Introduction
Colorectal cancer (CRC) is the third most common
malignancy worldwide and considered to be a major cause of
cancer-associated mortality (1,2). Despite
the rapid development of diagnostic and treatment methods, the
5-year survival rate of CRC remains poor, mostly due to recurrence
and metastasis. A number of genetic factors and factors that affect
the tumor microenvironment have been determined as key risk factors
for CRC, including gene mutations, poor dietary habits, obesity and
chronic intestinal inflammation. However, the exact mechanisms
leading to the initiation and development of colon cancer remain
unclear, and considerable scientific interest has been focused on
the molecular pathogenesis of CRC.
Emerging evidence has indicated that inflammation is
associated with tumor initiation and development, notably CRC
(3–6). In addition, epidemiological studies
have demonstrated that the development of CRC is closely associated
with chronic inflammation (7). The
inflammatory microenvironment facilitates tumorigenesis by a series
of dynamic and reciprocal interactions between inflammatory and
tumor cells (8). In this regulatory
process, nuclear factor-κB (NF-κB) is regarded as a critical
regulator that can promote the initiation and amplification of
inflammation (9). The activation of
NF-κB induces p65/p50 secretion into the nucleus, which regulates
the expression of target genes (10), such as the inflammation-associated
factors interleukin-1β (IL-1β), IL-6 and tumor necrosis factor-α
(TNF-α). Consequently, NF-κB activation may link inflammation to
tumor initiation and promotion by regulating the target genes of
NF-κB.
MicroRNAs (miRNAs) are a class of small non-coding
RNAs, approximately 17–24 nucleotides in length, which are involved
in several pathological and physiological processes by regulating
target gene expression. Recently, increasing evidence has confirmed
that miRNAs may serve vital roles in regulating pro-inflammatory
factor-induced initiation and progression of cancer (11–13).
Accumulating evidence has indicated that reduced expression of
miR-139-5p is a common characteristic of several types of cancer,
including CRC (14–16). It was also reported that miR-139-5p
was associated with abnormal inflammation in the animal models of
dextran sulfate salt-induced colon cancer and experimental colitis
(17). However, the association
between miR-139-5p and inflammation in CRC has not yet been fully
clarified.
In the present study, it was demonstrated that
overexpression of miR-139-5p was able to inhibit CRC cell
proliferation and invasion. Furthermore, it was also observed that
the mRNA and protein levels of the inflammatory cytokines IL-1β,
IL-6 and TNF-α were significantly decreased by reduced NF-κB
activity as a result of miR-139-5p overexpression. The results
indicated that miR-139-5p regulated chronic inflammation by
suppressing NF-κB activity in order to inhibit cell proliferation
and invasion in CRC. These findings undoubtedly illustrated a novel
molecular mechanism that may be applied in CRC therapeutics.
Materials and methods
Cell culture
The human CRC cell lines SW480, HT29, HCT-8, LoVo
and HCT116 were purchased from the American Type Culture Collection
(Manassas, VA, USA), whereas the human normal colon mucosal
epithelial cell line NCM460 was obtained from INCELL Corporation
LLC (San Antonio, TX, USA). The cell culture media of McCoy's 5A,
RPMI-1640, F12-K, Leibovitz's L-15 and DMEM were purchased from
Gibco (Thermo Fisher Scientific, Inc.). HCT116 and HT29 cells were
cultured in McCoy's 5A medium. HCT-8, LoVo, SW480 and NCM460 cells
were maintained in RPMI-1640, F12-K, Leibovitz's L-15 and DMEM,
respectively. All cell culture media aforementioned were
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and streptomycin. The cells
were incubated under 5% CO2 at 37°C.
miRNA transfection
Human hsa-miR-139-5p mimics (micrON™ hsa-miR-139-5p
mimics; cat. no. miR10000250-1-5) and miRNA negative control (NC)
mimics (micrON™ mimics Negative Control #24; cat. no. miR01201-1-5)
were synthesized by Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
The cells were respectively seeded in 24-well plates at a density
of 5×104 cells/well and then transfected with 60 nM
miR-139-5p mimics or NC mimics at 60% confluence using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol. The cells
transfected with the miR-139-5p or NC mimics were harvested at 48 h
post-transfection.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cell pellets using
the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol and subsequently quantified
using NanoDrop™ 2000 machine (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. For miRNA detection, the
TaqMan MicroRNA assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used according to the protocol supplied by
the manufacturer. The expression level of miR-139-5p was normalized
to that of the internal control, U6. For mRNA detection, the
synthesis of cDNA was performed using the PrimerScript™ RT Reagent
kit (Takara Bio Inc.) and qPCR was conducted by the FastStart
Universal SYBR Green Master kit (Roche Diagnostics), according to
the manufacturer's protocol. The mRNA expression levels of IL-6,
IL-1β and TNF-α were normalized to that of GAPDH, which was used as
an internal control. The primer sequences were listed in Table I. The thermal cycling conditions for
PCR were: Initial denaturation at 95°C for 15 min, followed by 35
cycles of denaturing at 95°C for 15 sec, annealing at 55°C for 30
sec and elongation at 72°C for 30 sec. The results of the detection
were calculated using the 2−ΔΔCq analysis method
(18).
