Introduction
Sepsis as a systemic inflammatory response syndrome
that is caused by severe infections (1). Patients with sepsis usually suffer from
insufficient blood flow and poor organ function, resulting in organ
failure, tissue hypoperfusion and mortality (2,3). It has
been reported that the prevalence of sepsis will be significantly
increased in the near future due to increased exposure to a number
of risk factors, such as systemic inflammatory diseases (4). In spite of efforts made to improve
sepsis therapeutic interventions, treatment outcomes remain
unsatisfactory. It is estimated that 30–70% patients will succumb
to sepsis during hospitalization and this unacceptable high
mortality rate did not decrease significantly during the past
several years (5,6).
Long non-coding RNAs (lncRNAs) are a group of
non-protein coding RNAs composed of >200 nucleotides (7). In spite of the lack of protein-coding
ability, lncRNAs directly participate in physiological and
pathological processes (8). The
involvement of lncRNAs in sepsis has also been reported by previous
studies, and certain lncRNAs are considered good diagnostic markers
or therapeutic targets for sepsis (9,10). In
particular, the lncRNA maternally expressed 3 (MEG3) is a well
characterized lncRNA in cancer biology (11,12).
Preliminary microarray data from our study suggested that lncRNA
MEG3 expression was also altered in the plasma of patients with
sepsis, indicating its involvement in this disease. The present
study revealed that lncRNA MEG3 overexpression may be involved in
sepsis, and the downregulation of lncRNA MEG3 may serve as a
potential therapeutic target for sepsis.
Materials and methods
Specimens and cell lines
Plasma samples were obtained before treatment from
82 patients with sepsis (sex, 45 males and 37 females; age range,
28.3–69.5 years; mean age, 49.1±6.1 years) who were admitted to the
Shanghai Jiaotong University Affiliated Sixth People's Hospital
South Campus (Shanghai, China) from March 2016 to March 2018.
Inclusion criteria: i) Patients with sepsis with complete medical
records including history of previous diseases and treatment; and
ii) patients understood the experimental protocol and signed
informed consent. Exclusion criteria: i) Patients additively
afflicted with other diseases, such as cancer and metabolic
diseases; and ii) patients who were treated by any therapies prior
to admission. This study also included 54 healthy controls (sex, 30
males and 24 females; age range, 29.1–66.2 years; mean age,
48.4±5.6 years) from Shanghai Jiaotong University Affiliated Sixth
People's Hospital South Campus recruited during the same time
period. All healthy controls also signed informed consent. This
study was approved by the Ethics Committee of Shanghai Jiaotong
University Affiliated Sixth People's Hospital South Campus
(Shanghai, China). All patients were followed-up for 6 months after
admission to record their survival.
Human primary renal mixed epithelial cells
(ATCC® PCS-400-012™) were purchased from American Type
Culture Collection. The AC16 human cardiomyocyte cell line was
purchased from Sigma-Aldrich (Merck KGaA). Cells were cultivated in
DMEM medium (Sigma-Aldrich; Merck KGaA) containing 10% FBS
(Sigma-Aldrich; Merck KGaA) at 37°C with 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from plamsa, A16
cardiomyocytes and renal mixed epithelial cells using
Monarch® Total RNA Miniprep kit (New England BioLabs,
Inc.) according to manufacturer's protocol, followed by reverse
transcription using SuperScript™ III Reverse Transcriptase (Thermo
Fisher Scientific., lnc.) and preparation of qPCR reactions using
SYBR® Green Quantitative RT-qPCR kit (Sigma-Aldrich;
Merck KGaA), according to the manufacturers' protocols. Primers of
lncRNA MEG3 and β-actin endogous control were designed and
synthesized by Shanghai GenePharma Co., Ltd. The following primers
were used: lncRNA MEG3 forward, 5′-CTGCCCATCTACACCTCACG-3′ and
reverse, 5′-CTCTCCGCCGTCTGCGCTAGGGGCT-3′; and β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′.
