Introduction
The liver is vulnerable to various factors, such as
bacteria, hepatitis viruses, alcohol, hepatotoxic drugs and
oxidative products leading to hepatic failure (1,2).
Fulminant hepatic failure (FHF) is a severe clinical syndrome
characterized by massive hepatocyte apoptosis with a high mortality
rate (60–80%) (3). The high
mortality rate of acute liver failure often requires liver
transplantation (4). Although liver
transplantation is a highly successful treatment, it is severely
limited by the shortage in donor organs (5). Therefore, identifying an effective
medical therapy is important. D-Galactosamine (D-GalN) and
lipopolysaccharide (LPS)-induced liver injury is a well-established
experimental model that closely resembles human fulminant hepatitis
in both morphological and functional features (6). Increasing evidence demonstrates that
inflammatory responses are important pathogenic factors that
contribute to LPS/D-GalN-induced FHF (7). D-GalN is a specific hepatotoxic agent
that increases the lethal effects of LPS (8). Stimulation by LPS can trigger the
Toll-like receptor 4 signalling pathway and can activate NF-κB to
release pro-inflammatory cytokines such as tumour necrosis factor-α
(TNF-α), interleukin (IL)-1β and IL-6, which have pivotal roles in
the pathogenesis of LPS/D-GalN-induced acute hepatitis (9). Therefore, inhibiting inflammation may
be a potential preventive measure for the development of FHF.
Crocetin, the major component present in saffron, is
a 20-carbon chain molecule containing six double bonds with a
carboxylic acid group at each end (10,11). In
the past few decades, an increasing amount of evidence has
identified that crocetin has promising anti-inflammatory,
antioxidant and neuroprotective properties (12). Investigation into the
hepatoprotective effects of crocetin determined that crocetin
administration increased the survival of rats during resuscitation
post-haemorrhage (13) and protected
against CCl4-induced liver damage (14). However, its role in FHF remains
poorly elucidated.
Therefore, to better understand the effects of
crocetin on FHF, the present study established an FHF model using
LPS/D-GalN and evaluated hepatic apoptosis and inflammation
following crocetin pre-treatment.
Materials and methods
Animals and their diets
A total of 60 male Wistar rats (63–70 days old;
180–200 g) and their food were obtained from Guangdong Medical
Laboratory Animal Centre. All rats were maintained in a 12-h
light/dark cycle at a constant temperature and humidity (22±3°C and
50±20%, respectively) with ad libitum access to food and
water.
Experimental protocol
Animals were randomly assigned to one of the three
following groups with 20 rats per group: i) Control; ii)
LPS/D-GalN; or iii) crocetin+LPS/D-GalN. Each group was then
further divided into four subgroups according to the time of
assessment: 0, 6, 12 or 48 h. Animals in the LPS/D-GalN group
received an intraperitoneal injection of 300 mg of D-GalN per kg of
body weight (Sigma-Aldrich; Merck KGaA) and then were injected
intradermally with 50 mg of LPS per kg of body weight
(Sigma-Aldrich; Merck KGaA) (15).
Rats were sacrificed after 0, 6, 12 and 48 h after LPS/D-GalN
injection. Animals in the crocetin+LPS/D-GalN group were
pre-treated with 200 µl of 5 µmol/l crocetin once, (Sigma-Aldrich;
Merck KGaA) (16,17) 1 day before the injection of
LPS/D-GalN. Furthermore, no treatment was performed on the rats in
the Control group. All the rats were anaesthetized with avertin
(250 mg/kg) and sacrificed by exsanguination from the femoral
artery. Liver and 100 µl blood samples were collected for
subsequent analysis. All rats received humane care according to the
Guidelines for the Care and Use of Research Animals established by
Southern Medical University and the experimental protocol was
approved by the Ethics Committee of the Fifth Affiliated Hospital
of Southern Medical University.
Haematoxylin and eosin (H&E)
staining
Livers were fixed in 4% paraformaldehyde solution
for 24–36 h at room temperature. The fixed samples were embedded in
paraffin. Microtome sections were prepared (5 µm thickness) and
stained with H&E according to Xin et al (18). The histology images (magnification,
×100 and ×400) were captured using a light microscope (Shanghai
Optical Instrument Factory No. 1).
Biochemical evaluation of serum
After centrifugation for 10 min at 4°C (300 × g;
Centrifuge 5804R), the activities of alanine transaminase (ALT),
aspartate aminotransferase (AST) and total bilirubin (TBIL) in the
rat serum were determined using an automatic biochemical blood
analyser (cat. no. 7600-210; Hitachi High-Technologies
Corporation). IL-6 (cat. no. ml002828), IL-1β (cat. no. ml003549)
and TNF-α (cat. no. ml002859) concentrations were detected using
commercial ELISA kits (Shanghai Enzyme-linked Biotechnology Co.,
Ltd.) according to the manufacturer's instructions. The activities
of malondialdehyde (MDA; cat. no. A003-1-2) and superoxide
dismutase (SOD; cat. no. A001-3-2) were detected using commercial
kits from Nanjing Jiancheng Bioengineering) according to the
instructions of the manufacturer.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Quantitative and qualitative analyses
of isolated RNA were assessed from the ratio of absorbance at 260
and 280 nm by a Biophotometer Plus (Eppendorf) and 1% agarose gel
electrophoresis. Complementary DNA was synthesized from 1 µg of
total RNA using the commercial Moloney Murine Leukaemia Virus
reverse transcriptase kit (Promega Corporation) according to the
manufacturer's instruction. Primer sequences for the rat genes were
designed and selected using Primer 5.0 (Premier Biosoft
International) and Oligo 7.0 software (Molecular Biology Insights,
Inc.), as presented in Table I.
