Introduction
Lower back pain (LBP) is the most common pain
syndrome that can cause disability (1,2).
Pathologies of the lumbar intervertebral discs, lumbar facet joints
(LFJs) and the sacroiliac joint are potential sources of chronic
LBP (3,4). Disorders of the facet joints represent
a common source of LBP. In previous studies, the prevalence of
chronic LBP caused by pathologies of the facet joints was reported
to be ~30%, which increased with age as LBP exhibits similar
characteristics with arthritis (5–8).
Osteoarthritis of the LFJs due to degenerative change(s) is
considered to be the main cause of facet joint-origin LBP (9). Degenerative changes in LFJs can lead to
abnormal stress and strain, resulting in increased load on the
facet joint (10). Additionally,
increased mechanical load and subsequent inflammation activate
nociceptors within and surrounding the joints, which further
exacerbate facet joint-origin LBP (10–12).
To manage facet joint-origin pain in clinical
practice, intra-articular (IA) LFJ corticosteroid injections are
widely and conventionally used, and the positive effect of this
treatment has been reported in a number of previous studies
(13–20). The effect of IA LFJ corticosteroid
injection may be different according to the degree of LFJ
degeneration. The prediction of treatment outcome after IA LFJ
corticosteroid injection may therefore enable clinicians to apply a
more appropriate therapeutic method to patients with LBP. However,
little is known about the treatment outcomes of IA LFJ
corticosteroid injections according to the severity of facet joint
osteoarthritis (FJOA). Therefore, the present study aimed to
evaluate whether the severity of FJOA affects IA LFJ corticosteroid
injection treatment.
Patients and methods
Patients
The current retrospective study recruited 50
patients who visited the spinal center of Yeungnam University
Hospital between March 2014 and Aug 2018 for the management of LFJ
pain and received IA LFJ corticosteroid injection treatment
(Table I). Patients were included
based on the following criteria: (i) ≥6-month history of axial LBP
without radicular symptoms (duration of pain was examined by asking
patients directly), (ii) aged between 20 and 79 years, (iii) local
paraspinalis tenderness with increased pain upon hyperextension,
rotation or lateral bending of the lower lumbar spine (iv) ≥80%
temporary pain relief following a diagnostic block with IA
injection of 0.5 ml of 1% lidocaine (temporary pain relief,
assessed using a numerical rating scale (NRS) (21), was evaluated using a survey that the
patients filled out) and (v) failure to respond to medication
(meloxicam 15 mg and/or acetaminophen/tramadol hydrochloride
325/37.5 mg) or physical therapy prior to IA LFJ corticosteroid
injection (LBP ≥4 on a NRS). Each patient underwent a lumbar spine
MRI. Patients with disc herniation, lumbar spinal stenosis, spinal
instability, coagulopathy, allergy to iodinated contrast, rheumatic
disorders or any uncontrolled medical or psychiatric conditions
were excluded from the current study. The present study was
approved by the Ethics Committee of Yeungnam University
Hospital.
 | Table I.Demographic characteristics and
baseline clinical data of the patients included in the current
study. Data regarding age and pain duration are presented as mean ±
standard deviation. |
Table I.
Demographic characteristics and
baseline clinical data of the patients included in the current
study. Data regarding age and pain duration are presented as mean ±
standard deviation.
