Introduction
Radical cystectomy is the standard treatment for
localized muscle-invasive bladder cancer (MIBC) (1,2). Urinary
diversion methods post-radical cystectomy include abdominal,
urethral and rectosigmoid diversions. Urinary diversion has evolved
along three distinct paths: Incontinent cutaneous diversion
(conduit); continent cutaneous diversion (pouch); and, most
recently, continent urinary diversion to the intact native urethra
(neobladder, orthotopic reconstruction) (2). Continent urinary diversion has been
revealed to improve the quality of life of patients post-cystectomy
and is preferred by most urologists and patients (3). Conduit urinary diversions fall into two
categories: Those using the small bowel, including the jejunum or
ileum; and those using a part of the colon (4).
The sigma-rectum pouch (Mainz pouch II), first
described by Fisch et al in 1993 (5), is a relatively safe and simple system
for urinary diversion. The procedure allows for continent urinary
diversion into a low-pressure, high-capacity reservoir created
using the rectosigmoid colon (6).
Hyperchloremic acidosis and electrolyte disturbances that lead to
metabolic disorders (owing to the absorption of urinary metabolites
by the intestinal mucosa) are one possible complication of
sigma-rectum pouch surgery and require treatment with oral
medication (5,7,8).
Patients with severe metabolic disorders, which do not respond to
drugs, can suffer extensive damage to renal function that
eventually threatens their life (8).
Between July 2011 and April 2015, a total of six
male patients afflicted with recurrent hyperchloremic metabolic
acidosis, hypokalemia and renal dysfunction after receiving
sigma-rectum pouch surgery, underwent urinary undiversion surgery
from a sigma-rectum pouch to a cutaneous urinary stoma at BenQ
Medical Center, with satisfactory results. All patients were
informed of the risk of unpredictable surgical outcomes and
complications prior to the surgery. On agreeing to participate they
signed informed consent forms and the surgery was approved by the
Medical Ethics Review Committee of BenQ Medical Center (approval
no. 2011072102).
Case report
Patient data
A total of six male patients, aged 58–82 years
(median age, 70 years), underwent radical cystectomy and
sigma-rectum pouch surgery due to MIBC 2–5 years prior to admission
to the Urology Department of BenQ Medical Center. Recurrent fatigue
and anorexia 1–4 years post-operatively had been complained by all
patients. Blood gas analysis (WERi-STAT300; Abbott Pharmaceutical
Co., Ltd.) and biochemical tests (Bs-390; MINDRAY Medical
International Co., Ltd.) indicated hyperchloremic metabolic
acidosis (hydrocarbonate <21 mmol/l; pH <7.35; chlorine
>106 mmol/l) and hypokalemia (potassium <3.5 mmol/l), with
two patients additionally suffering from hypocalcemia (calcium
<2.1 mmol/l). Oral administration of sodium bicarbonate (1.0 g;
three times per day) and potassium citrate (1.45 g; three times per
day) led to a poor therapeutic effect in all patients, who needed
additional intravenous bicarbonate (2–5 mmol/kg) and potassium salt
(40–60 mmol/day) supplements to correct the acidosis and
hypokalemia. The interval requiring intravenous rehydration was
gradually shortened. In one case, the patient suffered severe
paralytic ileus, leading to loss of consciousness. At 1 week prior
to the operation, the arterial blood pH, hydrocarbonate
(HCO3−), base excess, serum potassium level, chlorine,
urea nitrogen and creatinine were 7.16±0.08 (normal range
7.35–7.45), 7.57±4.25 mmol/l (normal range 21–26 mmol/l),
−19.68±4.85 mmol/l (normal range −3-3 mmol/l), 3.12±0.21 mmol/l
(normal range 3.5–5.5 mmol/l), 110.90±4.38 mmol/l (normal range
96–106 mmol/l), 20.15±3.77 mmol/l (normal range 3.2–7.1 mmol/l) and
304.67±55.58 µmol/l (normal range 53–106 µmol/l), respectively.
