Introduction
Psoriasis (PSO) skin over-expresses a myriad of
inflammatory mediators [e.g., interleukin (IL)-1α, IL-6, IL-17 and
tumor necrosis factor (TNF)-α], synthesized by different cell
types, which dynamically interact with melanocytes (1–4).
Synergistic action of these mediators down-regulates the
pigmentation signaling pathway and melanin production (4–6).
Previous studies have reported that primary human melanocytes
respond to IL-17 and/or TNF-α stimulation forming clusters and
modulating the expression of melanogenesis markers [e.g.,
microphthalmia-associated transcription factor (MITF) and
tyrosinase (Tyr)] (7,8). Indeed, therapeutic neutralization of
IL-17 and TNF-α with biologic agents is able to increase
pigmentation signaling in the areas corresponding to the psoriatic
plaques (8). However, the
relationship between psoriasis and melanogenesis remains to
clarify. Currently, melanocyte activity has been linked to the bone
morphogenetic proteins (BMPs), a group of more than 20 secreted
proteins also involved in the pathogenesis of PSO (9–11). In
particular, melanin synthesis is up-regulated by BMP-2 and BMP-6,
whereas controversial data exist on BMP-4 (5,11,12).
Yaar et al (13) showed that
BMP-4 supplementation of cultured human melanocytes decreased
melanin synthesis. According to Cichorek et al (6), BMP-4 secreted by keratinocytes after
ultraviolet (UV) radiation is able to increase melanogenesis. In
the present study, we aimed to investigate the effect of psoriatic
inflammatory network on melanogenesis uncovering a possible role of
BMP-4 in this scenario.
Materials and methods
Study population
The overall study enrolment comprised 40 psoriatic
and 40 healthy donors who had undergone plastic surgery. Psoriatic
subjects were enrolled at the Dermatology out-patients clinic of
the University of Naples Federico II whereas healthy ones were
recruited at the Plastic Surgery Unit of the University of Naples
Federico II. The study was approved by the Ethics Committee for
Biomedical Activities ‘Carlo Romano’ of University of Naples
Federico II, and conducted according to the Declaration of Helsinki
principles. Each participant gave written informed consent before
the onset of the study. Samples were collected between September
2017 and June 2018. Patients and controls were similar to each
other in terms of age (54±15 and 50±17, respectively) and male
distribution (67.5 and 62.5%, respectively). Inclusion criteria for
patients were: Diagnosis of moderate-to-severe PSO [Psoriasis Area
Severity Index (PASI) >10], disease duration of at least 6
months, age ≥18 years, topical and/or systemic treatment washout
period of at least 3 weeks, whereas for healthy subjects were: Age
≥18 years without a present- or past-positive history of PSO.
Adalimumab (ADL) was administered subcutaneously 80 mg at week
(W)-0 (baseline) to all psoriatic patients and successively 40 mg
every other week, starting from W-1 and up to W-16. Lesional (LS)
and non lesional skin (NLS) punch biopsies (3 mm diameter) were
performed on trunk at weeks 0 and 16. Normal skin from plastic
surgery remnants was used as control. Skin specimens were screened
through gene expression, immunohistochemistry, immunogold staining
and melanin content assay within 1 h of surgical intervention.
In vivo expression of Tyr, MITF and
BMPs family members
RNA was extracted from skin biopsies (RNeasy Mini
Protocol; Qiagen) and cDNA was prepared (Transcriptor High Fidelity
cDNA Synthesis; Roche) according to the manufacturer's
instructions. RT-qPCR (LightCycler; Roche) was used to analyze the
levels of expression of 18S, Tyr, MITF, BMP-2, BMP-4, BMP-6, BMP-7.
Relative mRNA levels were determined by the comparative threshold
cycle method 2−∆∆cq (14), and their expression was normalized to
the expression of 18S mRNA as previously reported (15). PCR primers (18S, Tyr, MITF, BMP-2,
BMP-4, BMP-6, BMP-7) were designed based on published sequences,
and their specificity was verified with BLAST alignment search. To
confirm amplification of the expected size fragment, amplification
products were characterized by agarose gel electrophoresis. Melting
curve analysis was carried out after completion to confirm the
presence of single amplified species.
