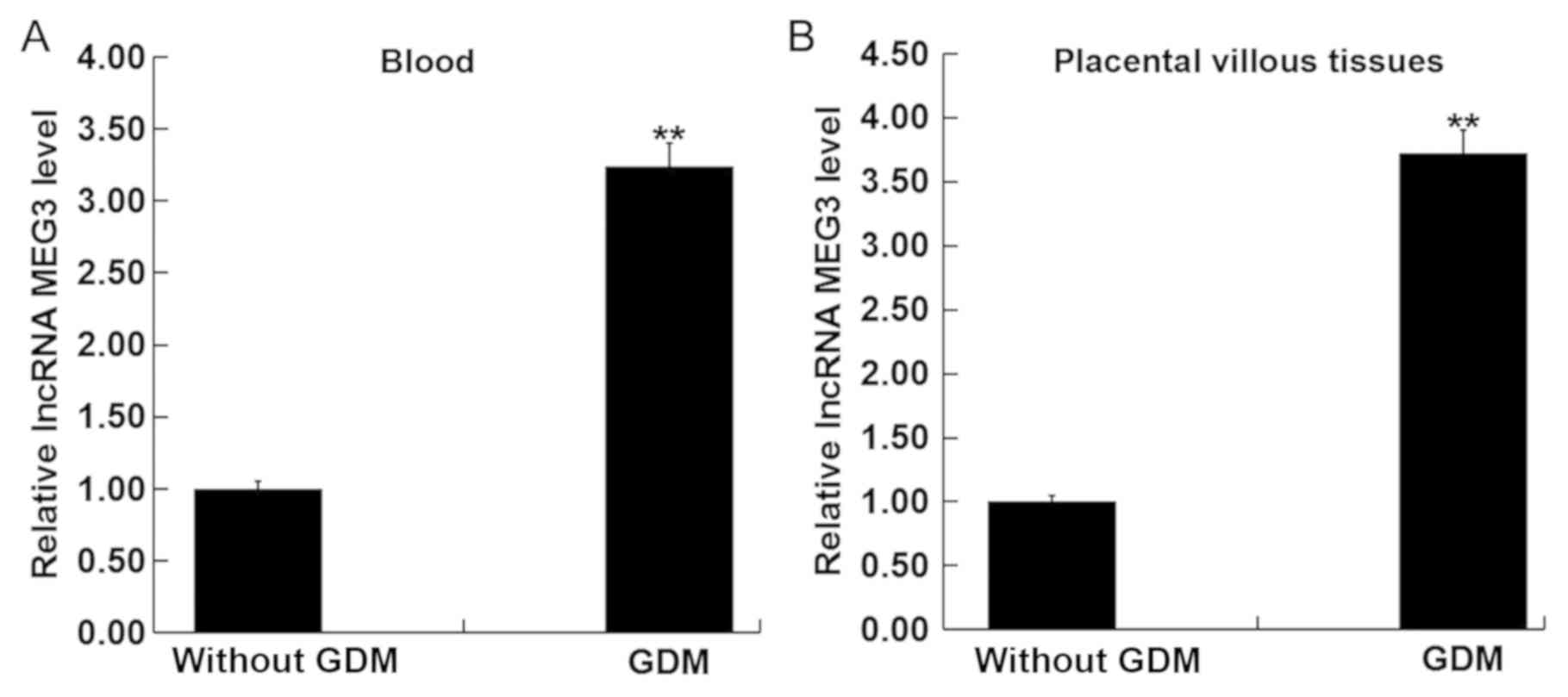
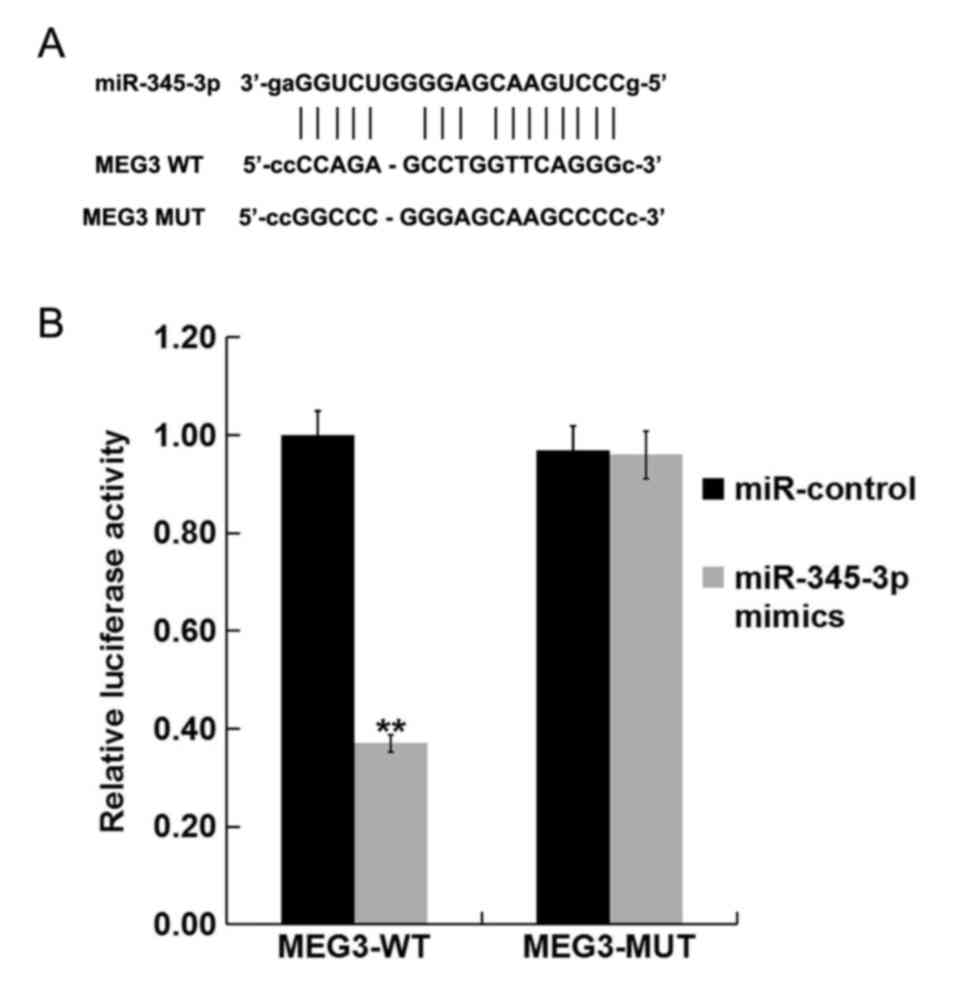
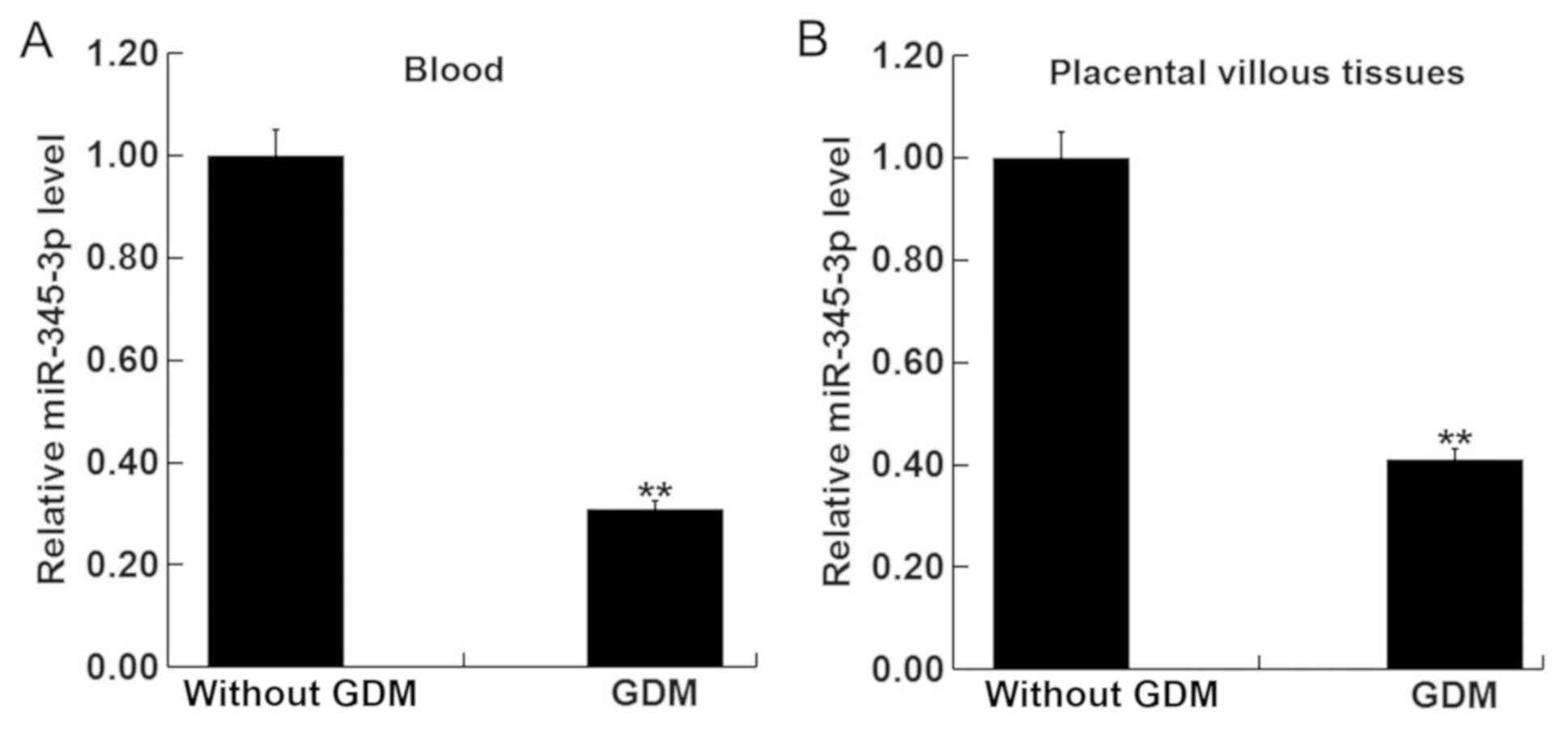
