Introduction
Microorganisms and toxic factors are the predominant
causative agents of periodontitis. In response to activated
tissues, macrophages and lymphocytes produce matrix
metalloproteinases and inflammatory factors, such as interleukin-1
(IL-1) and tumor necrosis factor (TNF) (1), which serve essential roles in
periodontal tissue destruction. Therefore, many inflammatory
mediators are considered to be crucial in the development of early
periodontal disease with nitric oxide (NO) the main inflammatory
factor in this disease (2).
NO is an extremely unstable, fat-soluble gas at room
temperature that can diffuse rapidly through biofilms. NO is mainly
generated by the catalysis of nitric oxide synthase (NOS) in an
organism, for example by the enzyme inducible nitric oxide synthase
(iNOS). iNOS expression and production levels are markedly
increased following injury when cells are activated by external
stimuli (3). Therefore, the present
study hypothesized that iNOS likely participates in the development
of periodontal disease (4). In 1993,
Bodis and Haregewoin (5) identified
the presence of NO in saliva, thereby providing the groundwork for
the study of NO in oral medicine and biology.
NO mediates the pathological effects of
lipopolysaccharide (LPS), TNF, IL-1, and other cytokines, regulates
leukocyte and epithelial cell adhesion, inhibits T cell
proliferation, and improves natural killer (NK) cell activity,
amongst other immune-related processes. These findings demonstrated
that NO is closely related to immune function. Given the
association of LPS, TNF, and IL-1 in the development of periodontal
disease, it was hypothesized that NO may be involved in the
progression of periodontal disease by regulating the action of
these and other cytokines (6).
To test this hypothesis, the present study
established an experimental periodontitis model in Sprague-Dawley
(SD) rats then examined the clinical manifestations and
pathological changes of periodontitis at different time periods,
and detected the expression of NO in the periodontium and serum.
The present findings provided a foundation for clarifying the role
of the NO signaling pathway in periodontitis development and its
mechanism of action.
Materials and methods
Establishment of the experimental
animal model and grouping
Forty-eight SD male rats (201.79±17.86 g; ~9 weeks
old) bought from Shanghai Slaccas were used in this study. All
animal experiments were approved by the Laboratory Animal Center of
Beihua University. The rats were randomly divided into the four
groups (n=12): Normal control group, 2 weeks post-operation, 4
weeks post-operation and 6 weeks post-operation. All the rats had
free access to water, and maintained under constant environmental
conditions with temperature of 25°C, 50% humidity, noise <85
decibels, ventilation once every 12 h, and a 12-h light/dark
cycle.
To establish the periodontal disease model, the rats
were routinely fed for one week, and then maxillary first molar
ligation was performed. The animals were monitored for signs of
peritonitis. In brief, rats were anesthetized with 10% chloral
hydrate (300 mg/kg). Rats were placed in the supine position and
fixed on the operating table, then the bilateral maxillary first
molars were ligated by simple ligation. The first maxillary molars
were wrapped by 0.2 mm orthodontic ligation, and the filaments were
placed as far as possible on the bottom of the gingival sulcus,
with the ligation tied to the palate. Each rat was labeled clearly.
All procedures were approved by the Animal Care and Use Committee
of The Beihua University and conformed to the guidelines of the
National Institute of Health.
Specimen collection
Following the operation at 2, 4 and 6 weeks, saliva,
abdominal aorta blood, and periodontal tissue samples were
collected under anesthesia. Salivation was induced by subcutaneous
administration of 10 mg/kg pilocarpine (Sigma-Aldrich; Merck KGaA)
in PBS. Saliva was collected with a pipette over a 15 min period
then transferred to a microcentrifuge tube containing protease
inhibitor (Complete Mini; Roche Diagnostics). Saliva was then
vortexed for 1 min, centrifuged at 16,000 × g for 5 min at 4°C and
transferred to a new tube, leaving behind any precipitated debris
from the mouth. This protocol ensures that intact microorganisms
present in the collected saliva are excluded from being processed
with the salivary proteins.
Determination of NO in the saliva by
the Griess method
Saliva was collected and stored at −20°C. When fully
thawed, the saliva was tested for NO content using the Nitric Oxide
(Enzymatic) kit (cat. no. ab65328; Abcam) instructions. The optical
density (OD) values of the measurement and standard tubes were
measured at 530 nm in a 0.5-cm cuvette, using a blank tube
standardized as zero. An average value was taken as the final
reading. NO content in each serum sample was calculated by the
following formula: NO content (µmol/l)=(sample OD value-blank OD
value)/(standard OD value-blank OD value) × standard concentration
(100 µmol/l).
Collection and treatment of serum
specimens
Anesthetized rats were injected intraperitoneally
with 10% chloral hydrate (300 mg/kg). All the animals were
monitored for any signs of peritonitis. Three rats in the control
group and all rats in the other groups were selected at 2, 4, and 6
weeks after the operation for blood collection. Abdominal aorta
blood was extracted with a single sterile needle head, and the
blood was centrifuged for 10 min (4°C; 3,800 × g). The supernatant
was collected and stored at −20°C until experimentation.
Extraction and treatment of tissue
specimens
Following blood collection, the rats were
sacrificed, and the bilateral maxillary bones containing the first
molar, the surrounding gingiva, and alveolar bone tissue were
dissected. The periodontal tissue specimens were fixed in 4%
paraformaldehyde solution for 16 h, at 4°C and then decalcified in
10% solution of pH 7.2–7.3. The solution was replaced every two
days until a pin could pass through the tissue without resistance.