Ex vivo expression of Tyr and
BMP4
Full-thickness skin, normal human epidermal sheets
and dermis were obtained from healthy donors, and stimulated with
recombinant human TNF-α protein (R&D Systems) at 20 ng/ml for
24 h. Next, samples were snap-frozen in liquid nitrogen and stored
at −70°C until RNA extraction. Major details are reported in
supplementary materials (Appendix S1).
Immunohistochemistry
The immunohistochemical detection of Tyr and BMP-4
was carried out on LS samples of 10 psoriatic patients before
(baseline) and after 16 weeks of ADL therapy. Healthy skin samples
were used as controls. Specimens were immediately placed in tissue
freezing medium (Jung; Leica) and stored at −80°C. Five micrometer
sections were cut with a cryostat and fixed with cold methanol for
10 min. The Vectastain Elite ABC Kit (Vector Laboratories) was used
as follows: Sections were incubated with blocking solution [horse
serum diluted in buffer: Phosphate buffered saline (PBS) + bovine
serum albumin 1%] for 20 min at 22°C. Biopsies were stained with
anti-tyrosinase (1 µg/ml; Gibco), anti-BMP-4 (10 µg/ml; Fitzgerald)
and incubated overnight at 4°C. In parallel, skin specimens were
incubated with specific isotype control antibodies (Mouse IgG1
Isotype Control, Mouse IgG2B Isotype Control, Goat IgG Control;
R&D System) used at the same concentration as the corresponding
primary antibody. The sections were then washed in buffer and
incubated with biotinylated secondary antibody for 30 min at room
temperature. Peroxydase activity was revealed using DAB substrate
(ImmPACT DAB). Counterstaining was performed with hematoxylin.
Staining was observed using Nikon Eclipse E600 epifluorescence
microscope (Nikon).
Immunogold staining
Tissue samples were fixed in a mixture of 0.5%
glutaraldehyde and 2% paraformaldehyde in PBS overnight at 4°C and
washed in the same buffer. Following dehydration, samples were
embedded in Epon resin, and ultrathin sections (60 nm) were
collected on 200 mesh nickel grids. Samples were washed 3 times in
distilled water for 5 min, equilibrated in PBS containing 1.5% goat
serum and 1% BSA for 15 min and incubated overnight at 4°C with
anti-Tyr (1 µg/ml; Gibco), anti-BMP-4 (10 µg/ml; Fitzgerald)
diluted in PBS/BSA 1%. Following 5 washings (2 min each) in PBS and
5 washings in PBS/BSA 0.5%, sections were incubated in 1% PBS/BSA
for 15 min and then for 1 h at room temperature with the goat
anti-mouse secondary antibody (H&L) labeled with 20-nm gold
particles (BB International). Sequential washings were performed in
1% PBS/BSA for 15 min, in 0.5% PBS/BSA 5 times for 2 min, in PBS 5
times for 2 min, and in distilled water twice for 1 min. After
staining with uranyl acetate, sections were analyzed using a Leo
912AB electron microscope (Carl Zeiss). Controls, based on the use
of only secondary antibody, were run in parallel.
Melanin content assay
Total cellular melanin content was performed in
healthy and psoriatic skin biopsies before and after 16 weeks of
ADL treatment using Fontana-Masson staining (AMTS Inc.) according
to the manufacturer's instructions.
Statistical analyses
All statistical analyses were performed using
GraphPad Prism 4.0 (GraphPad Software Inc.). The Kruskall Wallis
and Mann-Whitney tests were used for all intergroup comparisons
followed by post hoc corrections for multiple comparisons using
Bonferroni test. Wilcoxon test was used for paired samples. Values
of P<0.05 were considered significant and all data were
displayed as means ± standard deviation (SD).
Results
To better explore the link between psoriatic
inflammation and melanogenesis, we examined gene expression of Tyr,
MITF and some members of BMPs family (BMP-2, BMP-4, BMP-6 and
BMP-7) in PSO LS and NLS compared to healthy skin (HS). Our results
showed that Tyr and MITF were decreased in PSO LS compared to HS
(Fig. 1A and B). Likewise, BMP-4 and
BMP-7 were found significantly reduced in PSO LS (Fig. 1D and F), whereas no significant
difference was observed for BMP-2 and BMP-6 (Fig. 1C and E).