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
|
| Primer sequences
(5′-3′) |
|---|
|
|
|
|---|
| Gene name | Forward | Reverse |
|---|
| miR-139-5p |
ACACTCCAGCTGGGTCTACAGTGCACGTG |
CTCAACTGGTGTCGTGGA |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCCGT |
| IL-6 |
ACTCACCTCTTCAGAACGAATTG |
CCATCTTTGGAAGGTTCAGGTTG |
| IL-1β |
TTCGACACATGGGATAACGAGG |
TTTTTGCTGTGAGTCCCGGAG |
| TNF-α |
CCTCTCTCTAATCAGCCCTCTG |
GAGGACCTGGGAGTAGATGAG |
| GAPDH |
GGAGCGAGATCCCTCCAAAAT |
GGCTGTTGTCATACTTCTCATGG |
Cell proliferation assay
The effect of miR-139-5p on cell proliferation was
evaluated using the Cell Counting Kit-8 (CCK-8; Sangon Biotech Co.,
Ltd.). Briefly, cells transfected with the miR-139-5p or NC mimics
were plated into the 96-well plates (2×103 cells/well),
and 10 µl CCK-8 solution was added to each well following culturing
for 24, 48 and 72 h. The plate was then incubated at 37°C for 4 h,
and cell proliferation was evaluated by measuring the absorbance at
450 nm using Multiskan MS (Thermo Fisher Scientific, Inc.). A total
of three wells were used in each group, and the experiments were
repeated three times independently. The results are expressed as
the mean values ± standard deviation (SD).
Colony formation assay
The cells were seeded in a 24-well plate
(5×104 cells/well) and transfected with miR-139-5p or NC
mimics. After 48 h of incubation, the cells were collected and
seeded (1,000-1,500 cells/well) into a fresh 6-well plate for 10
days. The surviving colonies were counted following fixation with
methanol/acetone (1:1) and were finally stained with 0.5% methylene
blue (MedChemExpress). The experiments were repeated three
times.
Cell invasion assay
Cell invasion was examined by a Matrigel invasion
assay (BD Biosciences). In brief, the cells were transfected with
the miR-139-5p or NC mimics for 48 h, and then harvested and
suspended in serum-free medium. A total of 1×105 cells
were diluted in 500 µl serum-free medium and added to the upper
chamber of the Transwell apparatus (Corning Inc.) that was
precoated with 1 mg/ml Matrigel (BD Biosciences) for 2 h at 37°C
Subsequently, 0.6 ml serum-free media with 10 ng/ml hepatocyte
growth factor was added to the lower chamber. After 48 h of
incubation, the cells that invaded through the Matrigel membrane
were fixed with 4% paraformaldehyde at room temperature for 30 min,
stained with 0.5% crystal violet at room temperature for 30 min and
counted in five high-power fields (magnification, ×200). A total of
three independent experiments were conducted.
Western blot analysis
The total protein was extracted from the cells using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) with proteinase inhibitor cocktail (Roche
Diagnostics). Subsequently, the protein concentration was
quantified using the BCA Protein Assay Reagent kit (Thermo Fisher
Scientific, Inc,) and ~30 µg total protein was then separated by
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to a polyvinylidene difluoride membrane (EMD Millipore;
Merck KGaA). Non-specific binding was blocked by incubating the
membranes in 5% skim milk for 1 h at room temperature. Next, the
membranes were incubated overnight at 4°C with rabbit anti-matrix
metalloproteinase 9 (MMP9, cat. no. 13667), rabbit anti-MMP7 (cat.
no. 3801) and rabbit anti-GAPDH (cat. no. 5174) monoclonal
antibodies (dilution, 1:1,000; all purchased from Cell Signaling
Technology, Inc., Danvers, MA, USA). Following washing, the blots
were incubated with horseradish peroxidase-conjugated secondary
antibodies (cat. no. 5127; 1:10,000, Cell Signaling Technology,
Inc.) at room temperature for 30 min and visualized using the
automatic chemiluminescence image analysis system (Tanon 6100;
Tanon Science & Technology Co., Ltd.).
Enzyme-linked immunosorbent assay
(ELISA)
The cells were transfected with miR-139-5p or NC
mimics, and then seeded in 24-well plates (5×104
cells/well). After 48 h of incubation, the cell culture supernatant
was collected from the 24-well plates, and the content of the
inflammatory cytokines, including IL-1β, IL-6 and TNF-α, was
measured by ELISA kits (Sangon Biotech Co., Ltd.) according to the
protocols supplied the manufacturer. A total of three independent
experiments were performed in triplicate.
NF-κB activation assay
An electrophoretic mobility shift assay (EMSA) was
performed to assess NF-κB activation, as previously described
(19). Briefly, HCT116 cells were
transfected with miR-139-5p or NC mimics for 48 h prior to harvest.
The nuclear extract was prepared using a Nuclear Extract kit
(EpiGentek Group, Inc.). An EMSA kit (Thermo Fisher Scientific,
Inc.) was used for detection of DNA binding, and DNA fragments were
synthesized as described previously (20). Subsequently, the complexes of NF-κB
with the DNA were separated on 4% non-denaturing polyacrylamide gel
electrophoresis and transferred to PVDF membranes. The membranes
were exposed to UV light (120 mJ/cm2) at room
temperature for 10 min and then probed with a
streptavidin-horseradish peroxidase conjugate (cat. no. S911,
Thermo Fisher Scientific, Inc.). The membranes were then wrapped in
plastic film were placed in the middle of two X-ray films, placed
together in a cassette and developed at −80°C for 72 h. The bands
were analyzed using an ImageJ Software (version 1.8.0; National
Institutes of Health).
Xenograft mouse model
A xenograft mouse model was used to evaluate the
effects of miR-139-5p in regulating tumor growth. The experimental
protocol involving animals was approved by the Animal Ethics
Committee of the Kunming Medical University (Kunming, China).
Briefly, specific pathogen-free female BALB/c nude mice (n=9), aged
4–6 weeks old and weighing 20–30 g, were obtained from the
Guangdong Medical Laboratory Animal Center (Foshan, China). Mice
were housed in pathogen-free room at 24±2°C, with 60–80% humidity
and had free access to food and water with a 12-h light/dark cycle.
The animals were divided into three groups (3 animals/group), as
follows: Blank, NC mimics and miR-139-5p mimics groups. Transfected
HCT116 cells were injected subcutaneously into the left flank of
the mice (5×106 cells per mouse), respectively. The
tumor size was measured every 4 days using a vernier caliper, and
the tumor volume was calculated as follows: Volume=(length × width
× width)/2. The animals were sacrificed by cervical dislocation on
day 28, and the tumors were excised and snap-frozen.