GAPDH was used as a housekeeping gene to normalize target gene
transcript levels. qPCR was performed using SYBR-Green qPCR Super
Mix (Invitrogen; Thermo Fisher Scientific, Inc.) and the ABI
StepOne Real-Time PCR System or the 7500 Fast Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 2 min at 50°C, 2 min at
95°C, followed by 40 cycles of 15 sec denaturation at 95°C, 32 sec
annealing/extension at 60°C then a final melting curve analysis to
monitor the purity of the PCR product. The 2−∆∆Cq method
was used to quantify mRNA abundance (19). Relative gene expression levels were
normalized to GAPDH.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence |
|---|
| IL-1β | F:
5′-AGCATCCAGCTTCAAATCTC-3′ |
|
| R:
5′-AGCTCATGGAGAATACCACT-3′ |
| TNF-α | F:
5′-TGAAGTAGTGGCCTGGATTGC-3′ |
|
| R:
5′-GACATTCCGGGATCCAGTGA-3′ |
| iNOS | F:
5′-GAGCAAAAAAGGGCAACAC-3′ |
|
| R:
5′-CGCACTTCTGTCTCTCCAAA-3′ |
| p53 | F:
5′-GAGCTGACAAGACAATGCTAG-3′ |
|
| R:
5′-TCATACGATCTGTATCCTCCAG-3′ |
| GAPDH | F:
5′-CCCATTCTTCCACCTTTGAT-3′ |
|
| R:
5′-CAACTGAGGGCCTCTCTCTT-3′ |
Western blot analysis
Liver tissue lysates were prepared by adding 10 µl
phenylmethylsulfonyl fluoride and 10 µl protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA) into 1 ml radioimmunoprecipitation
assay lysis buffer (Beyotime Institute of Biotechnology). Liver
tissues (100 mg) were resuspended in 500 µl cell lysis buffer and
were homogenized and rocked for 30 min on ice. Crude lysates were
then centrifuged at 12,000 × g for 10 min at 4°C. Equal amounts (20
µg) of protein from each sample were subjected to 10% SDS-PAGE and
then proteins on the gel were transferred to polyvinylidene
difluoride membranes (EMD Millipore). Membranes were blocked with
5% skim milk at room temperature for 1 h and were then incubated
with the primary antibodies anti-GAPDH (1:1,000; cat. no. EPR16891;
Abcam), anti-caspase-8 (1:3,000; cat. no. ab32125; Abcam),
anti-truncated BH3 interacting domain death agonist (tBid)
(1:1,000; cat. no. sc-56025; Santa Cruz Biotechnology, Inc.),
anti-caspase-12 (1:2,000; cat. no. ab62484; Abcam), anti-BCL2 like
11 (Bim) (1:500; cat. no. ab32158; Abcam), anti-caspase-9 (1:1,000;
cat. no. ab32539; Abcam), anti-caspase-3 (1:500; cat. no. ab13847;
Abcam), anti-Bax (1:1,000; cat. no. ab32503; Abcam), anti-NF-κB
(1:500; cat. no. ab194729; Abcam) overnight at 4°C. After washing
with Tris-buffered saline and polysorbate 20, membranes were
incubated with the secondary antibodies conjugated to horseradish
peroxidase (SouthernBiotech), including Goat Anti-Rabbit IgG (H+L)