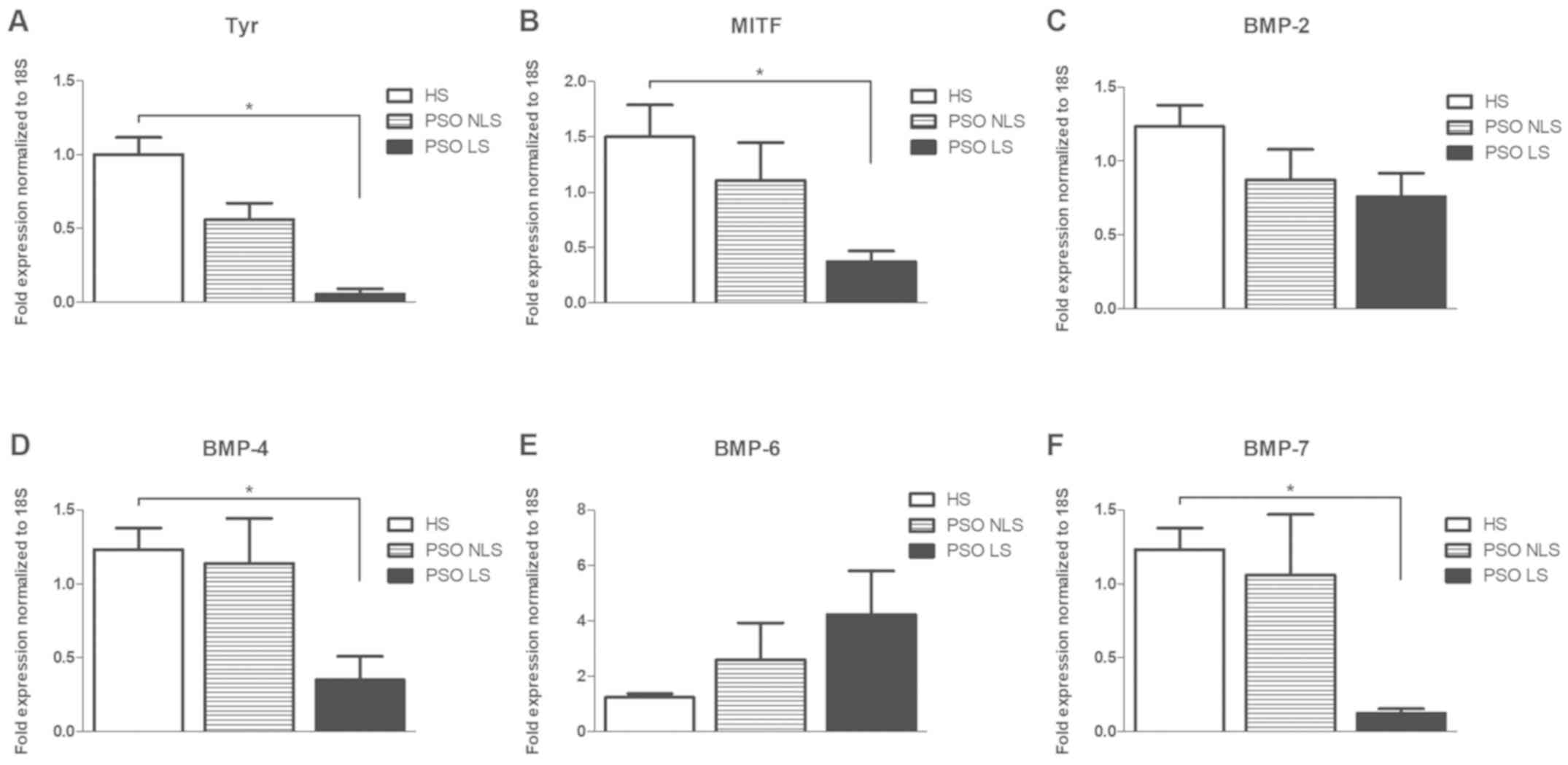 | Figure 1.Melanogenesis markers and BMP family
members are differentially expressed in HS, PSO LS and NLS. Gene
expressions of (A) Tyr, (B) MITF, (C) BMP-2, (D) BMP-4, (E) BMP-6
and (F) BMP-7 are presented. Data are presented as the mean ±
standard deviation. *P<0.05 as indicated. Tyr, tyrosinase; MITF,
microphthalmia-associated transcription factor; BMP, bone
morphogenetic protein; HS, healthy skin; PSO, psoriasis; LS,
lesional skin; NLS, non lesional skin. |
To verify the effects exerted by anti-TNF-α therapy
on melanogenesis markers in psoriatic lesions, we evaluated Tyr,
BMP-4 and melanin content in PSO LS before and after 16 weeks of
ADL treatment. Patients who did not achieve a 50% PASI reduction at
W-16 were defined as non responders. Immunohistochemical analysis
revealed that both Tyr (Fig. 2A) and
BMP-4 (Fig. 2B) were lower in PSO LS
(W-0) compared to HS, confirming gene expression analysis. At W-16,
Tyr (Fig. 2A) and BMP-4 (Fig. 2B) were enhanced in the lesions of
responder patients respect to W-0, whereas a lower intensity of
both markers was still observed in non responder subjects (Fig. 2A and B). The ability of TNF-α to
modulate Tyr and BMP-4 was further confirmed by ex vivo
experiments (Fig. S1). In
particular, TNF-α was able to reduce Tyr expression in normal human
epidermal sheet and HS organ culture, whereas a significant
reduction of BMP-4 was confirmed just in normal human epidermal
sheet (Fig. S1A-D). More evidence
on Tyr and BMP-4 in PSO LS was obtained through immunogold
staining. Both markers were lower in keratinocytes of PSO LS
compared to HS (Fig. 2C). Melanin
content produced by melanocytes was greater in PSO LS (W-0) than HS