Statistical analysis
The data are presented as the mean ± SD. All
experiments were repeated at least three times with duplicate or
triplicate samples in each assay. The statistical differences were
evaluated by conducting Student's t-test and one-way analysis of
variance using the SPSS software (version 22.0; IBM Corp.). A
P-value of <0.05 was considered to be an indicator of a
statistically significant difference.
Results
miR-139-5p is downregulated in human
CRC cell lines
Previous studies have reported that the expression
levels of miR-139-5p are downregulated in CRC tissues and are
associated with poor disease prognosis (15,21).
Although miR-139-5p shares the same precursor with miR-139-3p,
miR-139-3p expression has been reported to be undetectable in CRC
cells, the overexpression of which exhibited no significant effects
on the malignant behavior of CRC cells (15). Therefore, in the present study, the
function of miR-139-5p was examined. The data revealed significant
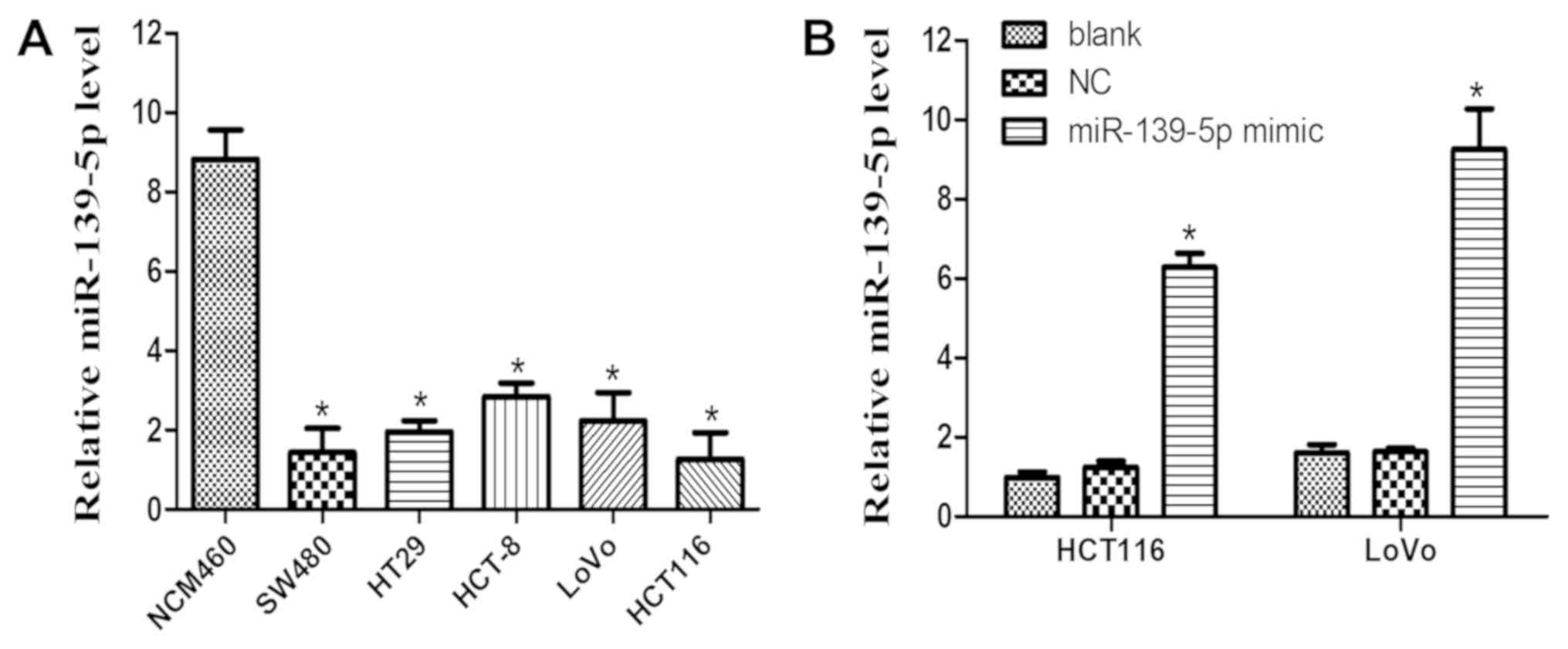
downregulation of miR-139-5p in five human CRC cell lines,
including SW480, HT29, HCT-8, LoVo and HCT116 cells, compared with
its expression in the normal human colon mucosal epithelial cell
line NCM460 (Fig. 1A). This
observation suggests that miR-139-5p may serve an important role in
CRC tumorigenesis. In accordance to these findings, a previous
study has reported that miR-139-5p sensitized LoVo and HCT-116
cells to 5-fluorouracil via NOTCH-1, with further increase in
apoptosis and reduced viability observed in these cells (22), indicating that miR-139-5p may serve
an important role in LoVo and HCT-116 cells. Thus, these two cell
lines were selected for the further investigation in the present
study.
