Introduction
Rapidly progressive glomerulonephritis (RPGN) has
been characterized by the rapid loss of kidney function and is
usually caused by crescentic glomerulonephritis (CrGN) within a few
weeks or months. CrGN is defined as crescents involving >50% of
the glomeruli (1). CrGN can be
divided into 3 types according to immunofluorescence microscopy:
Type I is defined as a linear deposition of immunoglobulins along
the glomerular basement membrane (GBM); type II is defined as
glomerular immune complex deposition; and type III is defined as
glomerular pauci-immune deposition (2). Epidemiologic data on CrGN have been
reported by large national kidney biopsy registries from India
(3), Japan (4), Saudi Arabia (5), the US (6), Spain (7)
and China (8,9). The prevalence of CrGN ranges from 1.56
to 10% of total kidney biopsies.
The exact mechanism of immune-pathogenesis in
crescent formation remains elusive. Studies have revealed that a
key stage is the breakage of the GBM, allowing plasma proteins to
enter the Bowman's capsule (10–12). A
second key stage in crescent formation is the accumulation of
fibrin within the Bowman's capsule, which provokes the
proliferation of parietal epithelial cells. In addition,
macrophages, CD4+ T cells and CD8+ T cells
are important molecules that are involved in the
immune-pathogenesis of crescent formation (10,13). The
standard induction therapy for CrGN includes oral prednisone
combined with cyclophosphamide (CTX) (10). Many physicians also frequently use
pulse methylprednisolone prior to the administration of high dose
oral steroids in addition to CTX. Plasmapheresis may be also used
to remove circulating auto-antibodies or immune complexes. Early
treatment is of vital importance for patients with CrGN (14). The prognosis of patients with CrGN is
regarded as having been improved over the past few years. The
5-year cumulative renal survival rates of patients with type I, II
and III CrGN have reached 17.6, 70.1 and 44.3%, respectively, in
China (8). Certain
clinicopathological features, including the subtype (8), oliguria and serum creatinine (SCr)
(3), age and an elevated percentage
of glomeruli with crescents (5),
have a significant impact on the prognosis of patients with CrGN.
The purpose of the present study was to assess the
clinicopathological features and outcomes of patients with
CrGN.
Patients and methods
Population and data collection
A total of 49 consecutive patients with
biopsy-proven CrGN diagnosed between December 2011 and July 2016 at
the Department of Nephrology of Xiangya Hospital of Central South
University (Changsha, China) were recruited for the present
retrospective study. During this period, 1,312 renal biopsies were
performed. Patients with <10 non-sclerotic glomeruli in sampled
renal tissue were excluded. CrGN was defined as the presence of
crescents within the Bowman's space in >50% of the total
glomeruli in the kidney biopsy (15).
Once patients provided written informed consent to
participate in the study, baseline demographic data, including age,
sex, duration of disease and clinicopathological, laboratory and
ultrasonography parameters, were obtained from the electronic
medical record system of the hospital. Details regarding the
process of treatment and outcome, including SCr levels, dependence
on dialysis, outcome concerning survival and cause of death, were
also collected. Patients were evaluated at the time of diagnosis,
at 1, 2 and 3 months, and then every 3 months until the end of the
study. Patients were followed up from diagnosis until end-stage
renal disease (ESRD) was reached or death or the final follow-up
date (July 30, 2017). All follow-up data were collected from the
hospital's electronic medical records and by contacting the
individual patients directly.
Classification of CrGN
As described previously, each renal specimen was
assessed by direct immunofluorescence, light and electron
microscopy (16). These experiments
had been performed with the samples at the time of diagnosis. CrGN
was defined as ≥50% of the total glomeruli having large crescents
as evaluated by light microscopy. On the basis of the
immunofluorescence microscopy results, CrGN was classified into 3
types. By definition, type I CrGN exhibited linear deposits of
immunoglobulins (Ig) along the GBM, which was always accompanied by
anti-GBM antibodies in the serum. Type II was characterized by
granular immune-complex deposition on the glomerular tuft, while in
type III, which is also referred to as pauci-immune CrGN,
immunofluorescence was negative or deposits of Ig were rare
(2).
Definitions
The definitions of acute kidney injury (AKI) and
chronic kidney disease (CKD) are widely accepted, while acute
kidney diseases and disorders (AKD) is a relatively novel concept
(17). Hence, the criteria for AKI,
AKD without AKI and CKD were used to illustrate the different
clinical characteristics of patients with CrGN. AKI was defined as
an increase in SCr by ≥0.3 mg/dl (26.5 µmol/l) within 48 h or an
increase in SCr to 1.5 times the baseline value within 7 days or a
urine volume of <0.5 ml/kg/h over 6 h. AKD without AKI was
defined as an estimated glomerular filtration rate (eGFR) of <60
ml/min per 1.73 m2 for <3 months, a decrease in eGFR
≥35%, an increase in SCr >50% or abnormalities of kidney
structure based on urinary markers and imaging studies for <3
months. CKD was defined as eGFR <60 ml/min per 1.73
m2 or kidney structural damage for >3 months
(8).
In addition to the major features of the kidney,
certain extrarenal manifestations coexisted, including
hematological disorders, serositis and pneumonia. For simplicity,
multiple-system involvement (MSI) was uniformly defined to
summarize the clinical manifestation, i.e. involvement of at least
one other organ in addition to the kidney. It is worth mentioning
that, since anemia in CrGN is one of the most common complications,
it was not considered to be an organ-involving manifestation.