(Fig. 2D). After 16 weeks of ADL
therapy, melanin was reduced in the lesional skin of responders
respect to baseline (W-0) (Fig. 2D),
whereas no melanin reduction was observed in non responder plaques
(Fig. 2D). The phenomenon of
hyper-pigmentation was clinically evident in responder patients at
W-16 (Fig. 2E).
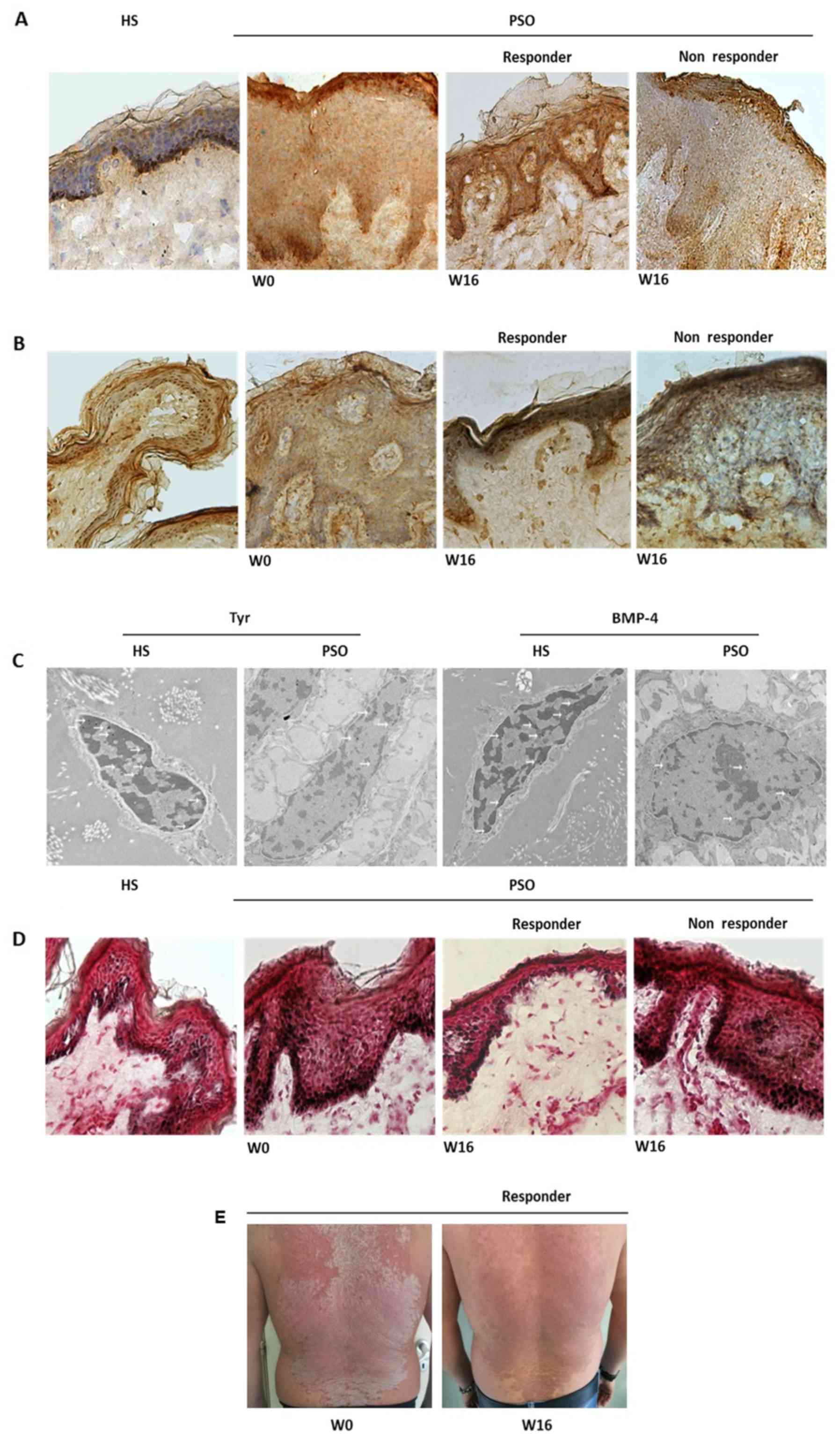 | Figure 2.Tyr, BMP-4 and melanin levels are
modulated by anti-tumor necrosis factor-α therapy in psoriasis.
Immunohistochemical detection of (A) Tyr and (B) BMP-4 in HS and
PSO LS samples before and after 16 weeks of adalimumab therapy.
Lower intensities of Tyr and BMP-4 were detected in PSO LS at W0
compared with HS. At W16, Tyr and BMP-4 were higher in the LS of
responder patients, whereas both markers remained low in lesions of
non-responder patients (magnification of each, ×20). (C) Immunogold
staining in HS and PSO LS. BMP-4 and Tyr were detected at the
keratinocyte level (white arrows). Reduced marker levels were
detected in PSO LS compared with HS (magnification, ×5000). (D)
Melanin content assay in HS and PSO LS before and after 16 weeks of
adalimumab therapy. At W0, a greater content of melanin was
exhibited in psoriatic skin when compared with HS. At W16, melanin
was reduced in the lesional skin of responders when compared with
W0, whereas no melanin reduction was observed in non-responder
plaques (magnification, ×20). (E) Clinical manifestation of
psoriasis at W0 and after 16 weeks of adalimumab therapy.
Representative image of a responder patient at W16 exhibiting
post-inflammatory hyper-pigmentation in areas of plaque. Tyr,
tyrosinase; BMP, bone morphogenetic protein; W, week; PSO,