miR-139-5p inhibits CRC cell
proliferation and tumor growth
To investigate the effects of miR-139-5p on the
proliferation and invasion of CRC cells, miR-139-5p mimics were
transfected into CRC cells (HCT116 and LoVo), and RT-qPCR was used
to determine the expression levels of miR-139-5p. The results
indicated an increased expression of miR-139-5p in HCT116 and LoVo
cells following transfection with miR-139-5p mimics, whereas NC
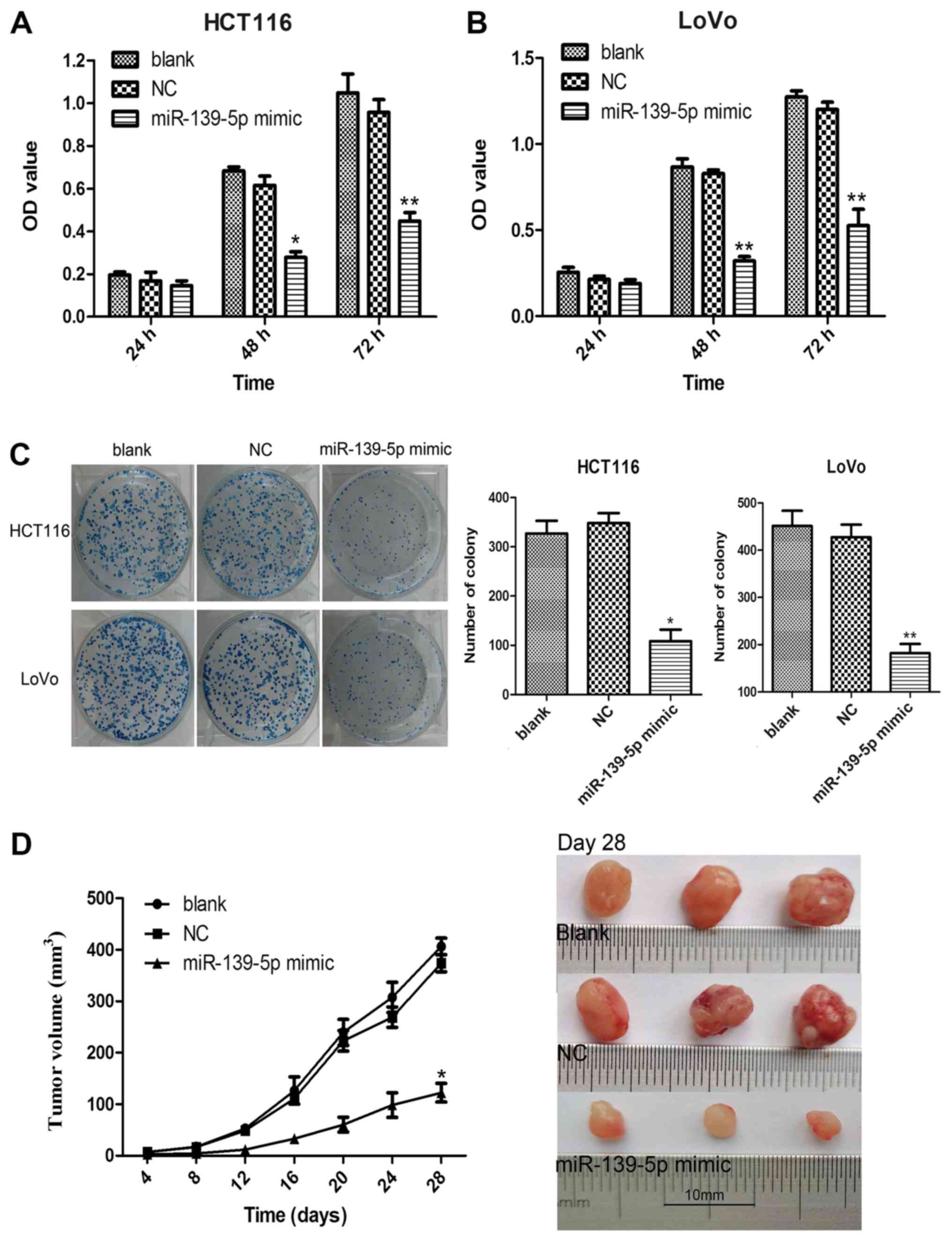
mimics had no marked effect compared with the blank group (Fig. 1B). It was observed that ectopic
expression of miR-139-5p significantly inhibited the proliferation
of HCT116 and LoVo cells when compared with the blank group
(Fig. 2A and B). The inhibitory
effects of miR-139-5p on colon cancer cell proliferation were
further confirmed by the colony formation assay. The results
indicated that overexpression of miR-139-5p markedly suppressed the
colony formation ability in miR-139-5p mimic-transfected HCT116 and
LoVo cells compared with that noted in the blank or NC mimics
groups (Fig. 2C). Subsequently, the
inhibitory effects of miR-139-5p on tumor growth were further
confirmed in vivo by conducting a xenograft tumor growth
assay. The subcutaneous tumor growth curve is shown in Fig. 2D and indicates that the tumor growth
rate of HCT116 cells transfected with miR-139-5p mimics was
significantly lower compared with that of the blank and NC mimics
cells on day 28. These results provided further evidence that
miR-139-5p serves a tumor suppressive role in colon cancer.
