Introduction
Hepatic fibrosis is a common pathological feature of
various chronic liver diseases, including viral hepatitis,
alcoholic liver disease and non-alcoholic fatty liver disease
(1). If untreated, hepatic fibrosis
ultimately develops into liver cirrhosis or hepatocellular
carcinoma (2). In the normal liver,
most hepatic stellate cells (HSCs) are in a quiescent state to
maintain a balance between synthesis and degradation of the
fibrillary extracellular matrix (ECM) (3). Hepatic fibrosis is characterized by the
progressive secretion and accumulation of ECM by activated HSCs
(4). A previous study demonstrated
that liver injury or culturing in vitro will result in HSC
activation and lead to the excessive accumulation of ECM
components, including fibronectin (FN) and various types of
collagens (5). In addition, a number
of chemokines, cytokines and growth factors have been reported to
contribute to HSC activation (6). In
particular, the platelet-derived growth factor (PDGF) family is one
of the most effective inducers of HSC activation, which further
exacerbates hepatic fibrosis (7).
Induction of activated HSC senescence, apoptosis and
reversion to the quiescent state has been reported to facilitate
the interruption of hepatic fibrosis (8,9).
Senescent cells display a large and flat morphology, exhibit
upregulated p21, p53 and HMGA1 expression (10), and exhibit enhanced
senescence-associated β-galactosidase (SA-β-gal) activity (11). During senescence, p21 expression is
increased and activated, which act as an inhibitor to
cyclin-dependent kinases, to arrest cell cycle progression
(12). In addition, high-mobility
group AT-hook 1 (HMGA) proteins accumulate on the chromatin of
senescent fibroblasts to stabilize the senescent state by
synergizing with p16 (13). In terms
of transcription, p53 is upregulated and cooperates with NF-κB to
promote cellular senescence (14).
The activation of NF-κB transcription factors serves as a driver
for the ageing process (15).
Telomerase reverse transcriptase (TERT), the catalytic subunit of
telomerase, elongates telomeres and maintains their structures.
However, declined activity or expression of TERT shortens telomeres
leading to senescence (16).
Activated HSCs can be depleted by the activation of programmed cell
death. Apoptotic cells undergo various morphological changes,
including cell shrinkage and convolution, pyknosis and karyorrhexis
(17). Phosphatidylserine
translocation to the cell surface is another apoptotic feature that
has been exploited for the detection of apoptosis (18). Activated HSCs can also be reverted to
a deactivated intermediate semi-quiescent state to reduce ECM
accumulation (19). Therefore,
investigations into determining the cell fate of activated HSCs
have become a focus of intense research for alleviating or
reversing hepatic fibrosis.
Astragalus membranaceus is one of the most
commonly used Traditional Chinese Medicine for hepatic fibrosis
treatment in China (20).
Astragaloside IV (ASIV) is the major active constituent of A.
membranaceus. At present, the degree of ASIV content is an
important parameter for the evaluation of A. membranaceus
quality (21). Previous in
vitro and in vivo studies have indicated that ASIV
possesses a variety of cytoprotective properties, including
anti-oxidative (22,23) and anti-cancer (24,25), in
addition to some anti-fibrotic roles in pulmonary (26,27),
cardiac (28,29), renal (30,31) and
hepatic fibrosis (32). However, the
molecular mechanism of ASIV-mediated anti-fibrosis remains poorly
understood.
The present study aimed to investigate the
protective mechanisms of ASIV on activated HSCs by using a
PDGF-BB-activated rat HSC-T6 cell line as a model. Reverse
transcription-quantitative PCR (RT-qPCR), western blotting and
immunofluorescence staining were performed to evaluate the
expression of fibrotic and senescence markers, and components of
the NF-κB signal pathway. SA-β-gal staining was implemented to
determine the degree of senescence at various concentrations of
ASIV. Flow cytometry was used for the analysis of apoptosis in
activated HSCs. In conclusion, it was demonstrated that ASIV
treatment suppressed PDGF-BB-induced HSC activation by activating
the NF-κB signaling pathway and promoting cellular senescence and
apoptosis. These data may provide new insights into the cell fate
determination of PDGF-BB-activated HSCs and implicated ASIV as a
potential treatment strategy for hepatic fibrosis.
Materials and methods
Reagents
DMEM, FBS, trypsin, penicillin-streptomycin (100X)
were purchased from Biological Industries. ASIV was purchased from
Beijing Solarbio Science & Technology Co., Ltd. (purity ≥98%,
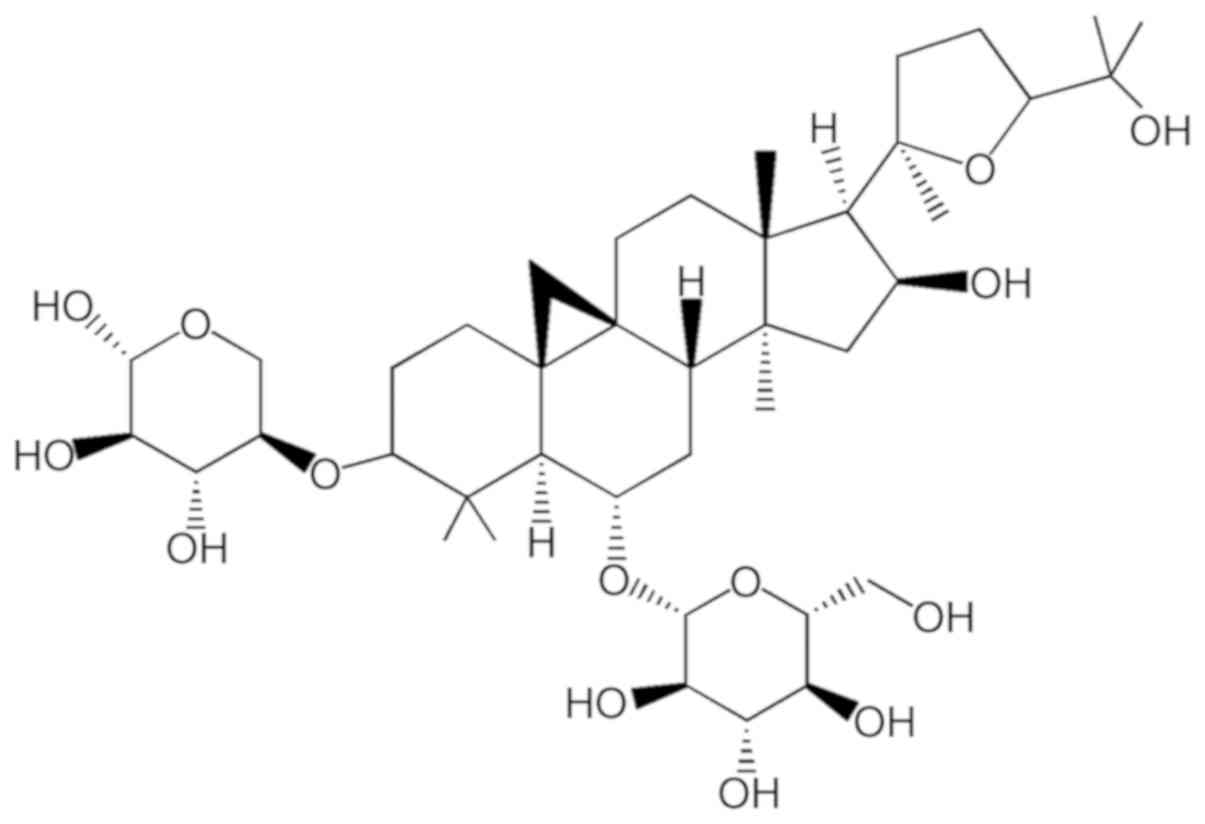
HPLC). The chemical structure of ASIV
(C41H68O14; molecular weight,
784.97) is illustrated in Fig. 1.
