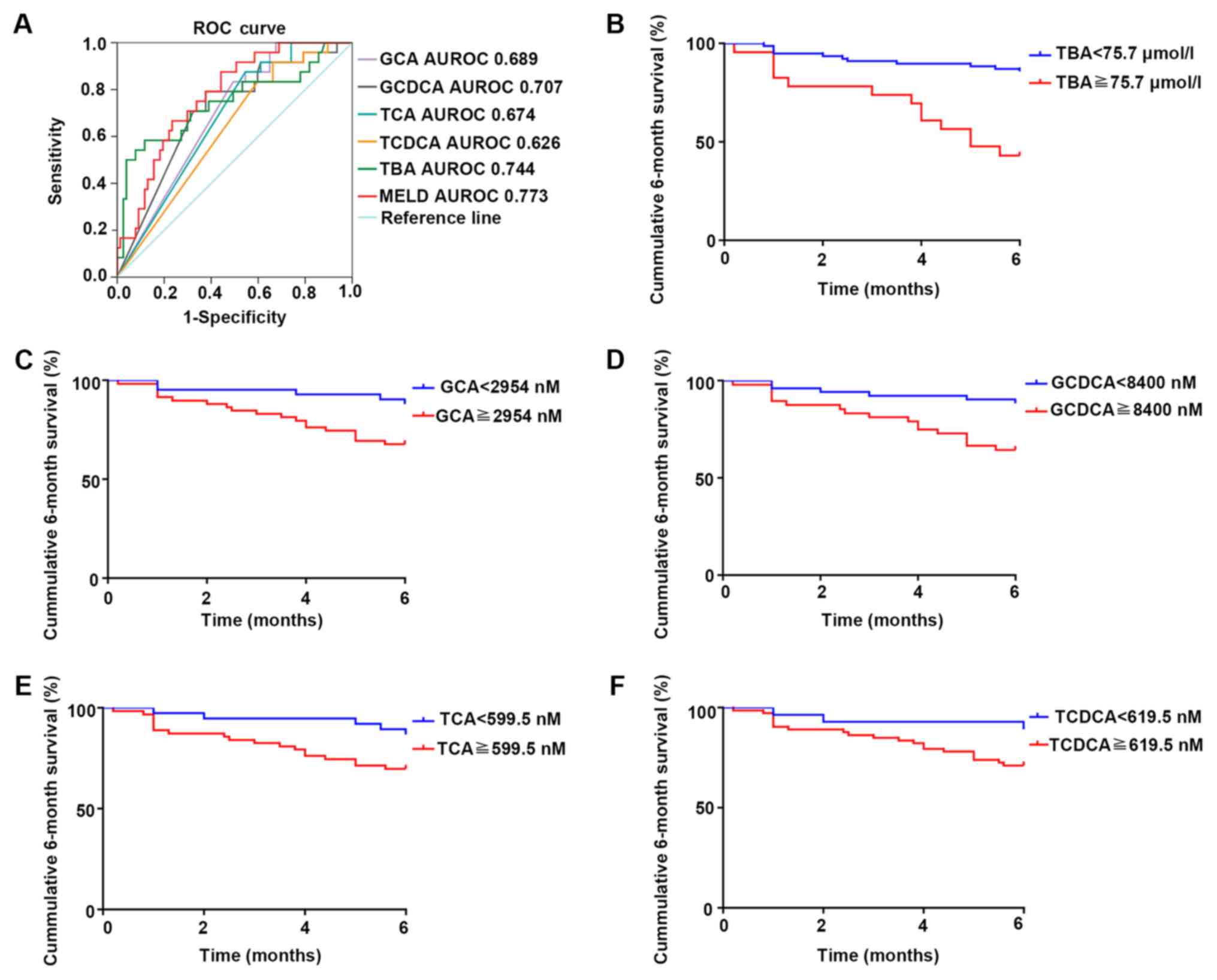
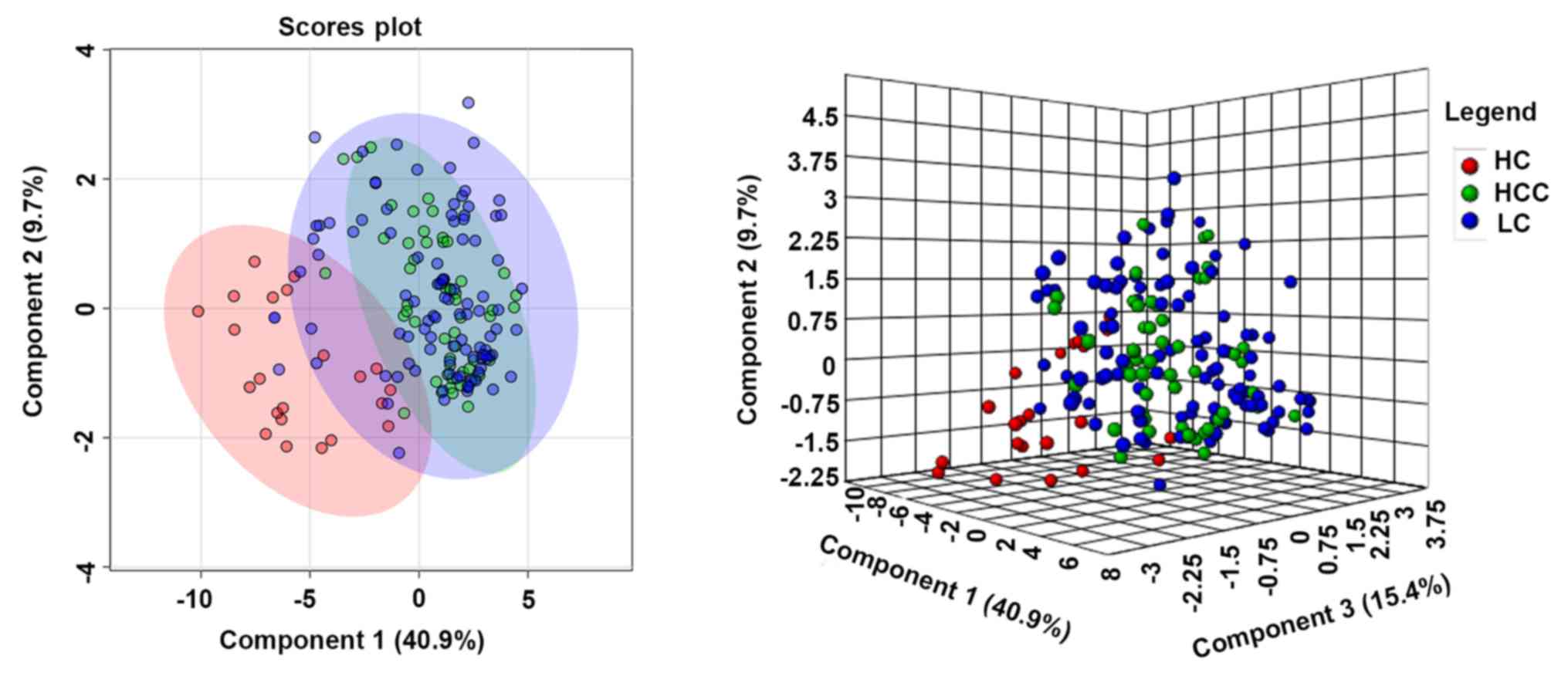
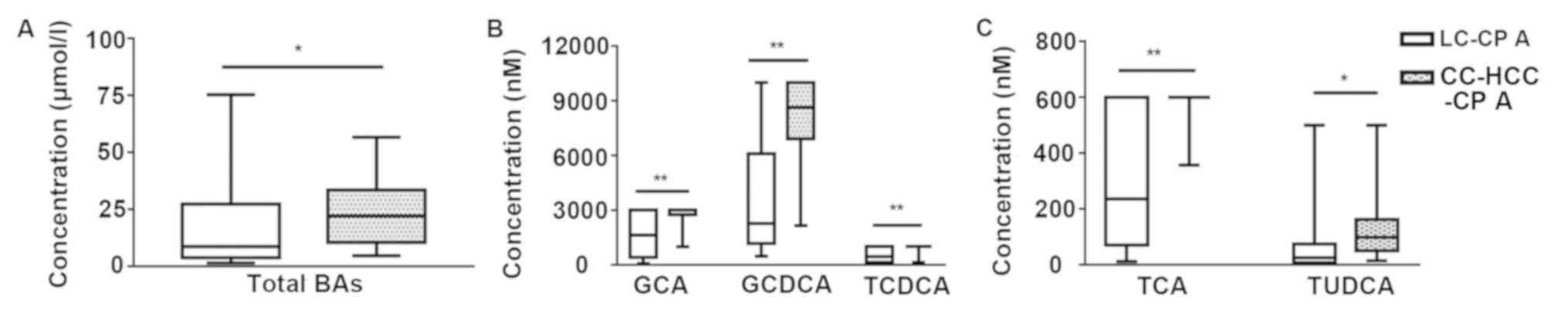
|
1
|
Asrani SK, Devarbhavi H, Eaton J and
Kamath PS: Burden of liver diseases in the world. J Hepatol.