miR-139-5p suppresses CRC cell
invasion
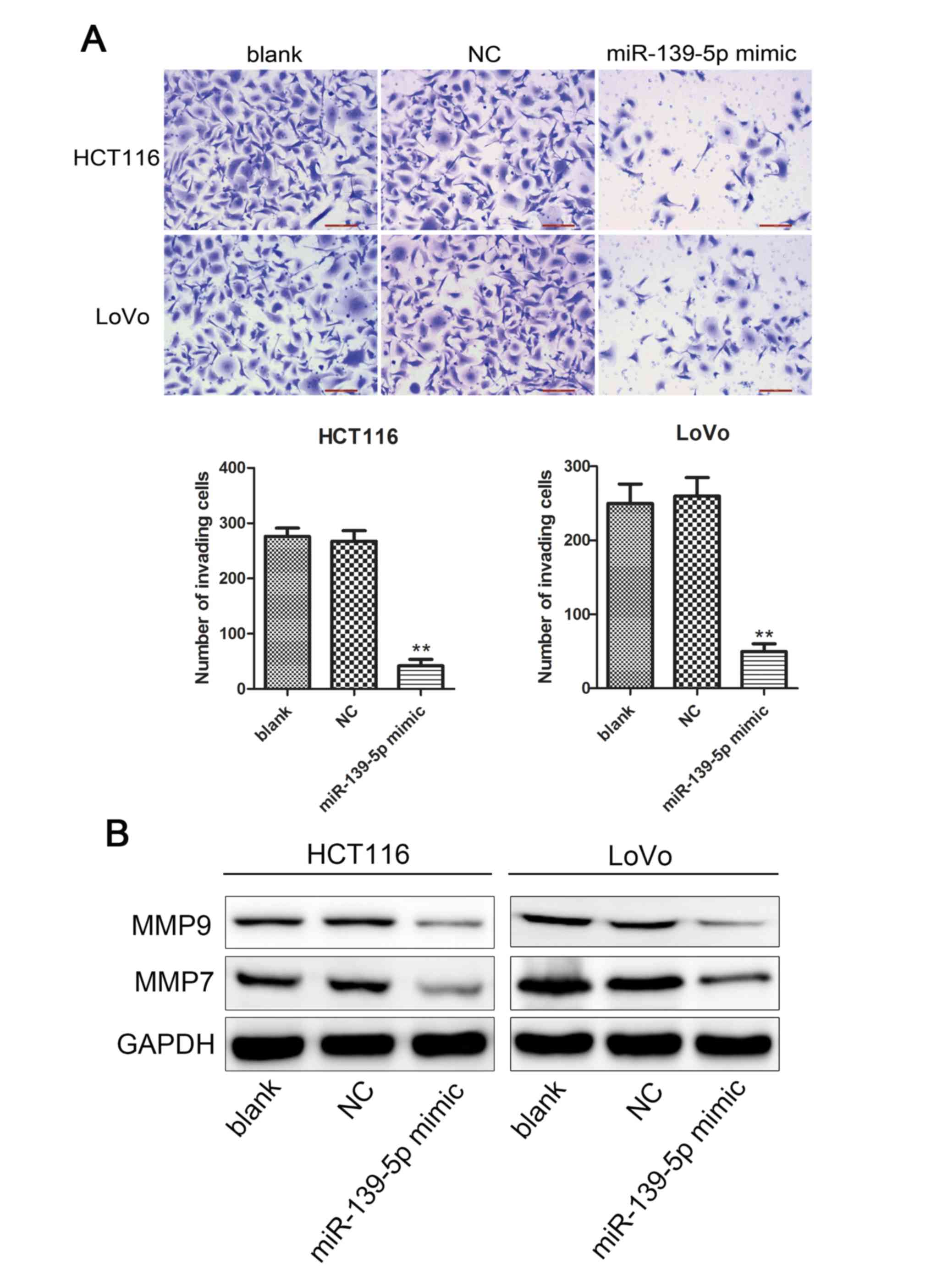
Following the initial results of miR-139-5p on colon
cancer cell growth, the current study further investigated whether
this miRNA affects the invasiveness of colon cancer cells in
vitro. The number of invading cells in the miR-139-5p
mimic-transfected HCT116 and LoVo cells were significantly
decreased compared with those in the blank or NC mimic-transfected
groups (Fig. 3A). The reduction in
the number of invading cells caused by the overexpression of
miR-139-5p further confirmed the effect of this miRNA in
suppressing cell invasion.
The MMP enzymes comprise a family of extracellular
proteinases enzymes that regulate basic cellular processes, such as
cell invasion. Two main members, namely MMP9 and MMP7, have been
demonstrated to play significant roles in cell invasion (23,24).
Therefore, the levels of MMP9 and MMP7 were measured by western
blot assays in the current study. The data demonstrated that
overexpression of miR-139-5p evidently inhibited the protein
expression levels of MMP7 and MMP9 (Fig.
3B). Taken collectively, these results strongly indicated that
miR-139-5p overexpression inhibited cell invasion.
miR-139-5p modulates chronic
inflammation by suppressing NF-κB activity
The inflammatory response contributes to malignant
tumor progression in the tumor microenvironment (25,26).
Changes in the inflammatory microenvironment are frequently
accompanied by molecular alternations in tumor tissues, and miRNAs
are usually considered as potential mediators of these processes
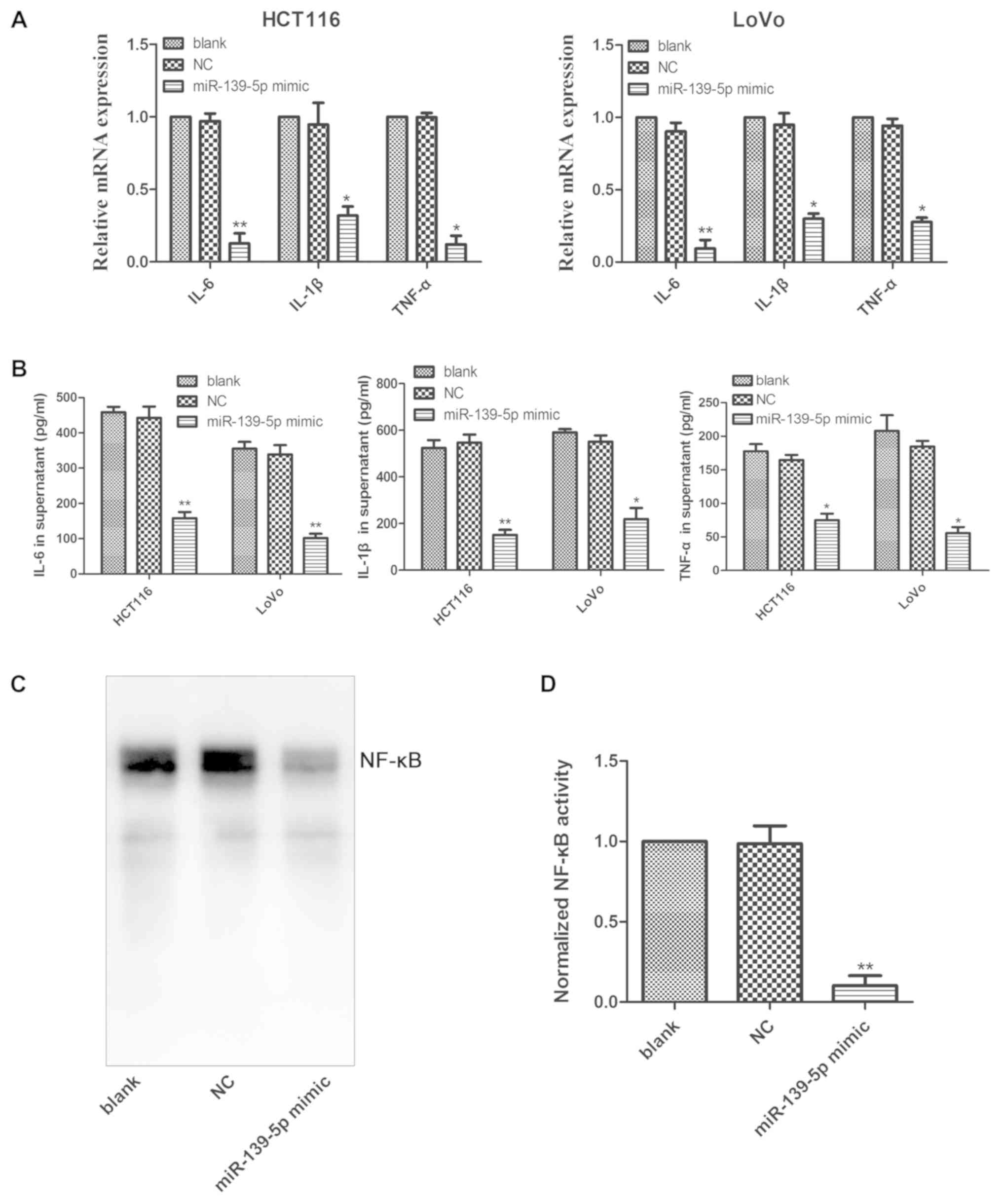
(27). In the present study,
overexpression of miR-139-5p significantly decreased the mRNA
expression levels of the inflammatory cytokines IL-1β, IL-6 and
TNF-α in HCT116 and LoVo cells transfected with miR-139-5p mimics
(Fig. 4A). Furthermore, transfection
of the cells with miR-139-5p mimics significantly decreased the
secretion of IL-1β, IL-6 and TNF-α in the culture supernatant of
HCT116 and LoVo cells (Fig. 4B). It
is well known that IL-1β, IL-6 and TNF-α are target genes of NF-κB,
and its activation has been demonstrated to boost the production of
these inflammatory cytokines (28).
The results of the present study indicated that activation of NF-κB
was apparent in HCT116 cells, whereas it was considerably
suppressed by overexpression of miR-139-5p in these cells (Fig. 4C and D). Taken collectively, the data
suggested that miR-139-5p was able to decrease the production of
IL-1β, IL-6 and TNF-α by suppressing NF-κB activation.
Discussion
Unstable amplification of inflammatory cytokines
secreted from the tumor microenvironment is a significant factor
that maintains the malignant behavior of tumors, and is further
associated with microRNA dysregulation. Accumulating evidence has
indicated that miR-139-5p is downregulated in inflammatory bowel