Statistical analysis
All data were analyzed using SPSS version 19.0 (IBM
Corp.). Quantitative data were expressed as the mean ± standard
deviation, n (%) or median with interquartile range. All parameters
were compared using the χ2 test or Fisher's exact test
for categorical data and one-way analysis of variance (ANOVA) or
Kruskal-Wallis test for continuous data. The least-significant
difference test and Bonferroni correction were used as post-hoc
tests that followed ANOVA and the Kruskal-Wallis test respectively.
Kaplan-Meier curves with log-rank tests were used to analyze
patient survival as well as renal survival. P<0.05 was
considered to indicate statistical significance.
Results
Demographics and kidney
manifestations
In the present retrospective study, CrGN accounted
for 3.73% (49/1312) of the total patients receiving renal biopsies
during the study period at the Department of Nephrology, Xiangya
Hospital of Central South University (Changsha, China). Of the 49
cases identified, 11 (22.45%) patients were classified as type I,
19 (38.78%) as type II and the remaining 19 (38.78%) as type III. A
total of 23 (46.94%) were females and 26 (53.06%) were males, with
an average age of 44.51±15.95 years at the time-point of diagnosis
(Table I). The duration of symptoms
prior to admission varied greatly from 7 days to 13 months, with a
median duration of 86 days. However, no significant differences in
the demographic data were determined among the 3 types of CrGN.
 | Table I.Baseline demographic and
clinicopathological characteristics of patients with crescentic
glomerulonephritis. |
Table I.
Baseline demographic and
clinicopathological characteristics of patients with crescentic
glomerulonephritis.
| Item | Total (n=49) | Type I (n=11) | Type II (n=19) | Type III
(n=19) | P-value |
|---|
| Age (years) | 44.51±15.95 | 37.09±15.14 | 43.74±18.06 | 49.58±12.80 | 0.113 |
| Female sex | 23 (46.94) | 3
(27.27) | 12 (63.16) | 8
(42.11) | 0.143 |
| Duration of disease
(days) | 86
(30–100) | 52
(17.5–60) | 90
(25.5–90) | 102
(32.5–120) | 0.16 |
| Hypertension | 29 (59.18) | 8
(72.73) | 10 (52.63) | 11 (57.89) | 0.531 |
| Oliguria | 9
(18.37) | 3
(27.27) | 2
(10.53) | 4
(21.05) | 0.484 |
| Anuria | 3 (6.12) | 1 (9.09) | 1 (5.26) | 1 (5.26) | 0.905 |
| AKI | 14 (28.57) | 8
(72.73) | 3
(15.79)a | 3
(15.79)a | 0.001 |
| AKD without
AKI | 23 (46.94) | 2
(18.18) | 11 (57.89) | 10 (52.63) | 0.09 |
| CKD | 12 (24.49) | 1 (9.09) | 5
(26.32) | 6
(31.58) | 0.404 |
| MSI | 48 (97.96) | 10 (90.91) | 19 (100) | 19 (100) | 0.224 |
Concerning the clinical characteristics, MSI was
observed in most CrGN patients (97.96%), 29 patients (59.18%) had
hypertension and 9 (18.37%) had oliguria. Furthermore, all CrGN
patients had hematuria, including microscopic or gross hematuria.
The proportions of patients with AKI, AKD without AKI, and CKD were
28.57, 46.94 and 24.49%, respectively. In addition, patients with
type I disease tended to have an acute onset, with 72.73% of
patients presenting with AKI, which was significantly different
from the other two groups (P=0.001; Table I).
Laboratory and ultrasonography
data
In general, patients with kidney diseases have
varying degrees of anemia (15), and
this was also observed in the CrGN patients of the present study.
As presented in Table II, patients
with type II CrGN had less severe anemia than the other two
subtypes (P<0.05 type II vs. type I; P<0.05 type II vs. type
III). The average baseline SCr level of all patients was 612±416
µmol/l. In accordance with the clinical presentation, the SCr level
and the kidney tubular injury parameter retinol binding protein of
patients with type I CrGN were markedly higher than those in
patients with the other two types (P<0.05). Of the patients with
type III CrGN, 84.21% had serum anti-neutrophilic cytoplasmic
autoantibodies (ANCA), and they had a lower amount of proteinuria.
As expected, circulating anti-GBM antibodies were detected in all
patients with type I CrGN. In addition, patients with type I CrGN
had a high level of serum complement 4 (P<0.05 type I vs. type
II; P<0.05 type I vs. type III) and patients with type III CrGN
had a relatively high level of serum IgG (P<0.05 type III vs.
type I; P<0.05 type III vs. type II). Finally, kidney
enlargement was identified in 57.14% of patients. However, there
were no obvious differences in kidney enlargement or serum albumin
levels among the different types of CrGN.
 | Table II.Laboratory and ultrasonography data
by type of crescentic glomerulonephritis. |
Table II.