Rat PDGF-BB was purchased from GenScript. Gene-specific primers
were synthesized by TsingKe Biological Technology Co., Ltd. DMSO
was purchased from Sigma-Aldrich (Merck KGaA).
Cell culture and ASIV treatment
The rat HSC line HSC-T6 was obtained from Procell
Life Science & Technology Co., Ltd. The cells were cultured
using DMEM supplemented with 10% (v/v) FBS and 100 µg/ml
penicillin-streptomycin at 37°C under 5% CO2. HSC-T6
cells were activated using PDGF-BB at a final concentration of 20
ng/ml, as previously reported (33).
ASIV powder was first dissolved in DMSO and subsequently diluted in
DMEM to a final concentration of 0, 20 and 40 µg/ml (34). The final concentration of DMSO was
not in excess of 0.1% (v/v).
Immunofluorescence staining
HSC-T6 cells were seeded onto coverslips placed in
24-well plates at 2×103 cells/well at 37°C for 12 h
prior to treatment with PDGF-BB and ASIV for 48 h. Following drug
treatment, the coverslips were rinsed three times with 1X PBS for 5
min each, before fixation in 4% paraformaldehyde for 30 min at room
temperature. Then, 0.2% Triton X-100 (Beijing Solarbio Science
& Technology Co., Ltd.) was used to permeabilize cells for 10
min at room temperature. After blocking with 5% BSA (Beyotime
Institute of Biotechnology) for 30 min at room temperature, the
coverslips were subsequently treated with primary antibodies
(1:100) for 2 h at room temperature. The primary antibodies used
were as follows: Collagen type I α1 (COL1A1; cat. no. ab6308;
Abcam), α-smooth muscle actin (α-SMA; cat. no. MAB1420; R&D
Systems, Inc.) and fibronectin (FN; cat. no. AF5335; Affinity
Biosciences). Cells were washed three times with 1X PBS, and the
coverslips were incubated with Cy®3-conjugated secondary
antibodies (1:200; cat. no. E031620-01; EarthOx Life Sciences) for
1 h at room temperature. After another rinsing three times with 1X
PBS, cells were finally stained using 10 µg/ml DAPI (Beijing 4A
Biotech Co., Ltd.) for 30 min at room temperature for microscopic
analysis using upright fluorescence microscopes at ×10 eyepiece and
×20 objective lens.
RNA isolation and RT-qPCR
HSC-T6 cells were seeded in 12-well plates at a
density of 5×104 cells/well. Following a 12-h culture,
cells were treated with PDGF-BB and ASIV for 48 h. Total RNA was
extracted from HSC-T6 cells using RNAiso Plus reagent (Takara Bio,
Inc.), according to manufacturer's protocols. A total of 500 ng
total RNA was reverse-transcribed into cDNA using PrimeScript™ RT
Master Mix (Takara Bio, Inc.). RT reaction steps consisted of 37°C
for 15 min and 85°C for 5 sec. Gene specific primers were designed
using NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast) and
are listed in Table I. qPCR analysis
was performed in a Bio-Rad CFX96 Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.) using TB Green™ Premix Ex Taq™
(Takara Bio, Inc.). The qPCR cycling conditions were as follows: 30
sec at 95°C, and 40 cycles of 10 sec at 95°C, 30 sec at 60°C and 20
sec at 72°C. Relative gene expression was quantified using the
2−ΔΔCq method (35).
GAPDH was used as internal control.
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5′-3′) | Product size
(bp) |
|---|
| GAPDH | F:
TTCAACGGCACAGTCAAGG | 114 |
|
| R:
CTCAGCACCAGCATCACC |
|
| COL1A1 | F:
GGAGAGAGCATGACCGATGG | 184 |
|
| R:
GGGACTTCTTGAGGTTGCCA |
|
| α-SMA | F:
CGAAGCGCAGAGCAAGAGA | 78 |
|
| R:
CATGTCGTCCCAGTTGGTGAT |
|
| FN | F:
TGGAGAGACAGGAGGAAATAGC | 122 |
|
| R:
CAGTGACAGCATACAGGGTGAT |
|
| p65 | F:
CCTGGAGCAAGCCATTAGCC | 99 |
|
| R:
CGGACCGCATTCAAGTCATAGT |
|
| p52 | F:
GGTCACCAAGCTCCATGCTA | 88 |
|
| R:
GGTTGGGGATCCAAGTCCAG |
|
| p50 | F:
TCTCTATGACCTGGACGACTC | 202 |
|
| R:
AGAGTTGCAGCCTCGTGTC |
|
| HMGA1 | F:
CAGGAAAAGGATGGGACTGA | 156 |
|
| R:
CTTGTTCTTGCTTCCCTTCG |
|
| p21 | F:
GAGCAAAGTATGCCGTCGTC | 127 |
|
| R:
CTCAGTGGCGAAGTCAAAGTTC |
|
| p53 | F:
ATATTCTGCCCACCACAGCG | 107 |
|
| R:
CACTTGGAGGGCTTCCTCTG |
|
| RelB | F:
TTGTGGTCCAGCACTCCATC | 96 |
|
| R:
CACCCCATCTCAACAGCACT |
|
| c-Rel | F:
CAGGCACCAGTTCCAGTTCT | 114 |
|
| R:
GTGCGTCGTTTGCTAATCCG |
|
| TERT | F:
TTTCGAACAGCAAACCAGCG | 81 |
|
| R:
GCTGTGTAACCGGAGCAAAC |
|
Western blot analysis
HSC-T6 cells were seeded in 6-well plates at a
density of 2×105 cells/well. Following a 12-h culture,
cells were treated with PDGF-BB and ASIV for 48 h. Total cellular
protein from HSC-T6 cells was extracted using RIPA lysis buffer
(Beyotime Institute of Biotechnology), and protein concentration
was determined using bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Proteins (40 µg/lane) were separated
using 10% SDS-PAGE, and subsequently transferred to PVDF membranes.
The membranes were blocked for 2 h at room temperature with 5%
skimmed milk powder dissolved in Tris-buffered saline containing
0.1% Tween-20. Following blocking the membranes were incubated with
primary antibodies (1:1,000) in 5% skimmed milk overnight at 4°C.
The primary antibodies used were as follows: α-smooth muscle actin
(α-SMA; cat. no. MAB1420; R&D Systems, Inc.), FN (cat. no.
AF5335; Affinity Biosciences), GAPDH (E021060-03; EarthOx Life
Sciences), HMGA1 (cat. no. ab129153; Abcam), p21 (cat. no.
ab109199; Abcam), p65 (cat. no. AF6387; Affinity Biosciences), p52
(cat. no. AF6373; Affinity Biosciences) and NF-κB inhibitor α
(IκBα; cat. no. NB100-56507; Novus Biologicals, Ltd.). Following
primary antibody incubation, the membranes were washed five times
with Tris-buffered saline containing 0.1% Tween-20. Then membranes
were subsequently incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin (Ig)G (cat. no.