disease-associated neoplastic transformation, in primary CRC and in
metastatic sites (29–31). In addition, it has been reported that
miR-139-5p is downregulated in CRC tissues and is associated with
poor disease prognosis (15,21). A previous study has also demonstrated
that deletion of miR-139-5p promotes intestinal inflammation and
CRC through activating the NF-κB signaling pathway in vivo
(17). It was further suggested that
miR-139-5p may be involved in the initiation and development of CRC
by regulating inflammation. In the present study, the results
demonstrated that the expression levels of miR-139-5p in CRC cells
were significantly downregulated, and that the overexpression of
miR-139-5p decreased the levels of the inflammatory cytokines
IL-1β, IL-6, and TNF-α by suppressing NF-κB activity. These
molecular events led to the inhibition of CRC cell proliferation
and invasion.
miR-139-5p was previously identified as a tumor
suppressor in endometrial cancer (32), hepatocellular carcinoma (33) and adult acute myeloid leukemia
(34). Furthermore, the aberrant
expression of miR-139-5p is a characteristic feature in the
development of CRC. For instance, Zhang et al (21) reported that the expression levels of
miR-139-5p in CRC tissues were significantly downregulated when
compared with those noted in the adjacent normal tissues. Similar
findings were also reported by Song et al (15). Based on these findings, the present
study aimed to detect the expression levels of miR-139-5p in CRC
cells and found that they were significantly downregulated in the
CRC cell lines compared with those noted in the human normal colon
mucosal epithelial cell line NCM460. These results indicated that
miR-139-5p may serve as a tumor suppressor in CRC.
Aberrant miRNA expression levels are associated with
the malignant progression of cancer. Shi and Guo (35) indicated that the overexpression of
miR-139-5p suppressed osteosarcoma cell growth, migration and
invasion by reducing DNA methyltransferase-1, or vice versa. In
addition, a study by Maoa et al (36) reported that loss of miR-139-5p
promoted colitis-associated tumorigenesis in a transgenic murine
model of colorectal carcinoma by activating the PI3K/AKT/Wnt
signaling. In esophageal squamous cell carcinoma, overexpression of
miR-139-5p inhibited cell proliferation, migration and invasion,
and induced apoptosis and cell cycle arrest (37). Therefore, in the present study, the
biological function of miR-139-5p in CRC cells was further
investigated. The results demonstrated that overexpression of
miR-139-5p markedly suppressed cell proliferation in vitro
and xenograft tumor growth in vivo, which were in agreement
with the findings of the aforementioned studies. Furthermore, the
data indicated that overexpression of miR-139-5p exhibited
significant inhibition of the invasion ability of CRC cells by
downregulating the protein expression levels of MMP9 and MMP7.