Ultrasound revealed two cases of mild hydronephrosis (renal sinus
separated 21 mm), three cases of moderate hydronephrosis (renal
sinus separated 32–38 mm) and one of unilateral severe
hydronephrosis (renal sinus separated 45 mm). Two patients suffered
retrograde infection and were treated with unilateral nephrostomy
and nephrectomy, respectively.
Preoperative preparation
At 1 week prior to the operation, intravenous
infusion of 5% sodium bicarbonate and 10% potassium chloride
solutions was administered to treat acidosis and hypokalemia.
Dynamic blood gas analysis and electrolyte and renal function
assessments were carried out to monitor patient status. From 2 days
prior to the surgery, the patients were allowed only a liquid diet.
Levofloxacin (500 mg) and tinidazole (1,000 mg) were administered
orally. A cleansing enema was organized the night prior to the
surgery. Intravenous infusion of 2.0 g ceftriaxone sodium was used
with general anesthesia to prevent infection.
Operation procedure
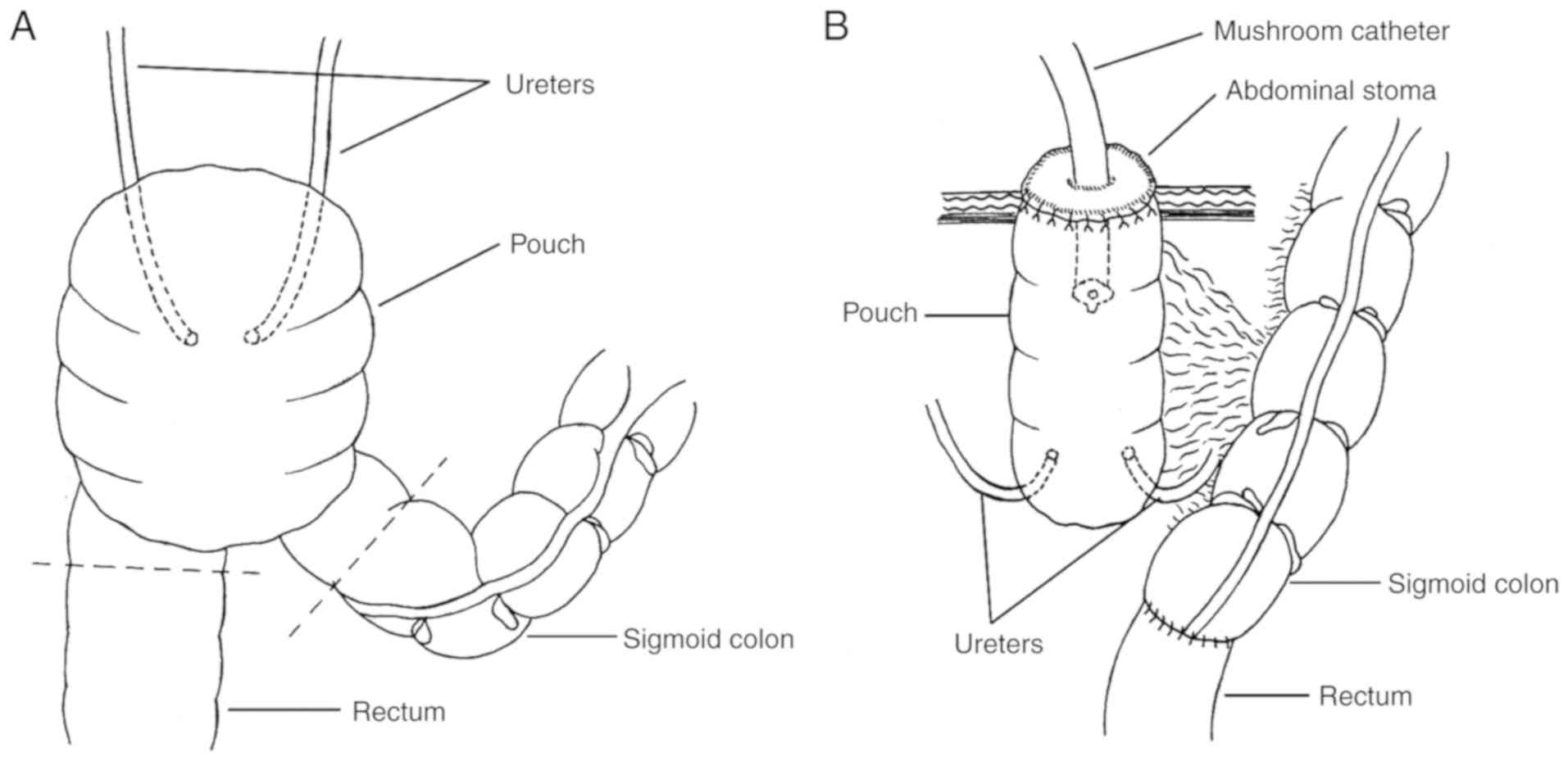
Pouch ostomy on the abdominal wall and
sigmoid-rectal anastomosis were performed under combined
intravenous and inhalation anesthesia (propofol 2.0 mg/kg
intravenous for induction; sevoflurane 1.0–2.0 vol% inhalation and
remifentanil 0.3 µg/kg/min intravenous for maintenance), in the
lithotomy position. A lower midline, 2-cm long, abdominal incision