|
| Group |
|
|---|
|
|
|
|
|---|
| Variable | A (n=10) | B (n=27) | C (n=13) | Total (n=50) | P-value |
|---|
| Age (years) | 66.0±11.0 | 62.4±11.8 | 68.5±7.8 | 64.7±10.9 | 0.229 |
| NRS
(pre-treatment) | 4.3±0.8 | 4.4±1.1 | 4.5±1.2 | 4.4±1.1 | 0.874 |
| Pain duration
(months) | 15.8±10.2 | 20.6±20.5 | 18.1±14.4 | 19.0±17.2 | 0.740 |
| Sex, n (%) |
|
|
|
|
|
| Male | 5 (50.0) | 12 (44.4) | 6 (46.2) | 23 (46.0) | 0.956 |
|
Female | 5 (50.0) | 15 (55.6) | 7 (53.9) | 27 (54.0) |
|
| Successful pain
relief, n (%) |
|
Failure | 4 (40.0) | 13 (48.2) | 7 (53.9) | 24 (48.0) | 0.805 |
|
Success | 6 (60.0) | 14 (51.9) | 6 (46.2) | 26 (52.0) |
|
The 50 patients recruited into the current study
were classified into three subgroups according to the results of
routinely performed lumbar axial MRI, and based on the study by
Maataoui et al (22)
(Fig. 1): Group A, the lumbar MRI
revealed narrowing of the LFJ space and the presence of small
osteophytes (mean age, 66.0±11.0 years; 5 males, 5 females); Group
B, the MRI revealed narrowing of the joint space, moderate
osteophytes and/or subchondral erosions (mean age, 62.4±11.8 years;
12 males, 15 females); Group C, the MRI revealed narrowing of the
joint space and the presence of large osteophytes and subchondral
erosion/cysts (mean age, 68.5±7.7 years; 6 males, 7 females).
Procedures
Treatment was administered using a posterior
approach, where patients were placed the prone position for C-arm
fluoroscopy (Siemens Healthineers; Table II) and a cushion was placed below
the lower abdomen to straighten the lumbar spine. The C-arm tube
was angled cephalad and rotated until it was at a tangent to the
LFJ space. Under C-arm fluoroscopy, after confirming IA access by
injecting 0.3 ml of contrast into the LFJ space, 10 mg (0.25 ml) of
dexamethasone mixed with 0.25 ml of 0.125% bupivacaine was injected
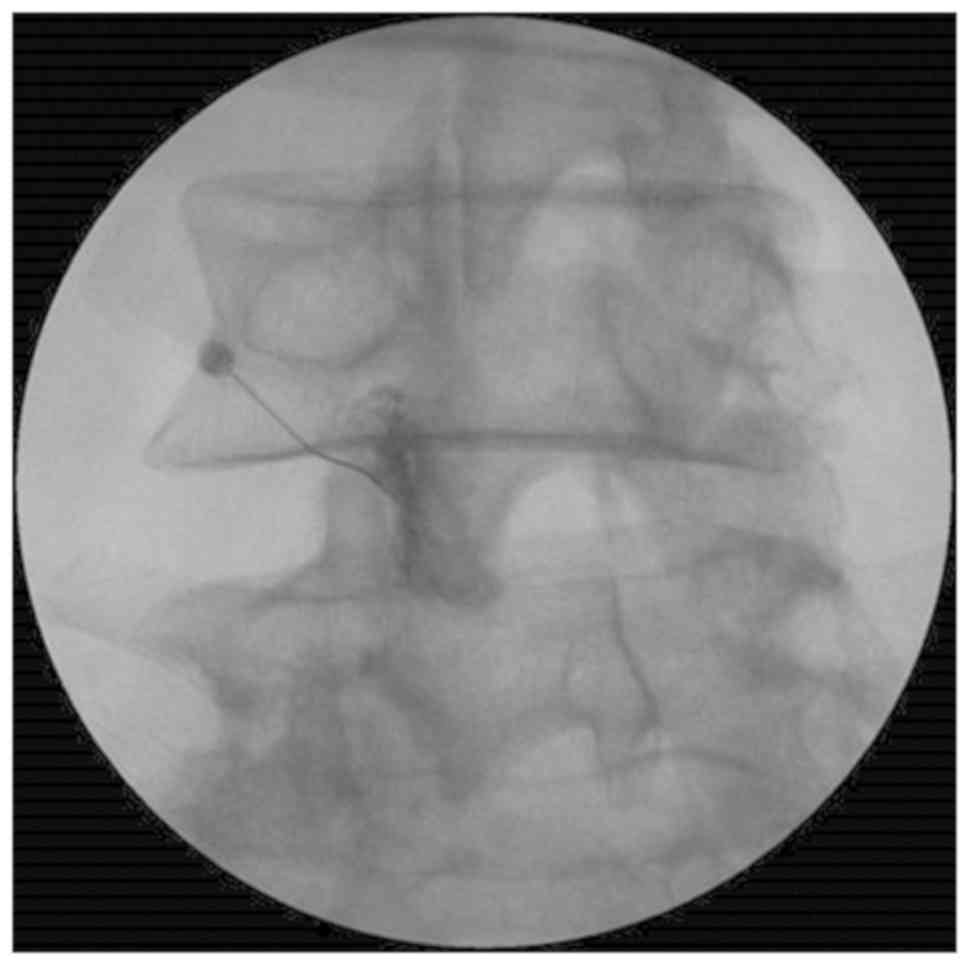
using a 26-gauge and a 90 mm spinal needle (Fig. 2). The IA LFJ corticosteroid injection
was administered once for each patient. During the follow-up
period, which occurred for a duration of 3 months, all the
recruited patients received no additional oral medication or
physical therapy.