Laboratory and ultrasonography data
by type of crescentic glomerulonephritis.
| Parameter | Total (n=49) | Type I (n=11) | Type II (n=19) | Type III
(n=19) |
|---|
| Hemoglobin
(g/l) | 83.98±20.87 |
78.18±22.35 |
94.74±19.98a |
76.58±16.87b |
| Albumin (g/l) | 28.1±5.05 | 26.98±3.77 | 28.15±5.61 | 28.71±5.64 |
| Proteinuria
(g/day) | 2.32±2.49 |
2.90±3.85 |
2.91±2.43 |
1.39±1.14a,b |
| Scr (µmol/l) | 612±416 |
928±381 |
502±410a |
537±365a |
| NAG (u/l) | 28.3±18.7 |
23.9±11.8 |
31.0±22.3 |
26.9±17.0 |
| RBP (mg/l) | 14.9±19.3 |
36.6±27.0 |
16.3±20.5a |
6.7±6.7a |
| C4 (mg/l) | 253
(208–281) | 309
(235–382) | 239
(208–274)a | 233
(194–264)a |
| C3 (mg/l) | 898
(744–1,045) | 1,039
(830–1,230) | 882
(758–984) | 829 (681–978) |
| IgG (g/l) | 12.7
(7.9–16.5) | 9.2
(7.0–10.0) | 10.8
(6.7–14.3) | 17.0
(13.9–20.2)a,b |
| IgA (mg/l) | 2,611
(1,675–3,285) | 2,193
(1,385–2,430) | 2,678
(1,790–3,255) | 2,807
(1,770–3,610) |
| IgM (mg/l) | 1,232
(811–1,540) | 1,124
(723–1,490) | 1,298
(843–1,710) | 1,228
(860–1,530) |
| ANCA positive | 19/46 (41.30) | 0/10 (0) | 3/17 (17.65) | 16/19
(84.21)a,b |
| GBM | 12/32 (37.50) | 11/11 (100) | 0/9
(0)a | 1/12
(8.33)a |
| MPO-ANCA
positive | 13/32 (40.63) | 0/11 (0) | 2/9 (22.22) | 11/12
(91.67)a,b |
| PR3-ANCA
positive | 1/32 (3.13) | 0/11 (0) | 1/9 (11.11) | 0/12 (0) |
| Kidney
enlargement | 28/49 (57.14) | 8/11(72.73) | 12/19 (63.16) | 8/19
(42.11) |
Primary diseases of patients with type
II CrGN
Regarding primary disease, the composition of the 19
patients with type II CrGN was as follows: 8/19 (42.11%) had IgA
nephropathy, 4/19 (21.05%) had lupus nephritis, 3/19 (15.79%) had
ANCA-associated glomerulonephritis, 2/19 (10.53%) had
Henoch-Schönlein purpura glomerulonephritis, 1/19 (5.26%) had
hepatitis B virus-associated nephritis and 1/19 (5.26%) had
idiopathic immune-complex CrGN (Table
