Introduction
Leukemia is recognized as one of the most common
types of human malignancy (1).
Globally, ~30,000 new cases are diagnosed and >23,000 patients
succumb annually as a result of this disease (2). Among children with cancer, almost
one-third of cases are diagnosed as a type of leukemia (3). In addition, leukemia is the second most
common form of cancer in infants (<1-year-old) and first most
common in older children (4).
Despite developments in various therapeutic strategies,
chemotherapy is administered as a first-line therapy and has a high
rate of remission. Of note, disease recurrence and drug resistance
often occur during the first year of treatment (5). As the overall survival rate of patients
with leukemia is poor, there is an urgent need to screen and
identify novel therapeutic targets for the treatment of this
disease (6).
Protein phosphatase type 2A (PP2A) is a broad
specificity serine/threonine phosphatase, which acts as a regulator
of various biological processes. Its loss of function has been
associated with signaling pathways involved in cancer, including
the mitogen-activated protein kinase (MAPK) kinase/extracellular
signal-regulated kinase and phosphatidylinositol 3-kinase
(PI3K)/protein kinase B (AKT) cascades (7). Template-activating factor Iβ (TAF-Iβ)
is a potent physiological inhibitor of PP2A and a multifunctional
protein with a role in various cellular processes, including DNA
replication, RNA splicing chromatin remodeling and nucleosome
assembly (8). In addition, TAF-Iβ
has been reported to inhibit the tumor suppressor NM23-H1, an
activator of the AP-1/MAPK signaling pathway (9); NM23-H1 regulates the production of
granzyme B and interferon-γ (10).
Additionally, it has been suggested that TAF-Iβ is an oncogene
involved in several types of solid tumor, including those in lung
cancer, cervical cancer, renal carcinoma, gastric carcinoma,
colorectal cancer and non-Hodgkin's lymphoma (11); however, the role of TAF-Iβ in the
development of leukemia requires further investigation.
Our previous study using comparative proteomics
revealed that TAF-Iβ is a differentially expressed protein that is
involved in arsenic trioxide-related cell survival in acute
promyelocytic leukemia (Fig. S1)
(12). In the present study, whether
TAF-Iβ is differentially expressed in other leukemia subtypes, and
its effects on cell proliferation and apoptosis were investigated.
Furthermore, the underlying molecular mechanism was determined,
which may provide a theoretical basis for the development of
targeted therapeutic approaches for treating leukemia.
Materials and methods
Cell line and culture
Leukemia can be divided into acute leukemia and
chronic leukemia. Both can be further divided into lymphocytic
leukemia and myeloid leukemia. In addition, acute myeloid leukemia
can be divided into M0-M7 according to the FAB classification
criteria (13). Therefore, leukemia
is a complex hematological malignant tumor, and each subtype has
its own unique features. In the present study, six leukemia cell
lines were used, namely the Jurkat human T cell acute lymphoblastic
leukemia cell line, the BALL-1 B cell acute lymphoblastic leukemia
cell line, the NB4, HL-60 and THP-1 acute myeloid leukemia cell
lines and the K562 chronic myeloid leukemia cell line. All cell
lines were donated by the Shanghai Institute of Hematology
(Shanghai, China). The cells were routinely cultured in RPMI 1640
medium supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) in a humidified incubator containing 5% CO2 at
37°C, as previously described (14).
SC79 was provided by Selleck Chemicals.
Patient samples
Primary bone marrow samples were obtained from the
inpatient and outpatient departments of Xiangya Hospital of Central
South University (Changsha, China). A total of 36 patients with
acute leukemia and 30 normal controls (healthy individuals without
hematological conditions or other solid tumors) were included for
tissue collection between 2016 and 2018; the patient
characteristics are presented in Table
I. Diagnosis was performed according to the National
Comprehensive Cancer Network Clinical Practice Guidelines in
Oncology: Acute lymphoblastic/myeloid leukemia (version 1.2015,
http://www.nccn.org). Clinical data were
collected from medical clinical records. The present study was
approved by the Ethics Committee of Xiangya Hospital (approval no.
201603063).
 | Table I.Clinical characteristics of a series
of 36 patients with acute leukemia. |
Table I.
Clinical characteristics of a series
of 36 patients with acute leukemia.
| Clinical and
molecular characteristics | N | % |
|---|
| Sex |
|
|
| Male | 15 | 41.7 |
|
Female | 21 | 58.3 |
| Age (years) |
|
|
|
<60 | 26 | 72.2 |
| ≥60 | 10 | 27.8 |
| ECOG score |
|
|
| 0–2 | 30 | 83.3 |
| 3–4 | 6 | 16.7 |
| Leukemia type |
|
|
| Acute myeloid
leukemia |
|
|
| M2 | 9 | 25.0 |
| M3 | 7 | 19.4 |
| M4 | 4 | 11.1 |
| M5 | 7 | 19.4 |
| Acute lymphoblastic
leukemia |
|
|
|
B-cell | 5 | 13.9 |
|
T-cell | 4 | 11.1 |
Cell transfection
Lentiviral vector construction was performed as
previously reported (15). Briefly,
short hairpin RNA (shRNA) against the human TAF-Iβ gene
[shRNA-knockdown (KD)] and scramble shRNA, which acts as a negative
control (shRNA-NC), were constructed by Shanghai GeneChem Co., Ltd.
(Shanghai, China). The leukemic cells were then transfected with
the shRNA-KD or shRNA-NC vectors using Lipofectamine®
2000. The green fluorescent protein (GFP)-positive cells were
counted under a fluorescence microscope. The RNA interference
efficiency was evaluated by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analyses.
