Introduction
Trigeminal neuralgia (TN) is a type of severe
chronic pain characterized by brief electric shock-like pains in
one or more divisions of the trigeminal nerve (1). The disorder typically occurs in the
middle- or advanced-aged population; however, young adults,
particularly those with multiple sclerosis, may still present with
TN (2). In general, TN is evoked by
stimulation in the ‘trigger points’ of the face and attacks may
occur repeatedly in a short period of time (3). A number of potential causes regarding
the etiology of TN have been proposed, including epileptic seizures
in the trigeminal structures of the brainstem, trigeminal root
compression, arteriovenous malformations and aneurysms (4). At present, two major treatment
modalities for TN are being applied in clinical settings:
Pharmacotherapy and neurosurgical procedures (5). Although surgical treatment may be more
likely to cure TN, the majority of patients opt for pharmacotherapy
due to the reduced risk associated with it. A number of
pharmacotherapies have been successfully applied in clinical
settings to relieve patients from the impairments of TN (6). Among the types of pharmacotherapy
offered, botulinum toxin A (BTX-A) has been increasingly reported
to successfully control TN in recent years, and has received
considerable attention in the subject area of pain management
(7).
BTX-A is one of the botulinum neurotoxins produced
by Clostridium botulinum. The agent exerts its function by
inhibiting acetylcholine release at nerve-muscle junctions, causing
relaxation of the muscle (8,9). Therefore, BTX-A has been widely used in
cosmetology and the treatment of dysmyotonia (10), and has exhibited promising effects in
the treatment of headaches (11,12). In
2002, Micheli et al (13)
initially reported the effect of BTX-A to ameliorate TN. A number
of subsequent studies supported the beneficial effects of BTX-A in
the treatment of TN (7,14). As a result, Medicines and Healthcare
Products Regulatory Agency in the UK and the US Food and Drug
Administration approved Botox® (Allergan, Inc.) for the
treatment of pains in adults with chronic migraine. However, the
application of BTX-A for the treatment of TN remains controversial,
with discrepancies concerning the optimal injection dose, starting
time of therapeutic effects, long-term therapeutic effects and side
effects (15,16). Although meta-analyses based on
randomized controlled trials have confirmed the benefits of BTX-A
in treating TN, the conclusions were limited by the small number of
trials and patients available that supported the safety and
efficacy of BTX-A in the treatment of TN (15,16).
Therefore, in the present study, a comprehensive retrospective
analysis was performed. The study cohort consisted of 152 subjects
and was established to assess the effect of BTX-A on TN by
different grouping strategies. The results of the present study
supplement the results of previous studies exploring the treatment
efficacy and side effects of BTX-A for TN.
Patients and methods
Patient recruitment
An open retrospective study was performed on 161 TN
patients treated with BTX-A. Patients who were treated with BTX-A
at the Department of Neurology of the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) from June 2011 to March
2016 were retrospectively enrolled in the current study. All
subjects enrolled in the analysis were diagnosed with classical TN
according to the current version of the International
Classification of Headache Disorders (17) and detailed information on the
clinicopathological characteristics, including age, sex, number of
branches affected by TN, course of disease, injection dose, number
of injections, starting time of therapeutic effect, duration of
therapeutic effect and side effects, was available. Each patient
underwent magnetic resonance imaging or computed tomography to
exclude the presence of structural pathology. Complete blood count,
electrocardiogram, liver function tests, renal function tests and
other diagnostic tests were performed prior to the trial to exclude
coagulopathy and severe dysfunction of major organs, including the
heart, liver and kidney. Patients with any disease that may have
represented a risk associated with BTX-A (including myasthenia
gravis, motor neuron disease or Lambert-Eaton syndrome), infections
or skin problems at any of the injection sites, use of drugs that
damaged the neuromuscular junction within 7 days prior to study
entry (e.g. quinine, aminoglycosides or penicillamine),
significantly unstable medical diseases, a history of significant
psychiatric disorder or a history of substance dependence or abuse
were excluded from the present study. Furthermore, females who were
pregnant, nursing, planning a pregnancy during the study or unable
or unwilling to use a reliable form of contraception during the
study were also excluded. Follow-up was performed for ≥6 months for
all patients and the longest follow-up time was 28 months. Based on
the data collected, 9 patients lost to follow-up within 6 months
following treatment were excluded from the database and the final
cohort for analysis consisted of 152 subjects.
The purpose and safety aspects of the study were
explained to all patients and written informed consent was included
in the documents of each patient. The present study was approved by
the Ethics Committee of the First Affiliated Hospital of Zhengzhou
University (Zhengzhou, China) for the screening, inspection and
data collection of the patients. All protocols were in accordance
with the provisions of the Declaration of Helsinki.
Treatment strategy
BTX-A crystals (HengLi®; Lanzhou
Institute of Biological Products Co., Ltd.; 100 units
Clostridium botulinum type A neurotoxin complex supplemented
with 5 mg gelatin, 25 dextran and 25 mg saccharose) was diluted
with 2 ml 0.9% saline to 50 U/ml prior to use. The injections of
BTX-A were administered intradermally and/or submucosally at the
trigger sites using a 1-ml syringe with a 0.45×16-mm needle
(Shandong Weigao Group Medical Polymer Co., Ltd.) by experienced
clinicians, and the injection depth was −0.1 cm. For patients
requiring injection at multiple sites, 15–25 injection sites in
total were located and a distance of 15–20 mm was set between them.