(1:20,000; cat. no. 4050-05) and Rabbit Anti-Mouse IgG (H+L)-HRP
(1:10,000; cat. no. 6170-05). The blots were then developed with an
enhanced chemiluminescence detection system (ChemiDoc MP; Bio-Rad
Laboratories, Inc.) according to the manufacturer's instructions.
Densitometric quantification of band intensities was determined
using Image J software (National Institutes of Health).
Flow cytometry
An Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit was utilized to detect early apoptosis
[Annexin V-FITC+/propidium iodide (PI)−; Q4],
late apoptosis (Annexin V-FITC+/PI+; Q2), and
necrosis (Annexin V-FITC−/PI+; Q1) according
to the manufacturer's instructions (Nanjing KeyGen Biotech. Co.
Ltd.). Briefly, fresh liver was removed and the liver mononuclear
was immediately isolated. Isolation of liver mononuclear cells was
achieved by cutting the organ into small pieces and then grinding
samples with glass rod on a 200 mesh (200 holes/cm) stainless steel
cell strainer. Following sufficient grinding, the cells were
collected by centrifugation at 300 × g for 5 min at 4°C. Cells were
washed twice with PBS to obtain the purified hepatocytes.
Subsequently, cells were digested with 0.25% trypsin and collected
by centrifugation at 300 × g for 5 min at 4°C. After being washed
twice with PBS, the cells were stained with Annexin V-FITC for 15
min and PI for 5 min at room temperature. The apoptotic cells were
identified by flow cytometry (LSRFortessa; BD Biosciences).
Statistical analysis
Statistical analysis was performed using the SPSS
18.0 software (SPSS, Inc.) and the figures were produced with
GraphPad Prism 5.0 software (GraphPad Software, Inc.). All data are
presented as the mean ± standard deviation. Differences between
groups were examined using one-way analysis of variance with
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Crocetin attenuates LPS/D-GalN-induced
FHF in the liver
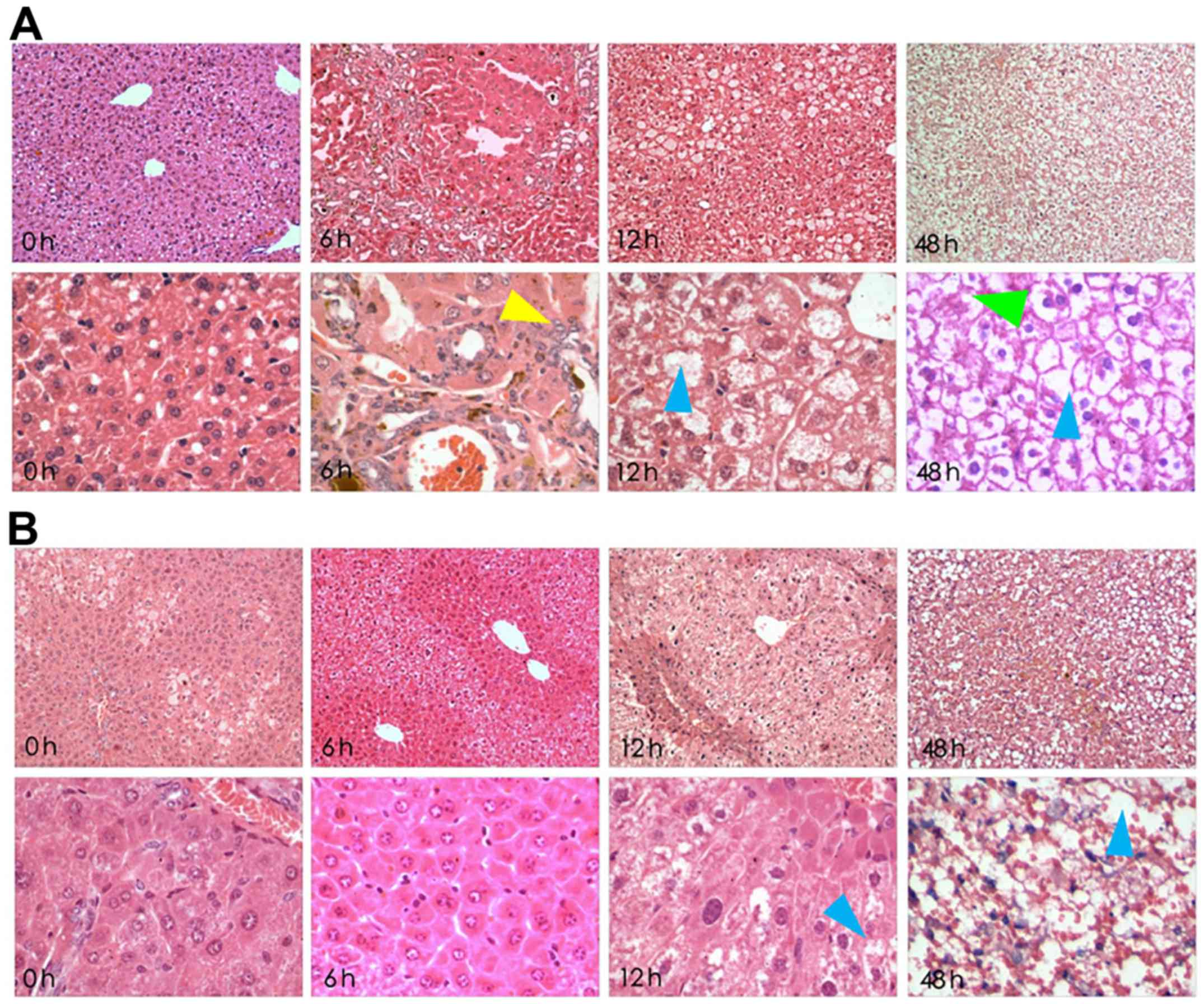
Histological slides were stained with H&E and
were analysed at ×100 and ×400 magnification (Fig. 1). In the LPS/D-GalN groups, severe
liver pathological changes were characterized by deformation and
irregular arrangement, but these alterations were attenuated with
crocetin pre-treatment. In detail, 6-h LPS/D-GalN treatment induced
acidophilic changes, inflammatory infiltration and few apoptotic
bodies. The 12-h LPS/D-GalN treatment induced nuclear dissolution,
nuclear fragmentation, massive haemorrhagic necrosis and massive
apoptotic bodies. Finally, 48-h LPS/D-GalN treatment induced a
fibre mesh stent collapse in addition to massive haemorrhagic