E030120-01; 1:10,000; EarthOx Life Sciences) and HRP-conjugated
goat anti-mouse IgG (cat. no. GB23301; 1:4,000; Wuhan Goodbio
Technology Co., Ltd.) in 1% skimmed milk at room temperature for 1
h, then the membranes were washed three times with Tris-buffered
saline containing 0.1% Tween-20. Finally, the protein bands were
visualized using UltraSignal Maximum Sensitivity ECL Substrate
(Beijing 4A Biotech Co., Ltd.) and detected using an imaging system
(Odyssey Fc Imaging System; Gene Company Ltd.). GAPDH was used as
the internal control.
SA-β-gal staining
HSC-T6 cells were seeded into 6-well plates at
6×104 cells/well prior to treatment with PDGF-BB and
ASIV for 48 h. SA-β-gal activity, a marker of senescence, was
evaluated using the SA-β-gal Staining kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocols.
Flow cytometry analysis of
apoptosis
Apoptosis analysis was performed using Annexin V/PI
assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to
manufacturer's protocols. Briefly, HSC-T6 cells treated with 20
ng/ml PDGF-BB and ASIV (0 or 40 µg/ml) for 48 h, and then cells
were collected for cell counting. A total of 1×106 cells
were rinsed once using ice-cold PBS and then with 1X Annexin V
binding buffer, before being subsequently suspended in 100 µl
binding buffer containing 5 µl Annexin V-Allophycocyanin (APC) and
5 µl propidium iodide (PI) followed by incubation at room
temperature for 15 min. Finally the cells were detected using a
flow cytometer no more than 30 min after the incubation. The data
were analyzed by FlowJo V10 (Expert Cytometry).
Statistical analysis
All data were presented as mean ± standard
deviation. All statistical analyses were performed using GraphPad
Prism software 5.0 (GraphPad Software, Inc.). A two-tailed t-test
was performed to assess differences between two data groups.
One-way ANOVA followed by Newman-Keuls' post hoc test was used to
compare differences between >2 data groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
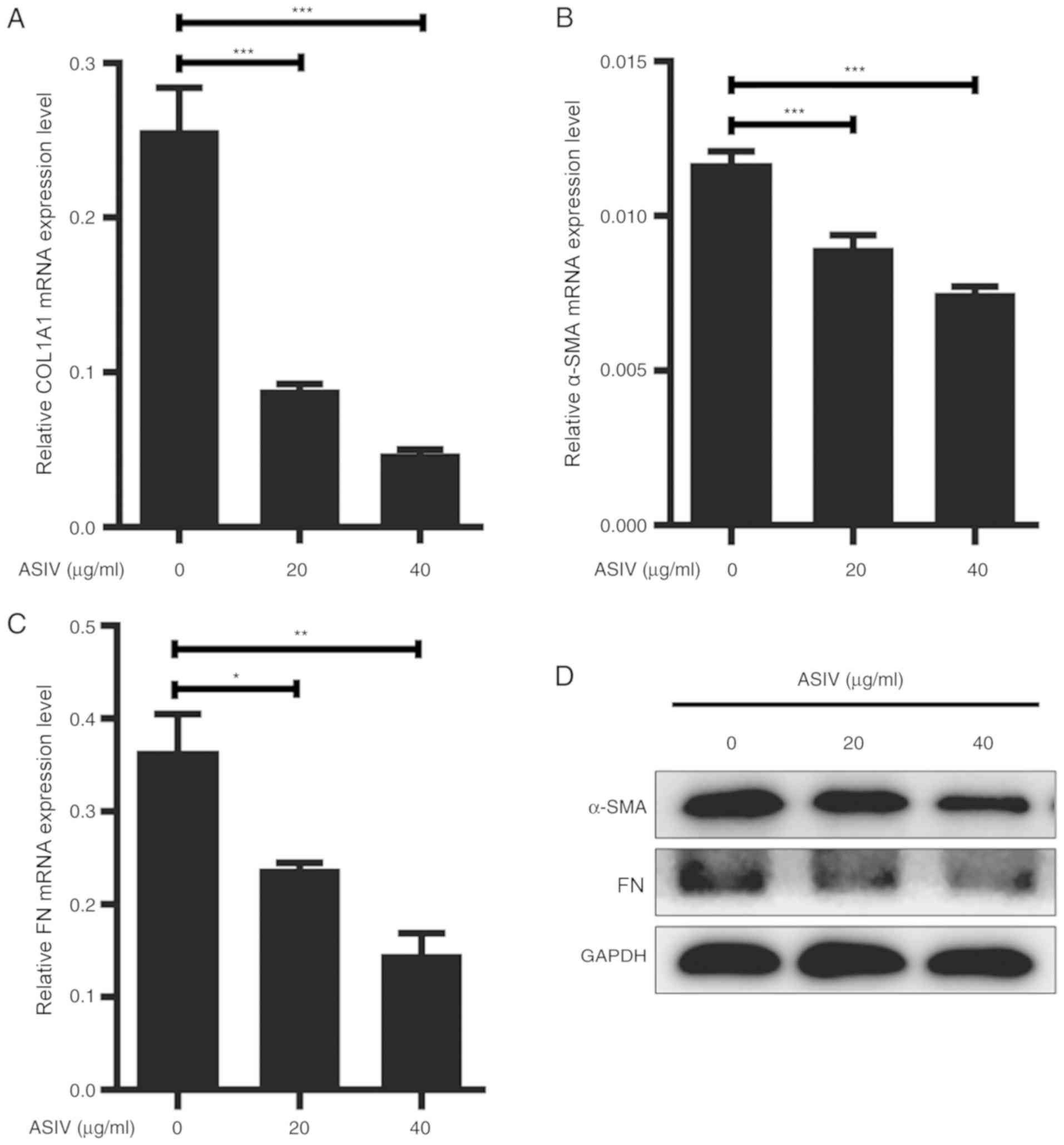
ASIV suppresses ECM expression in
PDGF-BB-activated HSC-T6
Hepatic fibrosis is characterized by the excessive
secretion and accumulation of ECM by activated HSCs (36). ECM components, mainly including
α-SMA, COL1A1 and FN, were used as the marker for HSC activation.