psoriasis; LS, lesional skin; HS, healthy skin. |
Discussion
Pigmentary changes in psoriasis may be modulated by
a myriad of inflammatory mediators that are overexpressed in
psoriatic lesions. Some of these mediators are known to have
hypo-pigmenting effects (e.g., IL-1α, IL-6, IL-17, TGF-β1, TNF-α),
and can independently regulate the expression of MITF, Tyr and
related enzymes (4,6,7). In the
present study, we have reported that MITF and Tyr were
significantly decreased in psoriatic lesions with respect to
healthy skin. Our data are consistent with research of Wang
(7). The authors showed a reduction
of main melanogenesis markers in contrast to a higher presence of
melanocytes in psoriatic lesions. Apart from a decrease of Tyr, we
observed a reduction of BMP-4 in psoriatic plaques. Moreover, an
increase of Tyr and BMP-4 was assessed in responder patients after
16 weeks of anti-TNF-α therapy, whereas levels similar to baseline
were encountered for non responder subjects. As hypothesized by Di
Cesare et al (8), high levels
of melanogenesis markers induced by the treatment with TNF-α
blockers could increase pigmentation signaling pathway. A possible
role of BMP-4 in psoriatic post-inflammatory hyper-pigmentation has
not been explored, even though controversial data exist on this
marker in the process of melanogenesis (6,13). We
have found that BMP-4 is linked to Tyr given that they shared the
same trend in response to anti-TNF-α treatment. Thus, this evidence
supports that BMP-4 is able to increase melanogenesis as reported
by Cichorek et al (6). In
particular, BMP-4 released by keratinocytes acts as a paracrine
factor that directly influences melanocytes activity.