necrosis and apoptotic bodies (Fig.
1A). However, preservation of liver tissue following 6 and 12-h
LPS/D-GalN induction was observed in crocetin-pre-treated rats.
However, 48-h LPS/D-GalN stimulation following crocetin
pretreatment still led to eosinophilic changes and inflammatory
infiltration with nuclear pyknosis and apoptotic features (Fig. 1B).
Crocetin decreases the activities of
ALT and AST and reduced the level of TBIL in serum
Exposure to LPS/D-GalN was associated with
significant increases in the liver damage markers ALT, AST and TBIL
at all assessment times (P<0.01; Table II). The highest values of these
parameters were detected following LPS/D-GalN treatment for 48 h.
Pre-treatment with crocetin significantly decreased the activities
of ALT, AST and TBIL at all the assessment times compared to their
counterpart LPS/D-GalN groups (P<0.01; Table II).
 | Table II.Crocetin decreases the
LPS/D-GalN-induced activities of ALT and AST and the level of
TBIL. |
Table II.
Crocetin decreases the
LPS/D-GalN-induced activities of ALT and AST and the level of
TBIL.
|
|
| LPS/D-GalN |
Crocetin+LPS/D-GalN |
|---|
|
|
|
|
|
|---|
| Parameters | Control | 12-h | 24-h | 48-h | 12-h | 24-h | 48-h |
|---|
| ALT, IU/l | 43.38±7.23 |
526.34±96.54a |
1131.23±198.76a |
1973.24±206.21a |
165.34±52.08b |
511.47±62.79b |
1001.27±137.55b |
| AST, IU/l | 59.01±8.17 |
467.89±93.91a |
927.89±79.58a |
1047.54±185.74a |
136.67±20.33b |
324.22±48.07b |
847.05±68.51b |
| TBIL, µmol/l | 0.68±0.25 |
2.65±0.53a |
36.46±6.63a |
61.73±9.71a |
1.55±1.04b |
12.35±5.02b |
51.78±8.61b |
Crocetin regulates LPS/D-GalN-induced
hepatocyte apoptosis
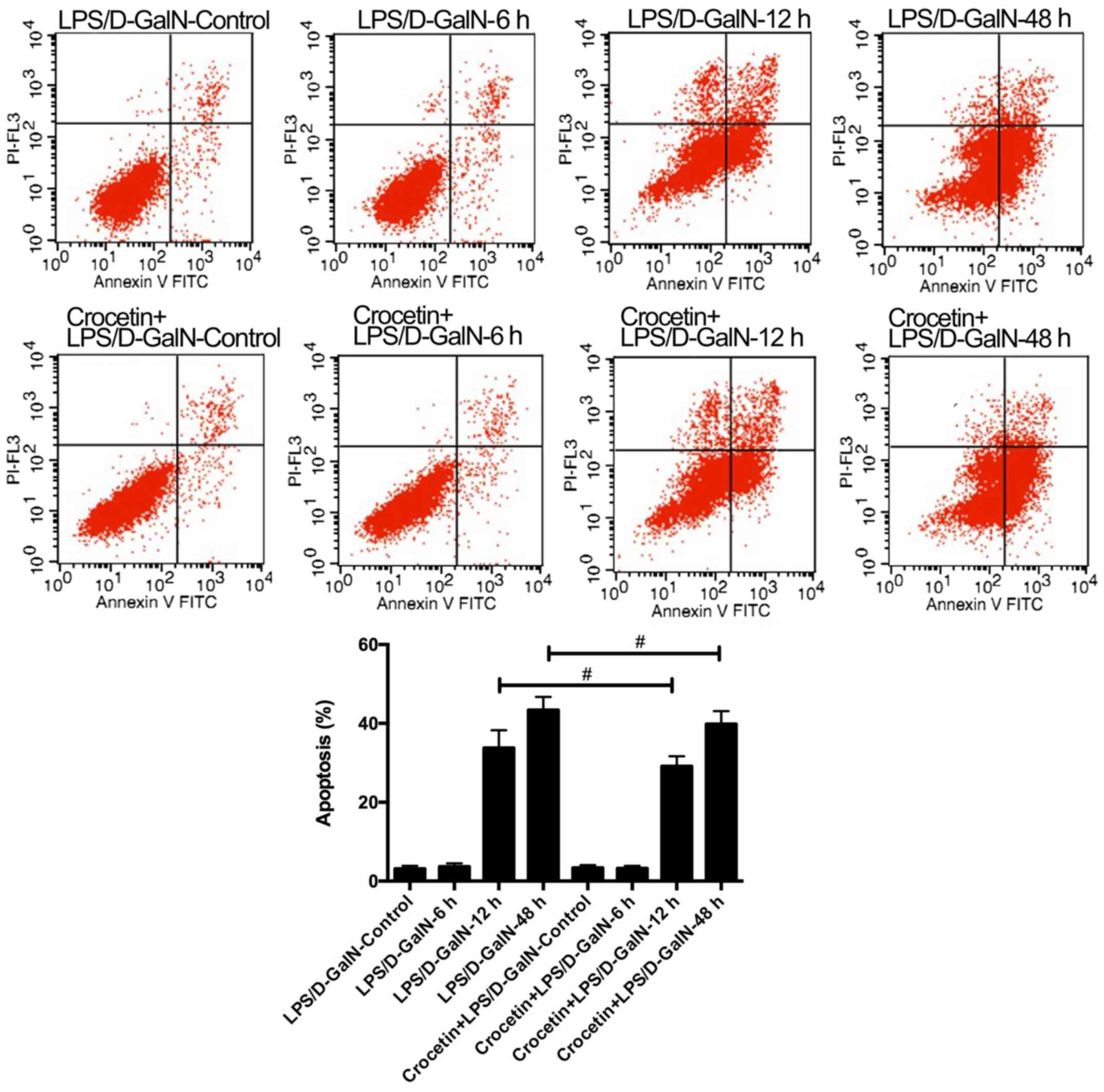
The percentage of apoptotic cells was augmented in
rats receiving LPS/D-GalN in a time-dependent manner (Fig. 2). Crocetin pre-treatment
significantly decreased cell apoptosis following LPS/D-GalN
treatment for 12 and 48 h (P<0.01; Fig. 2). To further detect the
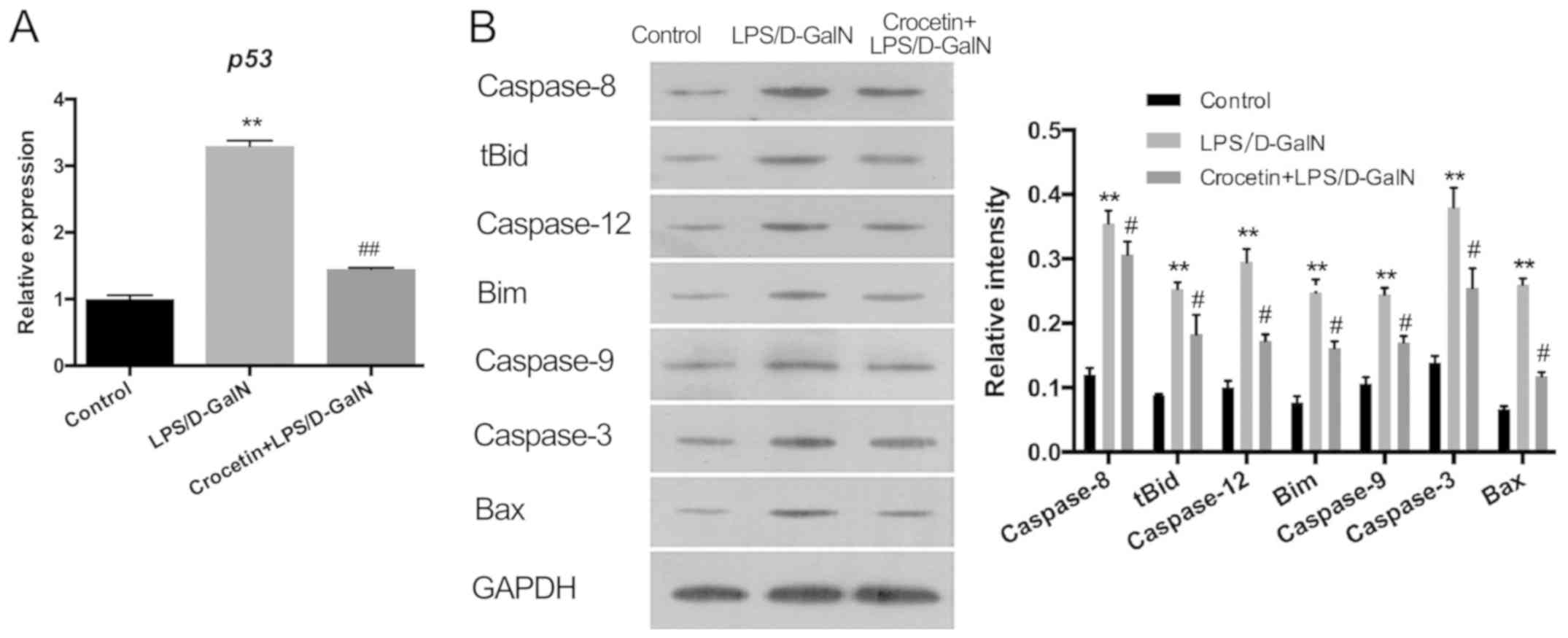
anti-apoptotic ability of crocetin, the expression levels of p53
and apoptosis-related proteins were measured in the LPS/D-GalN and
the crocetin+LPS/D-GalN groups following 48 h of stimulation
(Fig. 3). The results demonstrated
that p53 mRNA levels and the expression levels of apoptosis-related
proteins, including caspase-3, −8, −9 and −12, tBID, Bim, and BAX,
were significantly upregulated in the livers of rats following
LPS/D-GalN treatment (P<0.01). With crocetin treatment, both the
p53 level (P<0.01; Fig. 3A) and
the apoptotic protein levels (P<0.05; Fig. 3B) were significantly decreased.
