Introduction
Sepsis is a serious clinical condition caused by
multiple agents, including bacterial, viral and fungal infections,
which subsequently initiates the inflammatory response, leading to
organ failure in the affected host (1). Currently, sepsis remains the primary
cause of death in intensive care units (ICU), despite recent
advancements in medical technology (2,3). It has
been estimated that in 2017, the percentage of admissions to the
ICU caused by sepsis is ~25% and is associated with a mortality
rate >50% worldwide (2,4). Although the precise pathophysiology of
sepsis remains unclear, increasing evidence has indicated that it
may move from an early hyper-inflammatory phase characterized by
systemic inflammation induced by the excessive release of
pro-inflammatory factors, followed by a late immuno-suppressive
phase, characterized by the apoptosis of immune cells (including
monocytes and lymphocytes) (5,6).
Furthermore, apoptosis that occurs in the cells of tissues in the
site of primary infection during sepsis can result in microvascular
dysfunction, which subsequently leads to organ failure (7,8).
Therefore, inhibiting sepsis-induced apoptosis may be a potential
therapeutic approach for patients with sepsis.
Long non-coding RNAs (lncRNAs) and microRNAs
(miRNAs) are two important ncRNAs, which are characterized by a
lack of protein encoding ability (9–12). These
ncRNAs have been demonstrated to be involved in multiple biological
processes, including apoptosis, proliferation and differentiation
(13–15). miRNAs are a type of short RNA
molecule consisting of ~20–22 nucleotides that negatively regulate
gene expression by binding to the 3′-untranslated regions (3′-UTR)
of the mRNA of a target gene (16).
Aberrant miRNA expression has been observed in a number of human
diseases, including cancer, neurodegenerative disorders and
inflammatory-associated disorders (17–19).
LncRNAs also participate in the progression of various human
diseases, including cancer (20,21),
cardiovascular disease (22) and
rheumatic diseases (23) by acting
as a miRNA sponge (24). LncRNA-hox
transcript antisense RNA (HOTAIR) has been previously reported to
function as an oncogenic molecule in a number of human malignancies
including lung (25), prostate
(26), gastric (27) and colorectal cancer (28), etc.. Recently, HOTAIR was observed to
be upregulated in mice and cardiomyocytes following
lipopolysaccharide (LPS)-induced sepsis, in which silencing HOTAIR
protected the cardiac function of septic mice by downregulating
tumor necrosis factor-α (TNF-α) via the NF-κB signaling pathway
(29).
It has been well documented that sepsis may be
mediated by multiple inflammatory cytokines, including TNF-α,
interleukin-6 (IL)-6 and IL-1β (30,31). In
addition, emerging studies have revealed an association between
plasma inflammatory cytokine concentrations and mortality in
patients with sepsis (30,32). In particular, the upregulation of
IL-6 and its receptor, IL-6R, has been frequently observed in
patients with sepsis and the production of IL-6 was demonstrated to
be a good prognostic agent in the early phase of sepsis (33,34).
These results indicate that IL-6 and its receptor may function as
two potential therapeutic targets for patients with sepsis.
In the present study, HOTAIR and IL-6R were revealed
to be targeted by miR-211; however, since it remains unknown how
the interaction between HOTAIR, IL-6R and miR-211 contribute to the
etiology of sepsis, the aim of the present study was to investigate
the effects of the HOTAIR/miR-211/IL-6R axis on the pathogenesis of
sepsis.
Materials and methods
Establishment of an animal model of
sepsis
C57BL/6 mice (age, 8 weeks; mean weight, 23.4±0.92
g; weight range, 22–25 g) were purchased from the Animal Experiment
Center of the Institute of Radiation Medicine of the Chinese
Academy of Medical Sciences and all of the animal protocols used in
the present study were approved by the Institute of Radiation
Medicine of the Chinese Academy of Medical Sciences. All the
animals were raised for seven days to adapt to the environment
prior to experimentation. Animals were raised with sufficient water
and feed, at a temperature of 20–24°C, humidity of 50–60% with a
12-h light/dark cycle. A total of 8 male C57BL/6 mice (8 weeks old)
were used to induce sepsis via cecal ligation and puncture (CLP).
After anesthetizing the mice with 2% pentobarbital sodium (50
mg/kg, intraperitoneally), a small incision was made in the
abdomen. The cecum of the mice was then exposed and a sterile
21-gauge needle was used to puncture the cecum twice to extrude
fecal matter. Subsequently, the cecum was returned into the
abdominal cavity and the incision was closed in two layers. Control
mice (n=8) were treated the same as the experimental animals, but
without CLP.
Monocytes isolation
Following the establishment of the mouse model 48 h
following CPL, C57BL/6 mice in the sepsis and control groups were
decapitated and the spleen was subsequently removed. After washing
with PBS, the spleen was broken by collagenase (cat. no. 17104019;
Gibco; Thermo Fisher Scientific, Inc.) for 5 mins at 37°C, filtered
through a 74 µm pore size strainer (BD Biosciences) to create a
single-cell suspension. Cell concentration was determined using a
hemocytometer (Hausser Scientific) and adjusted to 1×108
cells/ml. After which the mouse monocytes were purified using CD11b
MicroBeads (cat. no. 130-049-001; Miltenyi Biotec GmbH) according
to the manufacturer's protocol.
Cell culture
Monocytes were isolated from the spleens as
aforementioned and maintained in DMEM medium (Sigma-Aldrich; Merck
KGaA) containing 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) with 1% penicillin and streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator
with 5% CO2.
Transfections of miRNA mimics and
inhibitors
Negative control (NC or scramble for mimics and
inhibitors; 5′-UUCUCCGAACGUGUCACGUTT-3′), miR-211 mimics
(5′-UUCCCUUUGUCAUCCUUUGCCU-3′) and miR-211 inhibitors
(5′-AGGCAAAGGATGACAAAGGGAA-3′) were synthesized by Shanghai
GenePharma Co., Ltd. Monocytes (5×104 cells/well) were
seeded in 6-well plates and transfected with NC (50 nM), miR-211
mimics (50 nM) and miR-211 inhibitors (50 nM) using
Lipofectamine® 2000 Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to manufacturer's protocol. Subsequent
experiments were performed 48 h following transfection.
Vector construction and
transfection
DNA was extracted from the 293T cells using TIANamp
Genomic DNA Kit (Tiangen Biotech Co., Ltd.) according to
manufacturer's protocol. HOTAIR was amplified using Taq PCR Master
Mix Kit (Qiagen, Inc.) with XhoI and BamHI
restriction sites. The temperature protocol for the PCR consisted
of 94°C for 3 min; followed by 30 cycles of 94°C for 30 sec, 55°C
for 30 sec and 72°C 1 min; and final extension at 72°C for 5 min.
The primers for amplification were forward,
5′-CCGCTCGAGACATTCTGCCCTGATTTCCGGAACC-3′ and reverse,
5′-CGCGGATCCCCACCACACACACACAACCTACAC-3′. HOTAIR DNAs were inserted
into the pcDNA3.0 vector (Invitrogen; Thermo Fisher Scientific,
Inc.) according to previous studies (35,36). A
total of 1×105 monocytes were seeded into 6-well plates
and transfected with the HOTAIR-expression vector or empty vector
(control) using Lipofectamine® 2000 Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) assay
The total RNA isolated from the splenic tissues of
septic and control mice, monocytes transfected with HOTAIR, miR-211
mimics, miR-211 inhibitor and corresponding controls were all
prepared using the TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
cDNA was subsequently synthesized using PrimeScript™ RT Master Mix
(Takara Biotechnology Co., Ltd.) using 50 ng total RNA with the
temperature protocol consisting of 95°C for 30 sec and 60°C for 30
mins. The amplification of interferon (IFN)-γ, IL-6, IL-17, TNF-α,
IL-1β, IL-6R, miR-211 and HOTAIR was performed using a
Bestar® SYBR Green qPCR master mix (DBI Bioscience;
Shanghai Xinghan Biotechnology Co., Ltd.) kit using an ABI PRISM
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The thermocycling conditions were: 95°C for 2 min; followed by 30
cycles of 95°C 10 sec, and 60°C 34 sec. The primer sequences used
were listed in Table I. The
expressions of IFN-γ, IL-6, IL-17, TNF-α, IL-1β, IL-6R and HOTAIR
were normalized to the level of GAPDH, whereas miR-211 expression
was normalized to the level of U6. The relative expression levels
were analyzed using 2−ΔΔCq method (37).
 | Table I.Primer sequences for RT-qPCR assay
used in this study. |
Table I.