III). All 4 patients diagnosed with lupus nephritis had serum
anti-nuclear antibodies, 3 of whom had anti-double stranded DNA
(anti-dsDNA) antibodies and 1 had positive anti-Smith
antibodies.
 | Table III.Primary diseases of patients with
type II CrGN. |
Table III.
Primary diseases of patients with
type II CrGN.
| Variables | n (% of Type II
CrGN) |
|---|
| IgA nephropathy, n
(%) | 8/19 (42.11) |
| Lupus nephritis, n
(%) | 4/19 (21.05) |
| Anti-nuclear
antibodies positive, n (%) | 4/4 (100) |
| Anti-double
stranded DNA antibodies positive, n (%) | 3/4 (75) |
| Anti-Smith
antibodies positive, n (%) | ¼ (25) |
| ANCA-associated
glomerulonephritis, n (%) | 3/19 (15.79) |
| H-S purpura
glomerulonephritis, n (%) | 2/19 (10.53) |
| Hepatitis B
virus-associated nephritis, n (%) | 1/19 (5.26) |
| Idiopathic
immune-complex CrGN, n (%) | 1/19 (5.26) |
Pathological characteristics
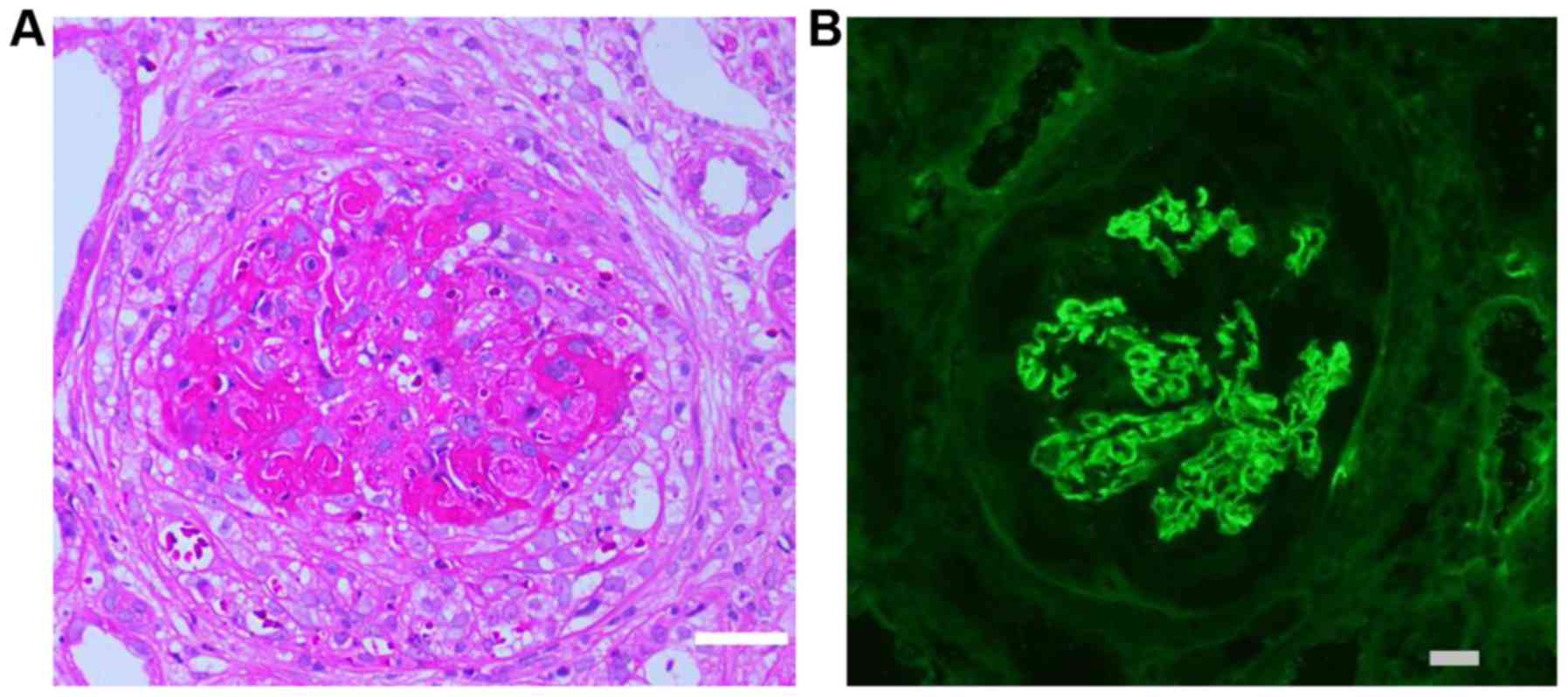
Renal biopsy from a representative case of type I
CrGN revealed predominant cellular crescents and linear deposits of
IgG along the GBM on immunofluorescence (Fig. 1). Among all of the subjects with
CrGN, the mean overall percentages of glomeruli with crescents,
cellular crescents and fibrous crescents were 65.84±13.25,
35.34±17.81 and 30.49±15.71%, respectively (Table IV). Compared with the other two
subtypes, patients with type I CrGN had a significantly higher
percentage of cellular crescents (P<0.05). Furthermore, patients
with type II CrGN had a lower percentage of sclerosis than type
III.
 | Table IV.Pathological characteristics of
patients with crescentic glomerulonephritis. |
Table IV.
Pathological characteristics of
patients with crescentic glomerulonephritis.
| Item | Total (n=49) | Type I (n=11) | Type II (n=19) | Type III
(n=19) |
|---|
| Cellular crescents
(%) | 35.34±17.81 | 48.31±18.69 |
32.67±16.61a |
30.52±15.51a |
| Fibrous crescents