Crocetin decreases LPS/D-GalN-induced
inflammation
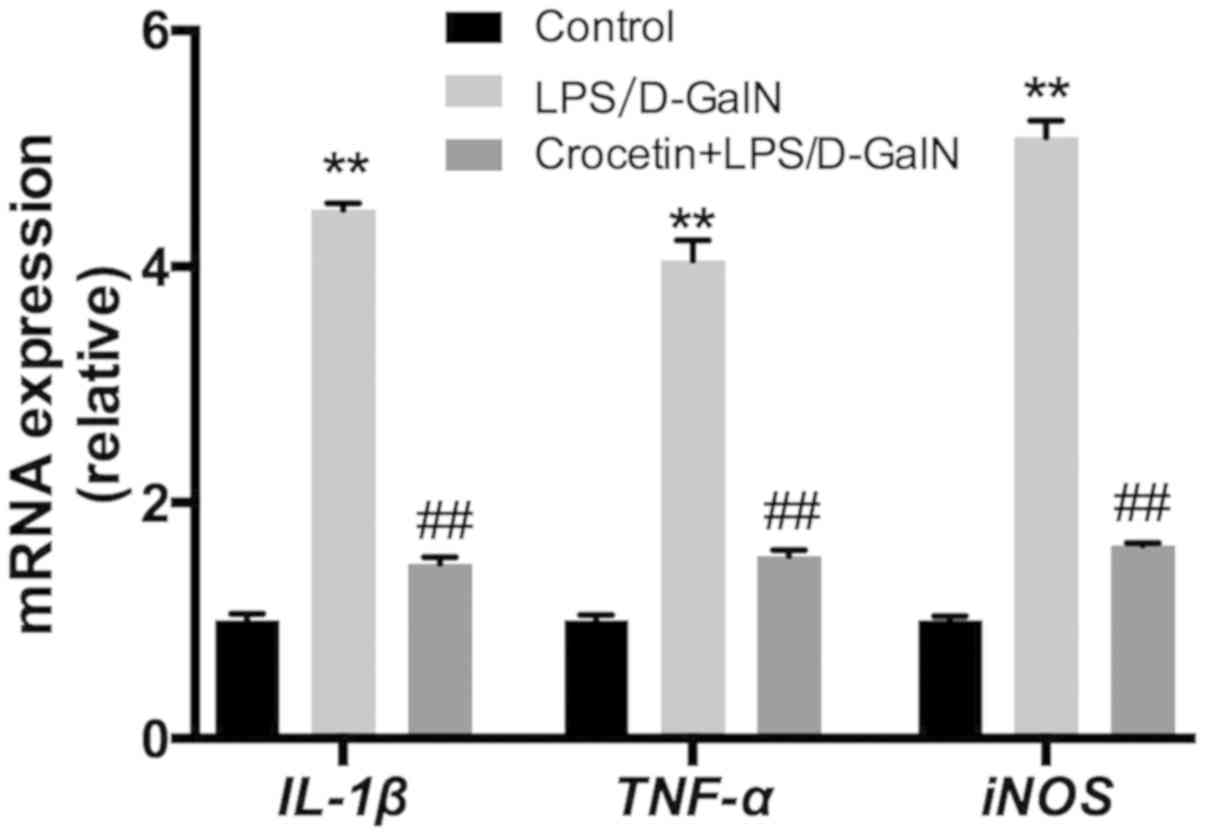
Following 48-h LPS/D-GalN treatment, the liver
inflammation level was investigated. mRNA expression levels of
IL-1β, TNF-α and inducible nitric oxide synthase (iNOS) were
significantly increased in the LPS/D-GalN treatment group
(P<0.01; Fig. 4). Conversely,
IL-1β, TNF-α and iNOS mRNA levels were significantly reduced
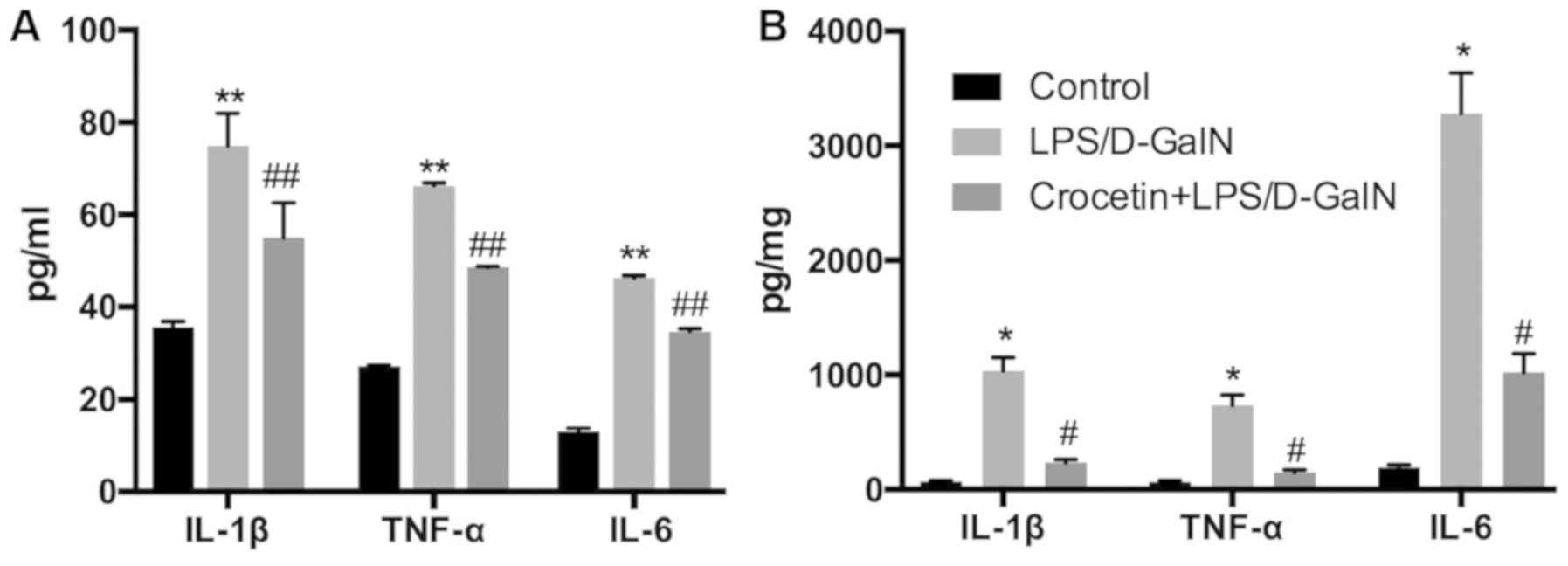
(P<0.01) in the crocetin+LPS/D-GalN treatment group (Fig. 4). ELISA data demonstrated that serum
and hepatic IL-1β, TNF-α and IL-6 concentrations were significantly
elevated in rats receiving LPS/D-GalN (P<0.05) but significantly
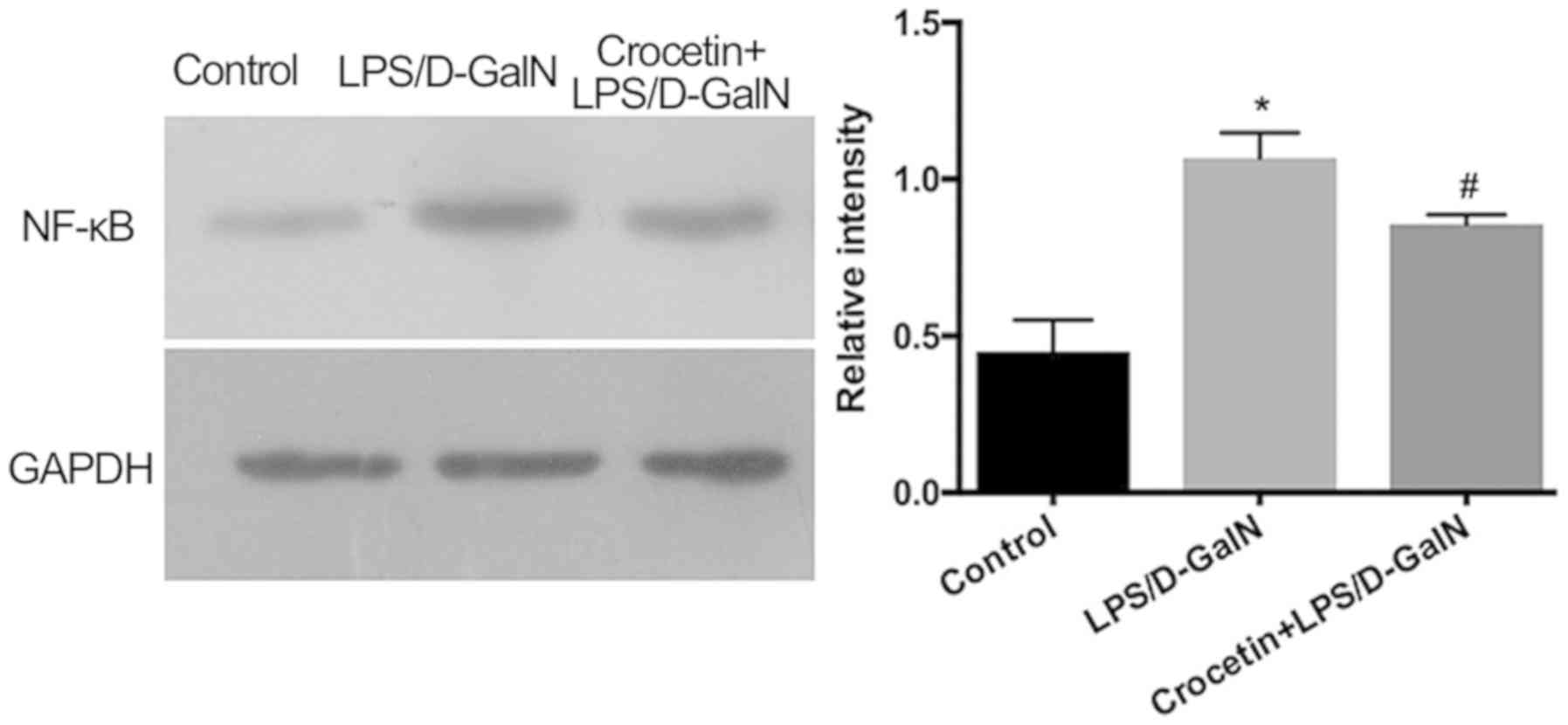
decreased by crocetin treatment (P<0.05; Fig. 5). Moreover, hepatic NF-κB expression
was significantly increased with LPS/D-GalN treatment (P<0.05)
but was significantly decreased following crocetin administration
(P<0.05; Fig. 6). The results of
the current study indicated that crocetin could decrease
LPS/D-GalN-induced hepatic inflammation.
Crocetin decreases LPS/D-GalN-induced
oxidative stress
As demonstrated in Table III, LPS/D-GalN treatment resulted
in higher malondialdehyde (MDA) levels (P<0.01) and lower
superoxide dismutase (SOD) activities in the liver (P<0.01)
compared with the control group. By contrast, compared to the
LPS/D-GalN group, crocetin significantly decreased the MDA
concentration (P<0.01) and increased the SOD activity
(P<0.05).
 | Table III.Crocetin decreases LPS/D-GalN-induced
oxidative stress. |
Table III.
Crocetin decreases LPS/D-GalN-induced
oxidative stress.
| Group | MDA, nmol/mg | SOD, U/mg |
|---|
| Control (n=6) | 15.03±1.09 | 127.32±15.71 |
| LPS/D-GalN
(n=6) |
126.39±15.26a |
63.26±6.58a |
| Crocetin+LPS/D-GalN
(n=6) |
58.37±5.18c |
82.07±8.33b |
Discussion
The LPS/D-GalN-induced animal model of FHF is
strongly relevant to human liver failure and has been widely used
to investigate the mechanisms of and potential therapeutic drugs
for clinical FHF (20). As
anticipated, the present results confirmed that LPS/D-GalN
stimulation for 6, 12 and 48 h induced severe liver damage, which
manifested as altered liver morphology, increased TBIL levels and
enhanced ALT and AST activities. The pathological condition of