was made from below the umbilicus to the symphysis pubis. The
pouch, sigmoid colon and rectum were mobilized, and the mesenteric
blood supply was carefully protected. The sigmoid colon was cut at
the junction of sigmoid colon and pouch using a disposable linear
cutter stapler (Sinolinks Medical Innovation, Inc.) and the rectum
below the uretero-pouch anastomosis was cut using a disposable
curved cutter (Sinolinks Medical Innovation, Inc.; Fig. 1A). End-to-end anastomosis of the
sigmoid colon and rectum was performed on the left of the pouch
with a tubular anastomat, after dilating the anal sphincter. The
integrity of removed bowels, whether the anastomosed intestine was
tension-free, and the patency of intestinal anastomosis were
assessed. To reinforce the intestinal anastomosis, interrupted
seromuscular inverting suture was performed. The pouch was opened
ventrally. The ureter-pouch anastomosis was dilated and a 7 Fr
single pig-tail stent (Well Lead Medical Co., Ltd.) was implanted
for patients with moderate to severe hydronephrosis. The pouch was
pulled out of the abdominal wall; interrupted suture was used to
suture the peritoneum with serosa and the aponeurosis of obliquus
externus abdominis muscle with seromuscular tissue. The entire
layer of the opening of the pouch was sutured to the skin and a 26
Fr mushroom catheter (Well Lead Medical Co., Ltd.) was inserted
into the pouch for drainage (Fig.
1B).