(%) | 30.49±15.71 | 22.46±13.18 | 31.58±16.65 | 34.06±15.18 |
| Sclerosis (%) | 19.45±18.16 | 21.53±25.48 | 12.63±13.84 |
25.05±15.56b |
| Crescents (%) | 65.84±13.25 | 70.77±14.67 | 64.24±13.22 | 64.58±12.43 |
Treatment, and overall and renal
survival
As presented in Table
V, only 14 patients (28.57%) of the present cohort received
plasma exchange, and even of the patients with type I CrGN, only
63.64% received plasma exchange. With regard to the treatment of
plasma exchange between type I CrGN and type II CrGN, the
difference was significant (P<0.05). Of the total patients, 48
(97.96%) and 41 (83.67%) received corticosteroids and
cyclophosphamide, respectively. A total of 35 patients (71.43%)
were treated with intravenous pulses of methylprednisolone at
diagnosis.
 | Table V.Treatment of patients with crescentic
glomerulonephritis. |
Table V.
Treatment of patients with crescentic
glomerulonephritis.
| Item | Total (n=49) | Type I (n=11) | Type II (n=19) | Type III
(n=19) | P-value |
|---|
| Plasma
exchange | 14 (28.57) | 7 (63.64) | 1
(5.26)a | 6 (31.58) | 0.003 |
|
Cyclophosphamide | 41 (83.67) | 10 (90.91) | 15 (78.95) | 16 (84.21) | 0.885 |
|
Corticosteroids | 48 (97.96) | 11 (100) | 19 (100) | 18 (94.74) | 1.000 |
| Methylprednisolone
pulse | 35 (71.43) | 10 (90.91) | 15 (78.95) | 10 (52.63) | 0.056 |
| Other
immunosuppressive agents | 9 (18.37) | 2 (18.18) | 5 (26.32) | 2 (10.53) | 0.522 |
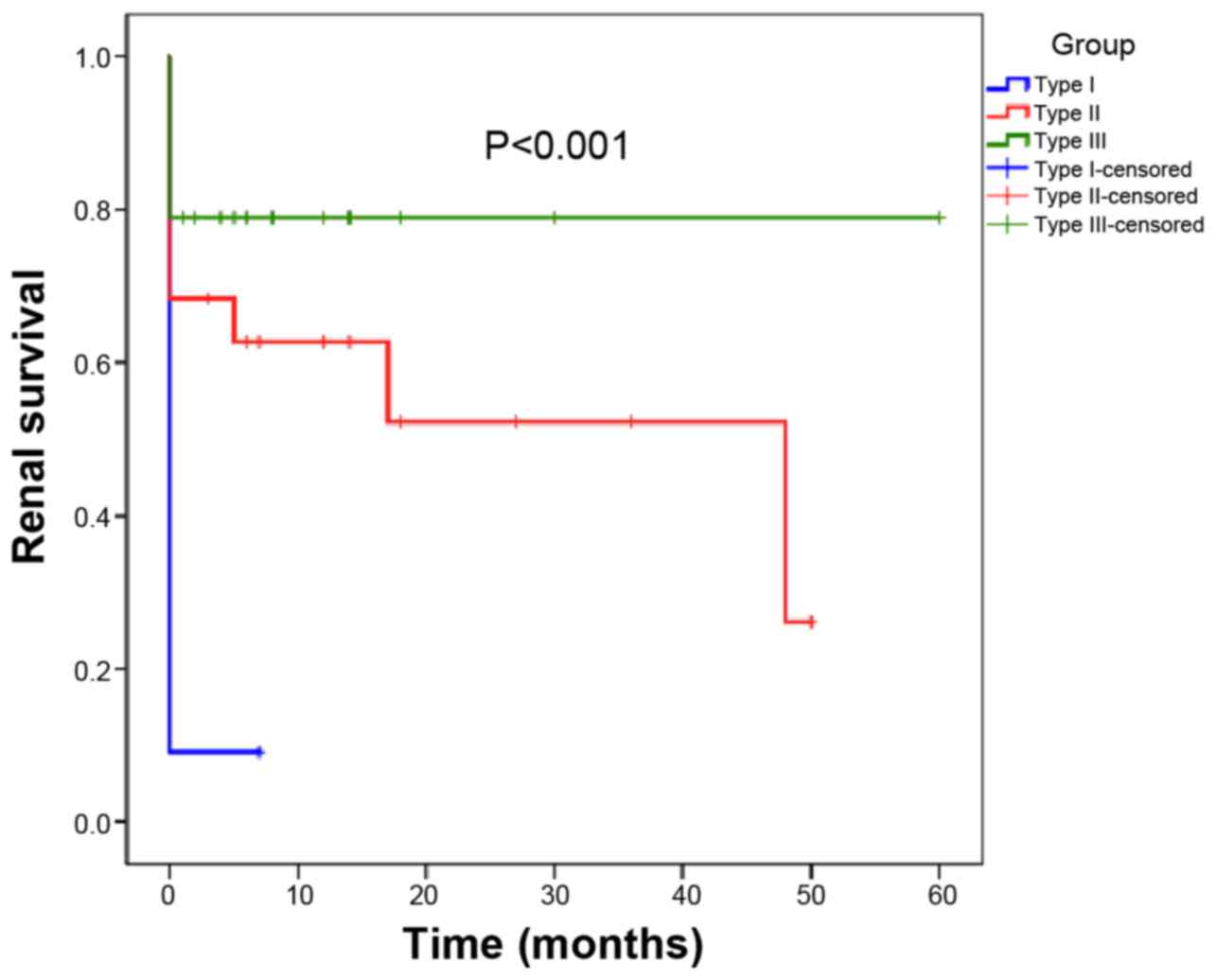
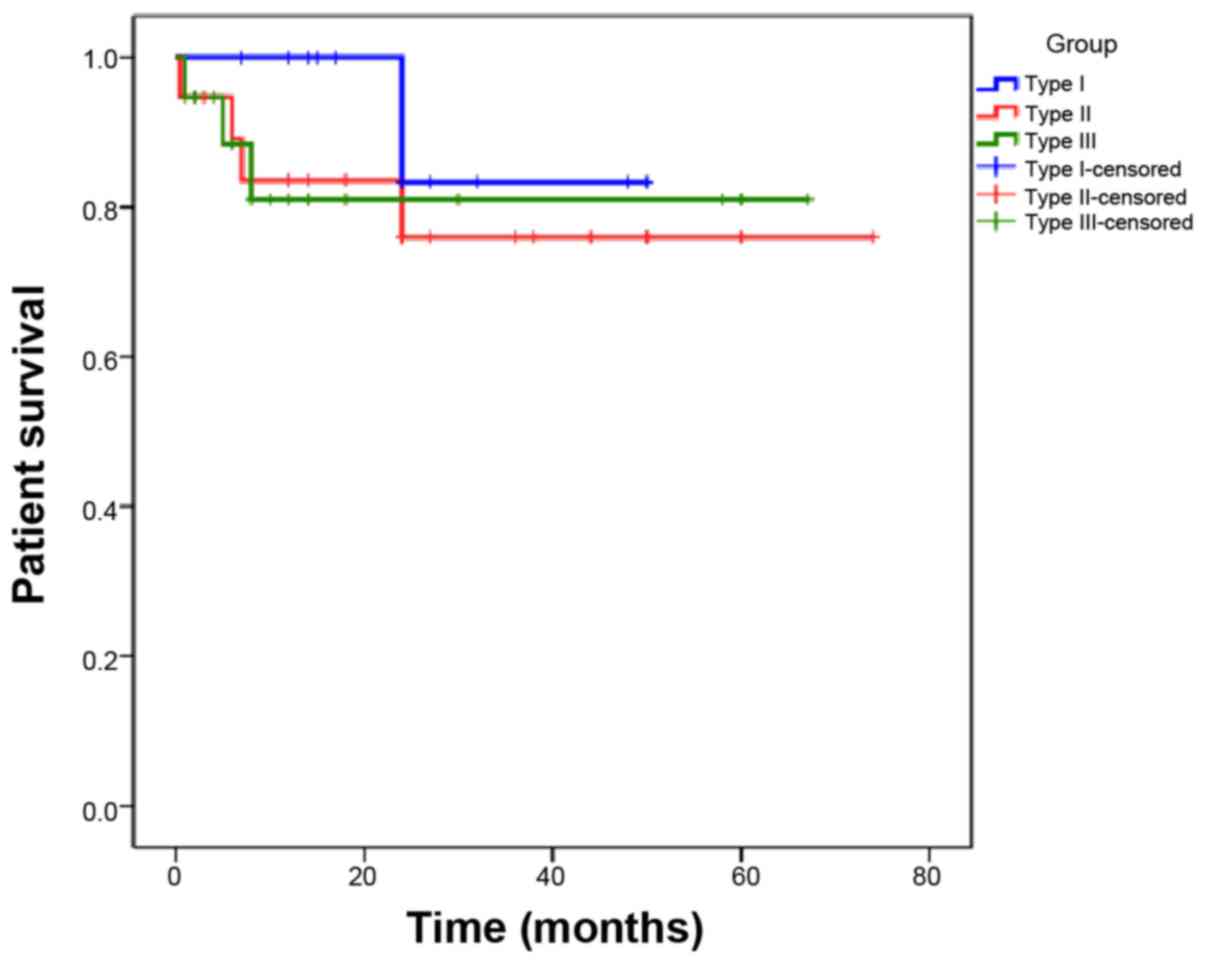
The renal survival of the patients is presented in
Fig. 2. Patients with type III CrGN
had superior renal survival, whereas those with type I CrGN had the
poorest renal prognosis (P=0.002, type I vs. type II; P<0.01,
type I vs. type III; P=0.15, type II vs. type III). Regarding
patient survival, no significant difference was present among the 3
types of CrGN (Fig. 3).