Using the 2−ΔΔCq method (13), lncRNA MEG3 expression was normalized
to β-actin endogenous control.
Cell transfection
Vectors (pcDNA3) expressing lncRNA MEG3, empty
pcDNA3 vectors, lncRNA MEG3 siRNA (5′-CCTCTTACCAAAAGACTTA-3′) and
scrambled negative control siRNA (5′-UGUGCAACGUCCGUCGAAGA-3′) were
designed and synthesized by Sangon Biotech Co., Ltd. Vectors (10
nM) and siRNAs sequences (40 nM) were transfected into renal
epithelial cells (1×105 cells/ml) and AC16 human
cardiomyocytes (1×105 cell/ml) using
Lipofectamine® 2000 reagent according to the
manufacturer's protocol. Cells only treated with Lipofectamine 2000
reagent were designated control cells whereas cells transfected
with either the empty vector or scrambled negative control siRNA
were were designated negative controls cells.
Cell apoptosis assay
Expression of lncRNA MEG3 was detected using RT-qPCR
24 h after transfection. Cell apoptosis was detected only in cases
where the overexpression rate reached 200% or the knockdown rate
reached 50% 24 h post-transfection. Briefly, cells were harvested
and cell suspensions at a density of 4×104 cells/ml were
prepared, which were then seeded into a six-well plate with 2
ml/well and treated with lipopolysaccharide (LPS, 10 mg/l;
Sigma-Aldrich; Merck KGaA) to induce apoptosis. Cells were then
cultured in 37°C under 5% CO2 for 48 h before digestion
with 0.25% trypsin, followed centrifugation at 1200 × g for 10 min
at room temperature. The harvested cells were subjected to staining
with Annexin V-fluorescein isothiocyanate and propidium iodide
using the Annexin V-FITC Apoptosis Staining/Detection kit (cat. no.
ab14085; Abcam) according to manufacturer's protocol (Dojindo
Molecular Technologies, Inc.). Apoptotic cells were detected and
counted using flow cytometry (FCS Express 6 flow cytometry