RT-qPCR analysis
Total RNA was extracted using an RNAfast200 kit
(Fastagen, Shanghai, China). RT was performed with a One Step SYBR
PrimeScript™ RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian,
China). The thermocycling conditions were as follows: 52°C for 5
min, 95°C for 10 sec. The primers sequences were as follows:
TAF-Iβ, forward 5′-AAATATAACAAACCTCCGCCAACC-3′, reverse,
5′-CAGTGCCTCTTCATCTTCCTC-3′ and GAPDH, forward
5′-TGCACCACCAATGCTTAG-3′ and reverse 5′-GGATGCAGGGATGATGTTC-3′. The
thermocycling conditions were as follows: 95°C for 5 sec, 60°C for
30 sec and 40 cycles, 4°C for 30 min and end of the PCR reaction.
GAPDH was used for normalization; expression levels were quantified
via the 2−ΔΔCq method (16).
Western blot analysis
The specific experimental process of western
blotting was performed according to our previous study (14). Briefly, the cells were washed with
pre-cooled PBS, following which protein (30 µg) was extracted with
radioimmunoprecipitation assay lysis buffer via centrifugation
(5,000 × g for 20 min at 4°C), and separated by 12% SDS-PAGE.
Following electrophoresis, the proteins were transferred onto
nitrocellulose membranes and incubated with primary antibodies at
room temperature for 2 h and 4°C overnight. The antibodies used
were as follows: Anti-TAF-Iβ (cat. no. ab181990, mouse monoclonal,
1:1,000; Abcam, Cambridge, MA, USA), anti-PP2A (cat. no. ab32141,
rabbit monoclonal, 1:5,000; Abcam), phosphorylated-glycogen
synthase kinase-3β (GSK-3β; Ser9; cat. no. 9336, rabbit monoclonal,
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
phosphorylated-AKT (Ser473; cat. no. 9271, rabbit monoclonal,
1:2,000; Cell Signaling Technology, Inc.), anti-caspase-3 (cat. no.
9662), anti-poly(ADP-ribose)polymerase (PARP; cat. no. 9542, rabbit
polyclonal, 1:1,000; Cell Signaling Technology, Inc.) and
anti-GAPDH (cat. no. sc-166574, mouse monoclonal, 1:5,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). Subsequently, the
washed membranes were incubated with secondary antibodies (cat. no.
sc-2005; goat anti-mouse or cat. no. sc-2004; goat anti-rabbit IgG,
1:10,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature and visualized via chemiluminescence (Bio-Rad
chemiluminescence imaging system; Bio-Rad Laboratories, Inc.).
Cell proliferation assay
Cell proliferation was analyzed using a Cell
Counting Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), which was performed according to the
manufacturer's protocol. A total of 100 µl of cell suspension
(5,000 cells/well) was inserted into a 96-well plate and the cells
were cultured at 37°C in 5% CO2 for 48 h. Following
culture, 10 µl CCK-8 solution was applied to each well of the plate
and cells were incubated for 3 h in an incubator at 37°C.
Subsequently, the absorbance was measured at 450 nm using a
multifunctional microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA).
Colony formation assay
This assay was performed using semisolid
methylcellulose medium (Stemcell Technologies, Inc., Vancouver, BC,
Canada) as previously described (17).
Flow cytometry (FCM)
Each group of cells were washed with pre-cooled PBS
three times, following which the cell cycle and apoptosis were
analyzed according to the manufacturer's protocols of the cell
cycle staining and Annexin V-phycoerythrin/7-aminoactinomysin D
apoptosis kits (Multi Sciences, Hangzhou, China), respectively,
with a BD FACScan flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). For cell cycle analysis, ~1×106 cells were
incubated with 1 ml DNA staining solution and 10 µl
permeabilization solution. The cells were vortexed for 10 sec and
incubated in the dark at room temperature for 30 min and analyzed.
For the detection of apoptotic cells, 3×106 untreated
cells were resuspended with 500 µl apoptosis-positive control
solution. Following incubation on ice for 30 min, the supernatant
was discarded. Subsequently, 1.5 ml 1X binding buffer was added.
This cell suspension was divided into three groups, labeled as the
blank control, single staining A (5 µl Annexin-fluorescein
isothiocyanate staining for 5 min without light) and single
staining B (10 µl propidium iodide staining for 5 min without
light) groups. Subsequently, the parameters for FCM (488 nm
excitation and band pass filters of 530/30 nm for FITC detection
and 585/42 nm for 7-AAD detection) were set for the analysis of the
aforementioned cell groups. As the six leukemia cell lines all
underwent the same treatment. The flow parameters of each cell line
were not set separately. Although this may result in a high
background state in certain cell lines, it does not cause a
qualitative change in the final statistical results. Based on these
criteria, all other analyses were performed.
Statistical analysis
All statistical evaluations were performed using
SPSS software (version 22.0; IBM Corp., Armonk, NY, USA); analyses
were repeated at least three times. Statistical significance was
determined using Student's t-test or one-way analysis of variance
(followed by the LSD post hoc test). P<0.05 was considered to
indicate a statistically significant difference.