The tissue was then trimmed, dehydrated, immersed in wax, and
embedded in paraffin. A series of tissue sections with thickness of
4 µm were made from the paraffin blocks of each specimen. The
pathological lesions were reported by an animal pathologist.
Hematoxylin-eosin (HE) staining
Histopathological sections of rats in each group
were stained with HE to observe the pathological changes of
periodontal tissues in each group. A light microscope was used to
observe the slices.
Expression of NO in the periodontal
tissues of rats with periodontitis by immunohistochemistry
Histopathological sections were stained with rabbit
anti-iNOS2 (1:1,000; cat. no. ab15323; Abcam) at 4°C overnight
using the streptavidin-biotin complex method for periodontal
tissue. Samples were incubated with secondary antibody
peroxidase-labeled streptavidin-biotin complex (1:1,000; Dako; cat.
no. P0447; Agilent Technologies, Inc.) for 30 min at 37°C.
Subsequently, the samples were observed using a fluorescence
microscope at ×20 magnification (Olympus Bio-fluorescence
Microscope BX53; Olympus Corporation). A total of three optical
fields were viewed per section. A total of 12 sections were
analyzed per sample and per group. Then the value was calculated by
Image J software (National Institute of Health).
Expression of NO in the serum of rats
with periodontitis by the nitrate reduction method
The expression of NO in the serum of rats with
periodontitis was determined through nitrate reduction, as NO is
quickly transformed into nitrate (NO3-) and nitrite
(NO2-) in vivo. In brief, a NO reduction kit
(Nanjing Institute of Bioengineering) was used to measure the
concentration of NO based on the color intensity, according to the
manufacturer's protocol. The mixed reagent, chromogenic agent, and
100 µmol/l standard application solution were prepared for NO
detection.
In brief, the serum supernatant and chromogenic
agent were mixed well, left at room temperature for 10 min, and
then the absorbance was determined at 550 nm and compared with the
standard of double-steamed water (OD adjusted to zero). The NO
content in each serum sample was calculated by the following
formula: NO content (µmol/l)=(sample OD value-blank OD
value)/(standard OD value-blank OD value) × standard concentration
(100 µmol/l). The experiments were repeated three times.
Gene chip technology to detect genes
associated with the NO signaling pathway in the course of
periodontitis
Selected tissue samples of periodontitis rats were
stored in liquid nitrogen and were detected using an NO signaling
gene chip that was obtained from Shanghai Kangcheng Bioengineering
Co., Ltd. This contained 113 genes associated with NO signaling
pathway. Furthermore, the chip contained target genes downstream of
the NO signaling pathway, which were involved in the biosynthesis
of NO, hyperoxidative metabolism and oxidative stress processes,
and genes that were promoted or suppressed by NO. These genes were
indicatives of whether the NO signaling pathway was activated.
Samples (50 mg) were immersed in 0.5 ml TRIzol
solution (Invitrogen; Thermo Fisher Scientific, Inc.), and the
supernatant of the tissue suspension was centrifuged at 4,900 × g.
The supernatant was discarded, the genomic RNA extracted and the
sample was transferred to a sterile 1.5-ml tube. Chloroform/isoamyl
alcohol (24:1; total 200 ml) was added, and the solution was shaken
vigorously for 30 sec. The supernatant was carefully transferred to
an RNase free 1.5-ml tube and centrifuged at 10,8000 × g at room
temperature for 5 min. The supernatant was carefully removed to
obtain tissue RNA. Sample concentration (µg/ml) was determined by
the following formula: A260×40×500 dilution factor.
The labeling of cRNA and the purification of
synthetic cRNA were determined according to the True
Labeling-AMP/linear RNA amplification kit and the SuperArray
ArrayGrade cRNA purification kit specification. The SuperArray
ArrayGrade cRNA purification kit instructions were used for the
operation. Sample concentration (µg/ml)=A 260×40×500 (dilution
multiple). The ratio of OD260/280 to OD260/230 was calculated to
determine the purity of the sample.
The expression level of NO-related genes in
experimental periodontitis tissues of rats can be detected
simultaneously using the nucleic acid hybridization technique, and
the key genes of NO signaling pathway in the course of
periodontitis can be screened (7).
The attachment loss was measured using radiographic measurements
(Ysio; Siemens Healthineers) and standardized with the Bioquant
software (Shanghai AB SCIEX Analytical Instrument Trading Co.)
based on the tooth width which was measured with a caliper.
Statistical analysis
All data were analyzed using SPSS v.20.0 software
(IBM Corp.) and expressed as the mean ± standard deviation.
Student's t-test was used to compare the difference between two
groups. One-way analysis of variance followed by Bonferroni post
hoc test was used for multiple comparison tests. Significant
differences were further evaluated between groups using the least
significant difference test. P<0.05 was considered to indicate
statistical significance.