software; De Novo Software).
Statistical analysis
GraphPad Prism software (version 6; GraphPad
Software, Inc.) was used for all statistical analyzes. Youden's
index was used to determine the cut-off values. All experiments in
this study were performed in triplicate and data are presented as
the mean ± standard deviation or rates. Comparison of mortality
frequencies was performed using the χ2 test. Comparisons
of lncRNA MEG3 expression levels between sepsis patients and
healthy controls were performed using unpaired t-test. Comparisons
of cell apoptotic rates between 3 groups were performed using
one-way ANOVA followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
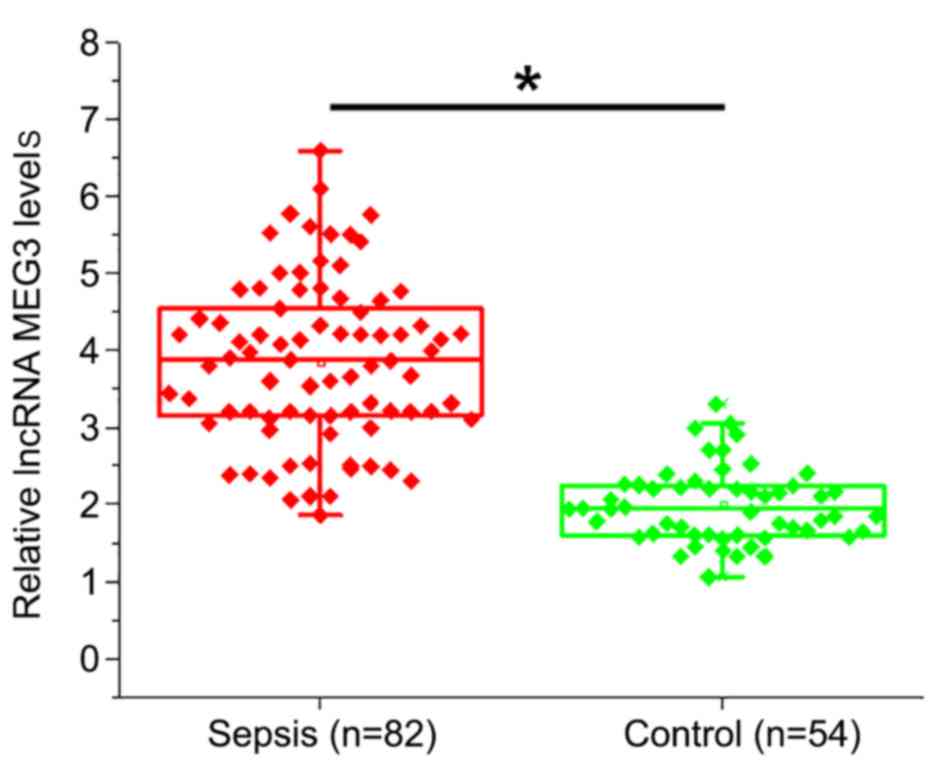
Plasma levels of lncRNA MEG3 are
significantly higher in sepsis patients compared with healthy
controls
Expression of lncRNA MEG3 in plasma samples of
patients with sepsis and healthy controls was detected using
RT-qPCR (Fig. 1). Compared with
healthy controls, plasma levels of lncRNA MEG3 were significantly
higher in patients with sepsis (P<0.05).
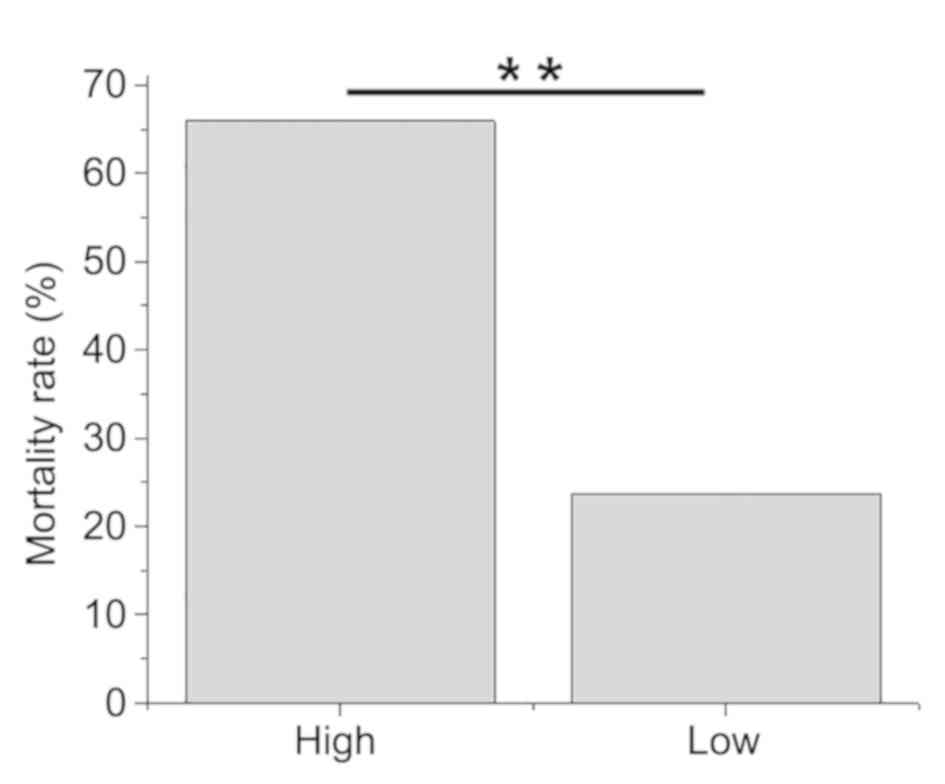
High lncRNA MEG3 plasma levels are
closely associated with higher mortality rates in sepsis
patients
According to Youden's index, patients were allocated
into high (n=44) and low (n=38) lncRNA MEG3 expression groups. A
total of 38 patients succumbed during hospitalization, including 29
cases in the high lncRNA MEG3 expression group, accounting for
65.9% of this group. A total of 9 cases of mortality were observed
in the low lncRNA MEG3 expression group, accounting for 23.7% of
this group. Patients from the high lncRNA MEG3 expression group
demonstrated significantly higher mortality rates compared with
those from the low lncRNA MEG3 expression group
(χ2=14.6201; P<0.01; Fig.