Discussion
Validation studies of epidemiologic data on CrGN
have been performed in certain countries (4). In the present retrospective study, CrGN
accounted for 3.73% of the total patients receiving renal biopsies
during the study period at our center, which is higher than
previously reported rates from China (8,9,15), but was similar to results from an
Indian study (3). In addition, an
equal proportion of pauci-immune and immune complex GN was
encountered in patients with CrGN at our center, followed by
anti-GBM disease. This result was different from that of several
previous studies, by which pauci-immune CrGN was reported to be the
predominant type (3,4,6,7). In Europe, the annual incidence of renal
vasculitis was reported to be 10–20 per 1 million individuals
(18); the incidence in China is
currently not available. According to a study by Chen et al
(19) >400 patients with
ANCA-associated vasculitis (AAV) were diagnosed during an 8-year
period in their referral diagnostic center in Peking University
First Hospital in China. Anti-GBM disease is considered a rare
disease, with an incidence of 1–2 cases per million per year in
China (20). While the incidence in
China is not available, >30 anti-GBM-positive sera from new
patients are screened annually at Peking University First Hospital
(21), indicating that, relative to
other hospitals worldwide, hospitals in China encounter more
patients with anti-GBM disease. The present study reported a higher
proportion of patients with type I CrGN among the CrGN cases
studied compared with the results of previous studies (3,4,6,7) and
further supports the notion that hospitals in China may encounter
more patients with anti-GBM disease. In type II CrGN, IgA
nephropathy was the most common primary disease, which is
consistent with previous studies (16,21–25).
In certain severely ill patients, renal biopsy
cannot always be smoothly and quickly performed. Thus, specific
serum markers [serum myeloperoxidase (MPO-ANCA) or proteinase 3
(PR3-ANCA)] are important and are correlated with AAV. Of those
patients with granulomatosis with polyangiitis (Wegener's
granulomatosis), 90% have PR3-ANCA and patients with microscopic
polyangiitis have the highest frequency of MPO-ANCA (26). ANCA themselves are deemed to be
pathogenic in these diseases (27).
Serum ANCA is considered a basis for the recognition of
ANCA-associated vasculitis, which frequently presents as type III
CrGN. However, it was also detected in a small number of patients
with type I and II CrGN. It was reported that patients with
negative ANCA had better renal outcomes (8,28,29).
However, others have reported that patients without ANCA had higher
levels of proteinuria, more severe glomerular lesions and poorer
renal outcomes (8). In the present
study, the majority of patients with type III CrGN were
MPO-ANCA-positive, which was similar to the result of a previous
study from China (8). In the present
cohort, serum ANCA was detected in 17.65% of cases with type II
CrGN, which is higher than the percentage reported in the above
previous study (8). Lin et al
(15) reported that glomerular
necrosis and crescent formation were associated with ANCA in
immune-complex CrGN. Furthermore, ANCA and immune complex
deposition possibly act synergistically (15). The renal survival in patients with
type II CrGN in the present study was lower than that previously
reported (3,8,9), perhaps
due to a higher ratio of serum ANCA in type II CrGN.
Several clinical characteristics were reported to be
associated with renal outcome in CrGN, including oliguria and SCr
(3,8). A clinical grading system for patients
with RPGN based on SCr and other clinical manifestations has been
established to predict the prognosis (4). The average SCr concentration of
patients at our center was much higher than that of patients from
Spain (7), India (3), the US (6) and Japan (4). Patients with pauci-immune CrGN had
higher SCr levels than those with immune complex CrGN at
presentation in certain studies from the US (6) and Saudi Arabia (5), which was consistent with the present
results. The medium proteinuria did not reach the standard of
nephrotic syndrome, which was lower than that in studies from Saudi
Arabia (5) and the US (6). Oliguria was similar to another study
from China (8).
The association between histopathological features
and prognosis has been previously reported (3,5). The
percentage of sclerosed glomeruli is one of the independent
predictors of renal death (5,8). The
percentage of sclerosed glomeruli was determined to be
significantly different between the pauci-immune and immune complex
CrGN, but renal survival was comparable in the two groups in the
presnt study. The average percentage of sclerosed glomeruli in the
current study was lower than that in another study from Saudi
Arabia (5). There were no
significant differences in the proportions of glomeruli exhibiting
crescents and fibrous crescents between the three groups in the
current study, which was consistent with the results of previous
studies (3,5,8).