Each site received one injection with 1.25–5.0 units (0.025–0.1 ml)
BTX-A during the first course of the treatment and the patients'
response to the treatment was monitored. For certain patients who
did not exhibit any symptom improvement within 2 weeks following
the initial injection, additional injections were administered at
the same sites (each site received one injection with 1.25–5.0
units BTX-A during the second course of the treatment). Patients
who received a total dose of <40 units were assigned to the
low-dose group, patients who received a total dose from 40–70 units
were stratified into the medium-dose group and those who received a
total dose of >70 units were classified into the high-dose
group.
Efficacy and safety assessment
The extent of pain was evaluated using a visual
analog scale (VAS) method (18):
Patients were requested to rate their pain by marking a
representative point on a panel with a length of 10 cm and 11
equidistant points, where 0 indicated ‘no pain’ and 10 represented
the ‘most severe pain’ (the difference between females and males in
rating/perceiving pain was not taken into consideration in the
present study). Regarding the treatment efficacy, a reduction in
the VAS score by <50% following treatment was classified as
‘ineffective’, 50–70% as ‘effective’ and >75% as ‘significantly
effective’ and 100% as ‘totally effective’. For different analysis
strategies, the overall treatment effect was defined as the
percentage of patients with a VAS score reduction by ≥50%. Side
effects (short-term facial asymmetry, twisted mouth, and ptosis)
associated with BTX-A treatment were also recorded in detail.
Statistical analysis
Continuous data with non-normal distribution
(assessed by Kolmogorov-Smirnov test) were reported as the median
(interquartile range). Categorical data were represented as the
number of cases (percentage). Wilcoxon's signed-rank test and the
Kruskal-Wallis test were employed for analysis of variables with a
non-normal distribution. χ2 and Fisher's exact tests
were used to determine whether differences existed between
different groups of categorical indices by sex, age, number of
branches affected by TN and dose of injections. All tests were
two-tailed and P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using R, version 3.3 (19).
Results
Patient characteristics
The patients were treated by BTX-A between June 2011
and March 2016, and a total of 161 patients were enrolled in the
present study. The follow-up period for each patient was ≥6 months
and 9 subjects lost to follow-up were excluded from the database.
The final cohort for analysis comprised 152 patients, including 87
females (57.2%) and 65 males (42.8%). The median age of the females
was 59 years (age range, 31–91 years) and that of males was 60
years (age range, 39–89 years); no statistically significant
difference was determined in the age distribution between different
sexes (P=0.65). The average disease course of the females was 68
months (range, 1–240 months) and that of the males was 60 months
(range, 2–300 months), and no significant difference was observed
between the two groups (P=0.11).
Efficacy results
The beneficial effect of BTX-A on TN, indicated by a
decrease in the degree of pain represented by the VAS score, was
observed as early as 1 week following the first injection. For
patients whose symptoms were not improved within 2 weeks following
the first injection, additional doses were administered at the same
site as the first injection. In the present study, 50 patients
received additional injections and 41 achieved pain alleviation
(82%). Following treatment with BTX-A, 136 patients exhibited