Results
Rat periodontal tissue clinical
changes following establishment of periodontitis model
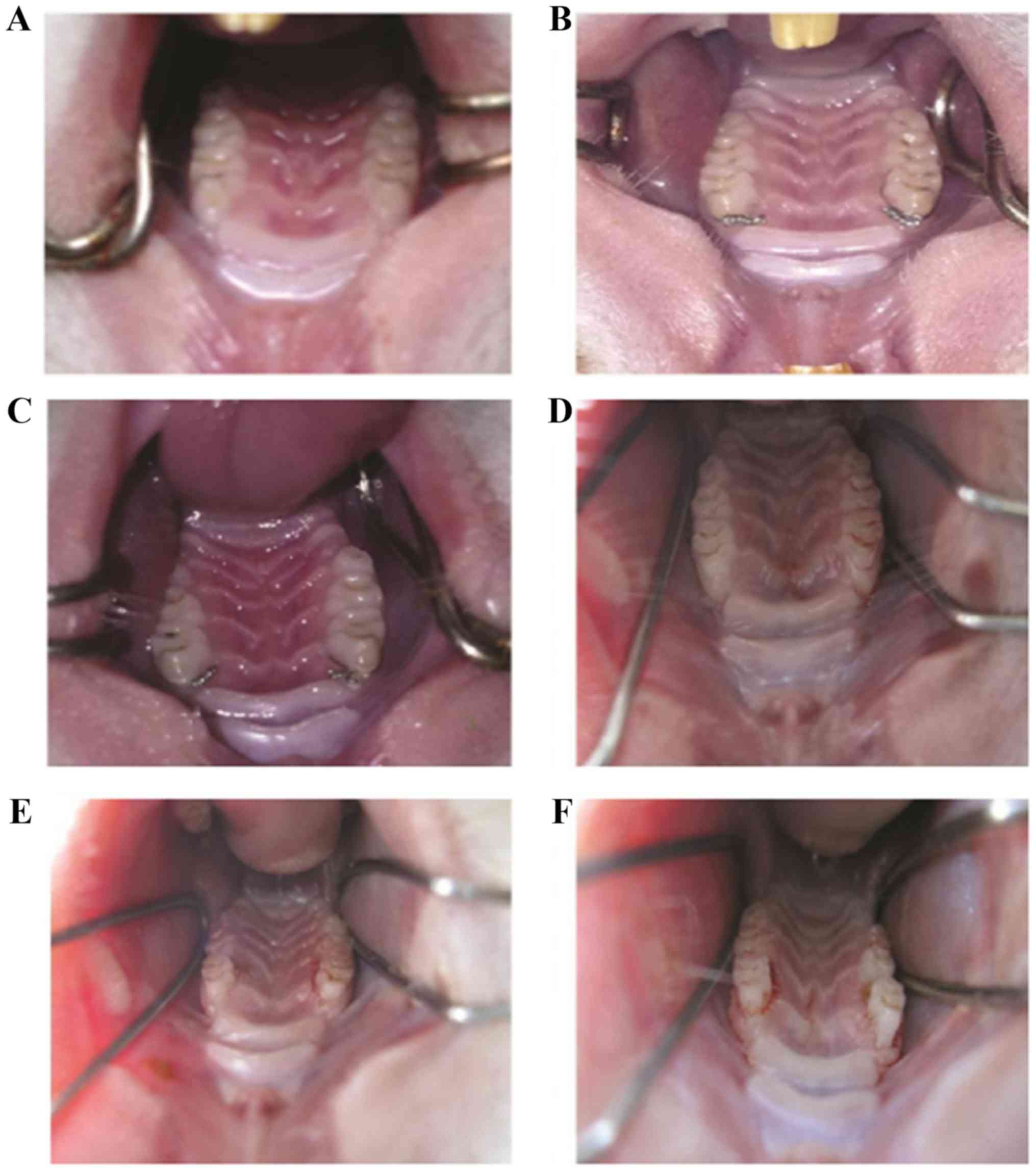
Before the operation, rats displayed healthy
periodontal tissue with pink gums (Fig.
1A). Surgery with ligation of the first molar and palatal
fixation, and food residue and soft scales are presented in
Fig. 1B and C, respectively. Two
weeks post-surgery, gingival edema and a small amount of bleeding
were observed (Fig. 1D). Four weeks
post-surgery, red gingival tissue with an irregular edge was
present with bleeding easily induced (Fig. 1E). Six weeks post-surgery, dull-red
atrophied gingival tissue was observed with bleeding easily induced
(Fig. 1F).
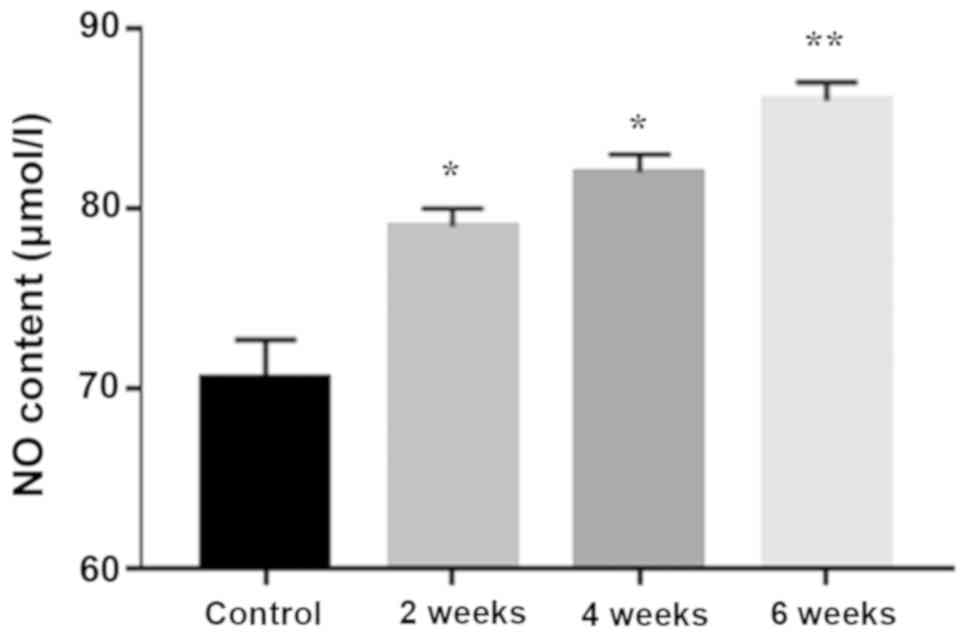
Saliva NO content increases with
periodontitis progression
A time-dependent significant increase in saliva NO
content was detected with progression of periodontitis (Fig. 2).