severe liver injury is characterised by significant elevations of
ALT, AST and TBIL in the serum (21–24).
However, crocetin pre-treatment effectively improved the structural
integrity of parenchymal hepatocytes and decreased these
biochemical indicators for liver injury, evidencing the protective
effect of crocetin against FHF.
Apoptosis is a complicated biological process that
regulates fulminant hepatic cell division and death (14,25). In
the present study, apoptotic hepatocytes were detected using flow
cytometry. The results demonstrated that the number of apoptotic
cells was increased by LPS/D-GalN treatment in a time-dependent
manner, whereas crocetin significantly decreased the number of
apoptotic cells following LPS/D-GalN treatment for 12 and 48 h.
Furthermore, the tumour suppressor p53 is a key protein in
preventing cell transformation and tumour progression. Activated by
a variety of stimuli, p53 regulates cell cycle arrest and apoptosis
(26). In the present study, the
elevated hepatic p53 expression in rats that had received
LPS/D-GalN for 48 h was decreased following crocetin pre-treatment.
In addition, massive activation of caspases is a crucial process
during the induction of apoptosis and is associated with the
pathogenesis of acute hepatic failure (27). Caspase-8 triggers the extrinsic
apoptotic pathway, whilst caspase-9 triggers the intrinsic pathway
(28). Caspase-12 is an endoplasmic
reticulum (ER)-specific caspase that is specifically activated by
disturbances to ER homeostasis (29). Caspase-3, the predominant downstream
effector, can be activated by caspase-8, −9 and −12 (30). In addition, the Bcl-2 family proteins
BAX, Bim and tBID (the activated form of BID) translocate to the
mitochondria and mediate the permeabilization of the outer
membrane, thereby facilitating apoptosis (31,32). In
the present study, western blot analysis demonstrated that the
expression of caspase-3, −8, −9 and −12, BAX, Bim and tBID was
upregulated following LPS/D-GalN treatment, but crocetin
effectively downregulated the expression of all the pro-apoptotic
proteins. Taken together, the aforementioned findings suggested
that crocetin can attenuate apoptosis by decreasing the expression
of p53 and pro-apoptotic proteins.