2).
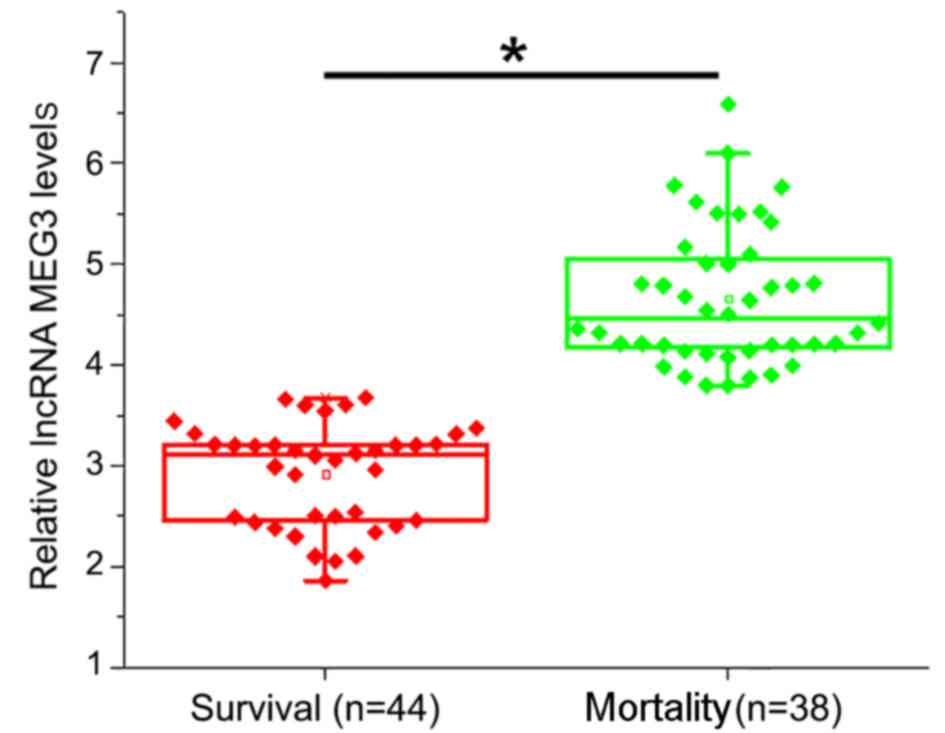
Higher pre-therapy lncRNA MEG3 plasma
levels are associated with higher mortality rates
According to treatment outcomes, patients were
divided into the survival (n=44) and mortality (n=38) groups. It
was observed that pre-therapy levels of lncRNA MEG3 in the plasma
were significantly higher in the mortality group compared with the
survival group (P<0.05; Fig.
3).