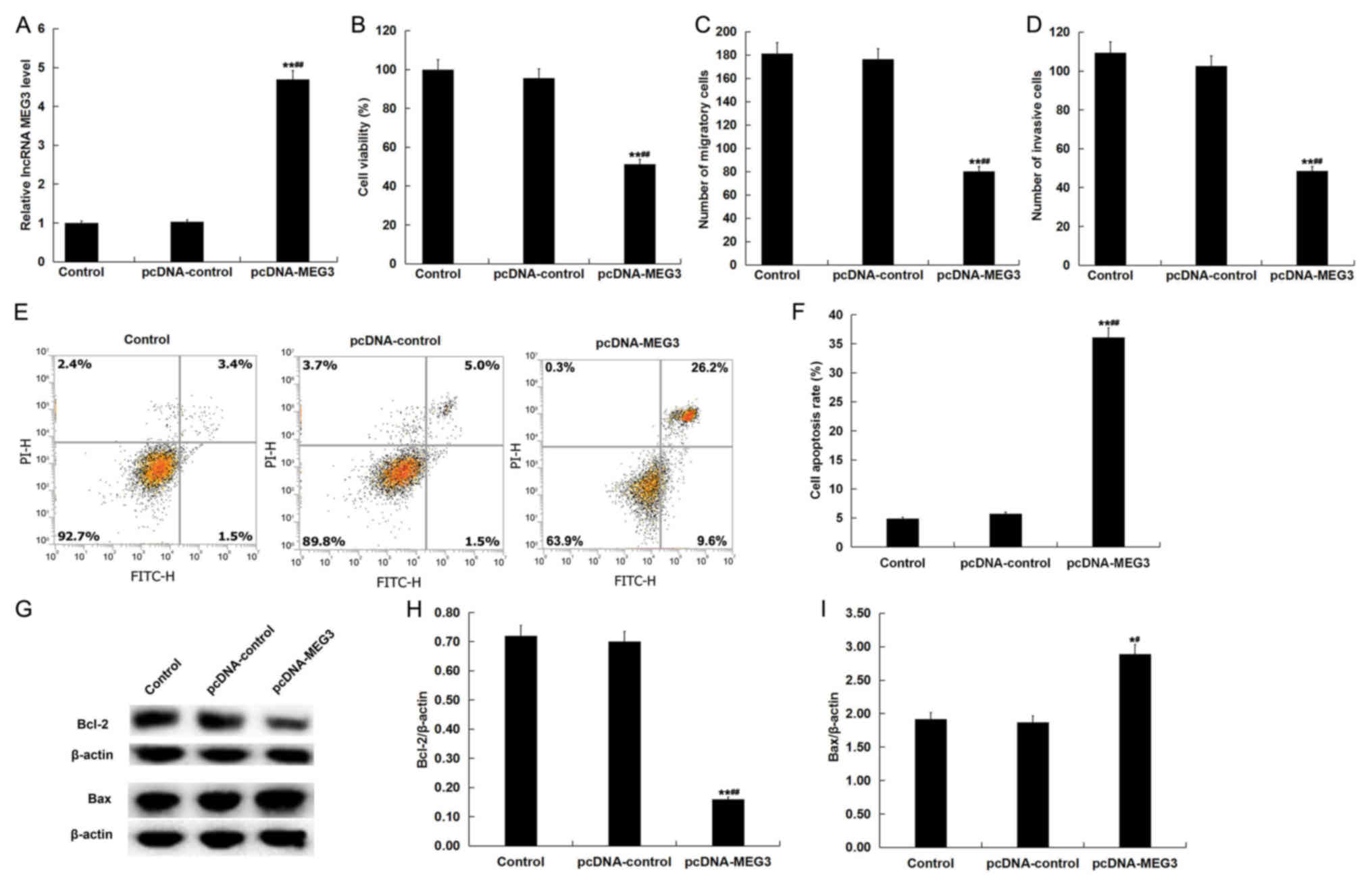
|
1
|
Surapaneni T, Nikhat I and Nirmalan PK:
Diagnostic effectiveness of 75 g oral glucose tolerance test for
gestational diabetes in India based on the international
association of the diabetes and pregnancy study groups guidelines.