Inflammation is considered to be a strongly
interrelated biological event involved in the pathogenesis of FHF
(33). Previous studies have
demonstrated that LPS/D-GalN-induced FHF was accompanied by the
release of multiple pro-inflammatory cytokines, including TNF-α,
IL-1β and IL-6, from liver cells (1,33). The
present study determined that crocetin pre-treatment effectively
decreased the expression of hepatic TNF-α and IL-1β as well as
reducing the secretion of serum TNF-α, IL-1β and IL-6 in an FHF rat
model. In addition, NO is a highly reactive oxidant produced by
parenchymal and nonparenchymal liver cells from L-arginine via the
action of iNOS (34). In animal
models, NO synthases are involved in the pathogenesis of
hyperdynamic circulation (35–38), and
the role of iNOS in causing liver damage in FHF has been
demonstrated in LPS/D-GalN-treated animals (38,39). In
the present study, increased iNOS mRNA expression was observed in
the LPS/D-GalN group, whilst crocetin treatment dramatically
downregulated the hepatic iNOS level. The expression of iNOS is
tightly regulated by the transcriptional factor NF-κB, which
promotes the secretion of cytokines including TNF-α, IL-1β and IL-6
and has a pivotal role in animals models of FHF (40,41).
Therefore, the present study analysed NF-κB expression in the
liver. In line with the cytokine level results, crocetin
pre-treatment also significantly inhibited the NF-κB level in rats
following LPS/D-GalN stimulation. Taken together, the present
findings indicated that crocetin attenuated inflammation in an FHF
rat model by decreasing the generation of cytokines by inhibiting
NF-κB.
FHF can induce oxidative stress (42). Therefore, the oxidative stress level
in the liver was analysed. MDA is a lipid peroxidation product and
SOD is a well-studied antioxidant enzyme (43). In the present study, LPS/D-GalN
treatment increased the MDA levels and decreased the SOD activity.
By contrast, crocetin pre-treatment reduced MDA generation and
elevated SOD activity. These results indicated that crocetin may
ameliorate oxidative stress caused by LPS/D-GalN.
In conclusion, the present study demonstrated the
beneficial effect of crocetin against LPS/D-GalN-induced FHF where
it decreased the secretion of pro-inflammatory cytokines via
inhibition of NF-κB, whilst also attenuating hepatocellular
apoptosis and oxidative stress. Thus, crocetin may be a potential
and promising agent for preventing FHF.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Traditional
Chinese Medicine Bureau Research Project of Guangdong Province
(grant no. 20171175) and Natural Scientific Fund of Guangdong
Province (grant no. 2018A030310479).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KG, XC and MC were responsible for performing the
experiments. QD and XZ were responsible for data analysis and
manuscript preparation. FL and HG were responsible for manuscript
writing and revision and experimental design. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Ethics Committee of The Fifth Affiliated Hospital of Southern
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
El-Agamy DS, Makled MN and Gamil NM:
Protective effects of agmatine against D-galactosamine and
lipopolysaccharide-induced fulminant hepatic failure in mice.
Inflammopharmacology. 22:187–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang X, Ding J, Gou C, Wen T, Li L, Wang
X, Yang H, Liu D, Lou J, Cehn D, et al: Qingchangligan formula
attenuates the in inflammatory response to protect the liver from
acute failure induced by d-galactosamine/lipopolysaccharide in
mice. J Ethnopharmacol. 201:108–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee SB, Kang JW, Kim SJ, Ahn J, Kim J and
Lee SM: Afzelin ameliorates Dgalactosamine and
lipopolysaccharide-induced fulminant hepatic failure by modulating
mitochondrial quality control and dynamics (150/150). Brit J
Pharmacol. 174:195–209. 2017. View Article : Google Scholar
|
|
4
|
Marzio HD and Sass DA: Fulminant hepatic
failure: Diagnosis and managementSpringer International Publishing;
Switzerland: pp. 229–245. 2014
|
|
5
|
Nasralla D, Coussios CC, Mergental H,
Akhtar MZ, Butler AJ, Ceresa CD, Chiocchia V, Dutton SJ,
Garcia-Valdecasas JC, Heaton N, et al: A randomized trial of
normothermic preservation in liver transplantation. Nature.
557:50–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duan GJ, Zhu J, Xu CY, Wan JY, Zhang L, Ge
XD, Liu LM and Liu YS: Protective effect of Go6976, a PKD
inhibitor, on LPS/d-GalN-induced acute liver injury in mice.
Inflamm Res. 6:357–366. 2011. View Article : Google Scholar
|
|
7
|
Jaeschke H: Reactive oxygen and mechanisms
of inammatory liver injury. J Gastroenterol Hepatol. 7:718–724.
2000. View Article : Google Scholar
|
|
8
|
Ben Ari Z, Avlas O, Pappo O, Veacheslav Z,
Cheporko Y, Bachmetov L, Zemel R, Asher S, Sharon E, Grief F and
Hochhauser E: Reduced hepatic injury in toll-like receptor
4-deficient mice following D-galactosamine/lipopolysaccharide
induced fulminant hepatic failure. Cell Physiol Biochem. 29:41–50.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Kim JK, Lee DU, Kwak JH and Lee
SM: Genipin protects lipopolysaccharide-induced apoptotic liver
damage in D-galactosamine-sensitized mice. Eur J Pharmacol.
635:188–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolhassani A, Khavari A and Bathaie SZ:
Saffron and natural carotenoids: Biochemical activities and
anti-tumor effects. Biochem Biophys Acta. 1845:20–30.
2014.PubMed/NCBI
|
|
11
|
Cao W, Cui J, Li S, Zhang D, Guo Y, Li Q,
Luan Y and Liu X: Crocetin restores diabetic endothelial progenitor
cell dysfunction by enhancing NO bioavailability via regulation of
PI3K/AKT-eNOS and ROS pathways. Life Sci. 181:9–16. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang X, Dong A, Wang R and Shi A:
Crocetin treatment inhibits proliferation of colon cancer cells
through down-regulation of genes involved in the inflammation.
Saudi J Biol Sci. 25:1767–1771. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang R, Vernon K, Thomas A, Morrison D,
Qureshi N and Van Way CW III: Crocetin reduces activation of
hepatic apoptotic pathways and improves survival in experimental
hemorrhagic shock. JPEN J Parenter Enteral Nutr. 35:107–113. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen P, Chen Y, Wang Y, Cai S, Deng L, Liu
J and Zhang H: Comparative evaluation of hepatoprotective
activities of geniposide, crocins and crocetin by CCl4-induced
liver injury in mice. Biomol Ther (Seoul). 24:156–162. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu LM, Zhang JX, Luo J, Guo HX, Deng H,
Chen JY and Sun SL: A role of cell apoptosis in lipopolysaccharide
(LPS)-induced nonlethal liver injury in D-galactosamine
(D-GalN)-sensitized Rats. Digest Dis Sci. 53:1316–1324. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao K, Guo H, Liu L, Ding Y, Kuang M and
Li J: Liver protection of crocetin against paraquat poisoning in
rats. Chin Crit Care Med. 28:876–880. 2016.(In Chinese).
|
|
17
|
Guo H, Gao K, Zou X, Deng Q, Chen M and
Liu F: Crocetin promotes autophagy in injured rat hepatocytes
induced by lipopolysaccharide and D-galactosamine in vitro. Nan
Fang Yi Ke Da Xue Xue Bao. 38:1121–1125. 2018.(In Chinese).