Primer sequences for RT-qPCR assay
used in this study.
| Gene | Sequence |
|---|
| GAPDH-F |
5′-TGTTCGTCATGGGTGTGAAC-3′ |
| GAPDH-R |
5′-ATGGCATGGACTGTGGTCAT-3′ |
| U6-F |
5′-CTCGCTTCGGCAGCACA-3′ |
| U6-R |
5′-AACGCTTCACGAATTTGCGT-3′ |
| IFN-γ-F |
5′-AGCGGATAATGGAACTCTTTTCTTAG-3′ |
| IFN-γ-R |
5′-AAGTTTGAAGTAAAAGAAGACAATTTGG-3′ |
| IL-6-F |
5′-AGTTGCCTTCTTGGGACTGA-3′ |
| IL-6-R |
5′-CAGAATTGCCATTGCACAAC-3′ |
| IL-17-F |
5′-CCGGACTGTGATGGTCAA-3′ |
| IL-17-R |
5′-CTCATTGCGGTGGAGATT-3′ |
| TNF-α-F |
5′-CGGGCAGGTCTACTTTGGAG-3′ |
| TNF-α-R |
5′-CAGGTCACTGTCCCAGCATC-3′ |
| IL-1β-F |
5′-CTTCTTCGACACATGGGATAAC-3′ |
| IL-1β-R |
5′-TTTGGGATCTACACTCTCCAGC-3′ |
| IL-6R-F |
5′-TGAGCTCAGATATCGGGCTGAAC-3′ |
| IL-6R-R |
5′-CGTCGTGGATGACACAGTGATG-3′ |
| miR-211-F |
5′-TTGTGGGCTTCCCTTTGTCATCCT-3′ |
| miR-211-R |
5′-TGCTGTGGGAAGTGACAACTGA-3′ |
| HOTAIR-F |
5′-CAGTGGGGAACTCTGACTCG-3′ |
| HOTAIR-R |
5′-GTGCCTGGTGCTCTCTTACC-3′ |
Western blot assay
The proteins were isolated from the spleens of
septic and control mice, monocytes transfected with HOTAIR, miR-211
mimics οr miR-211 inhibitor and corresponding control using RIPA
Lysis Buffer System (Santa Cruz Biotechnology, Inc.) supplemented
with 1.5 mM PMSF (Sigma-Aldrich; Merck KGaA). The lysates were then
subjected to centrifugation at 12,000 × g for 15 min at 4°C, after
which the supernatants were collected. The protein concentration
was determined using a bicinchoninic acid kit (Pierce; Thermo
Fisher Scientific, Inc.). A total of 30 µg proteins per lane were
isolated using 10% SDS-PAGE and transferred onto nitrocellulose
membranes (EMD Millipore; Merck KGaA) and incubated with 5% skimmed
milk at room temperature for 2 h. The membranes were then incubated
with primary rabbit antibodies against IFN-γ (1:1,000; cat. no.
ab77246), IL-6 (1:2,000; cat. no. ab6672), IL-17 (1:500; cat. no.
ab136668), TNF-α (1:500; cat. no. ab6671), IL-1β (1:1,000; cat. no.
ab200478), and IL-6R (1:200; cat. no ab128008; all Abcam) at 4°C
for 8 h. The membranes were incubated with horseradish
peroxidase-conjugated donkey anti-rabbit secondary antibodies
(1:2,000; cat. no. ab7083; Abcam) at room temperature for 2 h.
Finally, the signals were detected using enhanced chemiluminescent
(ECL) kit (Pierce; Thermo Fisher Scientific, Inc.). The grayscale
values of the membranes were counted using an ImageJ software (ver.
1.51d; National institutes of Health).
Bioinformatics analysis
TargetScan (http://targetscan.org/) was applied to analyze the
possible binding sites between HOTAIR and miR-211 and between
miR-211 and IL-6; TargetScan (http://targetscan.org/) (38), StarBase v2.0 (http://starbase.sysu.edu.cn/) (39) and miRDB (http://mirdb.org/miRDB/) (40) databases were utilized to analyze the
possible binding site of IL-6 as the downstream target of
miR-211.
Dual-luciferase reporter assay
The interaction between miR-211 and HOTAIR, as well
as miR-211 and IL-6R were verified with a dual-luciferase reporter
assay. Wild-type (WT) HOTAIR, mutant type (Mut) HOTAIR, WT IL-6R
and Mut IL-6R were purchased from Hanbio Co., Ltd. (Hanbio
Biotechnology Co., Ltd.). Briefly, total DNA was extracted from
293T cells using TIANamp Genomic DNA Kit (Tiangen Biotech Co.,
Ltd.), and the 3′-untranslated regions (3′-UTR) of the wild type
(WT) HOTAIR containing the miR-211 binding sites were amplified
using Taq PCR Master Mix Kit (Qiagen GmbH; cat. no. 201443). The
temperature protocol for the PCR consisted of 94°C for 3 min;
followed by 30 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C
1 min; and final extension at 72°C for 5 min. The mutant (Mut)
3′-UTR of HOTAIR was generated by changing the sequence from
‘AAAGGGAA’ to ‘UUUCCCUU’. The DNA products were sub-cloned into the
luciferase vector, psi-CHECK2 (Promega Corporation) to form a
recombinant reporter plasmid. The WT IL-6R (WT-IL-6R) and Mut IL-6R
(MUT-IL-6R) were constructed in the same manner as WT-HOTAIR and
Mut-HOTAIR. For the miR-211 and HOTAIR dual-luciferase reporter
assay, 293T cells were seeded into 24-well plates at a density of
1×104 cells/well. After culturing overnight at 37°C,
293T cells were co-transfected with WT-HOTAIR or Mut-HOTAIR
combined with miR-211 mimics or its negative control using a
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The collected 293T cells were seeded into
24-well plates at a concentration of 2×105 cells/well,
and cultured at 37°C overnight. Based on manufacturer's
instructions, the luciferase activities were measured with a Dual
Luciferase Assay System (Promega Corporation) to verify that the
interaction between miR-211 and IL-6R was the same as miR-211 and
HOTAIR. All luciferase activities were normalized to Renilla
luciferase activity.
Cell proliferation analysis
Cell Counting Kit-8 (CCK-8; Sigma-Aldrich; Merck
KGaA) was used to evaluate the effects of HOTAIR and miR-211 on
monocyte proliferation. The transfected monocytes (1×103
cell/well) were seeded into 96-well plates and transfected with
HOTAIR, miR-211 mimics or miR-211 inhibitors for 48 h at 37°C in a
humidified incubator with 5% CO2. The optical density
(OD) was then measured at 450 nm using a microtiter plate reader
(SpectraMax; Molecular Devices, LCC).
Cell apoptosis analysis
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining and flow cytometry
were performed to determine the effects of HOTAIR and miR-211 on
monocyte apoptosis. After culturing in DMEM for 48 h at 37°C,
monocytes transfected with either HOTAIR, miR-211 mimic or miR-211
inhibitor were harvested by centrifugation (1,000 × g for 5 min)
and washed twice with PBS. The monocytes were fixed in 70% ethanol
for 2 h at room temperature and then incubated with annexin V-FITC
and PI (Keygentec) for 10 min in the dark. Finally, the apoptotic
cells were evaluated using flow cytometry (BD Biosciences), and
analyzed using BD CellQuest software (Version 3.3; BD
Biosciences).