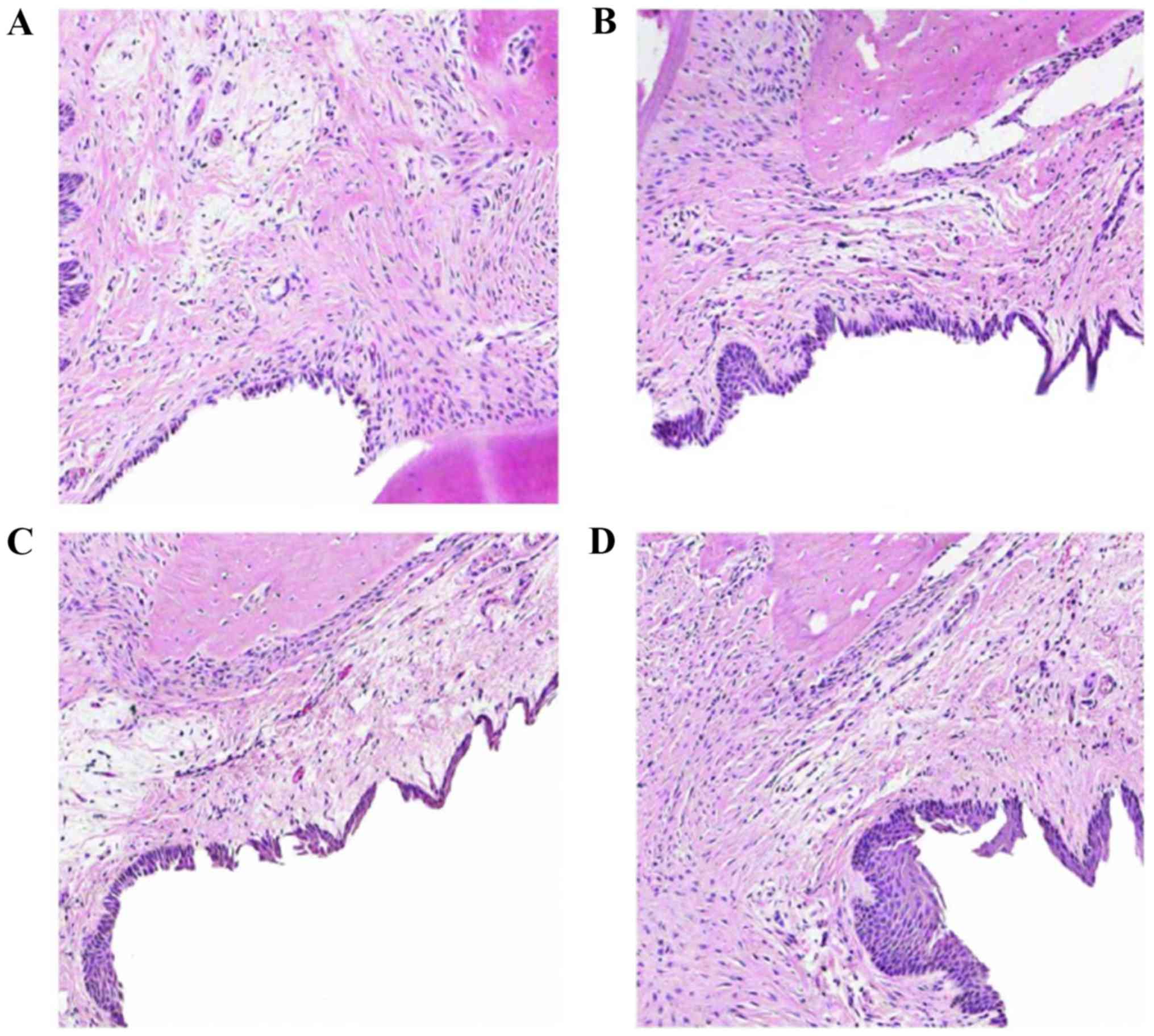
Tissue health declines with
periodontitis progression
Histopathological changes of periodontal tissue were
evaluated by HE staining. The normal control group demonstrated a
non-keratinized squamous epithelium, no epithelial spikes, an
intact smooth surface of the alveolar bone, and no formation of
osteocytes or lacunae (Fig. 3A).
Tissue samples from 2 weeks post-operation demonstrated
proliferation of epithelial rete pegs, invasion of the connective
tissue into the lower part, edema of the connective tissue,
infiltration of lymphocytes and neutrophils, and intact alveolar
bone (Fig. 3B). Tissue samples from
4 weeks post-operation demonstrated disordered collagen fibers,
edema, tissue degeneration and loss, with a large number of
infiltrated inflammatory cells (primarily neutrophilic
granulocytes), combined with epithelium proliferation, and a large
number of lymphocytes in the connective tissue of the subepithelial
tissue. Alveolar bone exhibited a few active osteoclastic
resorption lacuna with alveolar ridge top and destruction of the
inherent alveolar bone resorption (Fig.
3C). Tissue samples from 6 weeks post-operation demonstrated
dissolved subepithelial connective tissue, denatured collagen
fibers and prolific inflammatory cell infiltration into the
alveolar bone. The alveolar crest was also destroyed and vertical
absorption was observed (Fig. 3D).