Therefore, the present study aimed to investigate the effects of
ASIV on HSC activation. PDGF-BB-activated HSC-T6 were treated with
varying concentrations of ASIV (0, 20 or 40 µg/ml) for 48 h. The
expression levels of COL1A1, α-SMA and FN were subsequently
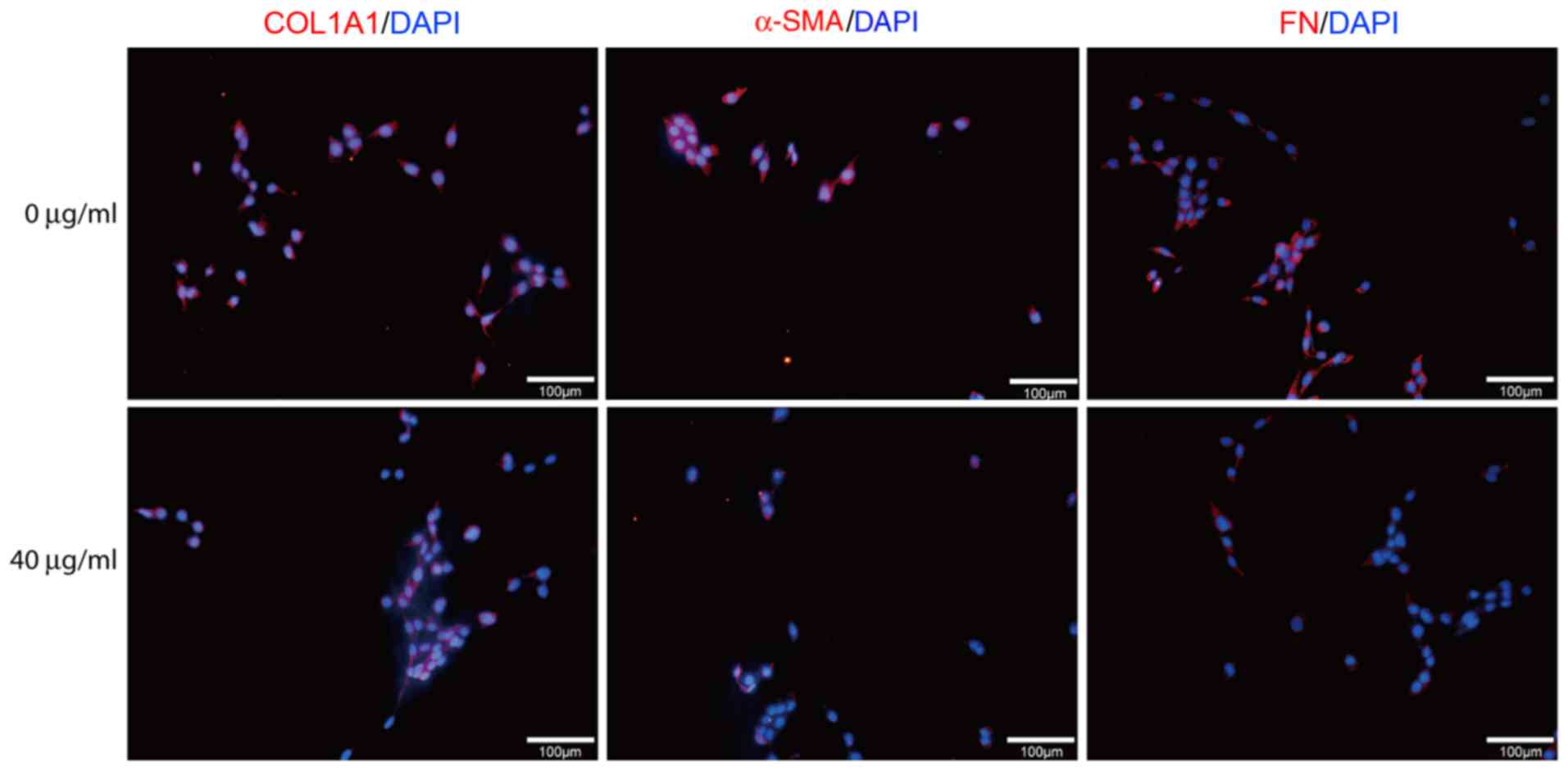
measured using RT-qPCR, western blotting or immunofluorescence
staining. The relative mRNA expression levels of COL1A1, α-SMA and
FN mRNA were significantly reduced following ASIV treatment in a
dose-dependent manner compared with untreated cells (Fig. 2A-C). On the protein level, data from
western blot analysis and immunofluorescence showed that ASVI
treatment downregulated the expression of COL1A1, α-SMA and FN in
HSC-T6. (Figs. 2D and 3).
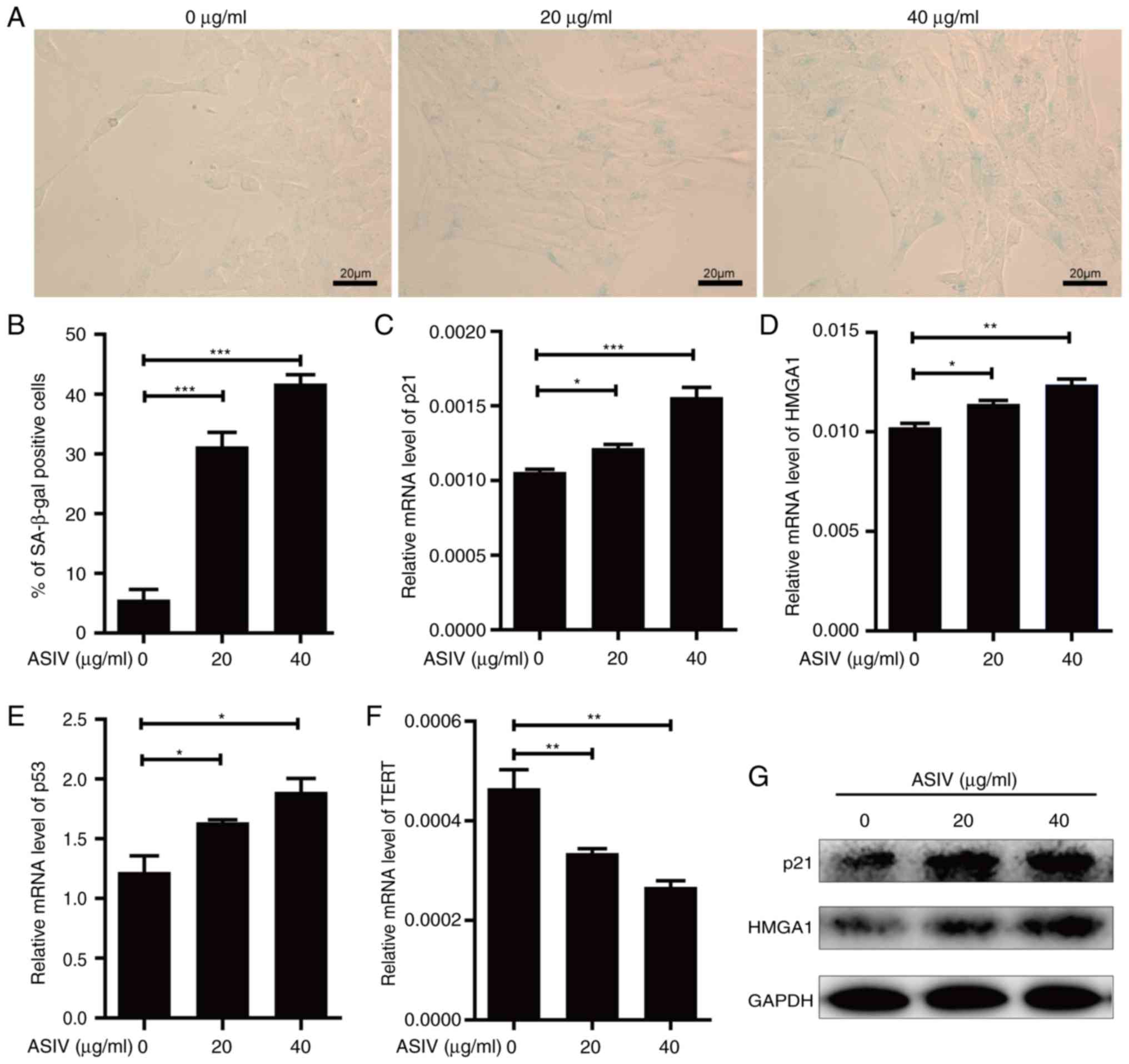
ASIV promotes cellular senescence in
PDGF-BB-activated HSC-T6
Induction of cellular senescence in activated HSCs
would be beneficial to the prevention of hepatic fibrosis (37). Therefore, the activity of SA-β-gal, a
common biomarker of senescence, was measured in activated HSCs in
the presence of ASIV. Following ASIV treatment, SA-β-gal activity
was revealed to be significantly enhanced in PDGF-BB-activated
HSC-T6 (Fig. 4A and B). Supporting
this, mRNA of senescence markers, p21, p53 and HMGA1 was
significantly upregulated (Fig.
