control group (Fig. 8A). However,
miR-10b inhibitor decreased the proliferation ability of esophageal
cancer cells compared with the control group. There was no
significant difference in proliferation between the miR-10b
inhibitor + TGF-β and control group. These findings indicated that
knocking down miR-10b expression in TE-1 cells decreased the
ability of TGF-β to promote cell proliferation.
Flow cytometry determined that the apoptosis (early
apoptosis + late apoptosis) rate in the miR-10b inhibitor group and
miR-10b inhibitor + TGF-β group was significantly increased,
whereas the TGF-β group was significantly decreased compared with
the control group (Fig. 8B and C).
The apoptosis rate in each group in descending order was as
follows: miR-10b inhibitor group > miR-10b inhibitor + TGF-β
group > control group > TGF-β group. Therefore, TGF-β
treatment attenuated the inhibitory effect of miR-10b inhibitor on
cell apoptosis.
miR-10b regulates the expression of
TGF-β and PTEN
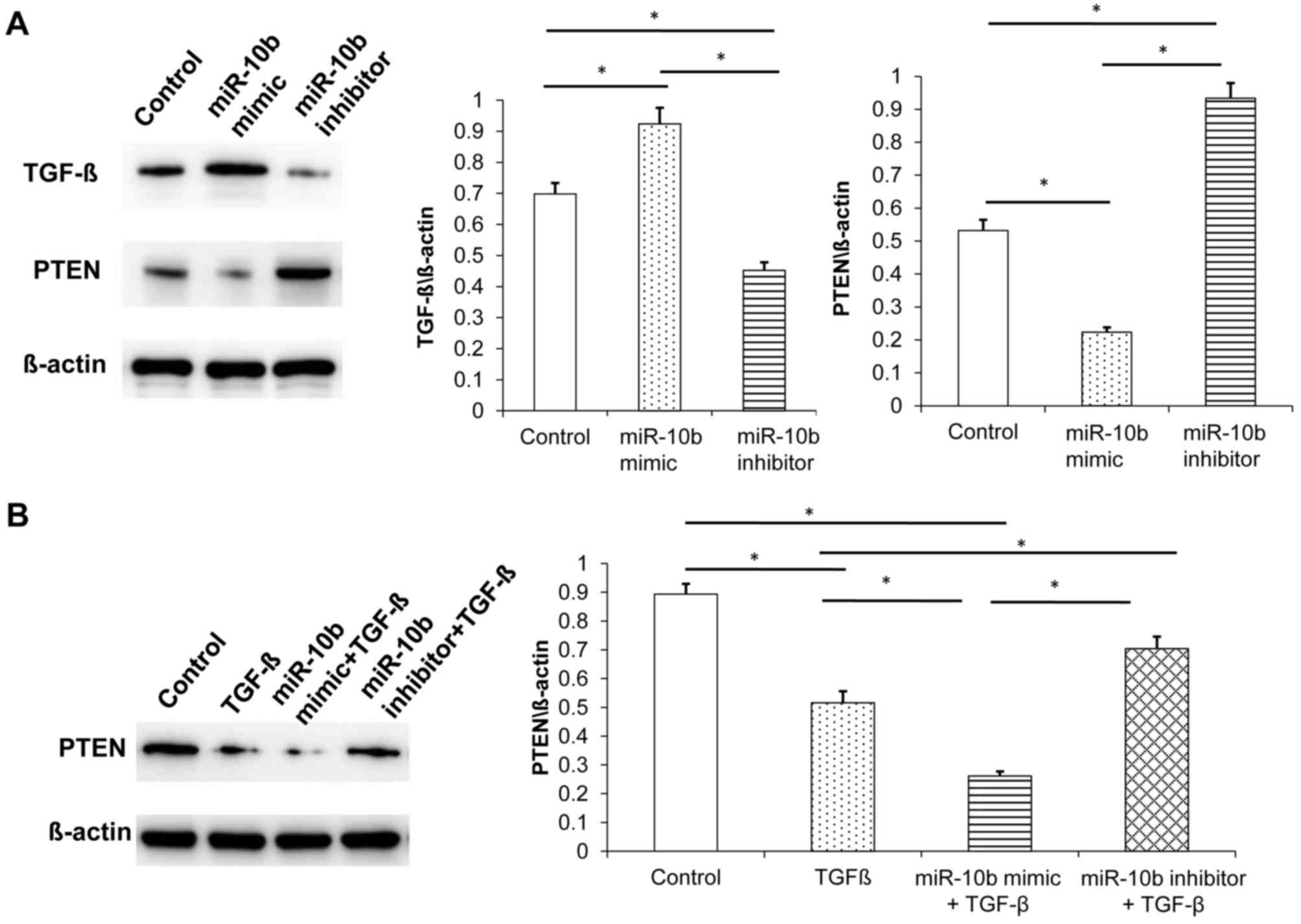
To detect the expression of TGF-β and PTEN following
miR-10b transfection, western blot analysis was performed. miR-10b
inhibitor downregulated the expression of TGF-β whereas miR-10b
mimic upregulated its expression in esophageal cancer cells,
indicating that miR-10b regulates the expression of TGF-β in
esophageal cancer cells (Fig.