Post-operative care
Ceftriaxone sodium (2.0 g) and parenteral nutrition
were given intravenously in the immediate days postoperatively,
until a liquid diet was permitted (4–5 days later). Arterial blood
gases, serum electrolytes and renal function were measured 1 week
post-operatively. The single J stent was removed 2 weeks
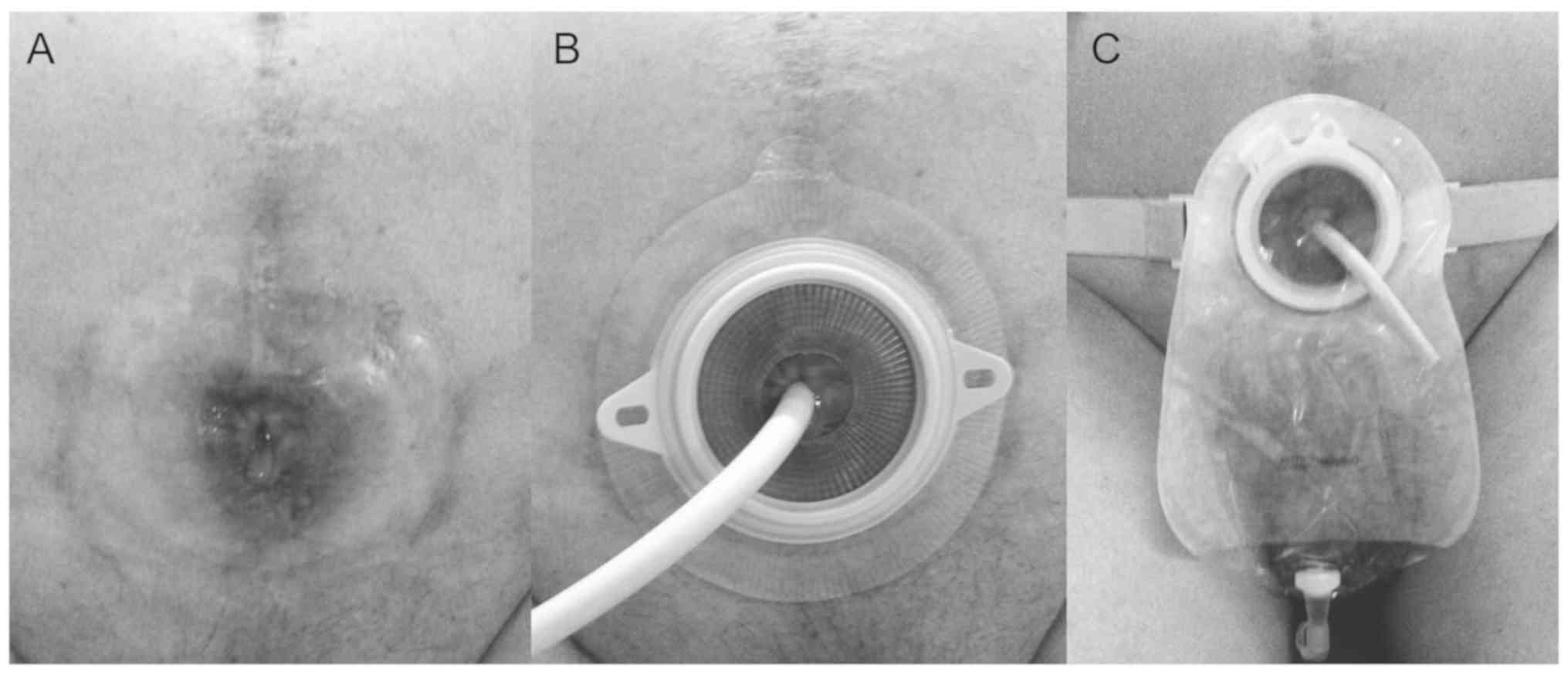
post-surgery. An EC two-piece urinary tract ostomy pocket (60 mm;
Coloplast, Ltd.) covered the stoma, and the catheter was shortened
so as to fit easily into the pocket (Fig. 2), and was replaced monthly.
Follow up
Follow-ups were carried out at 3 and 6 months, 1, 2,
and 3 years post-surgery. Arterial blood gas analysis, serum
electrolytes and renal function were monitored without sodium
bicarbonate and/or potassium citrate treatment. Preoperative and
postoperative data were compared. The quality of life index and
general health condition of the patients was evaluated at 1 year
post-surgery using the European Organization for Research and
Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) C-30
survey (9).
Statistical analysis
Data are presented as the mean ± standard deviation.
ANOVA of single-factor repeated measurement data was performed to
ascertain the significant differences, followed by bonferronis
method, between the pre- and post-surgery data using SPSS
statistics 20.0 software (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Surgical outcomes
The duration of the surgery was in the range of
230–290 min, averaging to 255.00±20.74 min. Intraoperative blood
loss was in the range of 40–300 ml, averaging to 161.67±89.09 ml.
No surgery-related complications, such as dehiscence of the bowel
anastomosis, urinary leakage, infections or thrombotic events, were
observed.