Statistical analysis
SPSS software (version 22.0; IBM, Corp.) was used
for all statistical analyses. Each experiment was repeated at least
three times and the data were expressed as the mean ± standard
deviation (SD). A Student's t-test was used for the statistical
analyses between two groups and the statistical differences between
more than two groups were analyzed using a one-way ANOVA followed
by Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
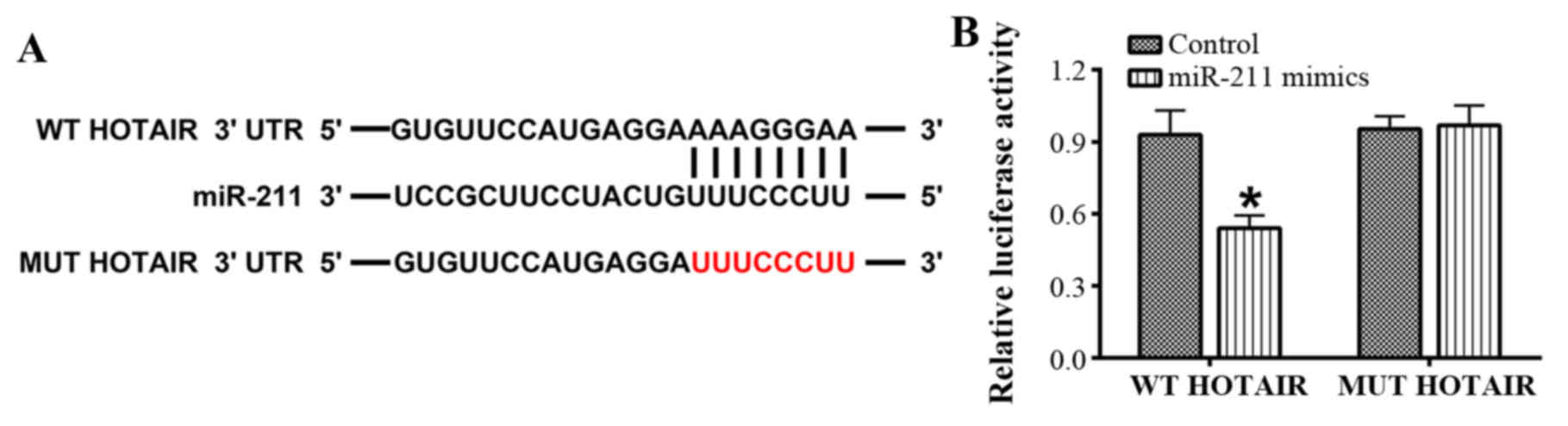
HOTAIR is directly targeted by
miR-211
The interaction between miR-211 and HOTAIR was
evaluated using online bioinformatics analysis and a
dual-luciferase reporter assay in 293T cells. The bioinformatics
analysis revealed that there were putative binding sites for
miR-211 in HOTAIR (Fig. 1). Further
analysis confirmed that luciferase activity was driven by WT-HOTAIR
as it was significantly attenuated by the miR-211 mimics. However,
no significant difference was observed with MUT-HOTAIR (P<0.05;
Fig. 1).
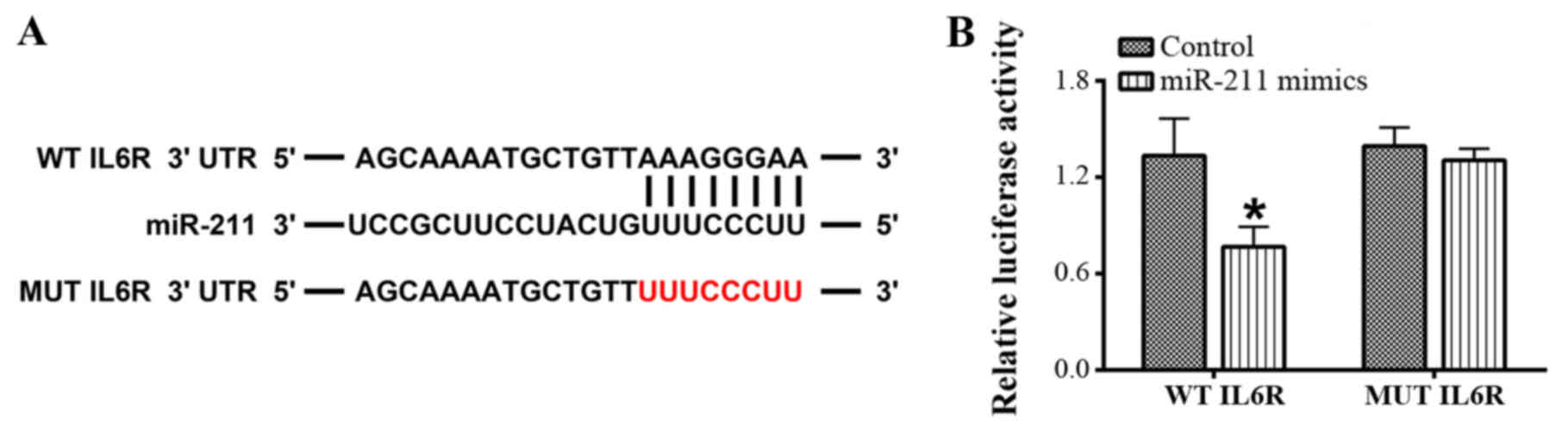
The 3′-UTR of IL-6R is targeted by
miR-211
The interaction between miR-211 and IL-6 using an
online bioinformatics analysis tool and dual-luciferase reporter
assay. The bioinformatics analysis indicated that there were
putative binding sites in the 3′-UTR of IL-6 for miR-211 (Fig. 2). Subsequently, a dual-luciferase
reporter assay was performed to verify the interaction between
miR-211 and IL-6 and the results revealed that the luciferase
activity driven by WT-IL-6R was significantly reduced by the
miR-211 mimics; however, there was no significant difference in
luciferase activity with MUT-IL-6R following treatment with the
miR-211 mimics (P<0.05; Fig.
2).