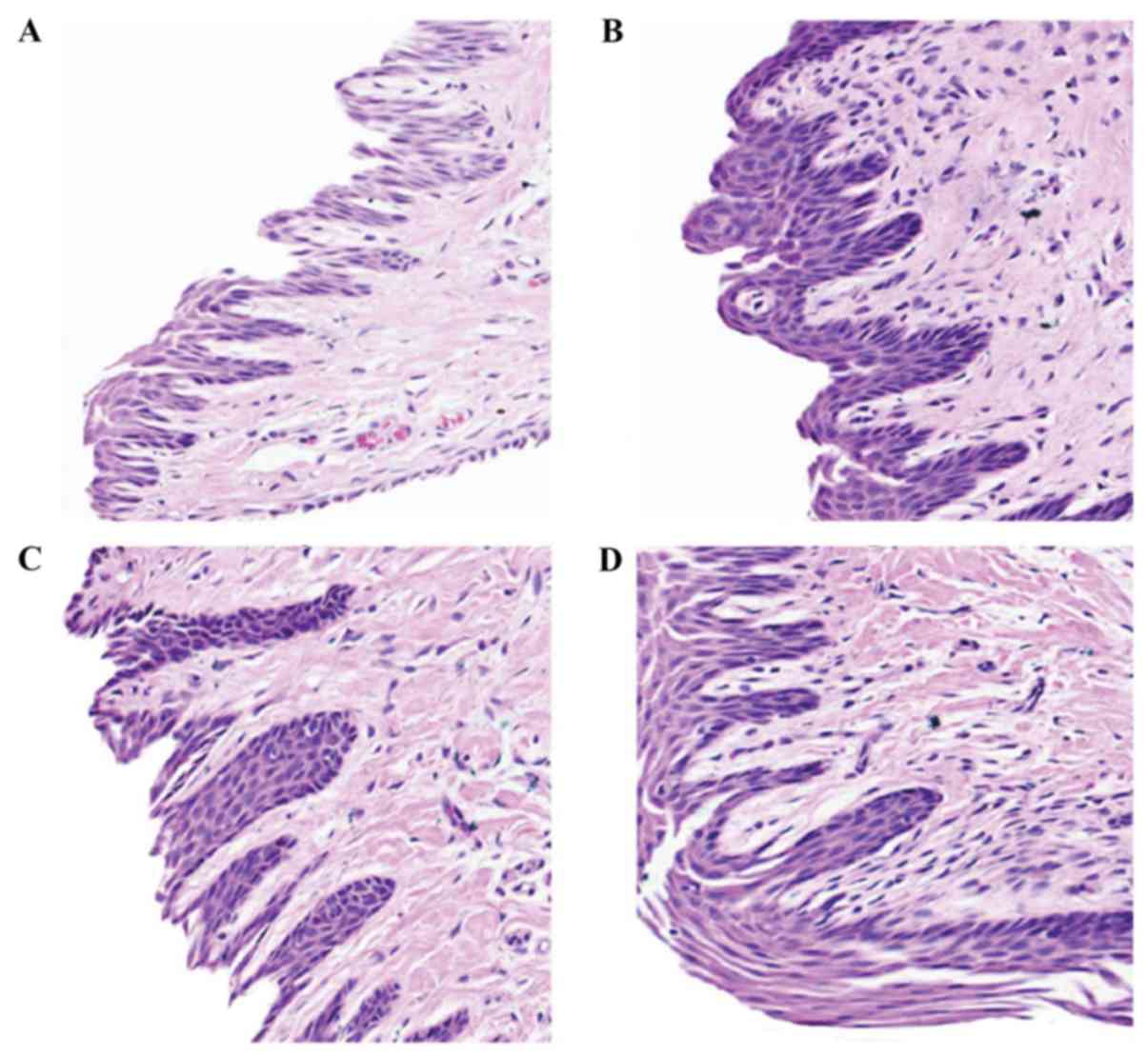
Representative examples of these histopathological changes are also
demonstrated at higher magnification in Fig. 4.