Obstet Med. 6:125–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Djelmis J, Pavic M, Mulliqi Kotori V,
Pavlić Renar I, Ivanisevic M and Oreskovic S: Prevalence of
gestational diabetes mellitus according to IADPSG and NICE
criteria. Int J Gynaecol Obstet. 135:250–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liao EY: Endocrine and metabolic diseases.
Beijing. People's Medical Publishing House. 2012.5 ISBN:
9787117151153. https://baike.so.com/doc/
|
|
4
|
Egan AM, Vellinga A, Harreiter J, Simmons
D, Desoye G, Corcoy R, Adelantado JM, Devlieger R, Van Assche A,
Galjaard S, et al: Epidemiology of gestational diabetes mellitus
according to IADPSG/WHO 2013 criteria among obese pregnant women in
Europe. Diabetologia. 60:1913–1921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song L, Shen L, Li H, Liu B, Zheng X,
Zhang L, Xu S and Wang Y: Socio-economic status and risk of
gestational diabetes mellitus among Chinese women. Diabet Med.
34:1421–1427. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang F, Dong L, Zhang CP, Li B, Wen J,
Gao W, Sun S, Lv F, Tian H, Tuomilehto J, et al: Increasing
prevalence of gestational diabetes mellitus in Chinese women from
1999 to 2008. Diabet Med. 28:652–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leng J, Shao P, Zhang C, Tian H, Zhang F,
Zhang S, Dong L, Li L, Yu Z, Chan JC, et al: Prevalence of
gestational diabetes mellitus and its risk factors in Chinese
pregnant women: A prospective population-based study in Tianjin,
China. PLoS One. 10:e01210292015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho NH, Whitng D, Forouhi N, et al: IDF
diabetes atlas, 8th edn. Brussels. Internatonal Diabetes Federaton.
2017.
|
|
9
|
Mitanchez D, Yzydorczy C, Siddeek B,
Boubred F, Benahmed M and Simeoni U: The offspring of the diabetic
mother-short- and long-term implications. Best Pract Res Clin
Obstet Gynaecol. 29:256–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bowers K, Laughon SK, Kiely M, Brite J,
Chen Z and Zhang C: Gestational diabetes, pre-pregnancy obesity and
pregnancy weight gain in relation to excess fetal growth:
Variations by race/ethnicity. Diabetologia. 56:1263–1271. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kristensen P, Susser E, Irgens LM, Mehlum
IS, Corbett K and Bjerkedal T: The association of high birth weight
with intelligence in young adulthood: A cohort study of male
siblings. Am J Epidemiol. 180:876–884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tieu J, McPhee AJ, Crowther CA and
Middleton P: Screening and subsequent management for gestational
diabetes for improving maternal and infant health. Cochrane
Database Syst Rev CD007222. 2014. View Article : Google Scholar
|
|
13
|
Kung JT, Colognori D and Lee JT: Long
noncoding RNAs: Past, present, and future. Genetics. 193:651–669.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez Y and Huarte M: Long non-coding
RNAs: Challenges for diagnosis and therapies. Nucleic Acid Ther.
23:15–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li CH and Chen Y: Targeting long
non-coding RNAs in cancers: Progress and prospects. Int J Biochem
Cell Biol. 45:1895–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carter G, Miladinovic B, Patel AA, Deland
L, Mastorides S and Patel NA: Circulatng long noncoding RNA GAS5
levels are correlated to prevalence of type 2 diabetes mellitus.