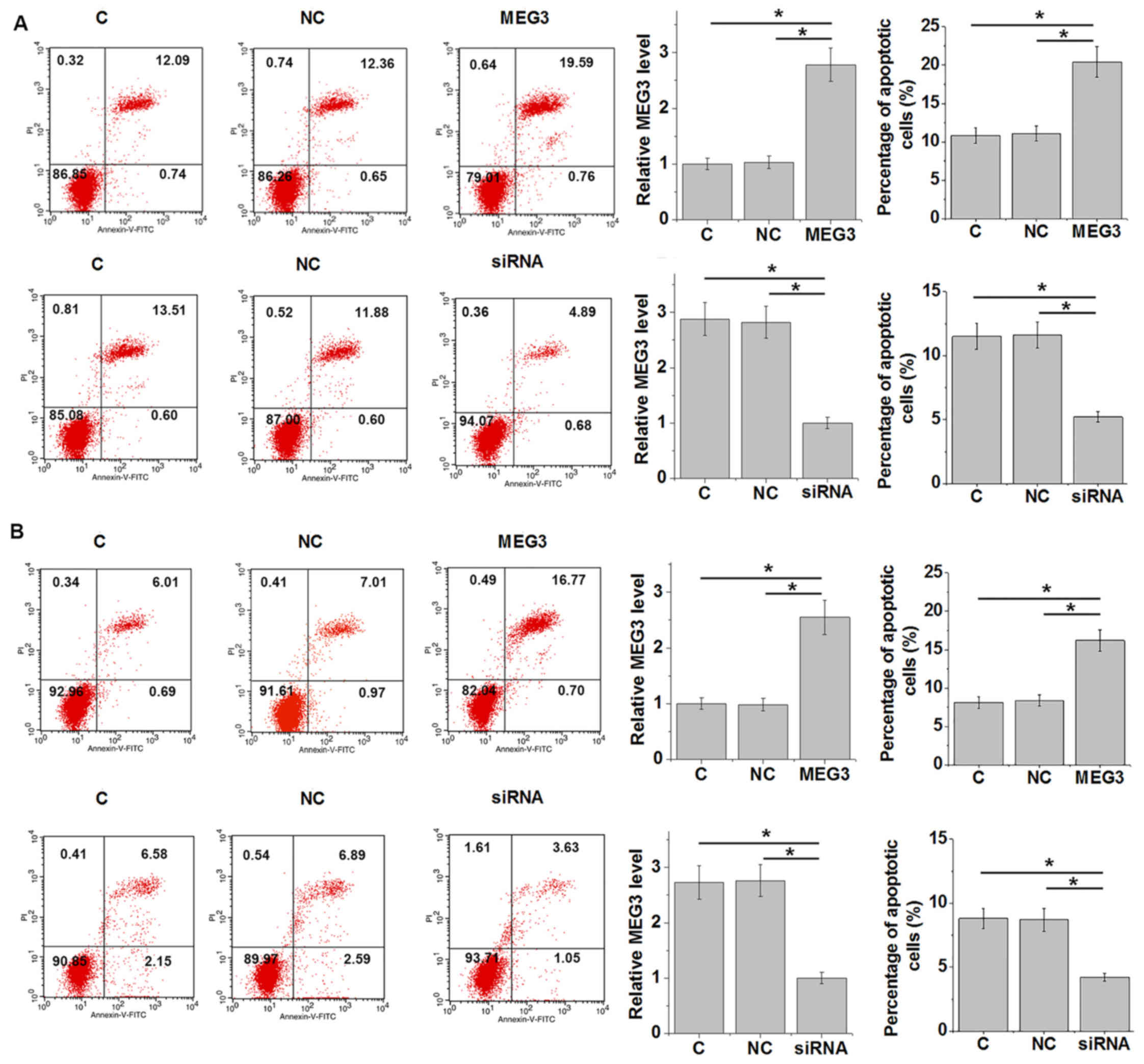
LncRNA MEG3 regulates renal epithelial
cell and cardiomyocyte apoptosis in the presence of
lipopolysaccharide (LPS)
Cell apoptosis serves an important role in organ
failure and death as a result of sepsis (1). Successful knockdown and overexpression
of MEG3 were confirmed (Fig. 4A and
B). In the presence of LPS treatment, lncRNA MEG3
overexpression and siRNA knockdown resulted in increased and
reduced renal epithelial cell (Fig.