70:151–171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127((5 Suppl 1)): S35–S50. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boyer JL: Bile formation and secretion.
Compr Physiol. 3:1035–1078. 2013.PubMed/NCBI
|
|
5
|
Arab JP, Martin-Mateos RM and Shah VH:
Gut-liver axis, cirrhosis and portal hypertension: The chicken and
the egg. Hepatol Int. 12 (Suppl 1):S24–S33. 2018. View Article : Google Scholar
|
|
6
|
Meier PJ and Stieger B: Bile salt
transporters. Annu Rev Physiol. 64:635–661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li T and Apte U: Bile acid metabolism and
signaling in cholestasis, inflammation, and cancer. Adv Pharmacol.
74:263–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Shang X, Wan X, Xiang X, Mao Q,
Deng G and Wu Y: Increased hepatocellular carcinoma risk in chronic
hepatitis B patients with persistently elevated serum total bile
acid: A retrospective cohort study. Sci Rep. 6:381802016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mouzaki M, Wang AY, Bandsma R, Comelli EM,
Arendt BM, Zhang L, Fung S, Fischer SE, McGilvray IG and Allard JP:
Bile acids and dysbiosis in non-alcoholic fatty liver disease. PLoS
One. 11:e01518292016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Xie G, Zhao A, Zheng X, Huang F,
Wang Y, Yao C, Jia W and Liu P: Serum bile acids are associated
with pathological progression of hepatitis B-induced cirrhosis. J
Proteome Res. 15:1126–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen T, Xie G, Wang X, Fan J, Qiu Y, Zheng
X, Qi X, Cao Y, Su M, Wang X, et al: Serum and urine metabolite
profiling reveals potential biomarkers of human hepatocellular
carcinoma. Mol Cell Proteomics. 10:M110.0049452011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singal AK and Kamath PS: Model for
end-stage liver disease. J Clin Exp Hepatol. 3:50–60. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamath PS and Kim WR; Advanced Liver
Disease Study Group, : The model for end-stage liver disease
(MELD). Hepatology. 45:797–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiesner R, Edwards E, Freeman R, Harper A,
Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, et al:
Model for end-stage liver disease (MELD) and allocation of donor
livers. Gastroenterology. 124:91–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krautbauer S and Liebisch G: LC-MS/MS
analysis of bile acids. Methods Mol Biol. 1730:103–110. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chong J, Yamamoto M and Xia J:
MetaboAnalystR 2.0: From raw spectra to biological insights.
Metabolites. 9(pii): E572019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mannes GA, Thieme C, Stellaard F, Wang T,
Sauerbruch T and Paumgartner G: Prognostic significance of serum
bile acids in cirrhosis. Hepatology. 6:50–53. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohkubo H, Okuda K, Iida S, Ohnishi K,
Ikawa S and Makino I: Role of portal and splenic vein shunts and
impaired hepatic extraction in the elevated serum bile acids in
liver cirrhosis. Gastroenterology. 86:514–520. 1984.PubMed/NCBI
|
|
19
|
Gilmore IT and Thompson RP: Plasma
clearance of oral and intravenous cholic acid in subjects with and
without chronic liver disease. Gut. 21:123–127. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bajaj JS, Heuman DM, Hylemon PB, Sanyal
AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR,
et al: Altered profile of human gut microbiome is associated with
cirrhosis and its complications. J Hepatol. 60:940–947. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cassard AM and Ciocan D: Microbiota, a key
player in alcoholic liver disease. Clin Mol Hepatol. 24:100–107.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Martinot E, Sèdes L, Baptissart M,
Lobaccaro JM, Caira F, Beaudoin C and Volle DH: Bile acids and
their receptors. Mol Aspects Med. 56:2–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Massafra V and van Mil SWC: Farnesoid X
receptor: A ‘homeostat’ for hepatic nutrient metabolism. Biochim
Biophys Acta Mol Basis Dis. 1864:45–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SG, Kim BK, Kim K and Fang S: Bile
acid nuclear receptor farnesoid X receptor: Therapeutic target for
nonalcoholic fatty liver disease. Endocrinol Metab (Seoul).