symptom improvement within 2 weeks, including 31 cases of effective
(20.4%), 21 cases of significantly effective (13.8%), and 83 cases
of completely effective treatment (54.6%). The overall effective
rate in the present study was 89.4%.
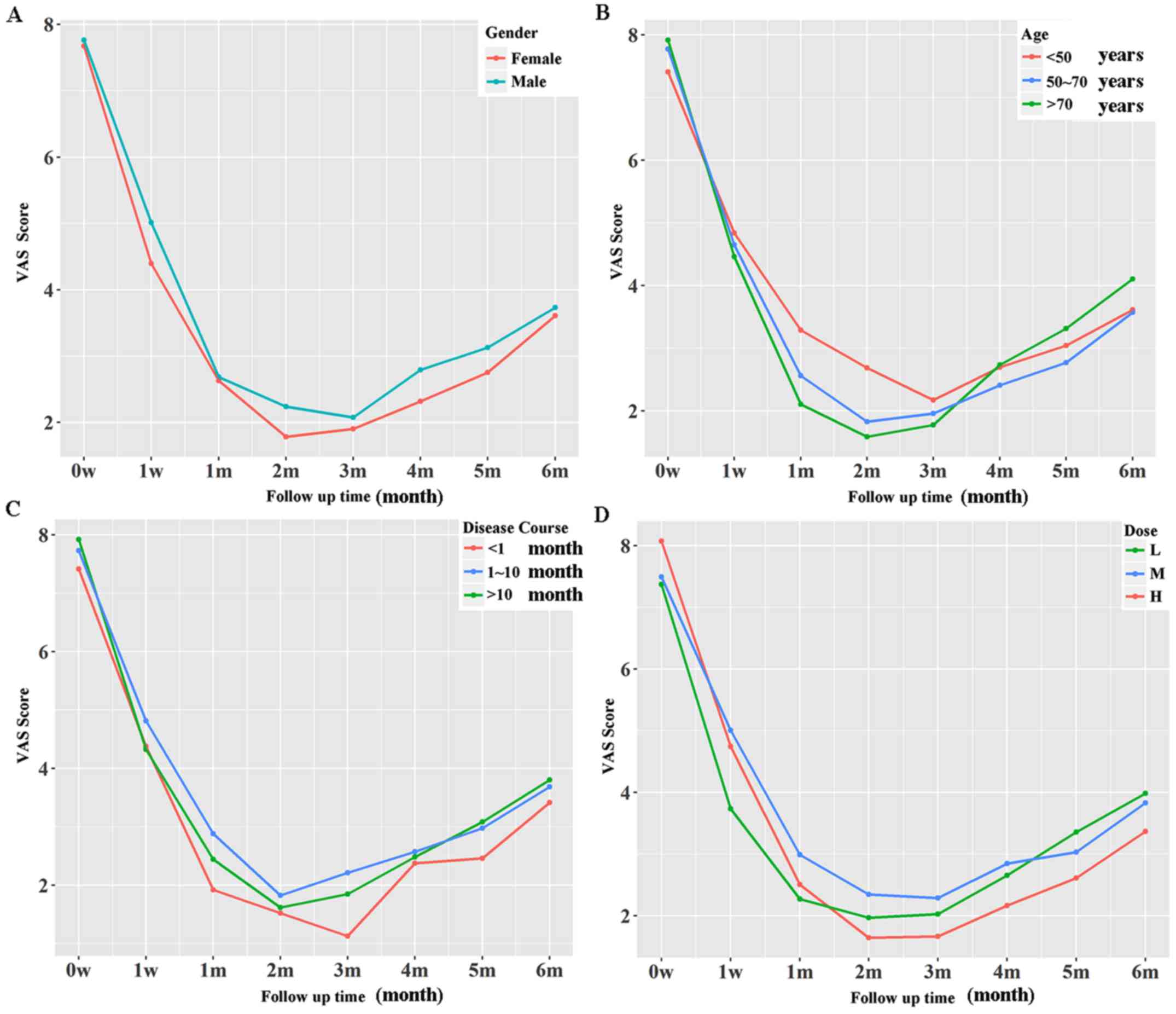
The effect of BTX-A was sustained throughout the
initial 6 months of the follow-up. No significant differences in
overall treatment efficacy were detected when the VAS data of the
initial 6 months were analyzed by sex, age, disease course or
injection dose (Fig. 1). However,
female sex, short disease course and high injection dose were
associated with lower VAS scores in a longer period (≥2 months),
although this was not statistically significant (Fig. 1).
In the present study, a reduction in the VAS score
by >50% was considered as ‘effective’. To further explore
factors influencing the treatment effect of BTX-A, changes in the
VAS score were analyzed with regard to sex, age, disease course,
branch number and injection dose. As presented in Table I, sex, age, disease course and branch
number did not influence the starting time of the effect, the peak
time of the effect, the average lasting time of the effect or the
overall efficacy of BTX-A. However, the injection dose affected the
treatment efficacy: patients who received injections of
medium-(21.1%) or high-dose (22.7%) BTX-A exhibited higher
completely treated rates compared with the low-dose group (11.2%)
although no statistical significance was observed regarding patient
distribution in treatment efficacy (Table I).
 | Table I.Therapeutic effect of botulinum toxin
A by sex, age, disease course, number of branches and injection
dose (n=152). |
Table I.
Therapeutic effect of botulinum toxin
A by sex, age, disease course, number of branches and injection
dose (n=152).
|
| Sex |
| Age (years) |
| Disease course
(months) |
| Number of
branches |
| Injection dose |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Item | Male | Female | P-value | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | 1 | >1 | P-value | L | M | H | P-value |
|---|
| Effect starting
timea | 7 | 7 | 0.33 | 7 | 7 | 7 | 0.44 | 7 | 7 | 7 | 0.71 | 7 | 7 | 0.99 | 7 | 7 | 7 | 0.97 |
|
| (6) | (1) |
| (1.7) | (4) | (4.5) |
| (5) | (4) | (2) |
| (3) | (4) |
| (3) | (5) | (7) |
|
| Effect peak
timea | 24 | 30 | 0.34 | 22.5 | 28 | 28 | 0.42 | 21.5 | 28 | 29 | 0.83 | 28 | 27 | 0.96 | 23.5 | 27.5 | 28 |
|
|
| (27) | (30) |
| (31) | (34) | (22) |
| (30) | (34) | (20) |
| (30) | (24) |
| (28) | (34) | (27) |
|
| Lasting
timea | 5 | 5 | 0.99 | 4 | 5.5 | 5 | 0.20 | 5 | 5 | 4.7 | 0.56 | 5 | 5 | 0.57 | 4 | 4.5 | 6 | 0.22 |
|
| (8) | (6.5) |
| (6) | (8) | (9) |
| (9) | (6) | (7) |
| (9) | (2) |
| (7) | (4) | (8) |
|
|
Efficacyb |
|
| 0.96 |
|
|
| 0.24 |
|
|
| 0.57 |
|
| 0.63 |
|
|
| 0.07 |
| Ineffective | 8 | 9 |
| 7 | 8 | 2 |
| 1 | 14 | 2 |
| 14 | 3 |
| 2 | 12 | 3 |
|
|
| (5.3%) | (5.9%) |
| (4.6%) | (5.3%) | (1.3%) |
| (1%) | (9.2%) | (1.3%) |
| (9.2%) | (2.0%) |
|
(1.3%) | (7.9%) | (2.0%) |
|
| Effective | 14 | 17 |
| 4 | 22 | 5 |
| 3 | 21 | 7 |
| 21 | 10 |
| 7 | 10 | 14 |
|
|
| (9.2%) | (11.2%) |
| (2.6%) | (14.5%) | (3.3%) |
| (2.0%) | (13.8%) | (4.6%) |
| (13.8%) | (6.6%) |
| (4.6%) | (6.6%) | (9.2%) |
|
| Significant | 9 | 12 |
| 2 | 16 | 3 |
| 5 | 12 | 4 |
| 14 | 7 |
| 2 | 6 | 13 |
|
|
| (5.9%) | (7.9%) |
| (1.3%) | (10.5%) | (2.0%) |
| (3.3%) | (7.9%) | (2.6%) |
| (9.2%) | (4.6%) |
| (1.3%) | (3.9%) | (8.6%) |
|
| Complete | 34 | 49 |
| 21 | 48 | 14 |
| 15 | 55 | 13 |
| 62 | 21 |
| 17 | 32 | 34 |
|
|
| (22.4%) | (32.2%) |
| (13.8%) | (31.6%) | (9.2%) |
| (9.9%) | (36.2%) | (8.6%) |
| (40.8%) | (13.8%) |
| (11.2%) | (21.1%) | (22.7%) |
|