Results
TAF-Iβ is upregulated in leukemic
cells and patients with leukemia
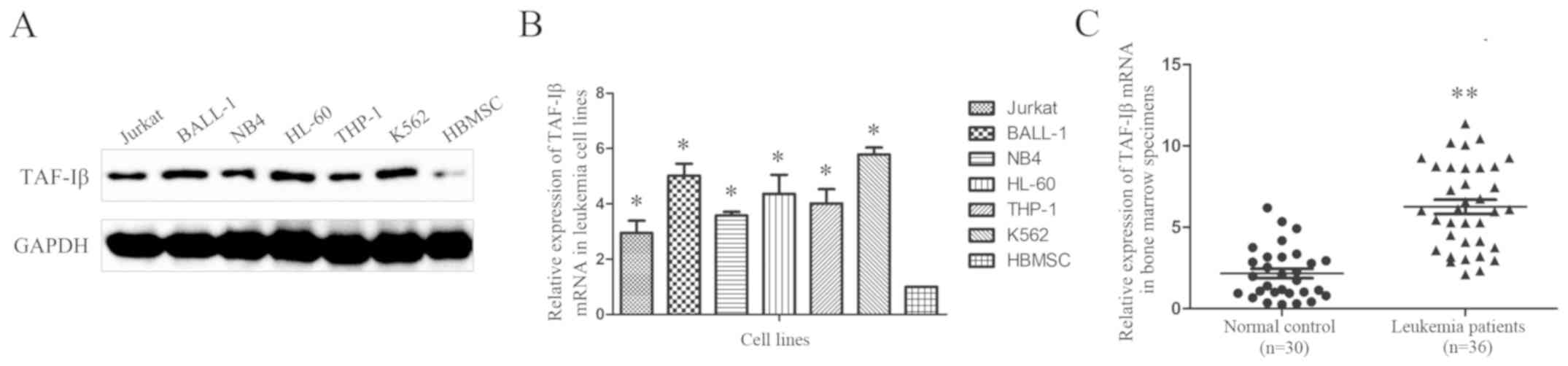
The expression of TAF-Iβ was examined in six typical
leukemic cell lines and normal human bone marrow mesenchymal stem
cells (HBMSCs). The western blot (Fig.
1A) and RT-qPCR (Fig. 1B)
analyses revealed increased expression levels of TAF-Iβ in almost
all leukemic cell lines compared with those in the HBMSCs.
Additionally, the RT-qPCR analysis of clinical samples from 36
patients with acute leukemia and 30 normal controls demonstrated
that 72.2% of the leukemic bone marrow specimens (26/36 samples)
exhibited upregulated expression of TAF-Iβ. By contrast, the normal
control specimens exhibited relatively low expression (Fig. 1C).
Generation of leukemic cells with
TAF-Iβ KD
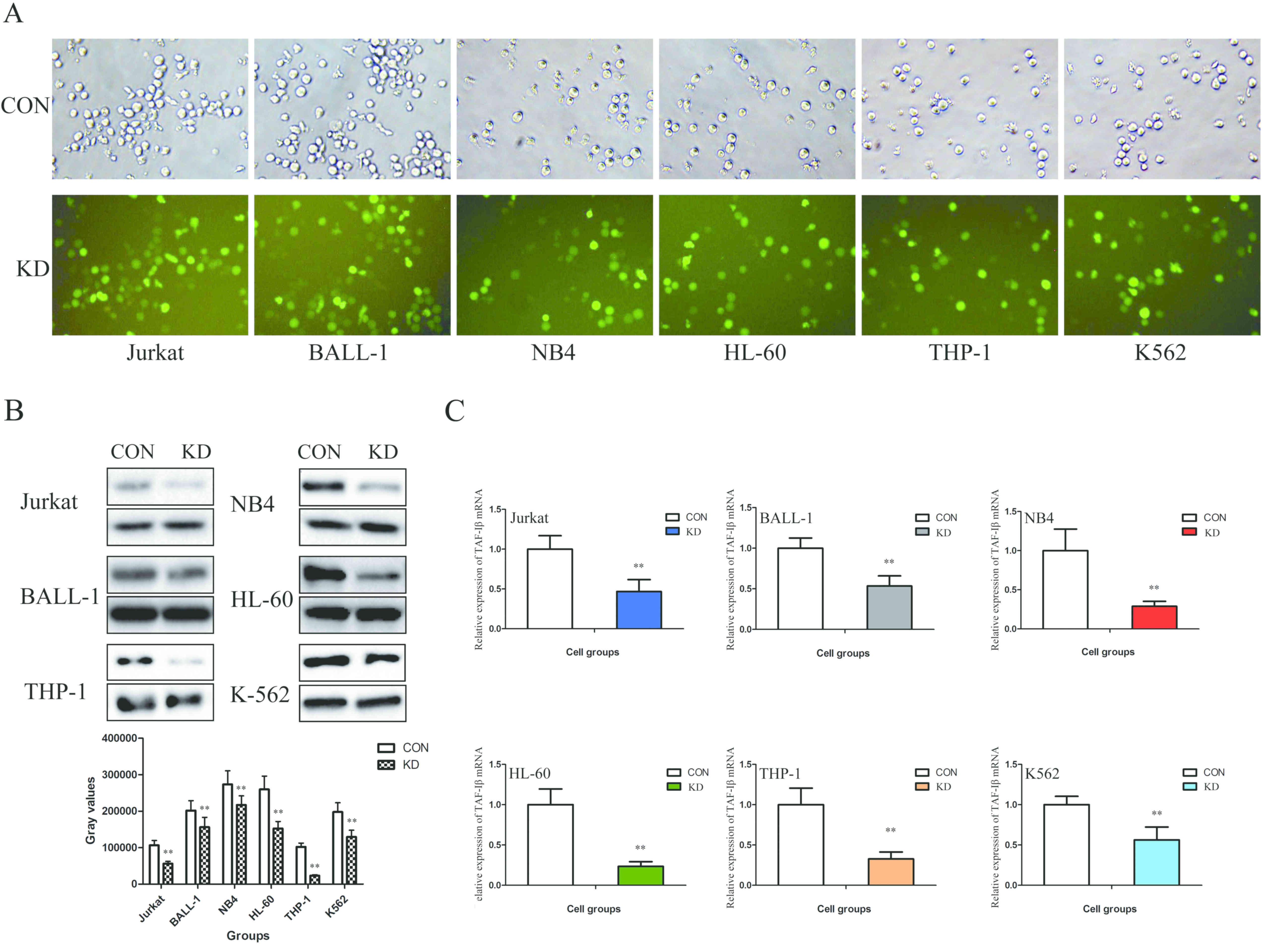
In order to determine whether TAF-Iβ serves a
functional role in leukemic cells, shRNA-KD or shRNA-NC were
transfected into the Jurkat, BALL-1, NB4, HL-60, THP-1 and K562
cells. The results demonstrated that the transfection efficiency
(GFP-positive cell count) was >80% (Fig. 2A). In addition, the mRNA and protein
expression levels of TAF-Iβ were significantly decreased in the KD
groups compared with those in the CON groups (P<0.01; Fig. 2B and C).