Chronic inflammation contributes to cancer
development and can cause predisposition to carcinogenesis. It has
been reported that ~20% of all cancer types are associated with
chronic infections (38). In a
previous study by Zou et al (17), the interaction of intestinal
inflammation and colitis-associated CRC was explored, revealing
that miR-139-5p knockout mice were highly susceptible to colitis
and colon cancer, accompanied by increased production of
inflammatory cytokines and activation of the NF-κB signaling
pathway. To further identify the types of inflammatory cytokines
regulated by miR-139-5p that are involved in CRC, the levels of
inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, were
evaluated in miR-139-5p overexpressing CRC cells in the current
study. The results indicated that overexpression of miR-139-5p
markedly decreased the secretion of cytokines IL-1β, IL-6 and TNF-α
in the culture supernatant of CRC cells. Concomitantly, IL-1β, IL-6
and TNF-α mRNA levels were also decreased. Therefore, the results
demonstrated that inhibition of cell proliferation and invasion by
miR-139-5p were associated with the induction of inflammation in
CRC.
The NF-κB pathway is a complex signaling pathway
that regulates oncogenesis to promote the initiation and
development of cancer (39). The
activation of the NF-κB pathway has been reported to boost the
production of pro-inflammatory cytokines, including TNF-α, IL-1β
and IL-6 (28). Further experiments
conducted in the current study confirmed our hypothesis, suggesting
that overexpression of miR-139-5p markedly suppressed the
activation of NF-κB. It has been reported that the activation of
NF-κB pathway mediates the proliferation and metastasis of CRC
cells (40), stimulates angiogenesis
in CRC cells (41), and promotes CRC
progression (42), while inhibition
of the NF-κB pathway suppresses CRC growth and metastasis (43). As a result, in the present study, it
can be concluded that miR-139-5p mediated the downregulation of
TNF-α, IL-1β and IL-6 by suppressing the activation of NF-κB, which
may be responsible for the suppressed proliferation of CRC cells
and stunted growth of CRC tumors.
In conclusion, the present study demonstrated that
miR-139-5p was significantly downregulated in CRC cells, and that
overexpression of miR-139-5p inhibited cell proliferation and
invasion by decreasing the secretion of inflammation-associated
cytokines. Furthermore, downregulation of the
inflammation-associated cytokines mediated by miR-139-5p was
associated with the suppression of NF-κB activity. These results
illustrated a novel molecular mechanism that can be used for the
development of CRC therapeutics.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL and BRY initiated and designed the present study.
YGD, QL and QZ analyzed and interpreted the results. MMZ, WZ and JM
performed the various experiments. MMZ wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol involving animals was
approved by the Animal Ethics Committee of the Kunming Medical
University (Kunming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schreuders EH, Ruco A, Rabeneck L, Schoen
RE, Sung JJ, Young GP and Kuipers EJ: Colorectal cancer screening:
A global overview of existing programmes. Gut. 64:1637–1649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Payne JK: State of the science: Stress,
inflammation, and cancer. Oncol Nurs Forum. 41:533–540. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rhodes JM and Campbell BJ: Inflammation
and colorectal cancer: IBD-associated and sporadic cancer compared.
Trends Mol Med. 8:10–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inflammatory bowel disease and cancer
risk. Arch Dis Child. 103:272017.PubMed/NCBI
|
|
6
|
Galvan-Roman JM, Curbelo J and Aspa J:
Inflammatory status and prognosis of locally advanced non-small
cell lung cancer. J Thorac Dis. 9:2782–2785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mayberry JF, Ballantyne KC, Hardcastle JD,
Mangham C and Pye G: Epidemiological study of asymptomatic
inflammatory bowel disease: The identification of cases during a
screening programme for colorectal cancer. Gut. 30:481–483. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bissell MJ, Radisky DC, Rizki A, Weaver VM
and Petersen OW: The organizing principle: Microenvironmental
influences in the normal and malignant breast. Differentiation.
70:537–546. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen Z, Zhou R, Liu C, Wang Y, Zhan W,
Shao Z, Liu J, Zhang F, Xu L, Zhou X, et al: MicroRNA-105 is
involved in TNF-α-related tumor microenvironment enhanced
colorectal cancer progression. Cell Death Dis. 8:32132017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baranwal S, Rawat SG and Gupta P: miR-301,
pleiotropic microRNA in regulation of inflammatory bowel disease
and colitis-associated cancer. Front Immunol. 9:5222018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anfossi S, Giordano A, Gao H, Cohen EN,
Tin S, Wu Q, Garza RJ, Debeb BG, Alvarez RH, Valero V, et al: High
serum miR-19a levels are associated with inflammatory breast cancer
and are predictive of favorable clinical outcome in patients with
metastatic HER2+ inflammatory breast cancer. PLoS One.
9:e831132014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen K, Mao R, Ma L, Li Y, Qiu Y, Cui D,
Le V, Yin P, Ni L and Liu J: Post-transcriptional regulation of the
tumor suppressor miR-139-5p and a network of miR-139-5p-mediated
mRNA interactions in colorectal cancer. FEBS J. 281:3609–3624.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song M, Yin Y, Zhang J, Zhang B, Bian Z,
Quan C, Zhou L, Hu Y, Wang Q, Ni S, et al: MiR-139-5p inhibits
migration and invasion of colorectal cancer by downregulating AMFR
and NOTCH1. Protein Cell. 5:851–861. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Liang X, Wang Y, Meng X, Xu Y, Cai
S, Wang Z, Liu J and Cai G: miR-139-5p inhibits the
epithelial-mesenchymal transition and enhances the chemotherapeutic
sensitivity of colorectal cancer cells by downregulating BCL2. Sci
Rep. 6:271572016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou F, Mao R, Yang L, Lin S, Lei K, Zheng
Y, Ding Y, Zhang P, Cai G, Liang X and Liu J: Targeted deletion of
miR-139-5p activates MAPK, NF-κB and STAT3 signaling and promotes
intestinal inflammation and colorectal cancer. FEBS J.