The long-term effect of BTX-A on TN was also
investigated in the present study. Cases from the BTX-A effective
cohort were followed up each month following the 7th month after
BTX-A treatment. Among the available patients (n=58) that were
followed up, no significant increases in the VAS score were
recorded, which may indicate the long-term control of TN by
BTX-A.
Safety
BTX-A treatment may cause facial asymmetry. In the
present study, 21 patients (13.8%) experienced short-term facial
asymmetry, whereas no cases of twisted mouth or ptosis were
observed (Table II). The incidence
of side effects did not vary with the sex, age or injection dose;
however, the data indicated that the disease course and number of
branches did influence the incidence of side effects, with patients
with a median disease course (duration, 1–10 months) exhibiting a
higher occurrence of side effects associated with BTX-A injection.
Furthermore, side effects of BTX-A were also influenced by the
number of branches, in that multiple branches contributed to a
higher incidence, later occurrence and longer duration of side
effects (Table II). Patients who
received one injection of BTX-A were also compared with those who
received >1 injections. Additional injections did not result in
a higher occurrence of side effects of BTX-A (P=0.76), since 13 out
of 102 patients who received no additional injections were
afflicted with side effects compared with 8 in 50 patients with
additional injections afflicted with side effects.
 | Table II.SEs associated with botulinum toxin A
treatment by sex, age, disease course, number of branches and
injection dose (n=152). |
Table II.
SEs associated with botulinum toxin A
treatment by sex, age, disease course, number of branches and
injection dose (n=152).
|
| Sex |
| Age (years) |
| Disease course
(months) |
| Number of
branches |
| Injection dose |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Item | Male | Female | P-value | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | 1 | >1 | P-value | L | M | H | P-value |
|---|
| SEsa |
|
| 0.48 |
|
|
| 0.60 |
|
|
| 0.05 |
|
| 0.01 |
|
|
| 0.16 |
| No | 58 | 73 |
| 28 | 81 | 22 |
| 23 | 83 | 25 |
| 102 | 29 |
| 26 | 54 | 51 |
|
|
| (38.2%) | (48.0%) |
| (18.4%) | (53.3%) | (14.5%) |
| (15.1%) | (54.6%) | (16.4%) |
| (67.1%) | (19.1%) |
| (17.1%) | (35.5%) | (33.6%) |
|
|
Yes | 7 | 14 |
| 6 | 13 | 2 |
| 1 | 19 | 1 |
| 9 | 12 |
| 2 | 6 | 13 |
|
|
| (4.6%) | (9.2%) |
| (3.9%) | (8.6%) | (13.2%) |
| (1.%) | (12.5%) | (1.0%) |
| (5.9%) | (7.9%) |
| (1.3%) | (3.9%) | (8.6%) |
|
| SE observed time
(days)b | 0 (0) | 0 (2) | 0.26 | 0 (0) | 0 (0) | 0 (0) | 0.63 | 0 (0) | 0 (0) | 0 (0) | 0.14 | 0 (0) | 0 (7) | 0.01 | 0 (0) | 0(0) | 0 (0) | 0.21 |
| SE lasting time
(days)b | 0 (0) | 0 (0) | 0.22 | 0 (0) | 0 (0) | 0 (0) | 0.60 | 0 (0) | 0 (0) | 0 (0) | 0.11 | 0 (0) | 0 (25) | 0.01 | 0 (0) | 0 (0) | 0 (0) | 0.21 |
Stratified analysis
To provide more comprehensive information regarding
the treatment of TN with BTX-A, a stratified analysis by sex and
age was performed. Based on the results, it was observed that the
age distribution of females influenced the treatment outcome of
BTX-A: Younger subjects (<70 years) exhibited higher sensitivity
to treatment with BTX-A (P=0.02; Table
III). It was also observed that the disease course was
significantly associated with the incidence of side effects in the
female patients (P<0.05; Table
III). In males, the disease course had no significant influence
on side effect-associated parameters (Table IV), indicating that the association
of the disease course with side effects in the entire cohort was
primarily due to the significant association in female patients.