TAF-Iβ KD significantly inhibits
leukemic cell growth and induces cell cycle arrest
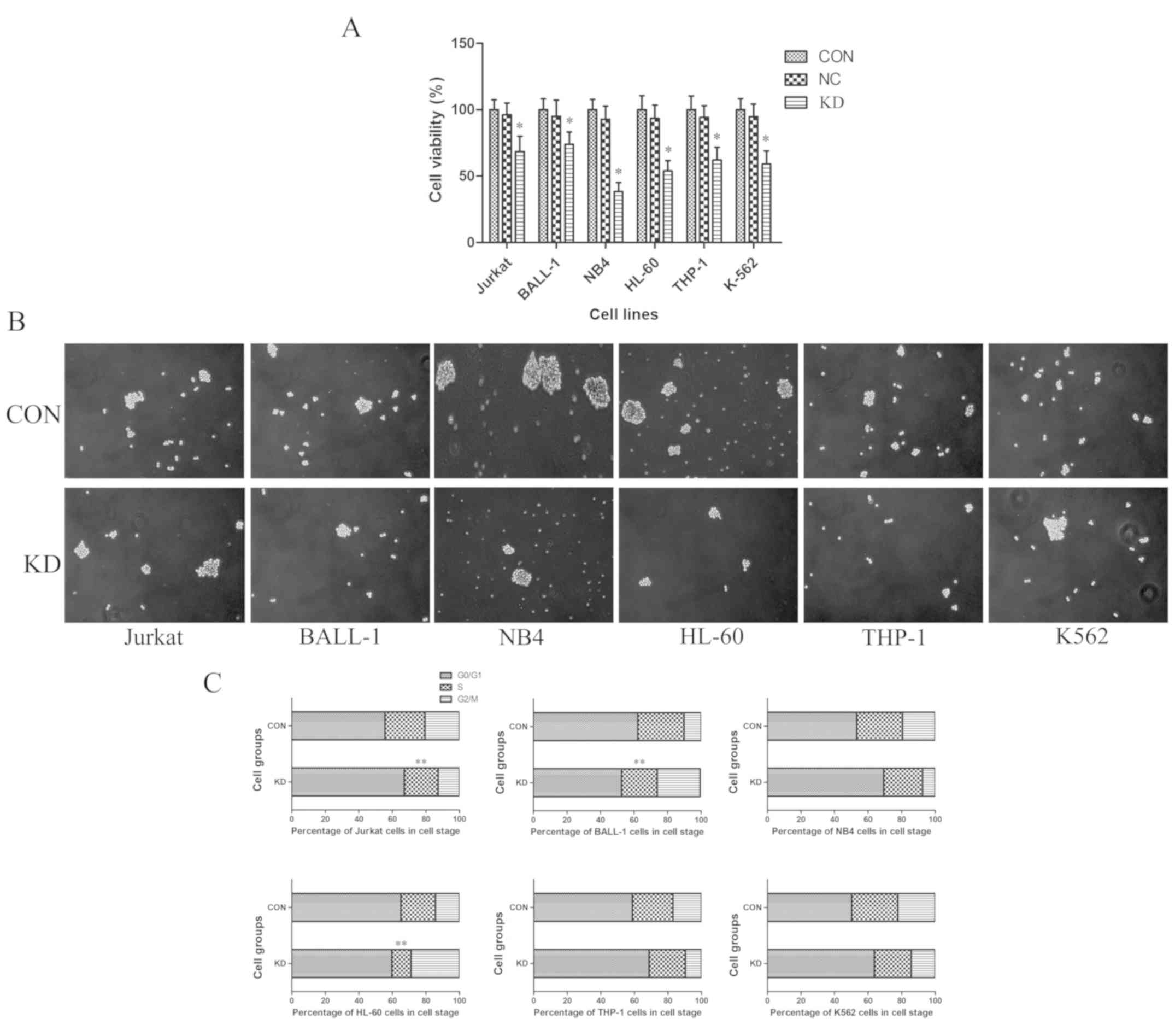
To evaluate the effects of TAF-Iβ KD on leukemic
cell proliferation in vitro, a CCK-8 assay was performed.