PubMed/NCBI
|
|
18
|
Xin J, Zeng D, Wang H, Ni X, Yi D, Pan K
and Jing B: Preventing non-alcoholic fatty liver disease through
Lactobacillus johnsonii BS15 by attenuating inflammation and
mitochondrial injury and improving gut environment in obese mice.
Appl Microbiol Biotechnol. 98:6817–6829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin X, Zhang S, Huang R, Tan S, Liang S,
Wu X, Zhuo L and Huang Q: Protective effect of tormentic acid from
Potentilla chinensis against lipopolysaccharide/D-galactosamine
induced fulminant hepatic failure in mice. Int Immunopharmacol.
19:365–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryan CJ, Aslam M and Courtney JM:
Transference of hepatic coma to normal rats from galactosamine
treated donors by reverse plasma exchange. Biomater Artif Cells
Artif Organs. 18:477–482. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pushpavalli G, Kalaiarasi V, Veeramani C
and Pugalendi KV: Effect of chrysin on hepatoprotective and
antioxidant status in D-galactosamine-induced hepatitis in rats.
Eur J Pharmacol. 631:36–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poojari R, Gupta S, Maru G, Khade B and
Bhagwat S: Chemopreventive and hepatoprotective effects of embelin
on N-nitrosodiethylamine and carbon tetrachloride induced
preneoplasia and toxicity in rat liver. Asian Pac J Cancer Prev.
11:1015–1020. 2010.PubMed/NCBI
|
|
24
|
Gavric A, Ribnikar M, Šmid L, Luzar B and
Stabuc B: Fat burner induced acute liver injury: Case series of
four patients. Nutrition. 47:110–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen B, Jiang L, Hao K, Wang L, Wang Y,
Xie Y, Shen J, Zhu M, Tong X, Li K and Wang Z: Protection of plasma
transfusion against lipopolysaccharide/d-galactosamine-induced
fulminant hepatic failure through inhibiting apoptosis of hepatic
cells in mice. J Zhejiang Univ Sci B. 19:436–444. 2018. View Article : Google Scholar
|
|
26
|
Giorgi C, Bonora M, Sorrentino G,
Missiroli S, Poletti F, Suski J, Galindo Ramirez F, Rizzuto R, Di
Virgilio F, Zito E, et al: p53 at the endoplasmic reticulum
regulates apoptosis in a Ca2+-dependent manner. Proc
Natl Acad Sci USA. 112:1779–1784. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leifeld L, Nattermann J, Fielenbach M,
Schmitz V, Sauerbruch T and Spengler U: Intrahepatic activation of
caspases in human fulminant hepatic failure. Liver Int. 26:872–879.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heather H, Kenneth H, Samik G and Tung KC:
Construction and analysis of a modular model of caspase activation
in apoptosis. Theor Biol Med Model. 5:262008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grosse L, Wurm CA, Brüser C, Neumann D,
Jans DC and Jakobs S: Bax assembles into large ring-like structures
remodeling the mitochondrial outer membrane in apoptosis. EMBO J.
35:402–413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan CT, Zhou QL, Su YC, Fu NY, Chang HC,
Tao RN, Sukumaran SK, Baksh S, Tan YJ, Sabapathy K, et al: MOAP-1
mediates Fas-induced apoptosis in liver by facilitating tBid
recruitment to mitochondria. Cell Rep. 16:174–185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv H, Qi Z, Wang S, Feng H, Deng X and Ci
X: Asiatic acid exhibits anti-inflammatory and antioxidant
activities against lipopolysaccharide and d-galactosamine-induced
fulminant hepatic failure. Front Immunol. 8:7852017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rockey DC and Chung JJ: Reduced nitric
oxide production by endothelial cells in cirrhotic rat liver:
Endothelial dysfunction in portal hypertension. Gastroenterology.
114:344–351. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shah V, Toruner M, Haddad F, Cadelina G,
Papapetropoulos A, Choo K, Sessa WC and Groszmann RJ: Impaired
endothelial nitric oxide synthase activity associated with enhanced
caveolin binding in experimental cirrhosis in the rat.
Gastroenterology. 117:1222–1228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Petermann H, Vogl S, Schulze E and Dargel
R: Chronic liver injury alters basal and stimulated nitric oxide
production and 3H-thymidine incorporation in cultured sinusoidal
endothelial cells from rats. J Hepatol. 31:284–292. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sass G, Koerber K, Bang R, Guehring H and
Tiegs G: Inducible nitric oxide synthase is critical for
immune-mediated liver injury in mice. J Clin Invest. 107:439–447.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang CC, Lin KJ, Cheng YW, Hsu CA, Yang
SS and Shyur LF: Hepatoprotective effect and mechanistic insights
of deoxyelephantopin, a phyto-sesquiterpene lactone, against
fulminant hepatitis. J Nutr Biochem. 3:516–530. 2013. View Article : Google Scholar
|
|
40
|
Kim YI, Park SW, Yoon YK, Lee KW, Lee JH,
Woo HJ and Kim Y: Orostachys japonicus inhibits the expression of
MMP-2 and MMP-9 mRNA and modulates the expression of iNOS and COX-2
genes in human PMA-differentiated THP-1 cells via inhibition of
NF-κB and MAPK activation. Mol Med Rep. 12:657–662. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gao K, Liu F, Guo H, Li J, Zhang Y and Mo
Z: miR-224 suppresses HBV replication posttranscriptionally through
inhibiting SIRT1-mediated autophagy. Int J Clin Exp Pathol.
11:189–198. 2018.
|
|
42
|
Reddy PV, Murthy ChR and Reddanna P:
Fulminant hepatic failure induced oxidative stress in nonsynaptic
mitochondria of cerebral cortex in rats. Neurosci Lett. 368:15–20.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Wu Y, Wang B, Xu H, Mei X, Xu X,
Zhang X, Ni J and Li W: Bacillus amyloliquefaciens SC06
protects mice against high-fat diet-induced obesity and liver
injury via regulating host metabolism and gut microbiota. Front
Microbiol. 10:11612019. View Article : Google Scholar : PubMed/NCBI
|