 | Table II.Treated facet joint level of each
group. |
Table II.
Treated facet joint level of each
group.
| Lumbar level, n
(%) | Group A | Group B | Group C |
|---|
| L3/4 | 2 (20.0) | 3
(11.1) | 2 (15.4) |
| L4/5 | 5 (50.0) | 16 (59.3) | 6 (46.2) |
| L5/S1 | 3 (30.0) | 8
(29.6) | 5 (38.4) |
Outcome measures
Pain intensity was assessed using an NRS, which was
administered prior to treatment and at 1, 2 and 3 months after IA
LFJ corticosteroid injection. NRS score was measured once for each
follow-up. Successful treatment was defined as a >50% reduction
in NRS score at 3 months compared with the pre-treatment NRS score.
To validate changes in pain reduction, NRS scores were evaluated by
assessing the difference between the pretreatment and the 3 month
follow-up treatment NRS scores [change in NRS (%) =(pretreatment
score-score at 3 months after treatment)/pretreatment score
×100].
Statistical analysis
The demographic characteristics and baseline
clinical variables were summarized using descriptive analysis.
Values of quantitative variables are presented as the mean ±
standard deviation, while qualitative variables are presented as
frequencies and percentages. Demographic data and successful pain
relief were compared among the three groups using the chi-squared
test for qualitative variables and one-way ANOVA for quantitative
variables. A multiple comparisons test was performed using the
Scheffe method. Analysis of transition aspect for NRS scores in all
recruited patients, by time, was performed using a repeated
measures ANOVA. The analysis of transition aspect for NRS scores by
time, group and interaction effects (time differences by group) was
performed using a repeated measure two (time and factor) factor
analysis. Multiple comparisons were performed by contrast with
Bonferroni's correction. P<0.05 was considered to indicate a
statistically significant difference. All tests were two-sided and
data were analyzed using SPSS version 22.0 (IBM Corp.).
Results