BBA Clin. 4:102–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie X, Tang B, Xiao YF, Xie R, Li BS, Dong
H, Zhou JY and Yang SM: Long non-coding RNAs in colorectal cancer.
Oncotarget. 7:5226–5239. 2016.PubMed/NCBI
|
|
20
|
Lin SP, Youngson N, Takada S, Seitz H,
Reik W, Paulsen M, Cavaille J and Ferguson-Smith AC: Asymmetric
regulation of imprinting on the maternal and paternal chromosomes
at the Dlk1-Gtl2 imprinted cluster on mouse chromosome 12. Nat
Genet. 35:97–102. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J and Xu Y: The lncRNA MEG3
downregulation leads to osteoarthritis progression via miR-16/SMAD7
axis. Cell Biosci. 7:692017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhan R, Xu K, Pan J, Xu Q, Xu S and Shen
J: Long noncoding RNA MEG3 mediated angiogenesis after cerebral
infarction through regulating p53/NOX4 axis. Biochem Biophys Res
Commun. 490:700–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qiu GZ, Tian W, Fu HT, Li CP and Liu B:
Long noncoding RNA-MEG3 is involved in diabetes mellitus-related
microvascular dysfunction. Biochem Biophys Res Commun. 471:135–141.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu X, Wu YB, Zhou J and Kang DM:
Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via
increasing FoxO1 expression. Biochem Biophys Res Commun.
469:319–325. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang N, Zhu Y, Xie M, Wang L, Jin F, Li Y,
Yuan Q and De W: Long noncoding RNA Meg3 regulates mafa expression
in mouse beta cells by inactivating Rad21, Smc3 or Sin3α. Cell
Physiol Biochem. 45:2031–2043. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu J, Wu J, Zhao Z, Wang J and Chen Z:
Circulating LncRNA serve as fingerprint for gestational diabetes
mellitus associated with risk of macrosomia. Cell Physiol Biochem.
48:1012–1018. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Ren M, Zhao Y, Lu X, Wang M, Hu J,
Lu G and He S: MicroRNA-26a inhibits proliferation and metastasis
of human hepatocellular carcinoma by regulating DNMT3B-MEG3 axis.
Oncol Rep. 37:3527–3535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Wu H, Wang F, Ye M, Zhu H and Bu
S: Long non-coding RNA MALAT1 expression in pregnant women with
gestational diabetes mellitus. Int J Gynaecol Obstet. 140:164–169.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buchanan TA, Xiang A, Kjos SL and Watanabe
R: What is gestatonal diabetes? Diabetes Care. 30 (Suppl
2):S105–S111. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
American Diabetes Association (ADA), .
Standards of medical care in diabetes. Diabetes Care. 37:14–80.
2014. View Article : Google Scholar
|
|
33
|
Loegl J, Nussbaumer E, Cvitic S, Huppertz
B, Desoye G and Hiden U: GDM alters paracrine regulation of
feto-placental angiogenesis via the trophoblast. Lab Invest.
97:409–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Esteves JV, Yonamine CY, Pinto-Junior DC,
Gerlinger-Romero F, Enguita FJ and Machado UF: Diabetes modulates
MicroRNAs 29b-3p, 29c-3p, 199a-5p and 532-3p expression in muscle:
Possible role in GLUT4 and HK2 repression. Front Endocrinol
(Lausanne). 9:5362018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chavali V, Tyagi SC and Mishra PK:
Differential expression of dicer, miRNAs, and inflammatory markers
in diabetic Ins2+/- Akita hearts. Cell Biochem Biophys. 68:25–35.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji L, Brkić J, Liu M, Fu G, Peng C and
Wang YL: Placental trophoblast cell differentiation: Physiological
regulation and pathological relevance to preeclampsia. Mol Aspects
Med. 34:981–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weiss G, Sundl M, Glasner A, Huppertz B
and Moser G: The trophoblast plug during early pregnancy: A deeper
insight. Histochem Cell Biol. 146:749–756. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang L, Li Y, Wang C, Li N, Hou Y, Chang
R, Sun M, Wang R, Zhu L and Qiao C: Overexpression of collapsin
response mediator protein 1 inhibits human trophoblastcells
proliferation, migration, and Invasion. Reprod Sci. 26:954–960.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li G, Lin L, Wang YL and Yang H:
1,25(OH)2D3 protects trophoblasts against insulin resistance and
inflammation via suppressing mTOR signaling. Reprod Sci.
26:223–232. 2019. View Article : Google Scholar : PubMed/NCBI
|