Furthermore, in males, a higher injection dose was associated with
a longer effect duration (P<0.01) and a higher incidence of
successful treatment (P=0.06; Table
IV). When the patients were stratified by age, the effect
duration of BTX-A increased with the injection dose in patients
younger than 50 years (P=0.09; Table
V).
 | Table III.Treatment outcomes of botulinum toxin
A by age, disease course, and injection dose in females (n=87). |
Table III.
Treatment outcomes of botulinum toxin
A by age, disease course, and injection dose in females (n=87).
|
| Age (years) |
| Disease course
(months) |
| Injection dose |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Item | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | L | M | H | P-value |
|---|
| Effect starting
timea | 7 (8) | 7 (3) | 7 (1) | 0.14 | 7 (2) | 7 (2) | 7 (1) | 0.69 | 7.5 (3) | 7 (5) | 7 (1) | 0.51 |
| Effect peak time
(days)a | 20 (31) | 30 (29) | 28.5 (36) | 0.14 | 21 (21) | 30 (33) | 31 (18) | 0.72 | 37 (30) | 30 (35) | 29 (21) | 0.70 |
| Lasting time
(days)a | 4 (6) | 6 (7) | 4.5 (3) | 0.17 | 5 (10) | 5 (5) | 4.5 (5) | 0.44 | 7.5 (7) | 5 (4) | 5 (5) | 0.50 |
|
Efficacyb |
|
|
| 0.02 |
|
|
| 0.71 |
|
|
| 0.49 |
|
Ineffective | 5 (3.3%) | 2 (1.3%) | 2 (1.3%) |
| 0 (0.0%) | 8 (5.3%) | 1 (1.0%) |
| 0 (0.0%) | 6 (3.9%) | 3 (2.0%) |
|
|
Effective | 1 (1.0%) | 15 (9.9%) | 1 (1.0%) |
| 3 (2.0%) | 11 (7.2%) | 3 (2.0%) |
| 3 (2.0%) | 7 (4.6%) | 7 (4.6%) |
|
|
Significant | 1 (1.0%) | 10 (6.6%) | 1 (1.0%) |
| 3 (2.0%) | 6 (3.9%) | 3 (2.0%) |
| 1 (1.0%) | 4 (2.6%) | 7 (4.6%) |
|
|
Complete | 13 (8.6%) | 28 (18.4%) | 8 (5.3%) |
| 7 4.6%) | 31 (20.4%) | 11 (7.2%) |
| 12 (7.9%) | 18 (11.8%) | 19 (12.5%) |
|
| SEsb |
|
|
| 0.39 |
|
|
| 0.05 |
|
|
| 0.19 |
| No | 15 (9.9%) | 48 (31.6%) | 10 6.6%) |
| 13 (8.5%) | 43 (28.3%) | 17 (11.2%) |
| 15 (9.9%) | 31 (20.4%) | 27 (17.8%) |
|
|
Yes | 5 (3.3%) | 7 (4.6%) | 2 (1.3%) |
| 0 (0.0%) | 13 (8.6%) | 1 (1.0%) |
| 1 (1.0%0 | 4 2.6%) | 9 (5.9%) |
|
| SE observed time
(days)a | 0 (2) | 0 (0) | 0 (0) | 0.57 | 0 (0) | 0 (0) | 0 (0) | 0.12 | 0 (0) | 0 (0) | 0 (0) | 0.26 |
| SE lasting time
(days)a | 0 (5) | 0 (0) | 0 (0) | 0.61 | 0 (0) | 0 (0) | 0 (0) | 0.12 | 0 (0) | 0 (0) | 0 (5) | 0.22 |
 | Table IV.Treatment outcomes of botulinum toxin
A by age, disease course, and injection dose in males (n=65). |
Table IV.
Treatment outcomes of botulinum toxin
A by age, disease course, and injection dose in males (n=65).