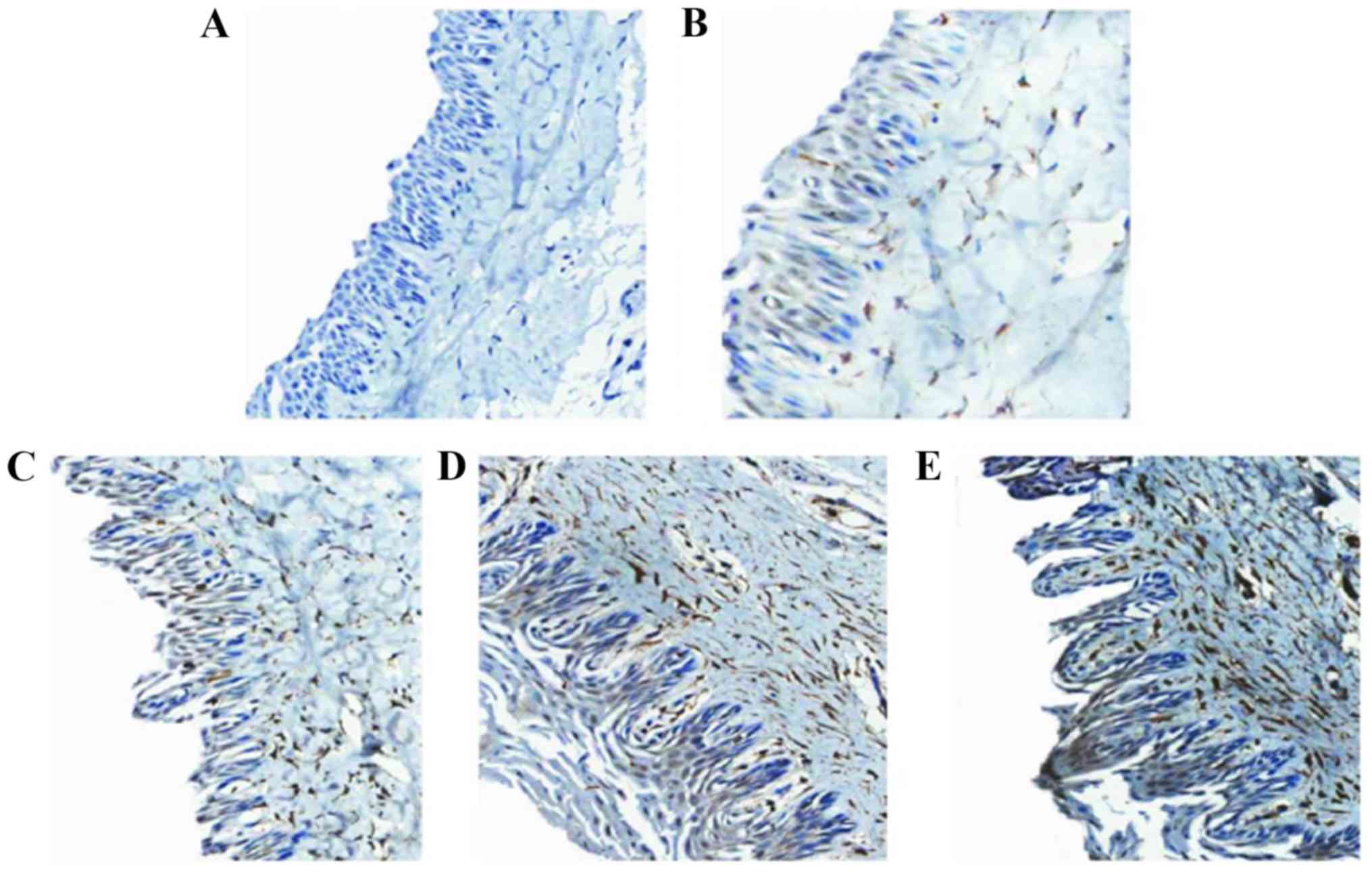
iNOS2 levels increase with
periodontitis progression
Tissue sections of the rats of each group were
analyzed to observe the expression of iNOS2 (brown staining) in the
periodontal tissue (Fig. 5). iNOS2
expression levels varied significantly between groups (P<0.001;
Table I).
 | Table I.NO expression levels in the
periodontal tissue of different groups. |
Table I.
NO expression levels in the
periodontal tissue of different groups.
| Comparison | Sum of squares | df | Mean square | F-test | P-value |
|---|
| Between groups | 1,523.43 | 3 | 507.820 | 101.824 | <0.001 |
| Within groups | 159.58 | 32 | 4.986 | 9.714 | >0.05 |
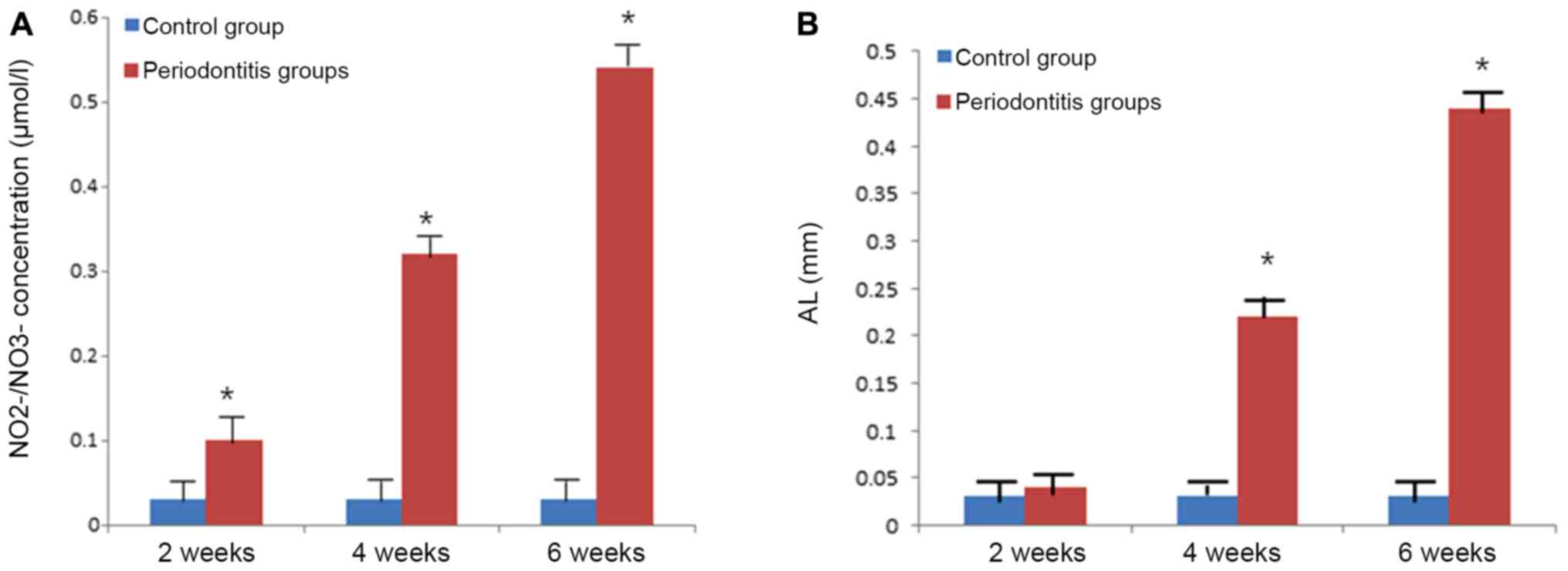
Serum NO expression and attachment
loss increases with periodontitis progression
NO contents in the serum significantly increased
with disease progression (P<0.001; Fig. 6A). There was a significant difference
in NO2-/NO3- levels (P<0.05) between the
periodontitis and the normal control group in the gingival tissue
at 2 weeks post-operation. By contrast, 4 and 6 weeks
post-operation, the NO2-/NO3- levels were
significantly higher compared with the normal control group
(P<0.001; Fig. 6A). Similar
results were observed for attachment loss with increasing
attachment loss during the progression of periodontitis (Fig. 6B).