The results revealed that, compared with those of the CON and NC
groups, the proliferative abilities of cells in the KD groups were
significantly inhibited (P<0.05; Fig.
3A). In accordance with these findings, TAF-Iβ KD in the cells
led to decreases in the number and size of colonies (P<0.05;
Fig. 3B). By contrast, analysis by
FCM indicated that the percentages of S-phase Jurkat, BALL-1 and
HL-60 cells in the KD groups were notably reduced compared with
those in the CON groups; the numbers of
G0/G1- or G2/M-phase cells were
significantly increased (P<0.01; Fig.
3C).
TAF-Iβ KD induces leukemia cell
apoptosis
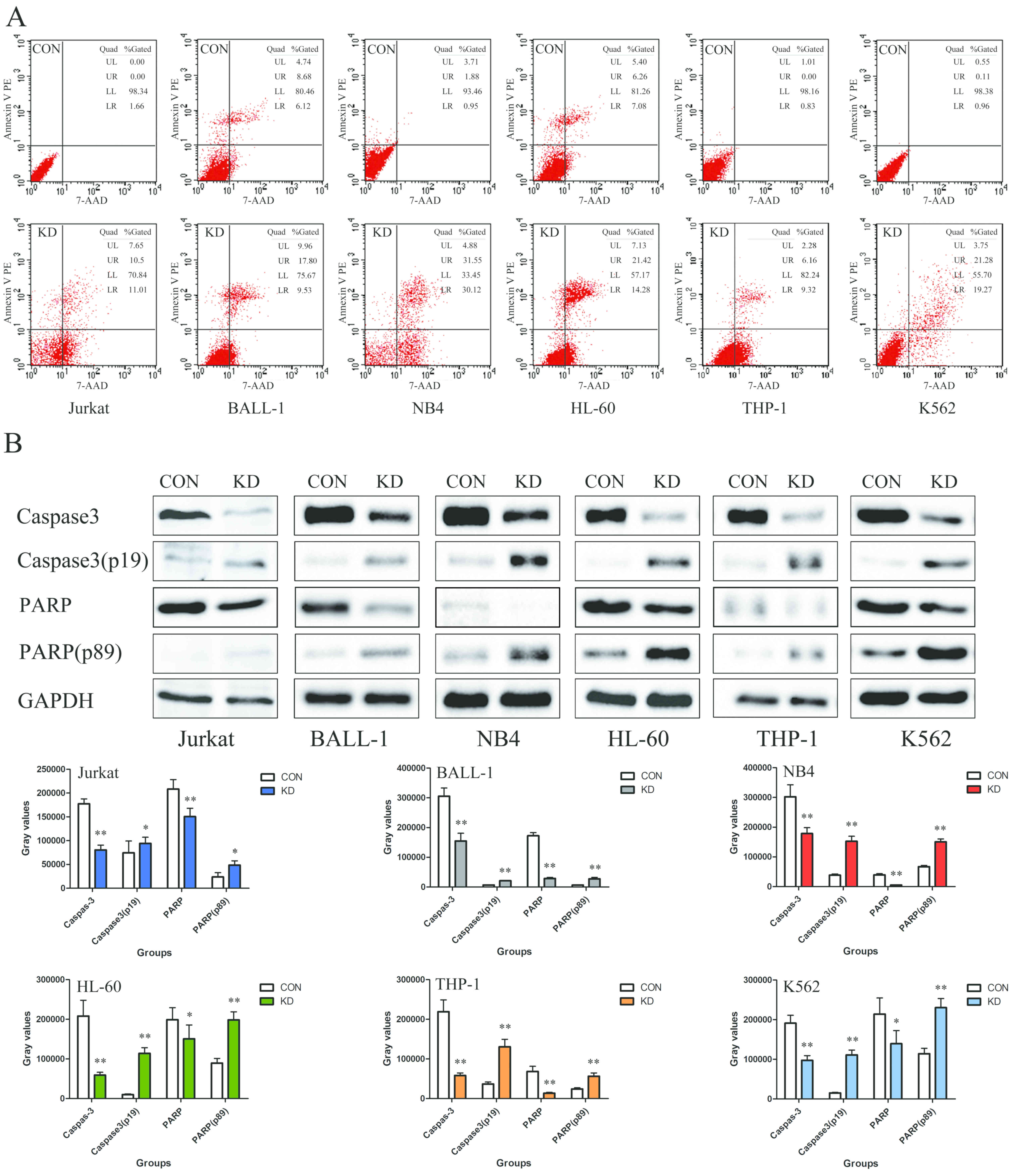
The results of the present study demonstrated that
the apoptotic rates of the Jurkat, BALL-1, NB4, HL-60, THP-1 and
K562 cells in the KD groups were 21.51±0.77, 27.33±0.77,
61.67±0.77, 35.70±0.77, 15.48±0.77 and 40.55±0.77%, respectively,
which was notably higher than those in the corresponding control
groups (Fig. 4A). Additionally, the
expression levels of cleaved caspase-3 (p19) and PARP (p89) were
significantly increased compared with those in the CON groups,
indicating the induction of apoptosis (P<0.01; Fig. 4B).