4C-E), while the protein expression of p53 and HMGA1 were
markedly upregulated (Fig. 4G).
Conversely, the corresponding mRNA levels of TERT were
significantly reduced by ASIV treatment (Fig. 4F). In addition, all the observations
made in this analysis appeared to display a dose-dependent trend
compared with the untreated control (Fig. 4). These results suggest that ASIV
treatment promoted senescence in activated HSC-T6.
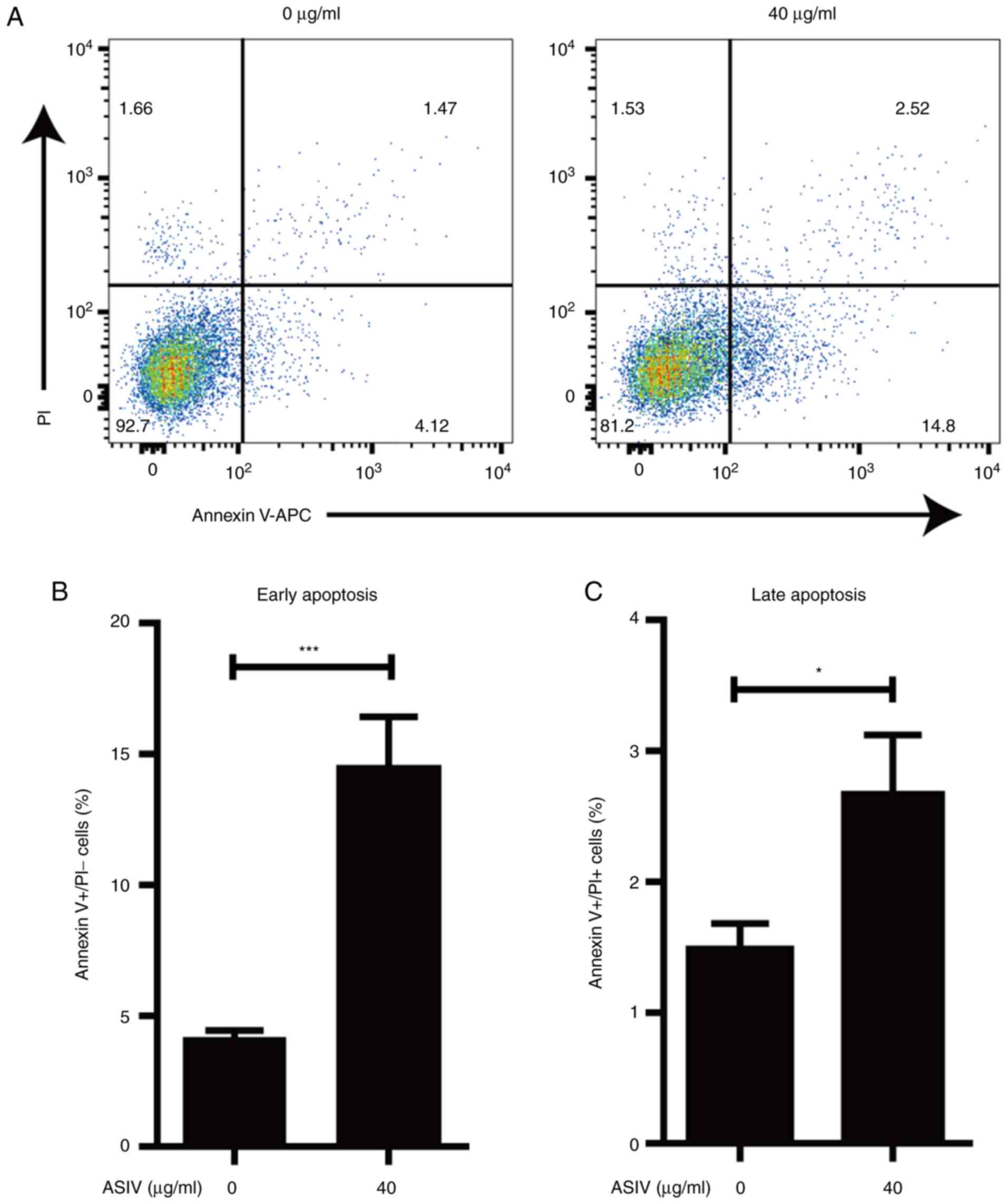
ASIV induces apoptosis in
PDGF-BB-activated HSC-T6
Apoptosis of activated HSCs contributed to the
resolution of hepatic fibrosis (38). To investigate the effect of ASIV on
the survival of PDGF-BB-activated HSC-T6, apoptosis was measured
using flow-cytometry. The percentage of early and late apoptotic
cells was revealed to be 2–3 times higher in the ASIV treatment
group compared with the control group (Fig. 5A-C). At the same time, the result
from flow-cytometry indicated that ASIV did not affect the necrotic
cell death in PDGF-BB-activated HSC-T6 (Fig. 5A). These data support the notion that
ASIV treatment accelerated apoptosis in addition to promoting
cellular senescence in activated HSC-T6.