It has to be taken into account that we have found a
decrease of melanocytes number upon ADL treatment. This might be in
contrast with post-inflammatory hyper-pigmentation, but the
concomitant increase of Tyr as well as BMP-4 may render local
melanocytes more active in the melanogenesis process. Taken
together, the innovative and intriguing results on BMP-4 sheds the
light on its possible involvement in hyper-pigmentation phenomenon
in the areas corresponding to psoriatic plaques during anti-TNF-α
therapy. Further studies on BMP-4 in this scenario would be
valuable.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the Electron
Microscopy facility at the Stazione Zoologica Anton Dohrn for their
valuable support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LDC, ES, GC, SL, RM, MM, AP, RDC and AB conceived
the current study, acquired, interpreted and analyzed the data, and
drafted the manuscript. LDC, ES, SL and AB revised the manuscript
for important intellectual content. All authors approved the final
version to be published and agreed to be accountable for all
aspects of the study.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Biomedical Activities ‘Carlo Romano’ University of
Naples Federico II (Protocol no. 160/010) and is in accordance with
the legal requirements of the Declaration of Helsinki. Each patient
provided written informed consent prior to enrolment.
Patient consent for publication
Consent for the publication of images was obtained
from patients included in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lowes MA, Suárez-Fariñas M and Krueger JG:
Immunology of psoriasis. Annu Rev Immunol. 32:227–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balato A, Scala E, Balato N, Caiazzo G, Di
Caprio R, Monfrecola G, Raimondo A, Lembo S and Ayala F: Biologics
that inhibit the Th17 pathway and related cytokines to treat
inflammatory disorders. Expert Opin Biol Ther. 17:1363–1374.
2017.PubMed/NCBI
|
|
3
|
Caiazzo G, Fabbrocini G, Di Caprio R,
Raimondo A, Scala E, Balato N and Balato A: Psoriasis,
cardiovascular events and biologics: Lights and shadows. Front
Immunol. 9:16682018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swope VB, Abdel-Malek Z, Kassem LM and
Nordlund JJ: Interleukins 1 alpha and 6 and tumor necrosis
factor-alpha are paracrine inhibitors of human melanocyte
proliferation and melanogenesis. J Invest Dermatol. 96:180–185.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balato N, Di Costanzo L, Balato A, Patruno
C, Scalvenzi M and Ayala F: Psoriasis and melanocytic naevi: Does
the first confer a protective role against melanocyte progression
to naevi? Br J Dermatol. 164:1262–1270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cichorek M, Wachulska M, Stasiewicz A and
Tymińska A: Skin melanocytes: Biology and development. Postepy
Dermatol Alergol. 30:30–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang CQF, Akalu YT, Suarez-Farinas M,
Gonzalez J, Mitsui H, Lowes MA, Orlow SJ, Manga P and Krueger JG:
IL-17 and TNF synergistically modulate cytokine expression while
suppressing melanogenesis: Potential relevance to psoriasis. J
Invest Dermatol. 133:2741–2752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Cesare A, Fargnoli MC, Marinucci A and
Peris K: Rationale for the development of speckled
hyperpigmentation in the areas of psoriatic plaques after treatment
with biologic agents. J Invest Dermatol. 135:318–320. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim M and Choe S: BMPs and their clinical
potentials. BMB Rep. 44:619–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh SK, Abbas WA and Tobin DJ: Bone
morphogenetic proteins differentially regulate pigmentation in
human skin cells. J Cell Sci. 125:4306–4319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blessing M, Schirmacher P and Kaiser S:
Overexpression of bone morphogenetic protein-6 (BMP-6) in the
epidermis of transgenic mice: Inhibition or stimulation of
proliferation depending on the pattern of transgene expression and
formation of psoriatic lesions. J Cell Biol. 135:227–239. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bilodeau ML, Greulich JD, Hullinger RL,
Bertolotto C, Ballotti R and Andrisani OM: BMP-2 stimulates
tyrosinase gene expression and melanogenesis in differentiated
melanocytes. Pigment Cell Res. 14:328–326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yaar M, Wu C, Park HY, Panova I, Schutz G
and Gilchrest BA: Bone morphogenetic protein-4, a novel modulator
of melanogenesis. J Biol Chem. 281:25307–25314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmittgen TD and Zakrajsek BA: Effect of
experimental treatment on housekeeping gene expression: Validation
by real-time, quantitative RT-PCR. J Biochem Biophys Methods.
46:69–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
