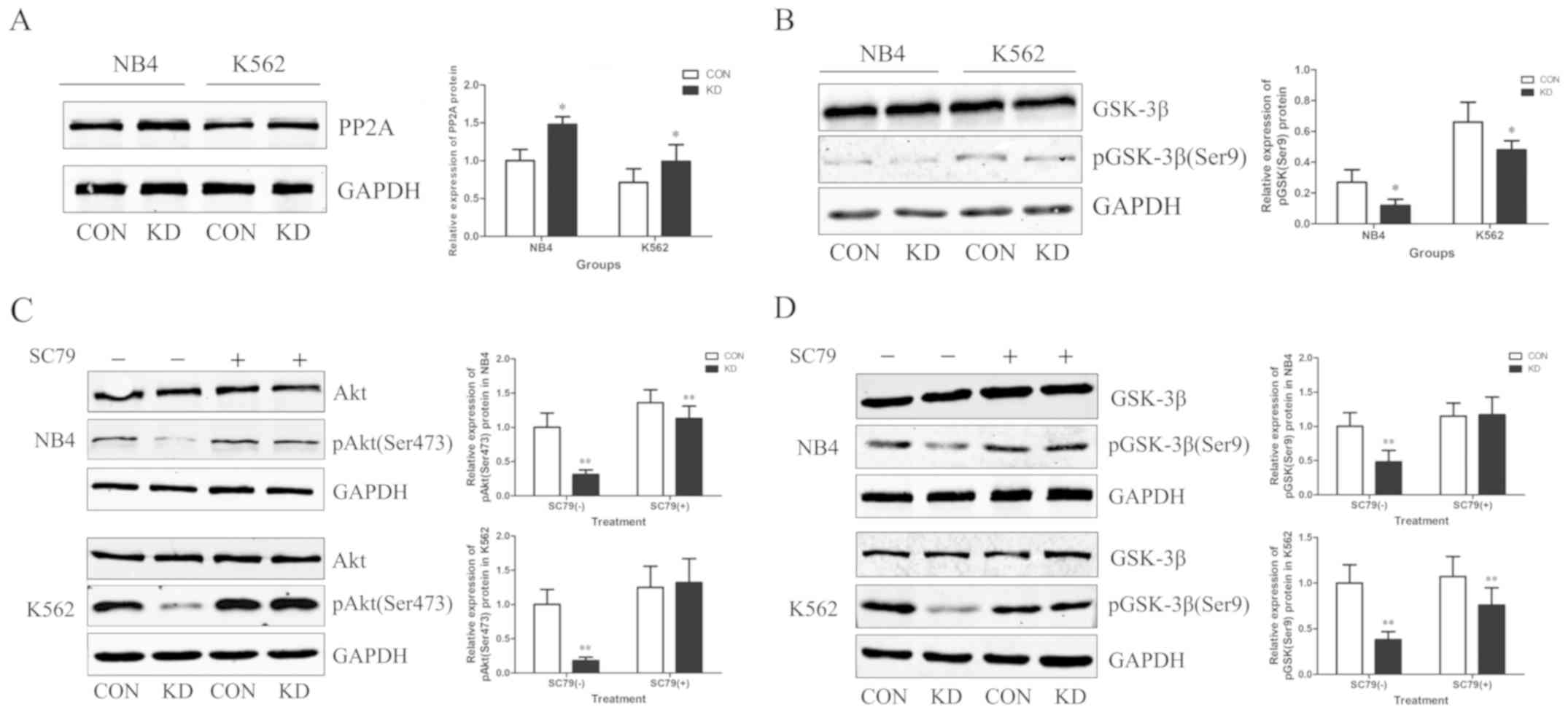
TAF-Iβ KD upregulates the expression
of PP2A and inhibits the AKT/GSK-3β signaling pathway
TAF-Iβ was originally identified as a potent
physiological inhibitor of PP2A; therefore, the expression levels
of PP2A were determined following TAF-Iβ silencing. As expected,
TAF-Iβ KD resulted in upregulated expression levels of PP2A
(P<0.05; Fig. 5A). Additionally,
as TAF-Iβ may be associated with the expression of GSK-3β in cancer
and diseases of the central nervous system (18), alterations in the expression of
GSK-3β were analyzed in NB4 and K562 cells. TAF-Iβ KD appeared to
inhibit the phosphorylation of GSK-3β, as demonstrated by
reductions in the levels of Ser9-phosphorylated GSK-3β (P<0.05;
Fig. 5B). To further investigate the
mechanism regulating the expression of GSK-3β, the expression of
AKT was analyzed, which is a well-known kinase that is activated by
various factors and is involved in the regulation of cell
proliferation, differentiation, metastasis and apoptosis (19). The results demonstrated that TAF-Iβ
KD markedly inhibited the expression of AKT; this effect was
reversed by SC79, an inducer of AKT. Additionally, the TAF-Iβ
KD-mediated downregulation of GSK-3β was suppressed following
treatment with SC79 (P<0.01; Fig. 5C
and D).
Discussion
The upregulation of TAF-Iβ has been detected in
several solid tumors and has been associated with increased
invasion and poor outcome (20). In
addition, TAF-Iβ deficiency has been shown to decrease cell growth
in various types of cancer (21);
however, this does not occur in all types of tumor. For example,
this inhibitory effect on cell growth has not been observed in
primary canine melanoma (22). This
suggests that TAF-Iβ may exhibit opposing effects on cell survival
in different types of cancer (23).
The role of TAF-Iβ has been reported extensively; however, the
expression of TAF-Iβ and the effects of its silencing in different
subtypes of leukemia require further investigation.