|
| Age (years) |
| Disease course
(months) |
| Injection dose |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Item | <50 | 50–70 | >70 | P-value | <1 | 1–10 | >10 | P-value | L | M | H | P-value |
|---|
| Effect starting
timea | 7.5 (3) | 7 (5) | 9 (7) | 0.56 | 8 (5) | 7 (5) | 7 (5) | 0.90 | 7 (5) | 7 (5) | 7 (6) | 0.50 |
| Effect peak time
(days)a | 26 (38) | 21 (31) | 28 (9) | 0.85 | 22 (38) | 25 (28) | 25 (17) | 0.86 | 18 (12) | 24 (27) | 28 (35) | 0.25 |
| Lasting time
(days)a | 3.5 (6) | 5 (8) | 6 (8) | 0.34 | 4 (9) | 5 (7) | 5 (9) | 0.97 | 3.5 (3) | 3.5 (5) | 6 (7) | 0.00 |
|
Efficacyb |
|
|
| 0.79 |
|
|
| 0.19 |
|
|
| 0.06 |
|
Ineffective | 2 (1.3%) | 6 (3.9%) | 0 (0.0%) |
| 1 (1.0%) | 6 (3.9%) | 1 (1.0%) |
| 2 (1.3%) | 6 (3.9%) | 0 (0.0%) |
|
|
Effective | 3 (2.0%) | 7 (4.6%) | 4 (2.6%) |
| 0 (0.0%) | 10 (6.6%) | 4 (2.6%) |
| 4 (2.6%) | 3 (2.0%) | 7 (4.6%) |
|
|
Significant | 1 (1.0%) | 6 (3.9%) | 2 (1.3%) |
| 2 (1.3%) | 6 (3.9%) | 1 (1.0%) |
| 1 (1.3%) | 2 (1.3%) | 6 (3.9%) |
|
|
Complete | 8 (5.2%) | 20 (13.2%) | 6 (3.9%) |
| 8 (5.2%) | 24 (15.8%) | 2 (1.3%) |
| 5 (3.3%) | 14 (9.2%) | 15 (9.8%) |
|
| SEsb |
|
|
| 0.47 |
|
|
| 0.83 |
|
|
| 0.87 |
| No | 13 (8.6%) | 33 (21.7%) | 12 (7.9%) |
| 10 (6.6%) | 40 (26.3%) | 8 (5.3%) |
| 11 (7,2%) | 23 (15.1%) | 24 (15.8%) |
|
|
Yes | 1 (1.0%) | 6 (3.9%) | 0 (0.0%) |
| 1 (1.0%) | 6 (3.9%) | 0 (0.0%) |
| 1 (1.0%) | 2 (1.3%) | 4 (2.6%) |
|
| SE observed time
(days)a | 0 (0) | 0 (0) | 0 (0) | 0.28 | 0 (0) | 0 (0) | 0 (0) | 0.55 | 0 (0) | 0 (0) | 0 (0) | 0.73 |
| SE lasting time
(days)a | 0 (0) | 0 (0) | 0 (0) | 0.31 | 0 (0) | 0 (0) | 0 (0) | 0.53 | 0 (0) | 0 (0) | 0 (0) | 0.76 |
 | Table V.Treating outcomes of botulinum toxin
A by sex, disease course, and injection dose in patients younger
than 50 years (n=34). |
Table V.
Treating outcomes of botulinum toxin
A by sex, disease course, and injection dose in patients younger
than 50 years (n=34).
|
| Sex |
| Disease course
(months) |
| Injection dose |
|---|
|
|
|
|
|
|
|
|---|
| Item | Male | Female | P-value | <1 | 1–10 | >10 | P-value | L | M | H | P-value |
|---|
| Effect starting
timea | 7.5 (3) | 7 (7) | 0.22 | 7.5 (1) | 7 (3) | 7 (5) | 0.87 | 7 (3) | 7 (1) | 8 (1) | 0.62 |
| Effect peak time
(days)a | 26 (17) | 20.5 (31) | 0.30 | 27 (29) | 23 (29) | 9 (24) | 0.58 | 22 (27) | 16.5 (44) | 30 (21) | 0.82 |
| Lasting time
(days)a | 3.5 (6) | 4 (6) | 0.83 | 7.5 (8) | 4 (6) | 3 (2) | 0.22 | 4 (5) | 3 (4) | 6 (6) | 0.09 |
|
Efficacyb |
|
| 0.54 |
|
|
| 0.88 |
|
|
| 0.49 |
|
Ineffective | 2 (1.3%) | 5 (3.3%) |
| 0 (0.0%) | 6 (3.9%) | 1 (1.0%) |
| 1 (1.0%) | 4 (2.6%) | 2 (1.3%) |
|
|
Effective | 3 (2.0%) | 1 (1.0%) |
| 0 (0.0%) | 4 (2.6%) | 0 (0.0%) |
| 1 (1.0%) | 0 (0.0%) | 3 (2.0%) |
|
|
Significant | 1 (1.0%) | 1 (1.05) |
| 0 (0.0%) | 2 (1.3%) | 0 (0.0%) |
| 1 (1.0%) | 0 (0.0%) | 1 (1.0%) |
|
|
Complete | 8 (5.3%) | 13 (8.6%) |
| 4 (2.6%) | 15 (9.9%) | 2 (1.3%) |
| 6 (3.9%) | 8 (5.2%) | 7 (4.6%) |
|
| SEsb |
|
| 0.36 |
|
|
| 0.76 |
|
|
| 0.39 |
| No | 13 (8.6%) | 15 (9.9%) |
| 4 (2.6%) | 12 (7.9%) | 3 (2.0%) |
| 8 (5.3%) | 11 (7.2%) | 9 (5.9%) |
|
|
Yes | 1 (1.0%) | 5 (3.3%) |
| 0 (0.0%) | 6 (3.9%) | 0 (0.0%) |
| 1 (1.0%) | 1 (1.0%) | 4 (2.6%) |
|
| SE observed time
(days)a | 0 (0) | 0 (1) | 0.18 | 0 (0) | 0 (0) | 0 (0) | 0.40 | 0 (0) | 0 (0) | 0 (7) | 0.30 |
| SE lasting time
(days)a | 0 (5) | 0 (0) | 0.21 | 0 (0) | 0 (0) | 0 (0) | 0.40 | 0 (0) | 0 (0) | 0 (21) | 0.33 |
Discussion
BTX-A has been widely used to protect patients
against neuropathic pain, including TN (20). A number of clinical studies and
meta-analyses have suggested that BTX-A is beneficial in the
treatment of TN when compared with placebo in terms of the
proportion of responders and the VAS score at the end of the
follow-up (15,16,21,22).