4A) and cardiomyocyte (Fig. 4B)
apoptosis, respectively, compared with their respective controls
(P<0.05). For renal epithelial cells, MEG3 overexpression
increased the cell apoptotic rate from ~13% at control to >20%,
whilst MEG3 knockdown decreased the cell apoptotic rate from ~12%
to <6% (Fig. 4A). For
cardiomyocytes, MEG3 overexpression increased the cell apoptotic
rate from ~8% at control to >17%, whereas MEG3 knockdown
decreased the cell apoptotic rate from ~9% to <5% (Fig. 4B).
Discussion
The role of MEG3 as a tumor suppressor lncRNA has
been well documented in a number of different malignancies
(11,12). The key finding of the present study
is that lncRNA MEG3 is upregulated during sepsis and downregulation
of this lncRNA may serve as a potential therapeutic strategy for
this disease by inhibiting apoptosis in different cell types.
Previous microarray studies revealed that the
development and progression of sepsis is accompanied by changes in
the expression patterns of a large set of lncRNAs (14), suggesting the involvement of lncRNAs
in this disease. The differentially expressed lncRNAs promote or
inhibit sepsis by participating in the gene interaction network
(15). Certain lncRNAs have been
proposed as potential biomarkers for sepsis (16). As a tumor suppressor, lncRNA MEG3 is
frequently downregulated in a variety of human malignancies, and
its overexpression has been demonstrated to inhibit tumor growth
and development by affecting physiological processes, including
cancer cell proliferation and apoptosis induction (17). The present study found that lncRNA
MEG3 expression was upregulated in the plasma samples of sepsis
patients compared with healthy controls, and high expression levels
of lncRNA MEG3 in the plasma were significantly associated with
high mortality rates. Therefore, these observations suggest that
pre-therapy levels of plasma lncRNA MEG3 may serve as a potentially
useful predictor of poor outcome from sepsis.
The progression of sepsis results in the apoptosis
of different cell types (18,19),
which in turn leads to multiple organ failure and mortality
(18,19). Therefore, development of therapeutic
interventions to inhibit cell apoptosis remains a challenge in the
treatment of sepsis. Heart failure and kidney dysfunction are
common complications in patients with sepsis (20). In the present study LPS was used to
establish a model of sepsis (21).
It was found that lncRNA MEG3 overexpression and knockdown
increased and inhibited LPS-induced apoptosis of renal epithelial
cells and cardiomyocytes, respectively. Therefore, lncRNA MEG3
knockdown may serve as a potential therapeutic target for the
treatment of sepsis by inhibiting cell apoptosis in important
organs. However, the molecular mechanism of the regulation of cell
apoptosis by MEG3 remains unclear, which warrants further
study.
Although lncRNA MEG3 exhibited different expression
patterns in cancer (downregulation) and sepsis (upregulation)
(17), lncRNA MEG3 promotes cell
apoptosis in both types of disease, indicating potentially similar
functions of lncRNA MEG3 in regulating cell apoptosis across
different diseases. Indeed, it has been previously shown that
lncRNA MEG3 may interact with p53 to regulate cancer cell apoptosis
(17). Therefore, lncRNA MEG3 may be
involved in the pathogenesis of sepsis by a similar mechanism.
In conclusion, lncRNA MEG3 is overexpressed in
sepsis and downregulation of lncRNA MEG3 may serve as a potential
therapeutic target for sepsis by inhibiting cell apoptosis in major
organs including the kidneys and the heart.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XS designed experiments. KC, XS and YJ performed
experiments. FW, QS and WX collected and analyzed data. XS drafted
the paper and all authors approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Shanghai Jiaotong University affiliated Sixth People's Hospital
South Campus (Shanghai, China). Individuals provided informed
consent for participation in the present study.