Renal replacement therapy in ESRD patients has a
high cost (30). In the present
study, patients with type I CrGN had the poorest renal prognosis,
which has also been confirmed by previous studies (3,6,8). The 1-year kidney survival rate in the
present study was lower than that reported previously (31,32).
This discrepancy in results may be explained by the higher initial
average SCr concentration in the present study compared to those in
the previous studies (8,31). The low proportion of patients who
received plasma exchange and less intensive immunosuppressive
therapy may be another important reason.
Determining which type of CrGN between pauci-immune
CrGN and immune complex CrGN is associated with better renal
survival remains elusive. Several studies have indicated that the
renal prognosis of patients with type II CrGN was superior to that
of patients with type III CrGN (3,8), which
is in contrast to the results reported by Han et al
(33). Of note, in the present
study, renal survival in patients with type III CrGN was better
than that in patients with type II CrGN, although the difference
did not reach statistical significance. This comparison warrants
further investigation in larger studies in the future.
The present study has several limitations. First,
due to inclusion of only a small number of patients in the present
study, there may have been a selection bias. Furthermore, follow-up
was limited and a longer follow-up may be required in future
studies. However, with the extension of the follow-up period, the
number of cases lost to follow-up is expected to increase.
In conclusion, the present retrospective study
reported that CrGN occurred in 3.37% of the total patients
receiving renal biopsies during the study period at our center,
which is higher than the rates reported by previous studies from
China (8,15). Type I CrGN had the poorest renal
prognosis. The 1-year kidney survival rate in the present study was
much lower than those reported by previous studies (31,32). The
results of the present study highlight the requirement for better
treatments for this disease. A large national investigation on CrGN
is required to improve the clinical management of these patients in
the near future.
Acknowledgements
Parts of the present study were presented at the ISN
World Congress of Nephrology, April 12–15, 2019 in Melbourne,
Australia.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81402453 and
81800641), the Natural Science Foundation of Hunan Province (grant
no. 2015JJ4058), the Project of Hunan Health Commission of Hunan
province (grant no. C2019184) and the Project of Hunan Development
and Reform Commission [grant no. (2013) 1199], China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, JBC, TW and JJP designed the study, analyzed the
data and wrote the manuscript. TM, QQL, XA, HLY, JXP, ZZP, WSP,
XZL, XCX, QLZ and PX contributed to patient enrollment and
follow-up. WL and HLY analyzed the pathological data. YZ and JBC
analyzed the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics review
committee of Xiangya Hospital Central South University (reference
no. 201403061). All patients provided written informed consent to
participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pettersson EE, Sundelin B and Heigl Z:
Incidence and outcome of pauci-immune necrotizing and crescentic
glomerulonephritis in adults. Clin Nephrol. 43:141–149.
1995.PubMed/NCBI
|
|
2
|
Couser WG: Rapidly progressive
glomerulonephritis: Classification, pathogenetic mechanisms, and
therapy. Am J Kidney Dis. 11:449–464. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta R, Singh L, Sharma A, Bagga A,
Agarwal SK and Dinda AK: Crescentic glomerulonephritis: A clinical
and histomorphological analysis of 46 cases. Indian J Pathol
Microbiol. 54:497–500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koyama A, Yamagata K, Makino H, Arimura Y,
Wada T, Nitta K, Nihei H, Muso E, Taguma Y, Shigematsu H, et al: A
nationwide survey of rapidly progressive glomerulonephritis in
Japan: Etiology, prognosis and treatment diversity. Clin Exp
Nephrol. 13:633–650. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oudah N, Al Duhailib Z, Alsaad K, Qurashi
S, Ghamdi G, Flaiw A, Hejaili F, Farooqui M and Al Sayyari A:
Glomerulonephritis with crescents among adult Saudi patients
outcome and its predictors. Clin Exp Med. 12:121–125. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jennette JC: Rapidly progressive
crescentic glomerulonephritis. Kidney Int. 63:1164–1177. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lopez-Gómez JM and Rivera F; Spanish
Registry of Glomerulonephritis, : Renal biopsy findings in acute
renal failure in the cohort of patients in the Spanish Registry of
Glomerulonephritis. Clin J Am Soc Nephrol. 3:674–681. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen S, Tang Z, Xiang H, Li X, Chen H,
Zhang H, Hu W, Zeng C and Liu Z: Etiology and outcome of crescentic
glomerulonephritis from a single center in china: A 10-year review.
Am J Kidney Dis. 67:376–383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang Z, Wu Y, Wang Q, Zeng C, Yao X, Hu W,
Chen H, Liu Z and Li L: Clinical spectrum of diffuse crescentic
glomerulonephritis in Chinese patients. Chin Med J (Engl).
116:1737–1740. 2003.PubMed/NCBI
|
|
10
|
Moroni G and Ponticelli C: Rapidly
progressive crescentic glomerulonephritis: Early treatment is a
must. Autoimmun Rev. 13:723–729. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen A, Lee K, Guan T, He JC and
Schlondorff D: Role of CD8+ T cells in crescentic
glomerulonephritis. Nephrol Dial Transplant. (pii): gfz0432019.doi:
10.1093/ndt/gfz043 (Epub ahead of print). PubMed/NCBI
|
|
12
|
McAdoo SP and Pusey CD: Anti-glomerular
basement membrane disease. Clin J Am Soc Nephrol. 13:1162–1172.