Discussion
The histopathological changes of human periodontitis
can be adequately replicated in an animal model using local
periodontal stimuli such as silk thread to knot the teeth and neck
of the animal (8). In the present
study, clinical observation of the periodontal changes and the
pathological examination of periodontal tissues demonstrated that a
rat model of periodontitis was successfully established using the
ligature method.
Immunohistochemical staining can be used to evaluate
the intensity of iNOS expression in periodontal tissues (9). It has been hypothesized that iNOS
levels increase in patients with periodontitis, depending on the
severity of inflammation. Macrophages demonstrate the greatest
expression of iNOS amongst cells (10). A recent study determined that NO
dilates blood vessels in the rabbit aorta (11). The increased level of NO in patients
with periodontitis is attributed to the increased levels of iNOS
expression in periodontal tissue cells during inflammation
(12). An increase in NO content
leads to vascular permeability and swelling of the gums leading to
the gingival redness and swelling characteristic of periodontitis
[14]. During periodontal probing, an increase in the bleeding index
of the gums may be caused by inhibition of platelet aggregation and
adhesion mediated by increased NO production. By contrast, the
decrease in alveolar bone height and increase in absorption is
likely caused by the increased osteoclast activity induced by NO
stimulation (12).
The present study determined that there was an
increased expression of iNOS2 in the gingival blood vessels, which
may be related to the vasodilation effect of NO. Moreover, iNOS2 is
abundantly produced by inflammatory cells such as macrophages
(13), with the concentration
determined by the extent of inflamed tissues, and bacterial
stimulation. The present immunohistochemical results supported the
hypothesis that iNOS2 expression in periodontal tissues increased
during the development of periodontitis (14).
Menaka et al (15), determined that NO3- and
NO2- in the serum is due to synthesis of NO by NOS from
L-arginine, and also from ingested food. Serum NO2-and
NO3- are mainly derived from the L-arginine/NO pathway
following fasting for 12 h. Moshage et al (16) identified that NO content can be fully
determined from measurement of NO2- and NO3-
in the serum after a blood sample is left to solidify at room
temperature for 30 min. Concentrations of serum NO2 has
been demonstrated to significantly increase in patients with
periodontitis compared with healthy controls, with a significant
increase in NO levels reported to contribute to the development of
periodontitis (17). Wadhwa et
al (18) demonstrated that NO
levels in the serum and saliva are good indicators to evaluate the
inflammatory status of periodontal tissues. It is also believed
that smoking increases the extent of periodontal injury, and
smokers also have a greater incidence of periodontal disease
expressing higher NO serum and saliva NO levels than non-smokers
(19). Increased salivary NO levels
were associated with a greater severity of periodontitis (20), and non-smoking patients with
periodontitis showed increased NO2- levels compared with
healthy patients (21). Hussain
et al (22) measured the
contents of NO2- and NO3- in the gingival
tissue of rats and indirectly determined the content of NO in
gingival tissue. Compared with the periodontitis group, the
NO2- and NO3- content in the periodontium
gingival tissue was significantly increased, and increased with
progression of periodontitis. Given that NO also displays
anti-inflammatory effects (4), it
likely acts bi-directionally where low concentrations inhibit
inflammation whilst high concentrations aggravate the development
of inflammation.
By contrast, one study demonstrated that the NO
metabolite NO2- content in the saliva of periodontitis
patients was reduced compared with healthy subjects (23). Moreover, Andrukhov et al
(24) determined that NO levels in
saliva and serum reflected a decreasing trend compared with the
normal control group. However, given that NO is clearly expressed
in periodontal disease tissues and its content increases with
disease progression, it evidently serves a role in the development
of periodontal disease.
Based on the results of genetic analysis, it was
hypothesized that NO and iNOS were involved in the progression of
periodontal disease. SNP rs2297518 of the iNOS gene, SNP rs1049255
of the cytochrome b-245 α chain gene, and SNP rs841 of the GTP
cyclohydrolase I are transcripted into mRNA, leading to expression
of iNOS protein. L-arginine and iNOS protein combine in NO
synthesis, which mediates the pathological effects of LPS (25). This in turn regulates leukocytes and
epithelial cell adhesion, thereby inhibiting T cell proliferation
to increase NK cell activity (26).
In addition, bacterial virulence along with the immune cell
response leads to tissue destruction, periodontitis, and ultimately
alveolar bone absorption and destruction (27).
In conclusion, the present results determined that
NO expression increases gradually with the progression of
periodontitis by inducing the action of certain cytokines.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YW and XH designed the study and interpreted results
of experiments. YW, XH and FH also contributed to the design of the
study and the interpretation of experimental results. XH and FH
performed experiments, analyzed data, prepared figures and drafted
the manuscript. YW, XH and FH approved final version of manuscript.