miR-211 and HOTAIR expression is
significantly upregulated in the spleens of mice with CLP-induced
sepsis
To explore whether miR-211 and HOTAIR were involved
in the pathogenesis of sepsis, a CLP-induced mouse model of sepsis
was established. The animal model of sepsis was initially verified
by detecting the levels of various inflammatory factors in the
spleens via RT-qPCR. The results indicated that the levels of
IFN-γ, IL-6, IL-17, TNF-α and IL-1β expression were significantly
upregulated in septic mice compared with control mice (Fig. 3A). In addition, there was a
significant upregulation in IL-6R expression in the septic mice
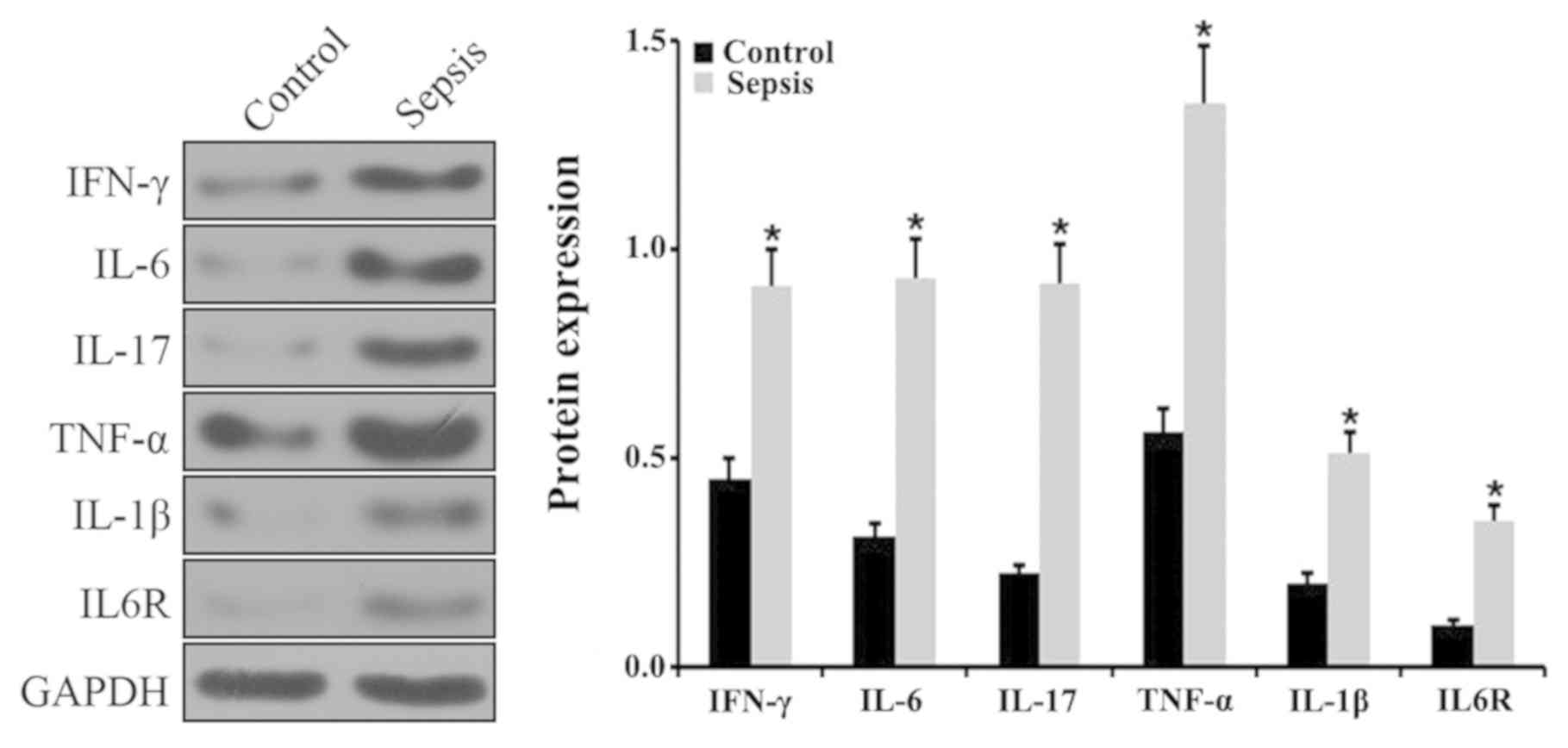
compared with control mice (P<0.05; Fig. 3A). The level of IFN-γ, IL-6, IL-17,
TNF-α, IL-1β and IL-6R expression was further examined via western
blotting. The results demonstrated a significant increase in IFN-γ,
IL-6, IL-17, TNF-α, IL-1β and IL-6R in the spleens of septic mice
compared with control mice (P<0.05; Fig. 4). The expression of miR-211 and
HOTAIR in the septic mice was subsequently examined using RT-qPCR.
The results indicated that the relative levels of miR-211 and
HOTIAR were significantly increased in the splenic tissues from the
septic mice compared with control mice (P<0.05; Fig. 3B).
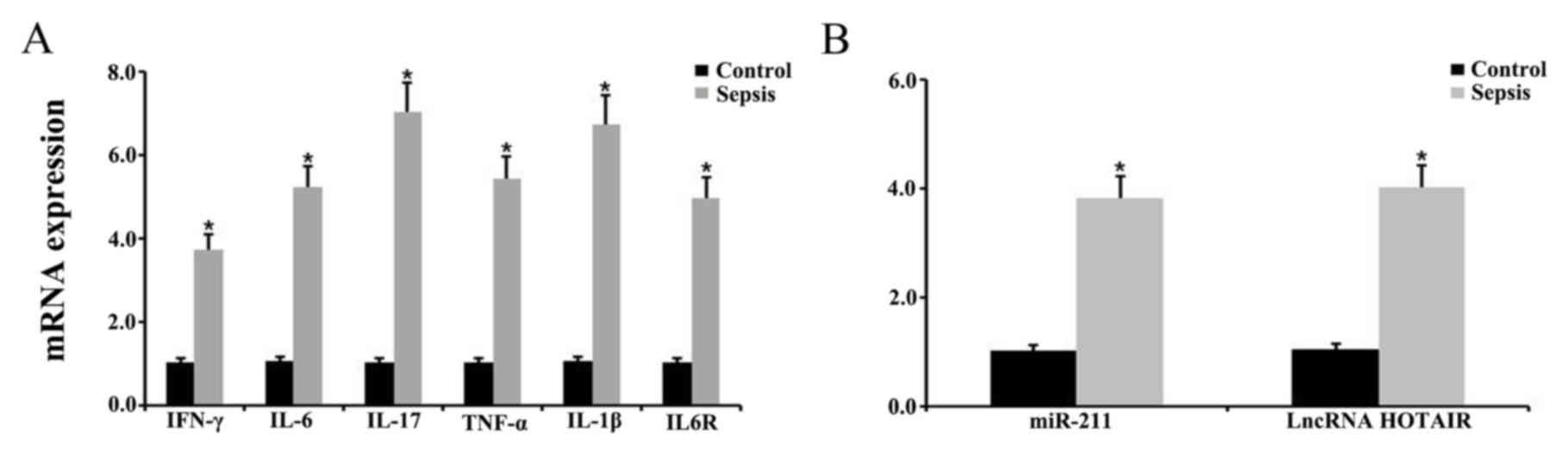 | Figure 3.Expression level of various
inflammatory factors, miR-211, and HOTAIR in the spleen. Relative
expression level of (A) IFN-γ, IL-6, IL-17, TNF-α, IL-1β and IL-6R
and (B) miR-211 and HOTAIR in the spleens of control and septic
mice, as determined via reverse transcription-quantitative PCR.
*P<0.05 vs. control group. Each experiment was repeated three
times. miR, microRNA; HOTAIR, hox transcript antisense RNA; IL,
interleukin; IFN, interferon; TNF, tumor necrosis factor. |
Overexpression of HOTIAR and knockdown
of miR-211 promotes the inflammatory response in monocytes
To further explore the biological function of
miR-211 and HOTAIR in sepsis, RT-qPCR was performed to determine
the levels of IFN-γ, IL-6, IL-17, TNF-α, IL-1β and IL-6R in the
monocytes transfected with HOTAIR, the miR-211 mimics and miR-211
inhibitors. The relative level of IFN-γ, IL-6, IL-17, TNF-α, IL-1β
and IL-6R mRNA and protein expression was demonstrated to be
significantly upregulated in the HOTAIR and miR-211
inhibitor-treated groups compared with that in the negative control
group (P<0.05; Figs. 5A and
6). However, there was a significant
downregulation in the level of IFN-γ, IL-6, IL-17, TNF-α, IL-1β and
IL-6R expression in monocytes treated with the miR-211 mimics
compared with the negative control (P<0.05; Figs. 5A and 6). In addition, miR-211 expression was
significantly decreased in the HOTAIR overexpressed group, and
HOTAIR expression was significantly increased in the
miR-211-silenced group. These results indicated that there was a
negative association between the expression of miR-211 and HOTAIR
in monocytes (P<0.05; Fig.
5B).