283:1438–1452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takada Y, Khuri FR and Aggarwal BB:
Protein farnesyltransferase inhibitor (SCH 66336) abolishes
NF-kappaB activation induced by various carcinogens and
inflammatory stimuli leading to suppression of NF-kappaB-regulated
gene expression and up-regulation of apoptosis. J Biol Chem.
279:26287–26299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo YC, Lin WC, Chiang IT, Chang YF, Chen
CW, Su SH, Chen CL and Hwang JJ: Sorafenib sensitizes human
colorectal carcinoma to radiation via suppression of NF-κB
expression in vitro and in vivo. Biomed Pharmacother. 66:12–20.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Dong Y, Zhu N, Tsoi H, Zhao Z, Wu
CW, Wang K, Zheng S, Ng SS, Chan FK, et al: microRNA-139-5p exerts
tumor suppressor function by targeting NOTCH1 in colorectal cancer.
Mol Cancer. 13:1242014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu H, Yin Y, Hu Y, Feng Y, Bian Z, Yao S,
Li M, You Q and Huang Z: miR-139-5p sensitizes colorectal cancer
cells to 5-fluorouracil by targeting NOTCH-1. Pathol Res Pract.
212:643–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh RD, Haridas N, Patel JB, Shah FD,
Shukla SN, Shah PM and Patel PS: Matrix metalloproteinases and
their inhibitors: Correlation with invasion and metastasis in oral
cancer. Indian J Clin Biochem. 25:250–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu JY, Pang ZJ and Yu YH: Regulation of
trophoblast invasion: The role of matrix metalloproteinases. Rev
Obstet Gynecol. 5:e137–e143. 2012.PubMed/NCBI
|
|
25
|
Shawki S, Ashburn J, Signs SA and Huang E:
Colon cancer: Inflammation-associated cancer. Surg Oncol Clin N Am.
27:269–287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mantovani A: The inflammation-cancer
connection. FEBS J. 285:638–640. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Markopoulos GS, Roupakia E, Tokamani M,
Alabasi G, Sandaltzopoulos R, Marcu KB and Kolettas E: Roles of
NF-κB signaling in the regulation of miRNAs impacting on
inflammation in cancer. Biomedicines. 6:2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther. 2:2017.
View Article : Google Scholar
|
|
29
|
Olaru AV, Selaru FM, Mori Y, Vazquez C,
David S, Paun B, Cheng Y, Jin Z, Yang J, Agarwal R, et al: Dynamic
changes in the expression of MicroRNA-31 during inflammatory bowel
disease-associated neoplastic transformation. Inflamm Bowel Dis.
17:221–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen K, Liang Q, Xu K, Cui D, Jiang L, Yin
P, Lu Y, Li Q and Liu J: MiR-139 inhibits invasion and metastasis
of colorectal cancer by targeting the type I insulin-like growth
factor receptor. Biochem Pharmacol. 84:320–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang KH, Miller N, Kheirelseid EA,
Lemetre C, Ball GR, Smith MJ, Regan M, McAnena OJ and Kerin MJ:
MicroRNA signature analysis in colorectal cancer: Identification of
expression profiles in stage II tumors associated with aggressive
disease. Int J Colorectal Dis. 26:1415–1422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Li C, Jiang Y, Wan Y, Zhou S and
Cheng W: Tumor-suppressor role of miR-139-5p in endometrial cancer.
Cancer Cell Int. 18:512018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hua S, Lei L, Deng L, Weng X, Liu C, Qi X,
Wang S, Zhang D, Zou X, Cao C, et al: miR-139-5p inhibits aerobic
glycolysis, cell proliferation, migration, and invasion in
hepatocellular carcinoma via a reciprocal regulatory interaction
with ETS1. Oncogene. 37:1624–1636. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krowiorz K, Ruschmann J, Lai C, Ngom M,
Maetzig T, Martins V, Scheffold A, Schneider E, Pochert N, Miller
C, et al: MiR-139-5p is a potent tumor suppressor in adult acute
myeloid leukemia. Blood Cancer J. 6:e5082016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi YK and Guo YH: MiR-139-5p suppresses
osteosarcoma cell growth and invasion through regulating DNMT1.
Biochem Biophys Res Commun. 503:459–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maoa R, Zou F, Yang L, Lin S, Li Y, Ma M,
Yin P, Liang X and Liu J: The loss of MiR-139-5p promotes
colitis-associated tumorigenesis by mediating PI3K/AKT/Wnt
signaling. Int J Biochem Cell Biol. 69:153–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu R, Yang M, Meng Y, Liao J, Sheng J, Pu
Y, Yin L and Kim SJ: Tumor-suppressive function of miR-139-5p in
esophageal squamous cell carcinoma. PLoS One. 8:e770682013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maletzki C and Emmrich J: Inflammation and
immunity in the tumor environment. Dig Dis. 28:574–578. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Geng R, Tan X, Wu J, Pan Z, Yi M, Shi W,
Liu R, Yao C, Wang G, Lin J, et al: RNF183 promotes proliferation
and metastasis of colorectal cancer cells via activation of
NF-κB-IL-8 axis. Cell Death Dis. 8:e29942017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang G, Chen C, Yang R, Cao X, Lai S, Luo
X, Feng Y, Xia X, Gong J and Hu J: p55PIK-PI3K stimulates
angiogenesis in colorectal cancer cell by activating NF-κB pathway.
Angiogenesis. 16:561–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu XB, Liu Y, Wang GH, Xu X, Cai Y, Wang
HY, Li YQ, Meng HF, Dai F and Jin JD: Mesenchymal stem cells
promote colorectal cancer progression through AMPK/mTOR-mediated
NF-κB activation. Sci Rep. 6:214202016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu YX, Ju HQ, Wang F, Chen LZ, Wu QN,
Sheng H, Mo HY, Pan ZZ, Xie D, Kang TB, et al: Inhibition of the
NF-κB pathway by nafamostat mesilate suppresses colorectal cancer
growth and metastasis. Cancer Lett. 380:87–97. 2016. View Article : Google Scholar : PubMed/NCBI
|