NRS scores were significantly
decreased at the follow-up evaluations of each patient
All patients completed the study and no adverse
effects were observed from treatment. No statistically significant
differences were observed in demographic data among groups
(Table I). Among all patients, mean
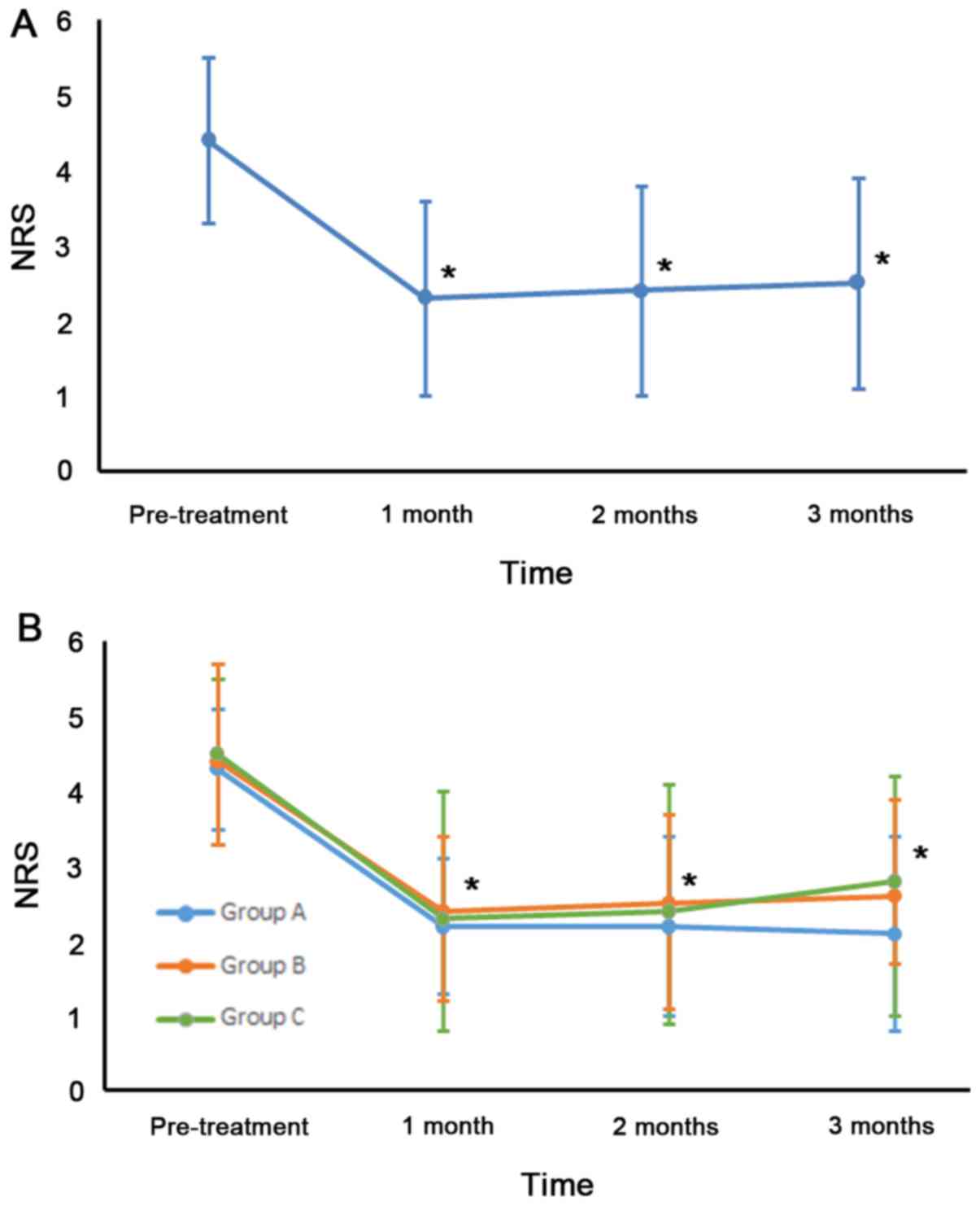
NRS scores were significantly reduced as follows: Pretreatment NRS,
4.4±1.1; NRS at 1 month, 2.3±1.3; NRS at 2 months, 2.4±1.4 and NRS
at 3 months, 2.5±1.4 (Fig. 3A). NRS
scores changed significantly over time (P<0.001). Compared with
pretreatment, the NRS scores at each evaluation time point were
significantly lower (P<0.001). A total of 26 (52%) patients
experienced successful pain relief (≥50%) after IA LFJ
corticosteroid injection (Table
I).
All groups exhibited a significant
decrease in NRS score at each follow-up evaluation
In group A, the mean NRS score significantly
decreased after treatment. The pretreatment NRS score was 4.3±0.8.
At 1 month, the mean NRS was 2.2±0.9, at 2 months, 2.2±1.2 and at 3
months, 2.1±1.3 (Fig. 3B). In group
B, the mean NRS decreased from 4.4±1.1 before treatment to 2.4±1.6
at 1 month, to 2.5±1.6 at 2 months, and to 2.6±1.6 at 3 months
after treatment. In group C, the mean NRS was 4.5±1.2 before
treatment, 2.3±1.1 at 1 month, 2.4±1.3 at 2 months, and 2.8±1.1 at
3 months after treatment. Among the three groups, the NRS scores at
1, 2 and 3 months were significantly lower than the pretreatment
scores (P<0.001). NRS scores from pretreatment to each
evaluation time point were significantly lower in all three groups
(P<0.001). However, changes in NRS scores were not significantly
different between groups (P=0.889). A period of 3 months after
treatment, 6 (60.0%) patients in group A, 14 (51.9%) in group B and
6 (46.2%) in group C reported successful pain relief (≥50%). The
rates of successful pain relief at 3 months after treatment were
not significantly different among the three groups (P=0.805;
Table I).