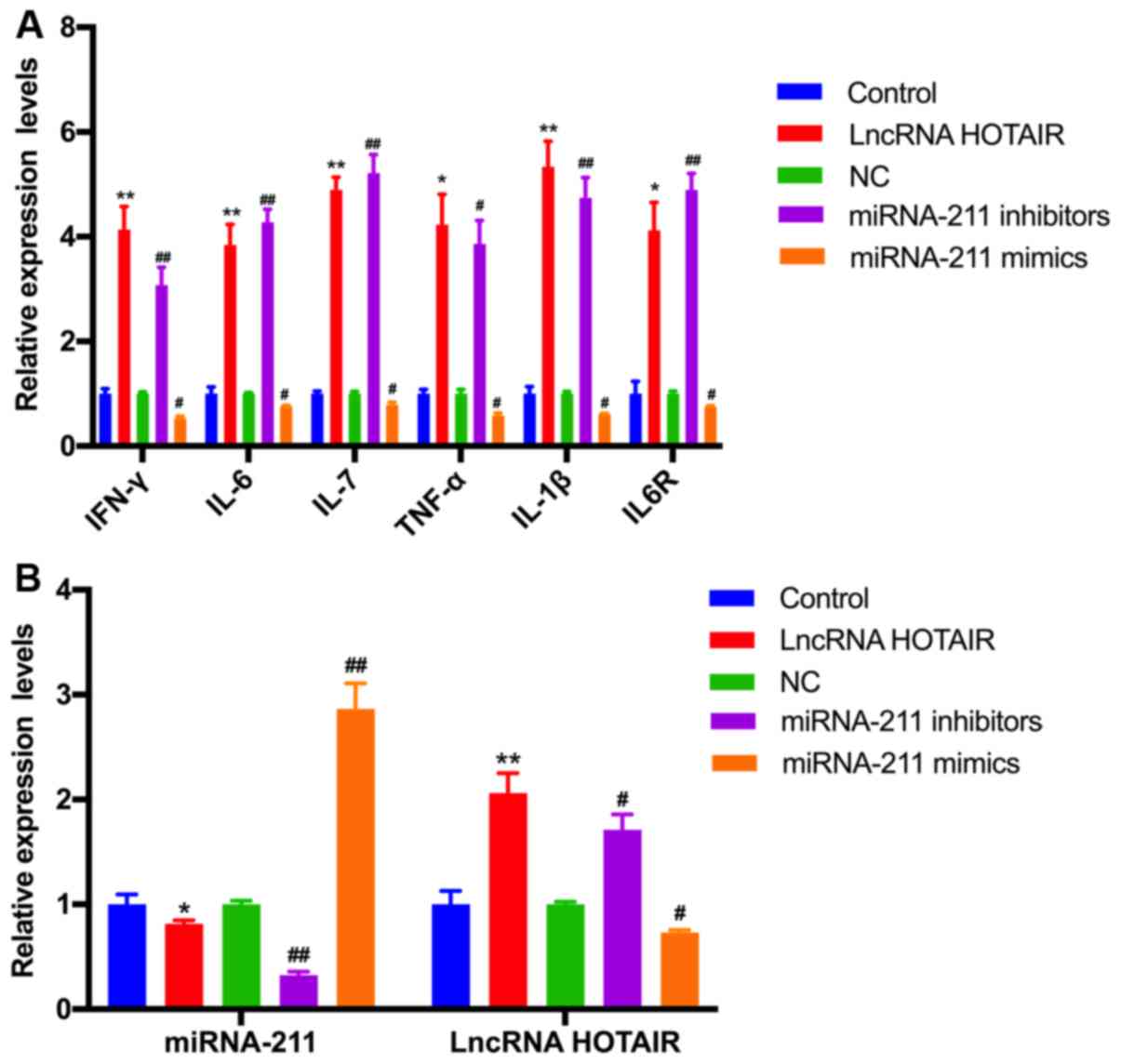 | Figure 5.Effects of HOTAIR and miR-211
overexpression or knockdown on the mRNA expression of various
inflammatory factors, miR-211 and HOTAIR. The relative level of (A)
IFN-γ, IL-6, IL-17, TNF-α, IL-1β, and IL-6R and (B) miR-211, and
HOTAIR expression in monocytes transfected with HOTAIR, miR-211
mimics, and miR-211 inhibitor was examined using reverse
transcription-quantitative PCR. *P<0.05, **P<0.01 vs. control
group; #P<0.05, ##P<0.01 vs. NC group.
Each experiment was repeated three times. HOTAIR, hox transcript
antisense RNA; miR, microRNA; IL, interleukin; TNF, tumor necrosis
factor; IFN, interferon; lncRNA, long non-coding RNA; NC, negative
control for miRNA mimic and inhibitor; Control, empty vector that
do not express HOTAIR. |
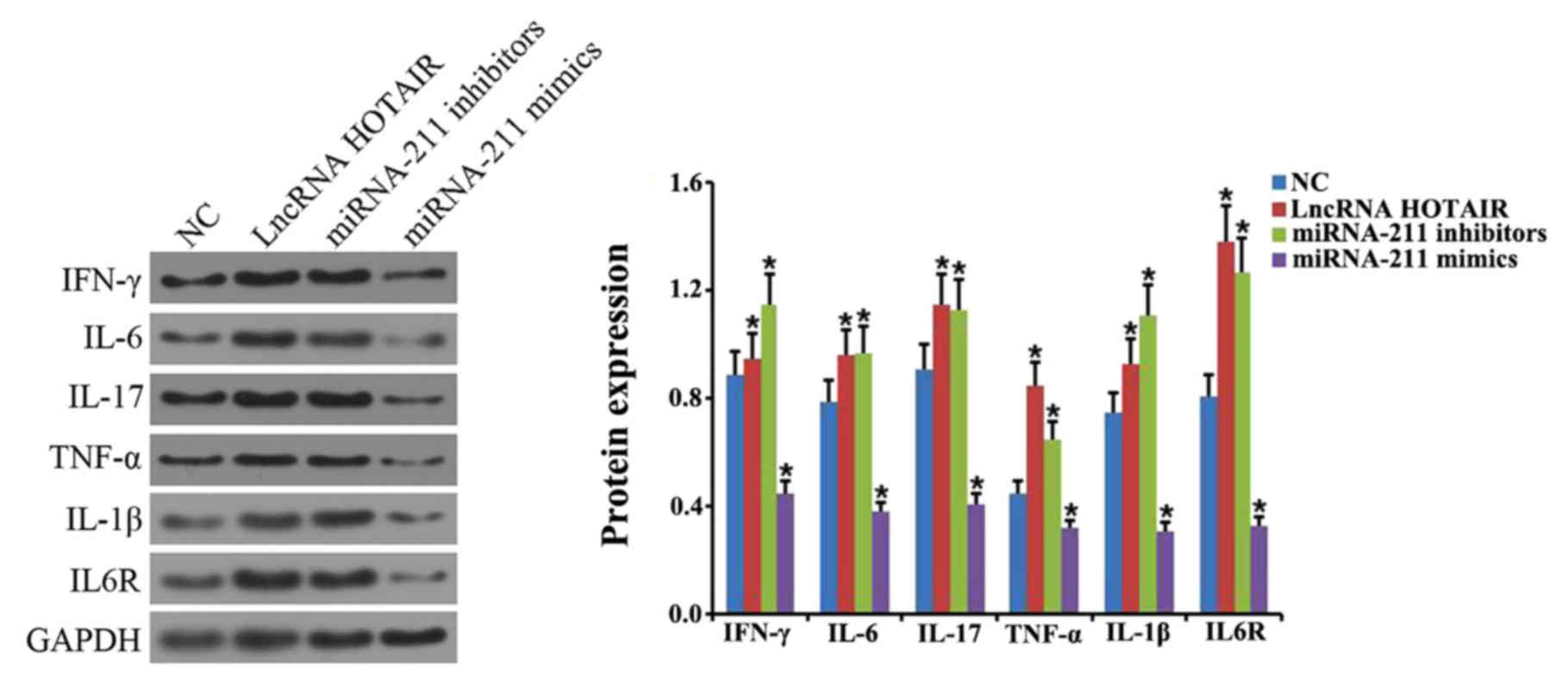 | Figure 6.Effects of HOTAIR and miR-211
overexpression or miR-211 knockdown on the protein expression of
various inflammatory factors. Western blot analysis was performed
to determine the level of IFN-γ, IL-6, IL-17, TNF-α, IL-1β and
IL-6R protein expression in cells transfected with HOTAIR, miR-211
mimics and miR-211 inhibitor. *P<0.05 vs. NC group. Each
experiment was repeated three times. HOTAIR, hox transcript
antisense RNA; miR, microRNA; IFN, interferon; IL, interleukin;
TNF, tumor necrosis factor; lncRNA, long non-coding RNA; NC,
negative control. |
Overexpression of HOTAIR and knockdown
of miR-211 inhibits proliferation and promotes apoptosis in
monocytes
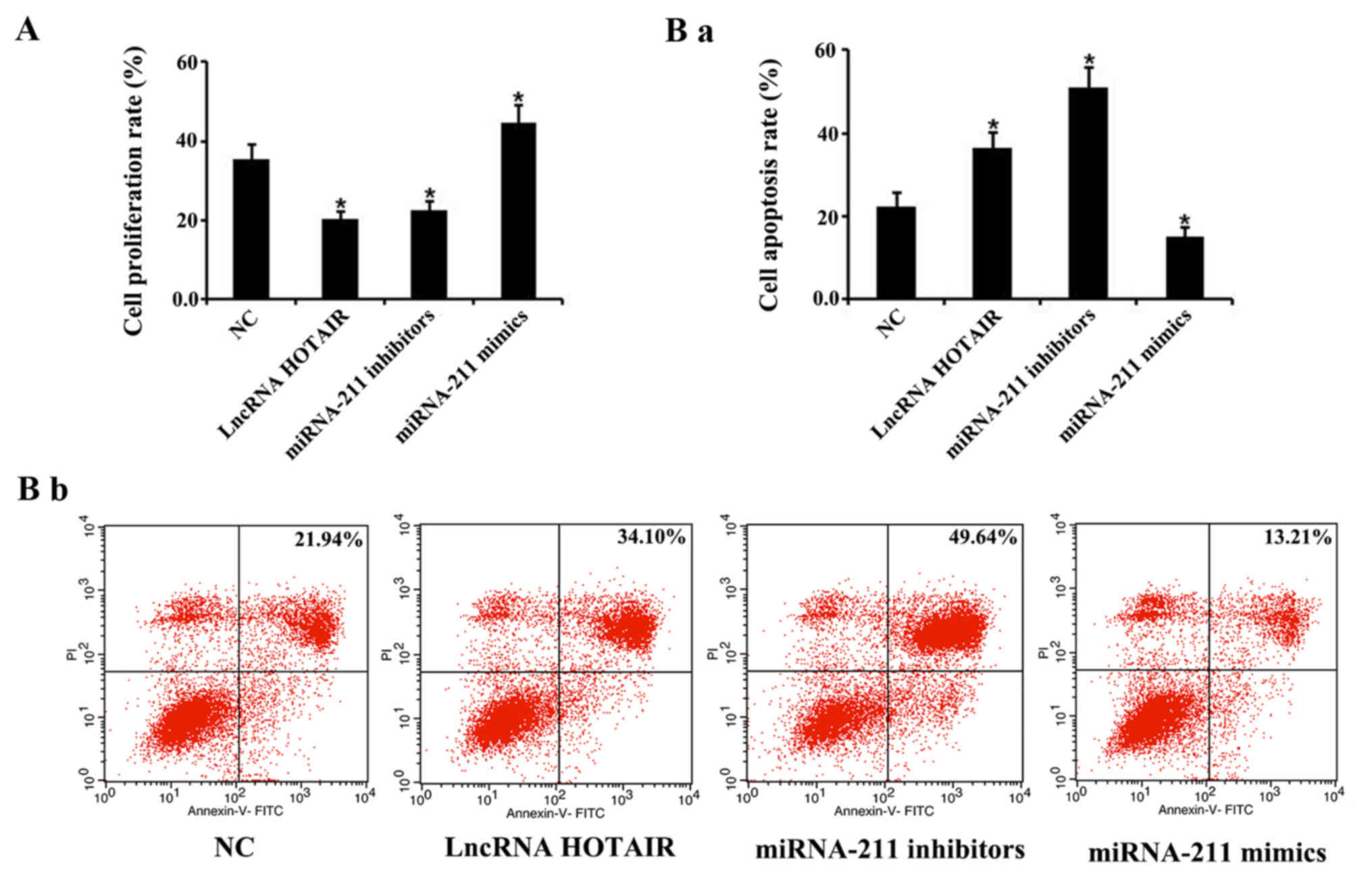
A CCK-8 assay and flow cytometry were performed to
investigate the effects of HOTAIR and miR-211 on cellular
proliferation and apoptosis, respectively, in monocytes. The
results revealed that the rate of cellular monocyte proliferation
transfected with HOTAIR and miR-211 inhibitors was significantly
reduced, whereas the cells transfected the miR-211 mimics exhibited
a significant increase in proliferation compared with the negative
control groups, respectively (P<0.05; Fig. 7A). Furthermore, monocytes transfected
with the HOTAIR and miR-211 inhibitors demonstrated a significant
increase in apoptosis, whereas those transfected with the miR-211
mimics showed a significant decrease in apoptosis, compared with
the negative control group (P<0.05, Fig. 7Ba and Bb).
Discussion
HOTAIR is transcribed by the antisense strand of the
HOXC gene located on chromosome 12 and is an important
lncRNA that was first identified by Rinn et al (41) in 2007, using a microarray assay. In
addition, evidence has indicated that HOTAIR regulates chromatin
dynamics and induces gene silencing by interacting with histone