31:500–504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Verbeke L, Farre R, Trebicka J, Komuta M,
Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F
and Laleman W: Obeticholic acid, a farnesoid X receptor agonist,
improves portal hypertension by two distinct pathways in cirrhotic
rats. Hepatology. 59:2286–2298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kakiyama G, Pandak WM, Gillevet PM,
Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon
JM, et al: Modulation of the fecal bile acid profile by gut
microbiota in cirrhosis. J Hepatol. 58:949–955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lorenzo-Zúñiga V, Bartolí R, Planas R,
Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA,
Ausina V and Gassull MA: Oral bile acids reduce bacterial
overgrowth, bacterial translocation, and endotoxemia in cirrhotic
rats. Hepatology. 37:551–557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seo YS and Shah VH: The role of gut-liver
axis in the pathogenesis of liver cirrhosis and portal
hypertension. Clin Mol Hepatol. 18:337–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marin JJ, Macias RI, Briz O, Banales JM
and Monte MJ: Bile acids in physiology, pathology and pharmacology.
Curr Drug Metab. 17:4–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gadaleta RM, van Mil SW, Oldenburg B,
Siersema PD, Klomp LW and Van Erpecum KJ: Bile acids and their
nuclear receptor FXR: Relevance for hepatobiliary and
gastrointestinal disease. Biochim Biophys Acta. 1801:683–692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nuño-Lámbarri N, Barbero-Becerra VJ, Uribe
M and Chávez-Tapia NC: Elevated cholesterol levels have a poor
prognosis in a cholestasis scenario. J Biochem Mol Toxicol. 31:1–6.
2017. View Article : Google Scholar
|
|
32
|
Gong Z, Zhou J, Zhao S, Tian C, Wang P, Xu
C, Chen Y, Cai W and Wu J: Chenodeoxycholic acid activates NLRP3
inflammasome and contributes to cholestatic liver fibrosis.
Oncotarget. 7:83951–83963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tripodi V, Contin M, Fernández MA and
Lemberg A: Bile acids content in brain of common duct ligated rats.
Ann Hepatol. 11:930–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horvatits T, Drolz A, Rutter K, Roedl K,
Fauler G, Müller C, Kluge S, Trauner M, Schenk P and Fuhrmann V:
Serum bile acids in patients with hepatopulmonary syndrome. Z
Gastroenterol. 55:361–367. 2017.PubMed/NCBI
|
|
35
|
Desai MS, Mathur B, Eblimit Z, Vasquez H,
Taegtmeyer H, Karpen SJ, Penny DJ, Moore DD and Anakk S: Bile acid
excess induces cardiomyopathy and metabolic dysfunctions in the
heart. Hepatology. 65:189–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pak JM and Lee SS: Vasoactive effects of
bile salts in cirrhotic rats: In vivo and in vitro studies.
Hepatology. 18:1175–1181. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Desai MS and Penny DJ: Bile acids induce
arrhythmias: Old metabolite, new tricks. Heart. 99:1629–1630. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cherqui D, Laurent A, Tayar C, Chang S,
Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D and Fagniez
PL: Laparoscopic liver resection for peripheral hepatocellular
carcinoma in patients with chronic liver disease: Midterm results
and perspectives. Ann Surg. 243:499–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fan M, Wang X, Xu G, Yan Q and Huang W:
Bile acid signaling and liver regeneration. Biochim Biophys Acta.
1849:196–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Altamirano-Barrera A, Uribe M, Lammert F
and Méndez-Sánchez N: Bile acids and the risk for hepatocellular
carcinoma in primary biliary cholangitis. Ann Hepatol. 15:453–454.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie G, Wang X, Huang F, Zhao A, Chen W,
Yan J, Zhang Y, Lei S, Ge K, Zheng X, et al: Dysregulated hepatic
bile acids collaboratively promote liver carcinogenesis. Int J
Cancer. 139:1764–1775. 2016. View Article : Google Scholar : PubMed/NCBI
|