9A).
PTEN is a tumor suppressor gene that has an
important regulatory role in cell proliferation and apoptosis
(18). PTEN is the target gene of
miR-10b (19). Western blot analysis
demonstrated that miR-10b mimic inhibited PTEN expression in tumor
cells and miR-10b inhibitor promoted PTEN expression (Fig. 9A).
The relationship between miR-10b, TGF-β and PTEN was
further investigated. In vitro experiments identified that,
compared with the control group, TGF-β-treated esophageal cancer
cells inhibited the expression of PTEN. miR-10b mimic + TGF-β group
exhibited a greater decrease in the expression of PTEN.
Furthermore, the miR-10b inhibitor + TGF-β group exhibited
increased PTEN expression compared with miR-10b mimic + TGF-β group
(Fig. 9B).
Discussion
miR-10b has varying roles in different cellular
backgrounds or tumor microenvironments (20,21). For
example, miR-10b promotes tumor invasion and metastasis in breast
and esophageal cancers and is therefore known as a pro-metastatic
factor (22). By contrast, miR-10b
has a tumor-suppressive role in clear-cell renal cell carcinoma
(23). Therefore, miR-10b may be
involved in the process of tumorigenesis and development. Further
study on the role of miR-10b in tumorigenesis and its mechanism may
provide experimental evidence for the clinical diagnosis and
treatment of tumors. The present study demonstrated that miR-10b
promoted cell proliferation. Inhibition of miR1-10b arrested the
cell cycle at S and G2/M phase, suggesting that miR-10b
promoted cell cycle progression. Furthermore, miR-10b inhibited
apoptosis and promoted tumor cell migration and invasion, which is
consistent with the role of miR-10b in breast cancer and
hepatocellular carcinoma (24,25).
TGF-β has an important role in cell proliferation,
differentiation, survival and apoptosis (26,27). It
also induces epithelial-mesenchymal transition by activating other
signaling pathways (28). In tumors,
TGF-β, once activated, promotes cell growth, migration and invasion
(29,30), therefore, the level of TGF-β
expression in tumors is also related to the degree of malignancy of
the tumor. A previous study demonstrated that TGF-β promotes the
migration of human glioma cells by promoting the expression of
miR-10b (31). In the present study,
it was demonstrated that the proliferation of esophageal cancer
cells increased along with an increase of TGF-β concentration.
TGF-β induced the high expression of miR-10b in esophageal cancer
cells. miR-10b also promotes the expression of TGF-β and invasion
of pancreatic cancer cells (32).
These results indicated that TGF-β is related to miR-10b. miRNA not
only mediates the role of the TGF-β/Smad signaling pathway in
tumors, but also has a role in promoting or suppressing tumor
progression by regulating important members of the TGF-β/Smad
signaling pathway (33). The present
study determined that upregulation of miR-10b expression by miR-10b
mimic enhanced the migration and invasion ability of esophageal
cancer cells and further upregulated the expression of TGF-β in
esophageal cancer cells. These results indicated that miR-10b
promoted the expression of TGF-β in tumor cells and serves a
crucial role in the occurrence and development of esophageal
cancer. These findings may be an important basis for the use of
miR-10b as a new target for cancer therapy.
The tumor suppressor protein PTEN can be regulated
by a variety of miRNAs. For example, miR-121 promotes tumor cell
proliferation, migration and invasion by targeting PTEN protein
(34). miR-10b can also target PTEN
to promote human glioma cell migration and invasion (5,35). This
present study demonstrated that upregulation of miR-10b expression
inhibited the expression of PTEN in tumor cells, whilst TGF-β also
inhibited the expression of PTEN. miR-10b overexpression together
with TGF-β treatment further inhibited PTEN expression, which may
have further enhanced the promotion effect of miR-10b on the
migration and invasion ability of esophageal cancer cells.