methylase and histone demethylase (42). Additionally, HOTAIR has been reported
to be involved in the etiology of multiple types of human cancer,
including hepatocellular, breast and lung cancer (43–45).
HOTAIR has also been demonstrated to regulate the expression of
miRNAs by acting as a competitive endogenous RNA (ceRNA) (42). For example, HOTAIR was revealed to
possess the binding sites for miR-130a, which were demonstrated to
be critical for the modulation of miR-130a by HOTAIR (46). In the present study, HOTAIR was
identified to function as a ceRNA of miR-211, and the expression of
HOTAIR and miR-211 were negatively associated in monocytes.
As a major public health issue, sepsis is frequently
accompanied by microbial infection, systemic inflammation and
cellular dysfunction, which can ultimately result in tissue damage,
organ failure and even death (47).
There are currently three main hypotheses used to explain the
pathogenesis of sepsis: i) Pro-inflammatory response; ii) impaired
compensatory anti-inflammatory responses; and iii)
immune-paralysis, all of which involve the excessive release of
inflammatory mediators responsible for the initiation and
development of systemic inflammation (1,48). TNF-α
was considered to be a central regulator of the immune response and
involved in the pathophysiological alterations associated with
sepsis (49,50). In addition, TNF-α was demonstrated to
promote the release of inflammatory mediators, including IL-6,
IL-8, IL-17 and IL-1β, which initiate the host inflammatory
response (51,52). IL-6 is primarily released by
activated monocytes and has been demonstrated to be negatively
associated with the prognosis of patients with sepsis (53). In the present study, since miR-211
was observed to bind to the 3′-UTR of IL-6R, it was hypothesized
whether HOTAIR was involved in the pathogenesis of sepsis by
indirectly regulating the expression of IL-6R through miR-211.
Currently, various animal models including zebrafish
(54), rat (55) and mice (56) have been established to investigate
the etiology of sepsis, including toxin treatments such as LPS,
zymosan or endotoxins, viable pathogens (including bacteria), as
well as altering the endogenous protective barrier in animals
(including the induction of colonic permeability leading to
bacterial translocation). In addition, CLP is the most frequently
applied model in rodents (57,58),
which can be used to create sepsis-inducing animal models (59–61).
Research has also demonstrated that CLP-induced murine sepsis does
not cause lung injury (62),
therefore CLP was used to induce murine sepsis in the present
study.