Regarding the mechanism suppressing the development
of leukemia, the most commonly reported in the literature is the
rescue of PP2A phosphatase activity (20). It is well known that PP2A is a
serine/threonine phosphatase and acts as a tumor suppressor. The
upregulation of PP2A decreases tumor cell growth and is inhibited
by TAF-Iβ (24). Consistent with
this hypothesis, the protein expression of TAF-Iβ was elevated in
patients with different subtypes of leukemia. In addition, TAF-Iβ
deficiency led to significantly increased expression of PP2A and
decreased leukemic cell proliferation. PP2A has also been reported
to serve as a switch that determines whether cells undergo
autophagy or apoptosis. Zhou et al (25) suggested that active caspase-3 cleaves
the A subunit of PP2A, following which AKT is inactivated by PP2A
to promote apoptosis. By contrast, the inactivation of caspase-3
leads to the dissociation of PP2A and AKT; unbound PP2A then
interacts with death-associated protein kinase to induce autophagy.
Similarly, the present study reported that TAF-Iβ silencing
upregulated the expression of cleaved caspase-3 (p19),
downregulated that of active AKT and induced apoptosis; however,
further verification by analyzing the co-localization of PP2A and
AKT is required.
Apart from the mitochondrial pathway of apoptosis,
leukemic cell death can be induced via TAF-Iβ deficiency-mediated
GSK-3β inhibition. Of note, GSK-3β is a downstream mediator of the
PI3K/AKT signaling cascade and can be phosphorylated by AKT
(26). Numerous studies have
reported that GSK-3β is essential for regulating a series of
cellular functions in tumors (27,28); the
suppression of GSK-3β results in the reduced binding of nuclear
factor-κB to its target gene promoters, inducing the apoptosis
and/or decreased growth of cells (29,30).
Collectively, the results of the present study
demonstrated that the downregulation of TAF-Iβ may suppress the
proliferation and promote the apoptosis of leukemic cells. The
mechanisms involved in these processes may be associated with the
rescue of PP2A phosphatase activity and inhibition of the
AKT/GSK-3β signaling pathway. These novel findings provide insight
into the oncogenic potential of TAF-Iβ and serve as a basis for
future investigations into the function of TAF-Iβ and the
mechanisms underlying its regulatory effects. Therefore, TAF-Iβ may
be considered as a therapeutic target in the treatment of leukemia.
A limitation of the present study was that no rescue experiments,
involving the overexpression of TAF-Iβ to overcome TAF-Iβ
deficiency and examine its effect on proliferation and apoptosis,
were performed. Rescue experiments warrant inclusion in future
investigations to consolidate and further the findings of the
present study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant nos. 81600135 and 81800680).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YFL designed the study and wrote the manuscript, YJ
performed the experiments and XF conducted data analysis; PCH was
responsible for data analysis and interpretation. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The use of human bone marrow specimens was approved
by the Ethics Committee of Xiangya Hospital (Changsha, China). All
patients provide written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang DY, Yuan XQ, Yan H, Cao S, Zhang W,
Li XL, Zeng H and Chen XP: Association between DCK 35708 T>C
variation and clinical outcomes of acute myeloid leukemia in South
Chinese patients. Pharmacogenomics. 17:1519–1531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Betz BL and Hess JL: Acute myeloid
leukemia diagnosis in the 21st century. Arch Pathol Lab Med.
134:1427–1433. 2010.PubMed/NCBI
|
|
3
|
Creutzig U, van den Heuvel-Eibrink MM,
Gibson B, Dworzak MN, Adachi S, de Bont E, Harbott J, Hasle H,
Johnston D, Kinoshita A, et al: Diagnosis and management of acute
myeloid leukemia in children and adolescents: Recommendations from
an international expert panel. Blood. 120:3187–3205. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schrappe M, Hunger SP, Pui CH, Saha V,
Gaynon PS, Baruchel A, Conter V, Otten J, Ohara A, Versluys AB, et
al: Outcomes after induction failure in childhood acute
lymphoblastic leukemia. N Engl J Med. 366:1371–1381. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang M, Zeng P, Kang R, Yu Y, Yang L, Tang
D and Cao L: S100A8 contributes to drug resistance by promoting
autophagy in leukemia cells. PLoS One. 9:e972422014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang H, Zheng QL, Fang P, Zhang J, Zhang
T, Liu W, Guo M, Robinson CL, Chen SB, Chen XP, et al: Targeting
the PI3K/AKT pathway via GLI1 inhibition enhanced the drug
sensitivity of acute myeloid leukemia cells. Sci Rep. 7:403612017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kauko O, Imanishi S, Kulesskiy E, Laajala
TD, Yetukuri L, Padzik A, Jumppanen M, Haapaniemi P, Yadaw B, Suni
V, et al: Abstract 5560: Systemic map of protein phosphatase 2A
(PP2A)-regulated phosphotargets and drug responses in cancer cells.
Cancer Res. 77:55602017.
|
|
8
|
Mody HR, Hung SW, Naidu K, Lee H, Gilbert
CA, Hoang TT, Pathak RK, Manoharan R, Muruganandan S and
Govindarajan R: SET contributes to the epithelial-mesenchymal
transition of pancreatic cancer. Oncotarget. 8:67966–67979. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu M, Yu G, Yan T, Ke D, Wang Q, Liu R,
Wang JZ, Zhang B, Chen D and Wang X: Phosphorylation of SET
mediates apoptosis via P53 hyperactivation and NM23-H1 nuclear
import. Neurobiol Aging. 69:38–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trotta R, Ciarlariello D, Dal Col J, Mao
H, Chen L, Briercheck E, Yu JH, Zhang JY, Perrotti D and Caligiuri
MA: The PP2A inhibitor SET regulates granzyme B expression in human
natural killer cells. Blood. 117:2378–2384. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Gu Y, Wang H, Yin J, Zheng G, Zhang
Z, Lu M, Wang C and He Z: Overexpression of PP2A inhibitor SET
oncoprotein is associated with tumor progression and poor prognosis
in human non-small cell lung cancer. Oncotarget. 6:14913–14925.