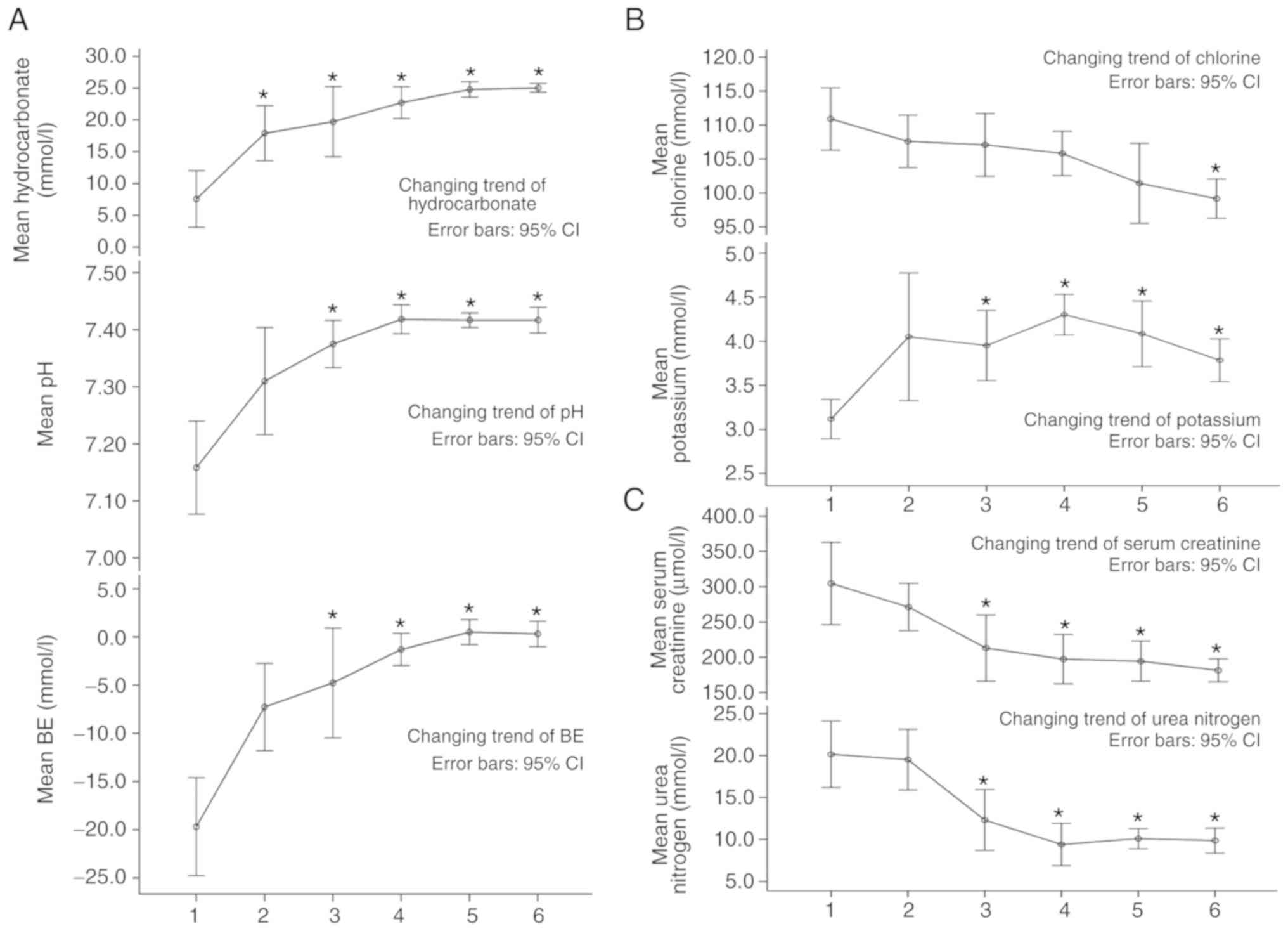
Data from the blood gas, electrolyte and renal
function analysis are shown in Table
I. Arterial blood gas analysis indicated: i) No significant
difference between the blood pH 3 months post-surgery and
preoperatively, though pH was found to recover and remain normal
(between 7.35 and 7.45) until 6 months later; and ii) the
hydrocarbonate and base excess levels were both significantly
different at 6 months and 3 years post-surgery compared with the
preoperative levels (P<0.05). At 1–2 years later, the data had
reached normal levels, with hydrocarbonate between 21 and 26 mmol/l
and base excess between −3 and 3 mmol/l.