Recently, the abnormal expression of lncRNAs has
been found in a number of animal models of sepsis, including mice
(29) and rat (63), indicating that lncRNAs may be
involved in the pathogenesis of sepsis (64,65). In
the present study, an animal model of sepsis was established using
CLP in mice, which was verified by detecting the increase in mRNA
and protein levels of IFN-γ, IL-6, IL-17, TNF-α, IL-1β and IL-6R.
In addition, HOTAIR expression was significantly upregulated,
whereas the expression of miR-211 was substantially downregulated
in the spleens of septic mice. Furthermore, both HOTAIR
overexpression and miR-211 knockdown upregulated the expression of
IFN-γ, IL-6, IL-17, TNF-α, IL-1β and IL-6R in monocytes. Treatment
with the miR-211 mimics exhibited the opposite effect in
monocytes.
Immune suppression caused by corresponding apoptosis
(in monocytes and lymphocytes), has been reported to be associated
with the pathogenesis of sepsis (5).
Therefore, the abrogation of immune cell apoptosis is considered to
be a potential therapeutic measure for patients with sepsis. In the
present study, HOTAIR overexpression and miR-211 knockdown were
revealed to inhibit cellular proliferation and promote apoptosis in
monocytes, whereas miR-211 overexpression was demonstrated to
induce the opposite effect in monocytes.
In conclusion, the results of the current study
indicated that HOTAIR promoted the progression of sepsis indirectly
by regulating IL-6R expression via miR-211. Therefore, HOTAIR may
be a potential therapeutic target for patients with sepsis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on a reasonable
request.
Authors' contributions
JC, FC, XG and LZ conceived, designed and
coordinated the study and prepared the draft of the manuscript. SW,
LZ and YH performed literature research, collected the data,
participated in the design of the study and performed the
statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All of the animal protocols in the present study
were approved by Institute of Radiation Medicine of the Chinese
Academy of Medical Sciences.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sagy M, Al-Qaqaa Y and Kim P: Definitions
and pathophysiology of sepsis. Curr Probl Pediatr Adolesc Health
Care. 43:260–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohamed AKS, Mehta AA and James P:
Predictors of mortality of severe sepsis among adult patients in
the medical Intensive Care Unit. Lung India. 34:330–335. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Vught LA, Wiewel MA, Hoogendijk AJ,
Frencken JF, Scicluna BP, Klein Klouwenberg PMC, Zwinderman AH,
Lutter R, Horn J, Schultz MJ, et al: The host response in sepsis
patients developing intensive care unit-acquired secondary
Infections. Am J Respir Crit Care Med. 196:458–470. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sánchez B, Ferrer R, Suarez D, Romay E,
Piacentini E, Gomà G, Martínez ML and Artigas A; Edusepsis Study
Group, : Declining mortality due to severe sepsis and septic shock
in Spanish intensive care units: A two-cohort study in 2005 and
2011. Med Intensiva. 41:28–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hotchkiss RS, Coopersmith CM, McDunn JE
and Ferguson TA: The sepsis seesaw: Tilting toward
immunosuppression. Nat Med. 15:496–497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang W, Zhong W, Deng Y, Chen C, Wang Q,
Zhou M, Li X, Sun C and Zeng H: Evaluation of a combination
‘lymphocyte apoptosis model’ to predict survival of sepsis patients
in an intensive care unit. BMC Anesthesiol. 18:892018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gill SE, Rohan M and Mehta S: Role of
pulmonary microvascular endothelial cell apoptosis in murine
sepsis-induced lung injury in vivo. Respir Res. 16:1092015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pool R, Gomez H and Kellum JA: Mechanisms
of organ dysfunction in sepsis. Crit Care Clin. 34:63–80. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Halimulati M, Duman B, Nijiati J and
Aizezi A: Long noncoding RNA TCONS_00024652 regulates vascular
endothelial cell proliferation and angiogenesis via microRNA 21.
Exp Ther Med. 16:3309–3316. 2018.PubMed/NCBI
|
|
10
|
Li TT, He RQ, Ma J, Li ZY, Hu XH and Chen
G: Long non-coding RNAs in small cell lung cancer: A potential
opening to combat the disease (Review). Oncol Rep. 40:1831–1842.
2018.PubMed/NCBI
|
|
11
|
Ling Z, Liu D, Zhang G, Liang Q, Xiang P,
Xu Y, Han C and Tao T: miR-361-5p modulates metabolism and
autophagy via the Sp1-mediated regulation of PKM2 in prostate
cancer. Oncol Rep. 38:1621–1628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni X, Liao Y, Li L, Zhang X and Wu Z:
Therapeutic role of long non-coding RNA TCONS_00019174 in
depressive disorders is dependent on Wnt/β-catenin signaling
pathway. J Integr Neurosci. 17:125–132. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang TN, Li D, Xia J, Wu QJ, Wen R, Yang
N and Liu CF: Non-coding RNA: A potential biomarker and therapeutic
target for sepsis. Oncotarget. 8:91765–91778. 2017.PubMed/NCBI
|
|
14
|
Diamantopoulos MA, Tsiakanikas P and
Scorilas A: Non-coding RNAs: The riddle of the transcriptome and
their perspectives in cancer. Ann Transl Med. 6:2412018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gibbons A, Udawela M and Dean B:
Non-coding RNA as novel players in the pathophysiology of
schizophrenia. Noncoding RNA. 4(pii): E112018.PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schoof CR, Botelho EL, Izzotti A and
Vasques Ldos R: MicroRNAs in cancer treatment and prognosis. Am J
Cancer Res. 2:414–433. 2012.PubMed/NCBI
|
|
19
|
Qiu L, Tan EK and Zeng L: microRNAs and
neurodegenerative diseases. Adv Exp Med Biol. 888:85–105. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kondo Y, Shinjo K and Katsushima K: Long
non-coding RNAs as an epigenetic regulator in human cancers. Cancer
Sci. 108:1927–1933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchida S and Dimmeler S: Long noncoding
RNAs in cardiovascular diseases. Circ Res. 116:737–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Y, Zhou T, Yu X, Xue Z and Shen N:
The role of long non-coding RNAs in rheumatic diseases. Nat Rev
Rheumatol. 13:657–669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rong D, Sun H, Li Z, Liu S, Dong C, Fu K,
Tang W and Cao H: An emerging function of circRNA-miRNAs-mRNA axis
in human diseases. Oncotarget. 8:73271–73281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loewen G, Jayawickramarajah J, Zhuo Y and
Shan B: Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol.
7:902014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ling Z, Wang X, Tao T, Zhang L, Guan H,
You Z, Lu K, Zhang G, Chen S, Wu J, et al: Involvement of
aberrantly activated HOTAIR/EZH2/miR-193a feedback loop in
progression of prostate cancer. J Exp Clin Cancer Res. 36:1592017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB,
Yin DD, Kong R, Xia R, Lu KH, Li JH, et al: Lnc RNA HOTAIR
functions as a competing endogenous RNA to regulate HER2 expression
by sponging miR-331-3p in gastric cancer. Mol Cancer. 13:922014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu X, Liu Z, Ning X, Huang L and Jiang B:
The long noncoding RNA HOTAIR promotes colorectal cancer
progression by sponging miR-197. Oncol Res. 26:473–481. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu H, Liu J, Li W, Liu G and Li Z:
LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of
LPS-induced sepsis mice by activating NF-κB pathway. Biochem
Biophys Res Commun. 471:240–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bosmann M and Ward PA: The inflammatory
response in sepsis. Trends Immunol. 34:129–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walborn A, Hoppensteadt D, Syed D, Mosier
M and Fareed J: Biomarker profile of sepsis-associated coagulopathy
using biochip assay for inflammatory cytokines. Clin Appl Thromb
Hemost. 24:625–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schulte W, Bernhagen J and Bucala R:
Cytokines in sepsis: Potent immunoregulators and potential
therapeutic targets-an updated view. Mediators Inflam.