Patient consent for publication
Patients signed informed consent for potential
publication of the present paper.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu V, Escobar GJ, Greene JD, Soule J,
Whippy A, Angus DC and Iwashyna TJ: Hospital deaths in patients
with sepsis from 2 independent cohorts. JAMA. 312:90–92. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seymour CW, Rea TD, Kahn JM, Walkey AJ,
Yealy DM and Angus DC: Severe sepsis in pre-hospital emergency
care: Analysis of incidence, care, and outcome. Am J Respir Crit
Care Med. 186:1264–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Micek ST, Hampton N and Kollef M: Risk
factors and outcomes for ineffective empiric treatment of sepsis
caused by gram-negative pathogens: Stratification by onset of
infection. Antimicrob Agents Chemother. 62:e00007–e000018. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conde KAP, Silva E, Silva CO, Ferreira E,
Freitas FG, Castro I, Rea-Neto A, Grion CM, Moura AD, Lobo SM, et
al: Differences in sepsis treatment and outcomes between public and
private hospitals in Brazil: A multicenter observational study.
PLoS One. 8:e647902013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K;
International Forum of Acute Care Trialists, : Assessment of global
incidence and mortality of hospital-treated sepsis. Current
estimates and limitations. Am J Respir Crit Care Med. 193:259–272.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ho J, Chan H, Wong SH, Wang MH, Yu J, Xiao
Z, Liu X, Choi G, Leung CC, Wong WT, et al: The involvement of
regulatory non-coding RNAs in sepsis: A systematic review. Crit
Care. 20:3832016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Wang X, Yan X, Cheng X, He X and
Zheng W: LncRNA MALAT1 regulates sepsis-induced cardiac
inflammation and dysfunction via interaction with miR-125b and p38
MAPK/NFκB. Int Immunopharmacol. 55:69–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Modali SD, Parekh VI, Kebebew E and
Agarwal SK: Epigenetic regulation of the lncRNA MEG3 and its target
c-MET in pancreatic neuroendocrine tumors. Mol Endocrinol.
29:224–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Q, Qian Z, Yan D, Li L and Huang L:
LncRNA-MEG3 inhibits cell proliferation of endometrial carcinoma by
repressing Notch signaling. Biomed Pharmacother. 82:589–594. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shu Q: Differential expression profile of
long non-coding RNAs from Gr-1+ CD11b+ myeloid cells in response to
sepsis in mice (INM2P. 424). J Immunol. 192:56–57. 2014.
|
|
15
|
Pellegrina DVS, Severino P, Barbeiro HV,
de Souza HP, Machado MCC, Pinheiro-da-Silva F and Reis EM: Insights
into the function of long noncoding RNAs in sepsis revealed by gene
co-expression network analysis. Noncoding RNA. 3(pii):
E52017.PubMed/NCBI
|
|
16
|
Dai Y, Liang Z, Li Y, Li C and Chen L:
Circulating Long Noncoding RNAs as Potential Biomarkers of Sepsis:
A Preliminary Study. Genet Test Mol Biomarkers. 21:649–657. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu KH, Li W, Liu XH, Sun M, Zhang ML, Wu
WQ, Xie WP and Hou YY: Long non-coding RNA MEG3 inhibits NSCLC
cells proliferation and induces apoptosis by affecting p53
expression. BMC Cancer. 13:4612013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ward PA: Sepsis, apoptosis and complement.
Biochem Pharmacol. 76:1383–1388. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hotchkiss RS and Karl IE: Endothelial cell
apoptosis in sepsis: A case of habeas corpus? Crit Care Med.
32:901–902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schefold JC, Filippatos G, Hasenfuss G,
Anker SD and von Haehling S: Heart failure and kidney dysfunction:
Epidemiology, mechanisms and management. Nat Rev Nephrol.
12:610–623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanaka KA, Kurihara S, Shibakusa T, Chiba
Y and Mikami T: Cystine improves survival rates in a LPS-induced
sepsis mouse model. Clin Nutr. 34:1159–1165. 2015. View Article : Google Scholar : PubMed/NCBI
|