Discussion
In the present study, the clinical effects of IA LFJ
corticosteroid injection were evaluated and the effects of
injection were compared according to the severity of FJOA. The
results demonstrated that pain severity, which was measured via the
NRS scoring system, was significantly decreased following IA LFJ
corticosteroid injection in all three groups, which were classified
according to the severity of FJOA. Furthermore, the pain-reducing
effect of IA LFJ corticosteroid injection was not significantly
different among the three groups.
FJOA-associated LBP may originate from inflammation
within the area surrounding the LFJ (23,24). The
anti-inflammatory properties of injected corticosteroids block the
production and release of inflammatory mediators, consequently
inhibiting processes associated with inflammation (25). Furthermore, corticosteroids inhibit
neural transmission within nociceptive C-fibers (26). The significant pain reduction
observed in the current study appears to have been induced by the
effects of the corticosteroid. Additionally, injection of
bupivacaine in patients may block pain transmission in the synovial
lining within nociceptive C-fibers and reduce ectopic discharge
(27). Therefore, bupivacaine, at
least in part, may have contributed to the extensive pain relief
reported in the current study. The results of the current study
were contrary to the expectation that the effect of IA LFJ
injection would be diminished in patients with severe FJOA.
Although some patients exhibited severe FJOA, there was no
statistically significant difference in the effect of injection
between patients with severe FJOA and patients with non-severe
FJOA. The corticosteroid can be suggested to exhibit
anti-inflammatory and analgesic effects that are sufficient to
alleviate all LFJ-origin LBP, regardless of the severity of FJOA
(28). Bupivacaine has also been
known to block transmissions in nociceptive C-fibers (27). Additionally, structures, including
lumbar paraspinalis muscles, paraspinal ligament and intervertebral
discs, may prevent or mitigate the severe mechanical loading on the
LFJ.
In the current study, the degree of pre-treatment
facet joint-origin pain was not significantly different among the
three groups. However, the present study included a relatively
small number of patients. Additionally, to the best of our
knowledge, no previous study has reported the association between
the level of pain and structural changes of the facet joint. Suri
et al (29) indicated that
severe FJOA is more often observed in patients exhibiting back pain
than in those without back pain. However, the aforementioned study
did not evaluate the degree of pain according to severity of FJOA.
Therefore, for this to be confirmed, further studies are
required.
To the best of our knowledge, a total of four
previous studies have reported the effects of IA LFJ corticosteroid
injection in controlling LFJ-origin pain (30–33). In
2011, Kawu et al (30)
performed IA LFJ corticosteroid injection treatment in 10 patients,
90% of whom experienced a pain reduction of >50% according to
visual analogue scale (VAS) scores 6 months after treatment. In the
same year, Celik et al (31)
performed IA LFJ corticosteroid injection treatment in 40 patients,
with the mean VAS scores being reduced by ~75% of pre-treatment
scores 6 months after treatment. In 2013, Ribeiro et al
(32) performed IA LFJ
corticosteroid injection treatment in 31 patients, who exhibited a
~50% reduction in mean VAS of pre-treatment scores 3 months after
treatment. In 2017, Do et al (33) performed IA LFJ corticosteroid
injection treatment in 30 patients and reported that the mean NRS
score was reduced from 5 at pretreatment to 3.2 at 6 months
following injection. In the current study, patients were classified
into 3 groups according to lumbar axial MRI results. Consistent
with the results of previous studies, a significant decrease was
observed in mean NRS scores, in all groups, after IA LFJ
corticosteroid injection (13–20).
Additionally, the current study is, to the best of our knowledge,
the first to evaluate and compare treatment outcomes of IA LFJ
corticosteroid injection according to the severity of FJOA.
In conclusion, the results of the current study
demonstrated that the IA LFJ corticosteroid injection treatment was
effective, regardless of FJOA severity. I IA LFJ corticosteroid
injections may be a useful clinical option for the management of
FJOA-mediated LBP. However, the present study had certain
limitations, including its retrospective design and relatively
small sample size. Additionally, the long-term effect of IA LFJ
corticosteroid injection were not evaluated. Therefore, further
studies are required to overcome these limitations and support the
results of the present study.