2013:1659742013. View Article : Google Scholar
|
|
33
|
Shao WX, Yu DJ, Zhang WY and Wang XJ:
Clinical significance of IL-6 in the diagnosis of sepsis and
discriminating sepsis induced by gram-negative bacteria. Pediatr
Infect Dis J. 37:801–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W and He J: Interleukin-6 is a key
factor for immunoglobulin-like transcript-4-mediated immune injury
in sepsis. J Intensive Care. 6:222018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen Y, Lin MC, Yao H, Wang H, Zhang AQ,
Yu J, Hui CK, Lau GK, He ML, Sung J and Kung HF:
Lentivirus-mediated RNA interference targeting enhancer of zeste
homolog 2 inhibits hepatocellular carcinoma growth through
down-regulation of stathmin. Hepatology. 46:200–208. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang L, Lai YK, Zhang J, Wang H, Lin MC,
He ML and Kung HF: Targeting S100P inhibits colon cancer growth and
metastasis by Lentivirus-mediated RNA interference and proteomic
analysis. Mol Med. 17:709–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu S, Li JH, Wu J, Zhou KR, Zhou H, Yang
JH and Qu LH: StarScan: A web server for scanning small RNA targets
from degradome sequencing data. Nucleic Acids Res. 43:W480–W486.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang JH, Li JH, Shao P, Zhou H, Chen YQ
and Qu LH: starBase: A database for exploring microRNA-mRNA
interaction maps from Argonaute CLIP-Seq and Degradome-Seq data.
Nucleic Acids Res 39 (Database Issue). D202–D209. 2011. View Article : Google Scholar
|
|
40
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res 43 (Database Issue). D146–D152. 2015. View Article : Google Scholar
|
|
41
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhan A and Mandal SS: LncRNA HOTAIR: A
master regulator of chromatin dynamics and cancer. Biochim Biophys
Acta. 1856:151–164. 2015.PubMed/NCBI
|
|
43
|
Hu ML, Wang XY and Chen WM: TGF-β1
upregulates the expression of lncRNA UCA1 and its downstream HXK2
to promote the growth of hepatocellular carcinoma. Eur Rev Med
Pharmacol Sci. 22:4846–4854. 2018.PubMed/NCBI
|
|
44
|
Perrot-Applanat M, Kolf-Clauw M, Michel C
and Beausoleil C: Alteration of mammary gland development by
bisphenol a and evidence of a mode of action mediated through
endocrine disruption. Mol Cell Endocrinol. 475:29–53. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han L, Zhang HC, Li L, Li CX, Di X and Qu
X: Downregulation of long noncoding RNA HOTAIR and EZH2 induces
apoptosis and inhibits proliferation, invasion, and migration of
human breast cancer cells. Cancer Biother Radiopharm. 33:241–251.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang J, Ke P, Guo L, Wang W, Tan H, Liang
Y and Yao S: Lentivirus-mediated RNA interference targeting the
long noncoding RNA HOTAIR inhibits proliferation and invasion of
endometrial carcinoma cells in vitro and in vivo. Int J Gynecol
Cancer. 24:635–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuki K and Murakami N: Sepsis
pathophysiology and anesthetic consideration. Cardiovasc Hematol
Disord Drug Targets. 15:57–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bone RC, Grodzin CJ and Balk RA: Sepsis: A
new hypothesis for pathogenesis of the disease process. Chest.
112:235–243. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ge L, Hu Q, Chen J, Shi M, Yang H and Zhu
G: Inhibition of TNF-α sepsis of lipopolysaccharide induction using
nano cerium oxide system. Mater Sci Eng C Mater Biol Appl.
77:405–410. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Y, Cui X, Ning L and Wei D: The
effects of tumor necrosis factor-α (TNF-α) rs1800629 and rs361525
polymorphisms on sepsis risk. Oncotarget. 8:111456–111469.
2017.PubMed/NCBI
|
|
51
|
Gao H, Liu L, Zhao Y, Hara H, Chen P, Xu
J, Tang J, Wei L, Li Z, Cooper DKC, et al: Human IL-6, IL-17,
IL-1β, and TNF-α differently regulate the expression of
pro-inflammatory related genes, tissue factor and swine leukocyte
antigen class I in porcine aortic endothelial cells.
Xenotransplantation. 24:2017. View Article : Google Scholar
|
|
52
|
Feng S, Yu H, Yu Y, Geng Y, Li D, Yang C,
Lv Q, Lu L, Liu T, Li G and Yuan L: Levels of inflammatory
cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in aqueous humour of
patients with diabetic retinopathy. J Diabetes Res.
2018:85464232018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
McGuire TR, Reardon NT, Bogard K, Plumb
TJ, Bultsma CJ, Nissen SW, Fuller PD and Olsen KM: IL6 plasma
concentrations in patients with sepsis receiving SLED and
antibiotics: A predictor for survival. In Vivo. 28:1131–1134.
2014.PubMed/NCBI
|
|
54
|
Philip AM, Wang Y, Mauro A, El-Rass S,
Marshall JC, Lee WL, Slutsky AS, dosSantos CC and Wen XY:
Development of a zebrafish sepsis model for high-throughput drug
discovery. Mol Med. 23:134–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen L, Sun Z, Zhao F, Wang W, Zhang W and
Zhu H: Expression of c-FLIP in a rat model of sepsis and its
effects on endothelial apoptosis. Mol Med Rep. 16:231–237. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Buras JA, Holzmann B and Sitkovsky M:
Animal models of sepsis: Setting the stage. 4:854–865.
2005.PubMed/NCBI
|
|
57
|
Rittirsch D, Hoesel LM and Ward PA: The
disconnect between animal models of sepsis and human sepsis. J
Leukoc Biol. 81:137–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chang R, Holcomb JB, Johansson PI, Pati S,
Schreiber MA and Wade CE: Plasma resuscitation improved survival in
a cecal ligation and puncture rat model of sepsis. Shock. 49:53–61.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Oberbaum M, Spira RM, Lukasiewicz E, Armon
Y, Samuels N, Singer SR, Barak V, Izbicki G, Einav S and Hersch M:
Effect of Traumeel S on cytokine profile in a cecal ligation and
puncture (CLP) sepsis model in rats. J Altern Complement Med.
17:909–913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang J, Bi J, Liu S, Pang Q, Zhang R,
Wang S and Liu C: 5-HT Drives Mortality in Sepsis Induced by Cecal
Ligation and Puncture in Mice. Mediators Inflamm. 2017:63742832017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sakai M, Suzuki T, Tomita K, Yamashita S,
Palikhe S, Hattori K, Yoshimura N, Matsuda N and Hattori Y:
Diminished responsiveness to dobutamine as an inotrope in mice with
cecal ligation and puncture-induced sepsis: Attribution to
phosphodiesterase 4 upregulation. Am J Physiol Heart Circ Physiol.
312:H1224–H1237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Iskander KN, Craciun FL, Stepien DM, Duffy
ER, Kim J, Moitra R, Vaickus LJ, Osuchowski MF and Remick DG: Cecal
ligation and puncture-induced murine sepsis does not cause lung
injury. Crit Care Med. 41:159–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Jiang ZJ, Zhang MY, Fan ZW, Sun WL and
Tang Y: Influence of lncRNA HOTAIR on acute kidney injury in sepsis
rats through regulating miR-34a/Bcl-2 pathway. Eur Rev Med
Pharmacol Sci. 23:3512–3519. 2019.PubMed/NCBI
|
|
64
|
Chen H, Wang X, Yan X, Cheng X, He X and
Zheng W: LncRNA MALAT1 regulates sepsis-induced cardiac
inflammation and dysfunction via interaction with miR-125b and p38
MAPK/NFκB. Int Immunopharmacol. 55:69–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang W, Lan X, Li X, Wang D, Sun Y, Wang
Q, Gao H and Yu K: Long non-coding RNA PVT1 promote LPS-induced
septic acute kidney injury by regulating TNFα and JNK/NF-κB
pathways in HK-2 cells. Int Immunopharmacol. 47:134–140. 2017.
View Article : Google Scholar : PubMed/NCBI
|