The present study has some limitations. For example,
due to limited time and materials, all experiments were not
performed with both cell lines, which warrants further study.
In summary, the present study demonstrated that
miR-10b promoted the invasion and metastasis of esophageal cancer
cells and has an important role in the occurrence and development
of esophageal cancer. TGF-β treatment induced high expression of
miR-10b with miR-10b overexpression also promoting high TGF-β
protein expression. miR-10b + TGF-β treatment synergistically
enhanced the invasion and metastasis ability of esophageal cancer
cells. The underlying mechanism might be via the inhibition of PTEN
expression. In the future, miR-10b may be used as a potential
target for the treatment of human esophageal cancer.
Acknowledgements
The authors would like to thank the Department of
Thoracic Surgery, Affiliated Tumor Hospital of Xinjiang Medical
University, Institute of Cancer Prevention and Treatment of
Affiliated Tumor Hospital of Xinjiang Medical University and
Department of Cardiothoracic Surgery, Shenzhen University General
Hospital for their techical support.
Funding
This study was supported by the Natural Science
Foundation of Xinjiang Uygur Autonomous Region (grant no.
2016D01C351).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL performed all the experiments and wrote, edited
and reviewed the manuscript. WZ performed the literature research
and data analysis. AS and DH provided help in flow cytometry
analysis. SG designed the study and reviewed the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stappert L, Roese-Koerner B and Brustle O:
The role of microRNAs in human neural stem cells, neuronal
differentiation and subtype specification. Cell Tissue Res.
359:47–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villegas-Ruiz V, Juárez-Méndez S,
Pérez-González OA, Arreola H, Paniagua-García L, Parra-Melquiadez
M, Peralta-Rodríguez R, López-Romero R, Monroy-García A,
Mantilla-Morales A, et al: Heterogeneity of microRNAs expression in
cervical cancer cells: Over-expression of miR-196a. Int J Clin Exp
Pathol. 7:1389–1401. 2014.PubMed/NCBI
|
|
3
|
Ye JJ and Cao J: MicroRNAs in colorectal
cancer as markers and targets: Recent advances. World J
Gastroenterol. 20:4288–4299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He B, Yin B, Wang B, Xia Z, Chen C and
Tang J: MicroRNAs in esophageal cancer (review). Mol Med Rep.
6:459–465. 2012.PubMed/NCBI
|
|
5
|
Ahmad A, Ginnebaugh KR, Yin S,
Bollig-Fischer A, Reddy KB and Sarkar FH: Functional role of
miR-10b in tamoxifen resistance of ER-positive breast cancer cells
through down-regulation of HDAC4. BMC Cancer. 15:5402015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knirsh R, Ben-Dror I, Modai S, Shomron N
and Vardimon L: MicroRNA 10b promotes abnormal expression of the
proto-oncogene c-Jun in metastatic breast cancer cells. Oncotarget.
7:59932–59944. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhen L, Li J, Zhang M and Yang K: MiR-10b
decreases sensitivity of glioblastoma cells to radiation by
targeting AKT. J Biol Res (Thessalon). 23:142016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Short MW, Burgers KG and Fry VT:
Esophageal cancer. Am Fam Physician. 95:22–28. 2017.PubMed/NCBI
|
|
10
|
Lin Y, Totsuka Y, Shan B, Wang C, Wei W,
Qiao Y, Kikuchi S, Inoue M, Tanaka H and He Y: Esophageal cancer in
high-risk areas of China: Research progress and challenges. Ann
Epidemiol. 27:215–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu H, Yao Y, Meng F, Qian X, Jiang X, Li
X, Gao Z and Gao L: Predictive value of serum miR-10b, miR-29c, and
miR-205 as promising biomarkers in esophageal squamous cell
carcinoma screening. Medicine (Baltimore). 94:e15582015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mishra L, Derynck R and Mishra B:
Transforming growth factor-beta signaling in stem cells and cancer.
Science. 310:68–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Madhunapantula SV and Robertson GP: The
PTEN-AKT3 signaling cascade as a therapeutic target in melanoma.
Pigment Cell Melanoma Res. 22:400–419. 2010. View Article : Google Scholar
|
|
15
|
Georgescu MM: PTEN tumor suppressor
Network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jamali L, Tofigh R, Tutunchi S, Panahi G,
Borhani F, Akhavan S, Nourmohammadi P, Ghaderian SMH, Rasouli M and
Mirzaei H: Circulating microRNAs as diagnostic and therapeutic
biomarkers in gastric and esophageal cancers. J Cell Physiol.