However, a number of controversies regarding the clinical
application of BTX-A remain. Compared with previous studies, the
present study performed a more comprehensively stratified analysis.
Consistent with previous results, the treatment of BTX-A decreased
the VAS score with only one administration. For patients
insensitive to one injection of BTX-A, repeated injection led to
better control of pain with no significant increase in the
incidence of side effects. The results also demonstrated that
patient sex, age or the number of branches had no significant
influence on the starting time of the effect, the peak time of the
effect, average effect duration, incidence of side effects,
starting time of side effects and side effect duration.
Furthermore, medium- and high-dose injections of BTX-A were more
likely to completely control the pain of the patients, even though
statistical significance was not observed (P=0.07). Conversely, a
median disease course (duration, 1–10 months) and multiple branches
contributed to a higher incidence of side effects, and multiple
branches were associated with later occurrence of side effects and
longer side effect duration.
Previous studies have indicated that the effect of
BTX-A may last as long as 2 years (23); however, whether the long-term effect
of BTX-A is influenced by the patients' characteristics has
remained elusive. Therefore, the follow-up was performed for up to
28 months. It was indicated that within the initial 6 months
following BTX-A treatment, females, patients with a short disease
course and patients with a high injection dose reported lower VAS
scores. In the short-term, higher injection doses of BTX-A did not
influence the VAS scores; however, the effect of high-dose BTX-A
receded at a slower rate than that of medium or low doses.
Considering that treatment with a higher dose of BTX-A did not lead
to a higher incidence of side effects, treatment of patients with
TN with relatively higher doses of BTX-A may contribute to a
greater duration of pain control. With regard to the dose range,
Fabregat et al (24)
suggested values from 50–100 units to achieve optimal treatment
outcomes. Zhang et al (21)
indicated that the lower (25 units) and the high dose (75 units)
were similar in terms of short-term efficacy. These conclusions
were validated in the present study: Different doses of BTX-A
injection did not influence the short-term treatment outcomes and
higher doses of BTX-A contributed to a more stable long-term
control of pain due to TN.
To provide more specific information for the
application of BTX-A in clinical settings, stratified analyses by
sex and age were also performed. The majority of parameters
investigated in the present study exhibited similar patterns with
different stratifications by sex or age. However, certain notable
trends were observed: For instance, female patients younger than 70
years were more sensitive to BTX-A treatment. Furthermore, the
disease course influenced the incidence of side effects primarily
in female patients, whereas in male patients, the disease course
was not significantly associated with changes in side effects,
indicating that the application of BTX-A in female patients with TN
with a median disease course (duration, 1–10 months) should be more
conservative. Higher BTX-A injection doses were more likely to
successfully treat TN in male patients. The mechanisms responsible
for the anti-TN effect of BTX-A remain to be elucidated. In
addition to its effect on muscle contraction by inhibiting the
release of acetylcholine, the direct analgesic action of BTX-A has
also been observed (25). Previous
studies have also indicated that BTX-A may alleviate neurogenic
inflammation in sensory terminals by reducing the release of
glutamate, substance P and calcitonin gene-associated peptide, and
selectively inhibiting C-fibers and the transient receptor
potential vanilloid (TRPV)1 receptor (26,27). The
distinct sensitivities to BTX-A under different stratifications may
also be attributable to the existence of BTX-A-sensitive and
-insensitive C-fibers and the TRPV1 receptor (27).
Collectively, the present results demonstrated that
the efficacy of BTX-A in the treatment of TN may persist for as
long as 28 months. Furthermore, based on the present stratified
analyses, it was demonstrated that female patients with a median
disease course (duration, 1–10 months) exhibited a higher incidence
of side effects following treatment with BTX-A and males achieved
better treatment outcomes with high BTX-A doses. Given that the
clinical response to treatment with BTX-A varies in all individual
patients, the present study provided insight regarding the
treatment outcomes of BTX-A and supported the long-term efficacy of
the treatment. To validate the present results, adequately powered
studies investigating the effect of BTX-A in other populations are
required in the future.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant nos. 81701271, 81771397
and 81701295).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HZ prepared the manuscript and analyzed the data. YL
analyzed the data. NX, XC, CC and HX performed the data collection.
YZ designed the experiment and revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China) for the screening, inspection and data
collection of the patients. All protocols were in accordance with
the provisions of the Declaration of Helsinki.
Patient consent for publication
The purpose and safety aspects of the study were
explained to all patients and written informed consent was included
in the documents of each patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Olesen J and Steiner TJ: The International
classification of headache disorders, 2nd edn (ICDH-II). J Neurol
Neurosurg Psychiatry. 75:808–811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katusic S, Beard CM, Bergstralth E and
Kurland LT: Incidence and clinical features of trigeminal
neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 27:89–95.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ngeow WC and Nair R: Injection of
botulinum toxin type A (BOTOX) into trigger zone of trigeminal
neuralgia as a means to control pain. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 109:e47–e50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devor M, Amir R and Rappaport ZH:
Pathophysiology of trigeminal neuralgia: The ignition hypothesis.