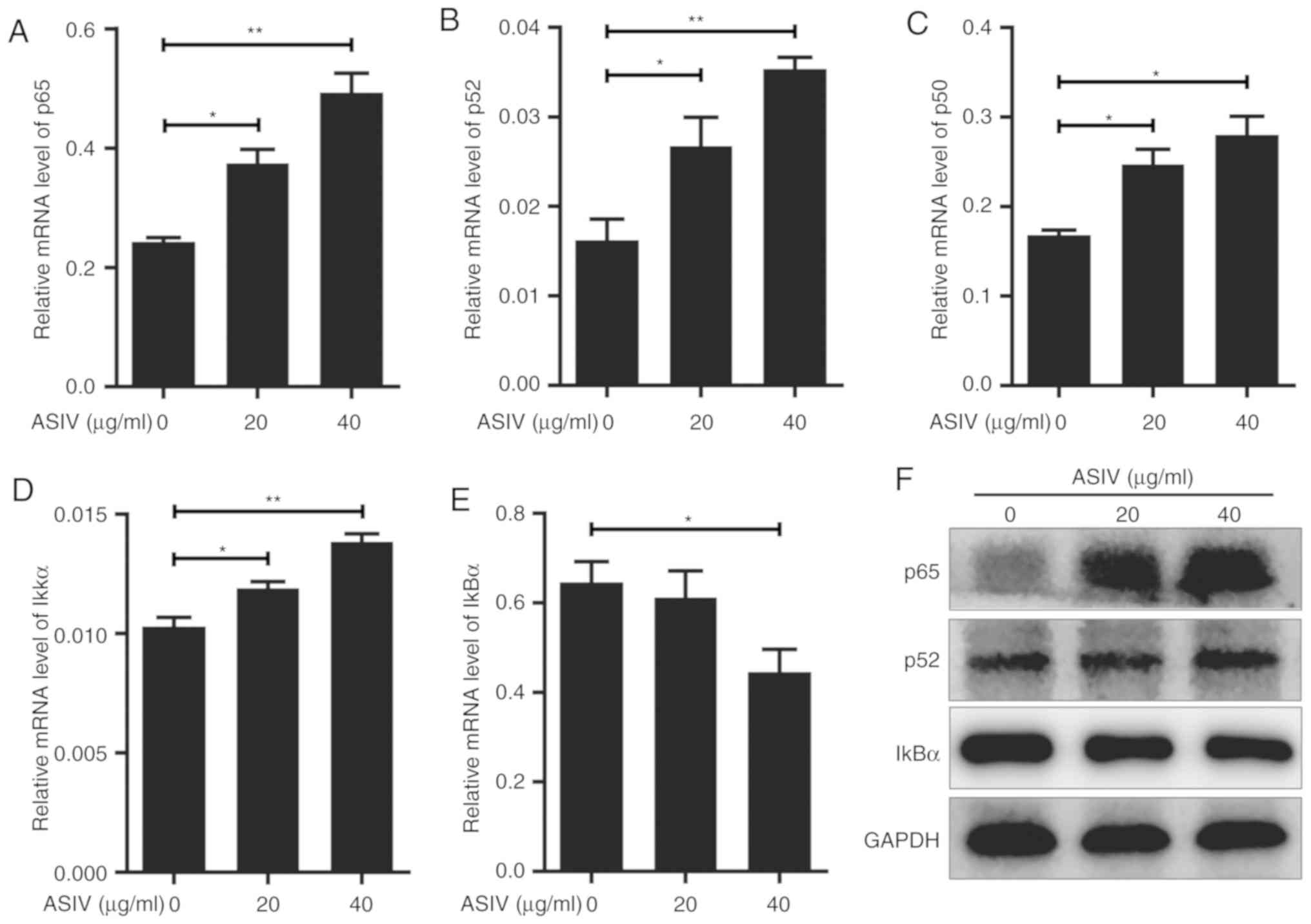
ASIV regulates the NF-κB signaling
pathway in PDGF-BB-activated HSC-T6
The NF-κB signaling pathway is involved in
significant physiological and biochemical processes including
cellular senescence and apoptosis (39,40). To
investigate the relationship between NF-κB signaling and
PDGF-BB-activated HSC-T6 in the presence of ASIV, expression levels
of p65, p52 and p50, components of the NF-κB protein family, were
analyzed. On mRNA level, p65, p52 and p50 were significantly
upregulated by ASIV treatment in a dose-dependent manner (Fig. 6A-C), whereas RelB and c-Rel mRNA
expression were not detectable in HSC-T6 using RT-qPCR. Western
blot analysis appeared to support the upregulation of p65 and p52
on protein level in PDGF-BB-activated HSC-T6 (Fig. 6F). To investigate the effect of ASIV
on the NF-κB signaling pathway further, the expression levels of
IκKα and IκBα, upstream regulatory components of the signaling
pathway, were measured. RT-qPCR results revealed that ASIV
treatment increased IκKα mRNA expression and decreased IκBα mRNA
expression (Fig. 6D and E,
respectively). ASIV also reduced IκBα protein expression, according
western blot analysis (Fig. 6F).
Discussion
The activation of HSCs marks a significant event in
the hepatic fibrosis process. Activated HSCs adversely disrupt the
balance between ECM production and proteolysis due to decreased
expression of matrix metalloproteinases and increased expression of
tissue inhibitors of metalloproteinases (37). Large amounts of collagen (types I,
III, IV, V and VI), α-SMA and FN are deposited by activated HSCs.
If the suppression of HSC activation can be achieved, the degree of
hepatic fibrosis could be considerably reduced or even reversed
(41). Therefore, investigations
into inhibiting HSC activation have become hotspots of research
into hepatic fibrosis. In the present study, ASIV treatment
downregulated the expression of fibrosis markers COL1A1, α-SMA and
FN to suppress PDGF-BB-induced HSC-T6 activation. Indeed, previous
studies demonstrated that ASIV inhibited collagen synthesis in
activated HSCs by regulating p38 MAPK pathway (32,34).
Evidence presented above demonstrated that ASIV can suppress a
variety of factors leading to HSCs activation. As the principal
mediator of hepatic fibrosis, data in the present study indicated
ASIV could be used as a potentially therapeutic agent for hepatic
fibrosis treatment.
Induction of cellular senescence and apoptosis are
the most effective methods of inhibiting HSC activation and
alleviating hepatic fibrosis (37).
During the senescence process, cell proliferation and ECM
expression become suppressed in activated HSCs (36). Further evidence exists demonstrating
that cellular senescence is involved in the upregulation of p53
(42). In particular, HSCs in
p53−/− mice exhibited senescence inhibition (8), and the knockdown of p53 in activated
HSCs suppressed the expression of p21, which lead to cellular
senescence (43). In the present
study, ASIV significantly increased the expression of p21 and p53
in PDGF-BB-activated HSC-T6, which indicated that cell senescence
was induced. In addition, ASIV enhanced the expression of the
senescence marker HMGA1 and activity of SA-β-gal, which have been
reported to result from cellular senescence (11,13). By
contrast, telomere shortening is a driving force behind replicative
senescence (44), and TERT is
involved in the maintenance of telomere length (45). ASIV treatment suppressed the mRNA
expression of TERT in PDGF-BB-activation HSC-T6 in the present
study. Taken together, these findings suggests that ASIV
upregulated the expression of senescence markers, concomitant with
downregulating fibrosis markers in activated HSC-T6 to attenuate
fibrosis.
Apoptosis is involved in the resolution of hepatic
fibrosis through a variety of intracellular signaling pathways
(46). A large number of
experimental and clinical chemotherapeutic agents including the
active components of Traditional Chinese Medicines have been
applied to inhibit hepatic fibrosis by inducing HSC apoptosis
(47–49). ASIV has been demonstrated to induce
apoptosis in osteosarcoma (50) and
vascular smooth muscle cells (51).
Data from the present study demonstrated that ASIV promoted
apoptosis but did not affect necrotic cell death in
PDGF-BB-activated HSC-T6. Crosstalk between cellular senescence and
apoptosis contributes to the inhibition of HSCs activation
(52). Therefore, these observations
suggested that ASIV suppressed the activation of HSC-T6 by
promoting cellular senescence and apoptosis.
A previous study demonstrated that NF-κB signaling
contributes to senescence, whereas decreasing the expression of
NF-κB components can bypass senescence (14). The main protein components that makes
up the NF-κB transcription factor include RelA/p65, RelB, c-Rel,
p52 (NF-κB2) and p50 (NF-κB1) (53).
The present study revealed that ASIV treatment upregulated the
expression of p65, p52 and p50 in PDGF-BB-activated HSC-T6; RelB
and c-Rel were undetectable possibly because of low expression.
This result suggested that ASIV increased NF-κB activity by
upregulating the expression of NF-κB components. In unstimulated
cells, inactive NF-κB heterodimers are sequestered into the
cytoplasm by associating with IκB proteins. In stimuli-activated
cells, the IKK complex induces the phosphorylation and degradation
of IκB, causing NF-κB heterodimers to be released and translocated
into the nucleus (54). In the
present study, ASIV treatment upregulated the expression of IKKα
and downregulated IκBα. This further indicated that ASIV promoted
HSCs senescence by upregulating the NF-κB signaling pathway. In
addition, ASIV induced apoptosis in activated HSCs, which indicated
a positive association between apoptosis and NF-κB signaling
activation in this cell type. This observation is consistent with
previous studies, where compounds including flavokawain B (55), daunorubicin (55), sodium benzoate (56) and nicotine (57) have been reported to induce apoptosis
by activating NF-κB signaling. The present study hypothesized that
ASIV promoted cellular senescence and apoptosis through regulating
expression of factors in NF-κB signaling pathway.