233:8538–8550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods Dec. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Xu W, Yang Z, Zhou SF and Lu N:
Posttranslational regulation of phosphatase and tensin homolog
(PTEN) and its functional impact on cancer behaviors. Drug Des
Devel Ther. 8:1745–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bahena-Ocampo I, Espinosa M,
Ceballos-Cancino G, Lizarraga F, Campos-Arroyo D, Schwarz A,
Garcia-Lopez P, Maldonado V and Melendez-Zajgla J: miR-10b
expression in breast cancer stem cells supports self-renewal
through negative PTEN regulation and sustained AKT activation. EMBO
Rep. 17:10812016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W: Study of miR-10b regulatory
mechanism for epithelial-mesenchymal transition, invasion and
migration in nasopharyngeal carcinoma cells. Oncol Lett.
14:7207–7210. 2017.PubMed/NCBI
|
|
21
|
Zhang Y, Liao RB, Hu LL, Tong BX, Hao TF
and Wu HJ: The microRNA miR-10b as a potentially promising
biomarker to predict the prognosis of cancer patients: A
meta-analysis. Oncotarget. 8:104543–104551. 2017.PubMed/NCBI
|
|
22
|
Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H
and Liu Z: MicroRNA-10b promotes migration and invasion through
KLF4 in human esophageal cancer cell lines. J Biol Chem.
285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carlsson J, Christiansen J, Davidsson S,
Giunchi F, Fiorentino M and Sundqvist P: The potential role of
miR-126, miR-21 and miR-10b as prognostic biomarkers in renal cell
carcinoma. Oncol Lett. 17:4566–4574. 2019.PubMed/NCBI
|
|
24
|
Zhang J, Yang J, Zhang X, Xu J, Sun Y and
Zhang P: MicroRNA-10b expression in breast cancer and its clinical
association. PLoS One. 13:e01925092018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hujie G, Zhou SH, Zhang H, Qu J, Xiong XW,
Hujie O, Liao CG and Yang SE: MicroRNA-10b regulates
epithelial-mesenchymal transition by modulating KLF4/KLF11/Smads in
hepatocellular carcinoma. Cancer Cell Int. 18:102018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moustakas A and Heldin CH: The regulation
of TGFbeta signal transduction. Development. 136:3699–3714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pickup M, Novitskiy S and Moses HL: The
roles of TGF beta in the tumour microenvironment. Nat Rev Cancer.
13:788–799. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng T and Yang J: Mechanism of
transforming growth factor β in patients with hepatocellular
carcinoma. Chin J Hepatobiliary Surg. 6:425–428. 2016.
|
|
29
|
He Z, Dong W, Li Q, Qin C and Li Y:
Sauchinone prevents TGF-β-induced EMT and metastasis in gastric
cancer cells. Biomed Pharmacother. 101:355–361. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katsuno Y, Lamouille S and Derynck R:
TGF-beta signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma C, Wei F, Xia H, Liu H, Dong X, Zhang
Y, Luo Q, Liu Y and Li Y: MicroRNA-10b mediates TGF-β1-regulated
glioblastoma proliferation, migration and epithelial-mesenchymal
transition. Int J Oncol. 50:1739–1748. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ouyang H, Gore J, Deitz S and Korc M:
MicroRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-beta
actions. Oncogene. 36:49522017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong H, Wang HR, Yang S, Zhong JH, Wang
T, Wang C and Chen FY: Targeting Smad4 links microRNA-146a to the
TGF-beta pathway during retinoid acid induction in acute
promyelocytic leukemia cell line. Int J Hematol. 92:129–135. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu MX, Liao J, Xie M, Gao ZK, Wang XH,
Zhang Y, Shang MH, Yin LH, Pu YP and Liu R: miR-93-5p transferred
by exosomes promotes the proliferation of esophageal cancer cells
via intercellular communication by targeting PTEN. Biomed Environ
Sci. 31:171–185. 2018.PubMed/NCBI
|
|
35
|
Liang HX, Sun LB and Liu NJ: Neferine
inhibits proliferation, migration and invasion of U251 glioma cells
by down-regulation of miR-10b. Biomed Pharmacother. 109:1032–1040.
2019. View Article : Google Scholar : PubMed/NCBI
|