Clin J Pain. 18:4–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheshire WP: Trigeminal neuralgia: For one
nerve a multitude of treatments. Expert Rev Neurother. 7:1565–1579.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merrison AF and Fuller G: Treatment
options for trigeminal neuralgia. BMJ. 327:1360–1361. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castillo-Alvarez F, Hernando de la Barcena
I and Marzo-Sola ME: Botulinum toxin in trigeminal neuralgia. Med
Clin (Barc). 148:28–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Humeau Y, Doussau F, Grant NJ and Poulain
B: How botulinum and tetanus neurotoxins block neurotransmitter
release. Biochimie. 82:427–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pearce LB, First ER, Maccallum RD and
Gupta A: Pharmacologic characterization of botulinum toxin for
basic science and medicine. Toxicon. 35:1373–1412. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lian YJ, Wei HL, Zhang BA, et al:
Treatment of 795 facial spasm and local dystonia with Botulinum
toxin A. J Zhengzhou Univ (Med Sci). 44:32009.
|
|
11
|
Aurora SK, Winner P, Freeman MC, Spierings
EL, Heiring JO, DeGryse RE, VanDenburgh AM, Nolan ME and Turkel CC:
OnabotulinumtoxinA for treatment of chronic migraine: Pooled
analyses of the 56-week PREEMPT clinical program. Headache.
51:1358–1373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diener HC, Dodick DW, Aurora SK, Turkel
CC, DeGryse RE, Lipton RB, Silberstein SD and Brin MF; PREEMPT 2
Chronic Migraine Study Group, : OnabotulinumtoxinA for treatment of
chronic migraine: Results from the double-blind, randomized,
placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia.
30:804–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Micheli F, Scorticati MC and Raina G:
Beneficial effects of botulinum toxin type a for patients with
painful tic convulsif. Clin Neuropharmacol. 25:260–262. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CJ, Lian YJ, Zheng YK, Zhang HF, Chen
Y, Xie NC and Wang LJ: Botulinum toxin type A for the treatment of
trigeminal neuralgia: Results from a randomized, double-blind,
placebo-controlled trial. Cephalalgia. 32:443–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, Guan X, Fan L, Li M, Liao Y, Nie Z
and Jin L: Therapeutic efficacy and safety of botulinum toxin type
A in trigeminal neuralgia: A systematic review. J Headache Pain.
14:722013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morra ME, Elgebaly A, Elmaraezy A, Khalil
AM, Altibi AM, Vu TL, Mostafa MR, Huy NT and Hirayama K:
Therapeutic efficacy and safety of botulinum toxin a therapy in
trigeminal neuralgia: A systematic review and meta-analysis of
randomized controlled trials. J Headache Pain. 17:632016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Headache Classification Subcommittee of
the International Headache Society, . The International
Classification of Headache Disorders: 2nd edition. Cephalalgia. 24
(Suppl 1):S9–S160. 2004.
|
|
18
|
Lee JS: Pain measurement: Understanding
existing tools and their application in the emergency department.
Emerg Med (Fremantle). 13:279–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ross I and Robert G: R: A language for
data analysis and graphics. J Computational Graphical Statistics.
5:299–314. 1996. View Article : Google Scholar
|
|
20
|
Zakrzewska JM: Botulinum toxin type A for
trigeminal neuralgia-we have the prima facie evidence. Cephalalgia.
32:1156–1157. 2012. View Article : Google Scholar
|
|
21
|
Zhang H, Lian Y, Ma Y, Chen Y, He C, Xie N
and Wu C: Two doses of botulinum toxin type A for the treatment of
trigeminal neuralgia: Observation of therapeutic effect from a
randomized, double-blind, placebo-controlled trial. J Headache
Pain. 15:652014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Lian YJ, Chen Y, Zhang HF, Ma YQ, He
CH, Wu CJ, Xie NC, Zheng YK and Zhang Y: Therapeutic effect of
botulinum toxin-a in 88 patients with trigeminal neuralgia with
14-month follow-up. J Headache Pain. 15:432014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen JH, Lian YJ and Zheng YK: Effect of
botulinum toxin type a on clssic trigeminal neuralgia. Chin J Rehab
Med. 26:22011.
|
|
24
|
Fabregat G, De Andrés J, Villanueva-Pérez
VL and Asensio-Samper JM: Subcutaneous and perineural botulinum
toxin type A for neuropathic pain: A descriptive review. Clin J
Pain. 29:1006–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klein AW: The therapeutic potential of
botulinum toxin. Dermatologic Surgery. 30:452–455. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aoki KR: Review of a proposed mechanism
for the antinociceptive action of botulinum toxin type A.
Neurotoxicology. 26:785–793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gazerani P, Pedersen NS, Staahl C, Drewes
AM and Arendt-Nielsen L: Subcutaneous Botulinum toxin type A
reduces capsaicin-induced trigeminal pain and vasomotor reactions
in human skin. Pain. 141:60–69. 2009. View Article : Google Scholar : PubMed/NCBI
|