Acknowledgements
Not applicable.
Funding
The present study was supported by a National
Research Foundation of Korea grant funded by the Korean government
(grant no. NRF-2019R1F1A1061348).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DGK and MCC performed wrote the manuscript and
acquired the data. SGK and AYL designed the current study, analyzed
the data and revised manuscript. MCC supervised the study and
conducted critical revision of manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yeungnam University Hospital, and written informed
consent was obtained from all patients.
Patient consent for publication
All participants provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LBP
|
low back pain
|
|
LFJ
|
lumbar facet joint
|
|
IA
|
intra-articular
|
|
FJOA
|
facet joint osteoarthritis
|
|
NRS
|
numerical rating scale
|
|
VAS
|
visual analogue scale
|
References
|
1
|
Vekaria R, Bhatt R, Ellard DR, Henschke N,
Underwood M and Sandhu H: Intra-articular facet joint injections
for low back pain: A systemic review. Eur Spine J. 25:1266–1281.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perolat R, Kastler A, Nicot B, Pellat JM,
Tahon F, Attye A, Heck O, Boubagra K, Grand S and Krainik A: Facet
joint syndrome: From diagnosis to interventional management.
Insights Imaging. 9:773–789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwarzer AC, Aprill CN, Derby R, Fortin
J, Kine G and Bogduk N: Clinical features of patients with pain
stemming from the lumbar zygapophysial joints. Is the lumbar facet
syndrome a clinical entity? Spine (Phila Pa 1976). 19:1132–1137.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bogduk N: The anatomical basis for spinal
pain syndromes. J Manipulative Physiol Ther. 18:603–605.
1995.PubMed/NCBI
|
|
5
|
Ashton IK, Ashton BA, Gibson SJ, Polak JM,
Jaffray DC and Eisenstein SM: Morphological basis for back pain:
The demonstration of nerve fibers and neuropeptides in the lumbar
facet joint capsule but not in ligamentum flavum. J Orthop Res.
10:72–78. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuslich SD, Ulstrom CL and Michael CJ: The
tissue origin of low back pain and sciatica: A report of pain
response to tissue stimulation during operations on the lumbar
spine using local anesthesia. Orthop Clin North Am. 22:181–187.
1991.PubMed/NCBI
|
|
7
|
Manchikanti L, Boswell MV, Singh V,
Pampati V, Damron KS and Beyer CD: Prevalence of facet joint from
in chronic spinal pain of cervical, thoracic, and lumbar regions.
BMC Musculoskelet Disord. 5:152004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marks R: Distribution of pain provoked
from lumbar facet joints and related structures during diagnostic
spinal infiltration. Pain. 39:37–40. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eubanks JD, Lee MJ, Cassinelli E and Ahn
NU: Prevalence of lumbar facet arthrosis and its relationship to
age, sex, and race: An anatomic study of cadaveric specimens. Spine
(Phila Pa 1976). 32:2058–2062. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gellhorn AC, Katz JN and Suri P:
Osteoarthritis of the spine: The facet joints. Nat Rev Rheumatol.
9:216–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lawrence RC: Estimates of the prevalence
of arthritis and selected musculoskeletal disordersin the United
States. Arthritis Rheum. 16:427–441. 1989.
|
|
12
|
Kang YM, Choi WS and Pickar JG:
Electrophysiologic evidence for an intersegmental reflex pathway
between lumbar paraspinal tissues. Spine (Phila Pa 1976).
27:E56–E63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cavanaugh JM, Ozaktay AC, Yamashita T,
Avramov A, Getchell TV and King AI: Mechanisms of low back pain: A
neurophysiologic and neuroanatomic study. Clin Orthop Relat Res.
166–180. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cohen SP, Huang JH and Brummett C: Facet
joint pain-advances in patient selection and treatment. Nat Rev
Rheumatol. 9:101–116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bellamy N, Campbell J, Robinson V, Gee T,
Bourne R and Wells G: Intraarticular corticosteroid for treatment
of osteoarthritis of the knee. Cochrane Database Syst Rev.