2015.PubMed/NCBI
|
|
12
|
Liu Y, He P, Liu F, Zhou N, Cheng X, Shi
L, Zhu H, Zhao J, Wang Y and Zhang M: Tetra-arsenic tetra-sulfide
(As4S 4) promotes apoptosis in retinoid acid-resistant human acute
promyelocytic leukemic NB4-R1 cells through downregulation of SET
protein. Tumour Biol. 35:3421–3430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sultan C, Deregnaucourt J, Ko YW, Imbert
M, D'Agay MF, Gouault-Heilmann M and Brun B: Distribution of 250
cases of acute myeloid leukaemia (AML) according to the FAB
classification and response to therapy. Br J Haematol. 47:545–551.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He P, Liu Y, Qi J, Zhu H, Wang Y, Zhao J,
Cheng X, Wang C and Zhang M: Prohibitin promotes apoptosis of
promyelocytic leukemia induced by arsenic sulfide. Int J Oncol.
47:2286–2295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, He P, Liu F, Cheng X and Zhang M:
Influence of I2PP2A gene silencing by RNA interference on
proliferation and apoptosis of human acute promyelocytic leukemia
cell line NB4-R1. Zhonghua Xue Ye Xue Za Zhi. 35:732–736. 2014.(In
Chinese). PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun QY, Ding LW, Tan KT, Chien W,
Mayakonda A, Lin DC, Loh XY, Xiao JF, Meggendorfer M, Alpermann T,
et al: Ordering of mutations in acute myeloid leukemia with partial
tandem duplication of MLL (MLL-PTD). Leukemia. 31:1–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y, Ma RH, Li XC, Zhang JY, Shi HR,
Wei W, Luo DJ, Wang Q, Wang JZ and Liu GP: Silencing I2PP2A rescues
tau pathologies and memory deficits through rescuing PP2A and
inhibiting GSK-3β signaling in human tau transgenic mice. Front
Aging Neurosci. 6:1232014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Sousa RT, Zanetti MV, Talib LL, Serpa
MH, Chaim TM, Carvalho AF, Brunoni AR, Busatto GF, Gattaz WF and
Machado-Vieira R: Lithium increases platelet serine-9
phosphorylated GSK-3β levels in drug-free bipolar disorder during
depressive episodes. J Psychiatr Res. 62:78–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bayarkhangai B, Noureldin S, Yu L, Zhao N,
Gu Y, Xu H and Guo C: A comprehensive and perspective view of
oncoprotein SET in cancer. Cancer Med. 2018:(Epub ahead of
print).
|
|
21
|
Jiang SW, Xu S, Chen H, Liu J and Duan P:
Oncogenic role of SET/I2PP2A for gynecologic cancers. Curr Drug
Targets. 18:1152–1157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Enjoji S, Yabe R, Fujiwara N, Tsuji S,
Vitek MP, Mizuno T, Nakagawa T, Usui T, Ohama T and Sato K: The
therapeutic effects of SET/I2PP2A inhibitors on canine melanoma. J
Vet Med Sci. 77:1451–1456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sobral LM, Coletta RD, Alberici LC, Curti
C and Leopoldino AM: SET/I2PP2A overexpression induces phenotypic,
molecular, and metabolic alterations in an oral keratinocyte cell
line. FEBS J. 284:2774–2785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herbert M and Toth A: How meiosis creates
the single-copy genome. Dev Cell. 40:3–4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou H, Luo W, Zeng C, Zhang Y, Wang L,
Yao W and Nie C: PP2A mediates apoptosis or autophagic cell death
in multiple myeloma cell lines. Oncotarget. 8:80770–80789.
2017.PubMed/NCBI
|
|
26
|
Lu Y, Lei S, Wang N, Lu P, Li W, Zheng J,
Giri PK, Lu H, Chen X, Zuo Z, et al: Protective effect of
minocycline against ketamine-induced injury in neural stem cell:
Involvement of PI3K/Akt and Gsk-3 beta pathway. Front Mol Neurosci.
9:1352016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye Z, Xia P, Cheng ZG and Guo Q:
Neuroprotection induced by sevoflurane-delayed post-conditioning is
attributable to increased phosphorylation of mitochondrial GSK-3β
through the PI3K/Akt survival pathway. J Neurol Sci. 348:216–225.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu J, Liao Q, He H, Zhong D and Yin K:
TWIST interacts with β-catenin signaling on osteosarcoma cell
survival against cisplatin. Mol Carcinog. 53:440–446. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ougolkov AV, Bone ND, Fernandez-Zapico ME,
Kay NE and Billadeau DD: Inhibition of glycogen synthase kinase-3
activity leads to epigenetic silencing of nuclear factor kappaB
target genes and induction of apoptosis in chronic lymphocytic
leukemia B cells. Blood. 110:735–742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ban JO, Kwak DH, Oh JH, Park EJ, Cho MC,
Song HS, Song MJ, Han SB, Moon DC, Kang KW and Hong JT: Suppression
of NF-kappaB and GSK-3beta is involved in colon cancer cell growth
inhibition by the PPAR agonist troglitazone. Chem Biol Interact.
188:75–85. 2010. View Article : Google Scholar : PubMed/NCBI
|