2017. View Article : Google Scholar
|
|
13
|
Kitching AR and Hutton HL: The players:
Cells involved in glomerular disease. Clin J Am Soc Nephrol.
11:1664–1674. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rovin BH, Caster DJ, Cattran DC, Gibson
KL, Hogan JJ, Moeller MJ, Roccatello D, Cheung M, Wheeler DC,
Winkelmayer WC, et al: Management and treatment of glomerular
diseases (part 2): Conclusions from a Kidney Disease: Improving
Global Outcomes (KDIGO) controversies conference. Kidney Int.
95:281–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin W, Chen M, Cui Z and Zhao MH: The
immunopathological spectrum of crescentic glomerulonephritis: A
survey of 106 patients in a single Chinese center. Nephron Clin
Pract. 116:c65–c74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao C, Xiao P, Li X, Li X, Li H, Chen Y,
Wang Y, Xu Y, Huang G and Zhou Q: Cordyceps sinensis may inhibit
Th22 cell chemotaxis to improve kidney function in lgA nephropathy.
Am J Transl Res. 10:857–865. 2018.PubMed/NCBI
|
|
17
|
Chawla LS, Bellomo R, Bihorac A, Goldstein
SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald
RL, et al: Acute kidney disease and renal recovery: Consensus
report of the acute disease quality initiative (ADQI) 16 workgroup.
Nat Rev Nephrol. 13:241–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watts RA and Scott DG: Epidemiology of the
vasculitides. Semin Respir Crit Care Med. 25:455–464. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen M, Cui Z and Zhao MH: ANCA-associated
vasculitis and anti-GBM disease: The experience in China. Nephrol
Dial Transplant. 25:2062–2065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Canney M, O'Hara PV, McEvoy CM, Medani S,
Connaughton DM, Abdalla AA, Doyle R, Stack AG, O'Seaghdha CM,
Clarkson MR, et al: Spatial and temporal clustering of
anti-glomerular basement membrane disease. Clin J Am Soc Nephrol.
11:1392–1399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen M, Cui Z and Zhao MH: ANCA-associated
vasculitis and anti-GBM disease: The experience in China. Nephrol
Dial Transplant. 25:2062–2065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan Q, Wang J, Peng Z, Zhou Q, Xiao X,
Xie Y, Wang W, Huang L, Tang W, Sun D, et al:
Neutrophil-to-lymphocyte ratio and incident end-stage renal disease
in Chinese patients with chronic kidney disease: Results from the
Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). J Transl
Med. 17:862019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gan L, Li X, Zhu M, Chen C, Luo H and Zhou
Q: Acteoside relieves mesangial cell injury by regulating Th22 cell
chemotaxis and proliferation in IgA nephropathy. Ren Fail.
40:364–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng Z, Wang J, Yuan Q, Xiao X, Xu H, Xie
Y, Wang W, Huang L, Zhong Y, Ao X, et al: Clinical features and
CKD-related quality of life in patients with CKD G3a and CKD G3b in
China: Results from the Chinese Cohort Study of Chronic Kidney
Disease (C-STRIDE). BMC Nephrol. 18:3112017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y, Li H, Xiao C, Zeng X, Xiao X, Zhou
Q and Xiao P: NLRC5: Potential novel non-invasive biomarker for
predicting and reflecting the progression of IgA nephritis. J
Transl Med. 16:3172018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gapud EJ, Seo P and Antiochos B:
ANCA-associated vasculitis pathogenesis: A commentary. Curr
Rheumatol Rep. 19:152017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao H, Hu P, Falk RJ and Jennette JC:
Overview of the pathogenesis of ANCA-associated vasculitis. Kidney
Dis (Basel). 1:205–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hedger N, Stevens J, Drey N, Walker S and
Roderick P: Incidence and outcome of pauci-immune rapidly
progressive glomerulonephritis in Wessex, UK: A 10-year
retrospective study. Nephrol Dial Transplant. 15:1593–1599. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hung PH, Chiu YL, Lin WC, Chiang WC, Chen
YM, Lin SL, Wu KD and Tsai TJ: Poor renal outcome of antineutrophil
cytoplasmic antibody negative Pauci-immune glomerulonephritis in
Taiwanese. J Formos Med Assoc. 105:804–812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Luo M, Xiao L, Zhu XJ, Wang C, Fu
X, Yuan SG, Xiao F, Liu H, Dong Z, et al: Exploration of
pathological prediction of chronic kidney diseases by a novel
theory of bi-directional probability. Sci Rep. 6:321512016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huart A, Josse AG, Chauveau D, Korach JM,
Heshmati F, Bauvin E, Cointault O, Kamar N, Ribes D, Pourrat J, et
al: Outcomes of patients with Goodpasture syndrome: A nationwide
cohort-based study from the French Society of Hemapheresis. J
Autoimmun. 73:24–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Daalen EE, Jennette JC, McAdoo SP,
Pusey CD, Alba MA, Poulton CJ, Wolterbeek R, Nguyen TQ,
Goldschmeding R, Alchi B, et al: Predicting outcome in patients
with anti-GBM glomerulonephritis. Clin J Am Soc Nephrol. 13:63–72.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han F, Chen L, Le J, Choong P, Xu Y, Wang
H and Chen J: The clinicopathologic spectrum of rapidly progressive
glomerulonephritis based on glomerular immune deposition and
antineutrophil cytoplasmic antibody. Appl Immunohistochem Mol
Morphol. 23:704–710. 2015. View Article : Google Scholar : PubMed/NCBI
|