PDGF-BB, a mitogen and fibrogenic cytokine,
activates HSCs effectively through intracellular signaling pathways
including Ras/Raf/MEK, PI3K/Akt, STATs, Rho/ROCK and NF-κB
(58). A number of preclinical and
clinical investigations were performed to suppress hepatic fibrosis
by targeting PDGF signaling. In particular, silybin has been
demonstrated to exert anti-fibrotic effects by inhibiting the
phosphorylation of Raf, MEK and ERK (59), whereas celecoxib promoted HSC
apoptosis by suppressing Akt activation (60). A. membranaceus has been widely
used to treat hepatic fibrosis or cirrhosis in China (20). However, its molecular anti-fibrotic
mechanism remains unclear. ASIV, extracted from the plant A.
membranaceus, significantly enhanced the NF-κB signaling
pathway. Results from the present study provided useful indications
that A. membranaceus may be capable of alleviating hepatic
fibrosis and cirrhosis possibly by activating the NF-κB signaling
pathway, resulting in HSC senescence and apoptosis. Therefore, it
is worth exploring the comprehensive anti-fibrotic mechanism of
ASIV to design combination therapies using ASIV and clinical
medicine in treating hepatic fibrosis or cirrhosis.
Acknowledgements
Not applicable.
Funding
This research project was supported by grants from
The National Natural Science Foundation of China (grant no.
81573860), Science and Technology Research Program of Chongqing
Municipal Education Commission (grant no. KJQN201800432) and China
Postdoctoral Science Foundation (grant no. 2018M633330).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC performed the experiments. ZC, YL and WC designed
the study and wrote the manuscript. GW collected the samples. LY,
ZP, SP, JC and JW analyzed the data and interpreted the results.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun M and Kisseleva T: Reversibility of
liver fibrosis. Clin Res Hepatol Gastroenterol. 39 (Suppl
1):S60–S63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albanis E and Friedman SL: Hepatic
fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis.
5:315–334, v-vi. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duarte S, Baber J, Fujii T and Coito AJ:
Matrix metalloproteinases in liver injury, repair and fibrosis.
Matrix Biol. 44-46:147–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bitto N, Liguori E and La Mura V:
Coagulation, microenvironment and liver fibrosis. Cells. 7:E852018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hernandez-Gea V and Friedman SL:
Pathogenesis of liver fibrosis. Annu Rev Pathol. 6:425–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higashi T, Friedman SL and Hoshida Y:
Hepatic stellate cells as key target in liver fibrosis. Adv Drug
Deliv Rev. 121:27–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wong L, Yamasaki G, Johnson RJ and
Friedman SL: Induction of beta-platelet-derived growth factor
receptor in rat hepatic lipocytes during cellular activation in
vivo and in culture. J Clin Invest. 94:1563–1569. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krizhanovsky V, Yon M, Dickins RA, Hearn
S, Simon J, Miething C, Yee H, Zender L and Lowe SW: Senescence of
activated stellate cells limits liver fibrosis. Cell. 134:657–667.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong X, Feng D, Wang H, Hong F, Bertola A,
Wang FS and Gao B: Interleukin-22 induces hepatic stellate cell
senescence and restricts liver fibrosis in mice. Hepatology.
56:1150–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Yao Z, Zhao S, Shao J, Chen A,
Zhang F and Zheng S: Interaction between autophagy and senescence
is required for dihydroartemisinin to alleviate liver fibrosis.
Cell Death Dis. 8:e28862017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee BY, Han JA, Im JS, Morrone A, Johung
K, Goodwin EC, Kleijer WJ, DiMaio D and Hwang ES:
Senescence-associated beta-galactosidase is lysosomal
beta-galactosidase. Aging Cell. 5:187–195. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leontieva OV and Blagosklonny MV:
CDK4/6-inhibiting drug substitutes for p21 and p16 in senescence:
Duration of cell cycle arrest and MTOR activity determine
geroconversion. Cell Cycle. 12:3063–3069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Narita M, Narita M, Krizhanovsky V, Nuñez
S, Chicas A, Hearn SA, Myers MP and Lowe SW: A novel role for
high-mobility group a proteins in cellular senescence and
heterochromatin formation. Cell. 126:503–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chien Y, Scuoppo C, Wang X, Fang X,
Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, et al:
Control of the senescence-associated secretory phenotype by NF-κB
promotes senescence and enhances chemosensitivity. Genes Dev.
25:2125–2136. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osorio FG, Lopez-Otin C and Freije JM:
NF-κB in premature aging. Aging. 4:726–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duci SB, Arifi HM, Ahmeti HR, Zatriqi VK,
Buja ZA, Hoxha ET and Mekaj AY: Outcomes of older adults with burn
injury: University clinical center of kosovo. World J Plast Surg.
4:153–158. 2015.PubMed/NCBI
|
|
17
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arur S, Uche UE, Rezaul K, Fong M,
Scranton V, Cowan AE, Mohler W and Han DK: Annexin I is an
endogenous ligand that mediates apoptotic cell engulfment. Dev
Cell. 4:587–598. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mallat A and Lotersztajn S: Reversion of
hepatic stellate cell to a quiescent phenotype: From myth to
reality? J Hepatol. 59:383–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun WY, Wang L, Liu H, Li X and Wei W: A
standardized extract from Paeonia lactiflora and Astragalus
membranaceus attenuates liver fibrosis induced by porcine serum in
rats. Int J Mol Med. 29:491–498. 2012.PubMed/NCBI
|
|
21
|
Liu Y, Liu J, Wu KX, Guo XR and Tang ZH: A
rapid method for sensitive profiling of bioactive triterpene and
flavonoid from Astragalus mongholicus and Astragalus membranaceus
by ultra-pressure liquid chromatography with tandem mass
spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci.
1085:110–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mei M, Tang F, Lu M, He X, Wang H, Hou X,
Hu J, Xu C and Han R: Astragaloside IV attenuates apoptosis of
hypertrophic cardiomyocyte through inhibiting oxidative stress and
calpain-1 activation. Environ Toxicol Pharmacol. 40:764–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hao M, Liu Y, Chen P, Jiang H and Kuang
HY: Astragaloside IV protects RGC-5 cells against oxidative stress.
Neural Regen Res. 13:1081–1086. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang S, Mou J, Cui L, Wang X and Zhang Z:
Astragaloside IV inhibits cell proliferation of colorectal cancer
cell lines through down-regulation of B7-H3. Biomed Pharmacother.
102:1037–1044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang PP, Luan JJ, Xu WK, Wang L, Xu DJ,
Yang CY, Zhu YH and Wang YQ: Astragaloside IV downregulates the
expression of MDR1 in Bel7402/FU human hepatic cancer cells by
inhibiting the JNK/cJun/AP1 signaling pathway. Mol Med Rep.
16:2761–2766. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li LC, Xu L, Hu Y, Cui WJ, Cui WH, Zhou WC
and Kan LD: Astragaloside IV improves bleomycin-induced pulmonary
fibrosis in rats by attenuating extracellular matrix deposition.
Front Pharmacol. 8:5132017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qian W, Cai X, Qian Q, Zhang W and Wang D:
Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal
transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med.