YW edited and revised manuscript.
Ethics approval and consent to
participate
The animal study was approved by the Animal Care and
Use Committee of The Beihua University and conformed with the
guidelines of National Institute of Health.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vonholdt J, Gonzáles JR, Michel J,
Herrmann JM and Meyle J: Interleukin-1 polymorphism in patients
with early onset-and adult periodontitis. Int Poster J Dent Oral
Med. 3:722001.
|
|
2
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reeves SR, Simakajornboon N and Gozal D:
The role of nitric oxide in the neural control of breathing. Respir
Physiol Neurobiol. 164:143–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schmidt HH, Nau H, Wittfoht W, Gerlach J,
Prescher KE, Klein MM, Niroomand F and Böhme E: Arginine is a
physiological precursor of endothelium-derived nitric oxide. Eur J
Pharmacol. 154:213–216. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bodis S and Haregewoin A: Evidence for the
release and possible neural regulation of nitric oxide in human
saliva. Biochem Biophys Res Commun. 194:347–350. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Do MJ, Kim K, Lee H, Cha S, Seo T, Park
HJ, Lee JS and Kim TI: Development of animal experimental
periodontitis models. J Periodontal Implant Sci. 43:147–152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lockhart PB, Bolger AF, Papapanou PN,
Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW,
Gornik HL, Gewitz MH, et al: Periodontal disease and
atherosclerotic vascular disease: Does the evidence support an
independent association? A scientific statement from the American
heart association. circulation. 125:2520–2544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Achong R, Nishimura I, Ramachandran H,
Howell TH, Fiorellini JP and Karimbux NY: Membrane type (MT)
1-matrix metalloproteinase (MMP) and MMP-2 expression in
ligature-induced periodontitis in the rat. J Periodontol.
74:494–500. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Albandar JM and Rams TE: Global
epidemiology of periodontal diseases: An overview. Periodontol
2000. 29:7–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lappin DF, Kjeldsen M, Sander L and Kinane
DF: Inducible nitric oxide synthase expression in periodontitis. J
Periodontal Res. 35:369–373. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poorsattar Bejeh Mir A: Focusing on
periodontitis as a vasculupathy: The therapeutic possibilities from
the perspective of a dentistry student. J Pharm Biomed Sci.
13:2011.
|
|
12
|
López NJ, Smith PC and Gutierrez J: Higher
risk of preterm birth and low birth weight in women with
periodontal disease. J Dent Res. 81:58–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teng YT: Protective and destructive
immunity in the periodontium: Part 1-innate and humoral immunity
and the periodontium. J Dent Res. 85:198–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Förstermann U, Schmidt HH, Pollock JS,
Sheng H, Mitchell JA, Warner TD, Nakane M and Murad F: Isoforms of
nitric oxide synthase. Characterization and purification from
different cell types. Biochem Pharmacol. 42:1849–1857. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Menaka K, Ramesh A, Thomas B and Kumari
NS: Estimation of nitric oxide as an inflammatory marker in
periodontitis. J Indian Soc Periodontol. 13:75–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moshage H, Kok B, Huizenga JR and Jansen
P: Nitrite and nitrate determinations in plasma: A critical
evaluation. Clin Chem. 41:892–896. 1995.PubMed/NCBI
|
|
17
|
Lamster IB and Novak MJ: Host mediators in
gingival crevicular fluid: Implications for the pathogenesis of
periodontal disease. Crit Rev Oral Biol Med. 3:31–60. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wadhwa D, Bey A, Hasija M, Moin S, Kumar
A, Aman S and Sharma VK: Determination of levels of nitric oxide in
smoker and nonsmoker patients with chronic periodontitis. J
Periodontal Implant Sci. 43:215–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gautam DK, Jindal V, Gupta SC, Tuli A,
Kotwal B and Thakur R: Effect of cigarette smoking on the
periodontal health status: A comparative, cross sectional study. J
Indian Soc Periodontol. 15:383–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reher VG, Zenóbio EG, Costa FO, Reher P
and Soares RV: Nitric oxide levels in saliva increase with severity
of chronic periodontitis. J Oral Sci. 49:271–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sreedevi M, Ramesh A and Dwarakanath C:
Periodontal status in smokers and nonsmokers: A clinical,
microbiological, and histopathological study. Int J Dent.
2012:5715902012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hussain QA, McKay IJ, Gonzales-Marin C and
Allaker RP: Regulation of adrenomedullin and nitric oxide
production by periodontal bacteria. J Periodontal Res. 50:650–657.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aurer A, Aleksić J, Ivić-Kardum M, Aurer J
and Culo F: Nitric oxide synthesis is decreased in periodontitis. J
Clin Periodontol. 28:565–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andrukhov O, Haririan H, Bertl K, Rausch
WD, Bantleon HP, Moritz A and Rausch-Fan X: Nitric oxide
production, systemic inflammation and lipid metabolism in
periodontitis patients: Possible gender aspect. J Clin Periodontol.
40:916–923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Förstermann U and Sessa WC: Nitric oxide
synthases: Regulation and function. Eur Heart J. 33:829–837. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chiossone L, Dumas PY, Vienne M and Vivier
E: Natural killer cells and other innate lymphoid cells in cancer.
Nat Rev Immunol. 18:671–688. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartold PM and Van Dyke TE: Periodontitis:
A host-mediated disruption of microbial homeostasis. Unlearning
learned concepts. Periodontol 2000. 62:203–217. 2013. View Article : Google Scholar : PubMed/NCBI
|