19:CD0053282006.
|
|
16
|
Buchbinder R, Green S and Youd JM:
Corticosteroid injections for shoulder pain. Cochrane Database Syst
Rev. CD0040162003.PubMed/NCBI
|
|
17
|
Machado E, Bonotto D and Cunali PA:
Intra-articular injections with corticosteroids and sodium
hyaluronate for treating temporomandibular joint disorders: A
systemic review. Dental Press J Orthod. 18:128–133. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carette S, Marcoux S, Truchon R, Grondin
C, Gagnon J, Allard Y and Latulippe M: A controlled trial of
corticosteroid injections into facet joints for chronic low back
pain. N Engl J Med. 325:1002–1007. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schulte TL, Pietilä TA, Heidenreich J,
Brock M and Stendel R: Injection therapy of lumbar facet syndrome:
A prospective study. Acta Neurochir (Wien). 148:1165–1172. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manchikanti L, Manchikanti KN, Manchukonda
R, Cash KA, Damron KS, Pampati V and McManus CD: Evaluation of
lumbar facet joint nerve blocks in the management of chronic low
back pain: A preliminary report of a randomized, double-blind
controlled trial: Clinical trial NCT00355914. Pain Physician.
10:425–440. 2007.PubMed/NCBI
|
|
21
|
Farrar JT, Young JP Jr, LaMoreaux L, Werth
JL and Poole RM: Clinical importance of changes in chronic pain
intensity measured on an 11-point numerical pain rating scale.
Pain. 94:149–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maataoui A, Voql TJ, Middendorp M,
Kafchitsas K and Khan MF: Association between facet joint
osteoarthritis and the Oswestry Disability Index. World J Radiol.
6:881–885. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Groen GJ, Baljet B and Drukker J: Nerves
and nerve plexuses of the human vertebral column. Am J Anat.
188:282–296. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C, Lu Y, Kallakuri S, Patwardhan A
and Cavanaugh JM: Distribution of A-delta and C-fiber receptors in
the cervical facet joint capsule and their response to stretch. J
Bone Joint Surg Am. 88:1807–1816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee DG, Ahn SH and Lee J: Comparative
effectiveness of pulsed radiofrequency and transforaminal steroid
injection for radicular pain due to disc herniation: A prospective
randomized trial. J Korean Med Sci. 31:1324–1330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olmarker K, Byröd G, Cornefjord M,
Nordborg C and Rydevik B: Effects of methylprednisolone on nucleus
pulposus-induced nerve root injury. Spine (Phila Pa 1976).
19:1803–1808. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee DG, Cho YW, Cho KH and Chang MC:
Management of refractory sciatic neuropathic pain using
ultrasound-guided pulsed radiofrequency. J Back Musculoskelet
Rehabil. 30:1141–1145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barnes PJ: Anti-inflammatory actions of
glucocorticoids: Molecular mechanisms. Clin Sci (Lond). 94:557–572.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suri P, Hunter DJ, Rainville J, Guermazi A
and Katz JN: Presence and extent of severe facet joint
osteoarthritis are associated with back pain in older adults.
Osteoarthritis Cartilage. 21:1199–1206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawu AA, Olawepo A and Salami AO: Facet
joints infiltration: A viable alternative treatment to
physiotherapy in patients with low back pain due to facet joint
arthropathy. Niger J Clin Pract. 14:219–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Celik B, Er U, Simsek S, Altug T and
Bavbek M: Effectiveness of lumbar zygapophysial joint blockage for
low back pain. Turk Neurosurg. 21:467–470. 2011.PubMed/NCBI
|
|
32
|
Ribeiro LH, Furtado RN, Konai MS, Andero
AB, Rosenfeld A and Natour J: Effect of facet joint injection
versus systemic steroids in low back pain: A randomized controlled
trial. Spine (Phila Pa 1976). 38:1995–2002. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Do KH, Ahn SH, Cho YW and Chang MC:
Comparison of intra-articular lumbar facet joint pulsed
radiofrequency and intra-articular lumbar facet joint
corticosteroid injection for management of lumbar facet joint pain:
A randomized controlled trial. Medicine (Baltimore). 96:e65242017.
View Article : Google Scholar : PubMed/NCBI
|