22:4354–4364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan Y, Xu L, Wang Y, Tuerdi N, Ye M and Qi
R: Preventive effects of astragaloside IV and its active sapogenin
cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3
inflammasome. Eur J Pharmacol. 833:545–554. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen P, Xie Y, Shen E, Li GG, Yu Y, Zhang
CB, Yang Y, Zou Y, Ge J, Chen R and Chen H: Astragaloside IV
attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in
coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol.
658:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu W, Shao X, Tian L, Gu L, Zhang M, Wang
Q, Wu B, Wang L, Yao J, Xu X, et al: Astragaloside IV ameliorates
renal fibrosis via the inhibition of mitogen-activated protein
kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp
Ther. 350:552–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH,
Zhang XM, Peng W and Cai H: Astragaloside IV inhibits renal
tubulointerstitial fibrosis by blocking TGF-β/Smad signaling
pathway in vivo and in vitro. Exp Biol Med (Maywood).
239:1310–1324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Wei W, Sun WY and Li X: Protective
effects of astragaloside IV on porcine-serum-induced hepatic
fibrosis in rats and in vitro effects on hepatic stellate cells. J
Ethnopharmacol. 122:502–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ping J, Li JT, Liao ZX, Shang L and Wang
H: Indole-3-carbinol inhibits hepatic stellate cells proliferation
by blocking NADPH oxidase/reactive oxygen species/p38MAPK pathway.
Eur J Pharmacol. 650:656–662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Wang X, Han C, Wang X, Xing G, Zhou
L, Li G and Niu Y: Astragaloside IV suppresses collagen production
of activated hepatic stellate cells via oxidative stress-mediated
p38 MAPK pathway. Free Radic Biol Med. 60:168–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsuchida T and Friedman SL: Mechanisms of
hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol.
14:397–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang Y, Deng X and Liang J: Modulation of
hepatic stellate cells and reversibility of hepatic fibrosis. Exp
Cell Res. 352:420–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Oliveira da Silva B, Ramos LF and
Moraes KCM: Molecular interplays in hepatic stellate cells:
Apoptosis, senescence, and phenotype reversion as cellular
connections that modulate liver fibrosis. Cell Bio Int. 41:946–959.
2017. View Article : Google Scholar
|
|
39
|
Kang C, Xu Q, Martin TD, Li MZ, Demaria M,
Aron L, Lu T, Yankner BA, Campisi J and Elledge SJ: The DNA damage
response induces inflammation and senescence by inhibiting
autophagy of GATA. Science. 349:aaa56122015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thirukkumaran C, Shi ZQ, Thirukkumaran P,
Luider J, Kopciuk K, Spurrell J, Elzinga K and Morris D: PUMA and
NF-κB are cell signaling predictors of reovirus oncolysis of breast
cancer. PLoS One. 12:e01682332017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zoubek ME, Trautwein C and Strnad P:
Reversal of liver fibrosis: From fiction to reality. Best Pract Res
Clin Gastroenterol. 31:129–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rufini A, Tucci P, Celardo I and Melino G:
Senescence and aging: The critical roles of p53. Oncogene.
32:5129–5143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Pan J, Wang J, Song K, Zhu D,
Huang C and Duan Y: Soluble egg antigens of schistosoma japonicum
induce senescence in activated hepatic stellate cells by activation
of the STAT3/p53/p21 pathway. Sci Rep. 6:309572016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Campisi J and d'Adda di Fagagna F:
Cellular senescence: When bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao Z, Pan X, Liu L and Liu N: Telomere
length maintenance, shortening, and lengthening. J Cell Physiol.
229:1323–1329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duval F, Moreno-Cuevas JE, Gonzalez-Garza
MT, Rodriguez-Montalvo C and Cruz-Vega DE: Liver fibrosis and
protection mechanisms action of medicinal plants targeting
apoptosis of hepatocytes and hepatic stellate cells. Adv Pharmacol
Sci. 2014:3732952014.PubMed/NCBI
|
|
47
|
Meng D, Li Z, Wang G, Ling L, Wu Y and
Zhang C: Carvedilol attenuates liver fibrosis by suppressing
autophagy and promoting apoptosis in hepatic stellate cells. Biomed
Pharmacother. 108:1617–1627. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Senoo T, Sasaki R, Akazawa Y, Ichikawa T,
Miuma S, Miyaaki H, Taura N and Nakao K: Geranylgeranylacetone
attenuates fibrogenic activity and induces apoptosis in cultured
human hepatic stellate cells and reduces liver fibrosis in carbon
tetrachloride-treated mice. BMC Gastroenterol. 18:342018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kuo LM, Chen PJ, Sung PJ, Chang YC, Ho CT,
Wu YH and Hwang TL: The bioactive extract of pinnigorgia sp.
induces apoptosis of hepatic stellate cells via
ROS-ERK/JNK-caspase-3 signaling. Mar Drugs. 16:E192018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu T, Fei Z and Wei N: Chemosensitive
effects of astragaloside IV in osteosarcoma cells via induction of
apoptosis and regulation of caspase-dependent Fas/FasL signaling.
Pharmacol Rep. 69:1159–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yuan W, Zhang Y, Ge Y, Yan M, Kuang R and
Zheng X: Astragaloside IV inhibits proliferation and promotes
apoptosis in rat vascular smooth muscle cells under high glucose
concentration in vitro. Planta Med. 74:1259–1264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Childs BG, Baker DJ, Kirkland JL, Campisi
J and van Deursen JM: Senescence and apoptosis: Dueling or
complementary cell fates? EMBO Rep. 15:1139–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xia Y, Shen S and Verma IM: NF-κB, an
active player in human cancers. Cancer Immunol Res. 2:823–830.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hayden MS and Ghosh S: Regulation of NF-κB
by TNF family cytokines. Semin Immunol. 26:253–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lee JJ, Koh KN, Park CJ, Jang S, Im HJ and
Kim N: The combination of flavokawain B and daunorubicin induces
apoptosis in human myeloid leukemic cells by modifying NF-κB.
Anticancer Res. 38:2771–2778. 2018.PubMed/NCBI
|
|
56
|
Yilmaz B and Karabay AZ: Food additive
sodium benzoate (NaB) activates NFkB and induces apoptosis in
HCT116 cells. Molecules. 23:E7232018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim CS, Choi JS, Joo SY, Bae EH, Ma SK,
Lee J and Kim SW: Nicotine-induced apoptosis in human renal
proximal tubular epithelial cells. PLoS One. 11:e01525912016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ying HZ, Chen Q, Zhang WY, Zhang HH, Ma Y,
Zhang SZ, Fang J and Yu CH: PDGF signaling pathway in hepatic
fibrosis pathogenesis and therapeutics (Review). Mol Med Rep.
16:7879–7889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Trappoliere M, Caligiuri A, Schmid M,
Bertolani C, Failli P, Vizzutti F, Novo E, di Manzano C, Marra F,
Loguercio C and Pinzani M: Silybin, a component of sylimarin,
exerts anti-inflammatory and anti-fibrogenic effects on human
hepatic stellate cells. J Hepatol. 50:1102–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Paik YH, Kim JK, Lee JI, Kang SH, Kim DY,
An SH, Lee SJ, Lee DK, Han KH, Chon CY, et al: Celecoxib induces
hepatic stellate cell apoptosis through inhibition of Akt
activation and suppresses hepatic fibrosis in rats. Gut.
58:1517–1527. 2009. View Article : Google Scholar : PubMed/NCBI
|