 | Table I.Results for arterial blood gas
analysis, serum electrolytes and renal function at 1 week
preoperatively and 3 months to 3 years postoperatively (mean ±
SD). |
Table I.
Results for arterial blood gas
analysis, serum electrolytes and renal function at 1 week
preoperatively and 3 months to 3 years postoperatively (mean ±
SD).
| Analysis | Parameters | Follow-up time | Results | P-value |
|---|
| Arterial blood gas
analysis | pH | 1 week
pre-operation | 7.16±0.08 | – |
|
|
| 3 months
post-operation | 7.31±0.09 | 1.000 |
|
|
| 6 month
post-operation |
7.36±0.04a | 0.028 |
|
|
| 1 year
post-operation |
7.42±0.02a | 0.005 |
|
|
| 2 years
post-operation |
7.42±0.01a | 0.009 |
|
|
| 3 years
post-operation |
7.42±0.02a | 0.006 |
|
| Hydrocarbonate,
mmol/l | 1 week
pre-operation | 7.57±4.25 | – |
|
|
| 3 months
post-operation |
17.90±4.12a | 0.026 |
|
|
| 6 month
post-operation |
19.72±5.26a | 0.022 |
|
|
| 1 year
post-operation |
22.72±2.39a | 0.005 |
|
|
| 2 years
post-operation |
24.78±1.17a | 0.001 |
|
|
| 3 years
post-operation |
25.03±0.67a | 0.001 |
|
| BE, mmol/l | 1 week
pre-operation | −19.68±4.85 | – |
|
|
| 3 months
post-operation | −7.27±4.31 | 0.064 |
|
|
| 6 month
post-operation |
−4.77±5.42a | 0.022 |
|
|
| 1 year
post-operation |
−1.30±1.59a | 0.003 |
|
|
| 2 years
post-operation |
0.50±1.25a | 0.001 |
|
|
| 3 years
post-operation |
0.32±1.26a | 0.001 |
| Serum
electrolytes | Potassium,
mmol/l | 1 week
pre-operation | 3.12±0.21 | – |
|
|
| 3 months
post-operation | 4.05±0.69 | 0.481 |
|
|
| 6 month
post-operation |
3.95±0.38a | 0.032 |
|
|
| 1 year
post-operation |
4.30±0.22a | 0.012 |
|
|
| 2 years
post-operation |
4.08±0.35a | 0.038 |
|
|
| 3 years
post-operation |
3.78±0.23a | 0.039 |
|
| Chlorine, mmol/l | 1 week
pre-operation | 110.90±4.38 | – |
|
|
| 3 months
post-operation | 107.60±3.68 | 1.000 |
|
|
| 6 month
post-operation | 107.08±4.39 | 1.000 |
|
|
| 1 year
post-operation | 105.82±3.12 | 0.526 |
|
|
| 2 years
post-operation | 101.43±5.61 | 0.184 |
|
|
| 3 years
post-operation |
99.17±2.75a | 0.038 |
| Renal function | Urea nitrogen,
mmol/l | 1 week
pre-operation | 20.15±3.77 | – |
|
|
| 3 months
post-operation | 19.52±3.45 | 0.745 |
|
|
| 6 month
post-operation |
12.32±3.46a | 0.004 |
|
|
| 1 year
post-operation |
9.40±2.40a | 0.001 |
|
|
| 2 years
post-operation |
10.10±1.16a | 0.041 |
|
|
| 3 years
post-operation |
9.87±1.42a | 0.026 |
|
| Serum creatinine,
µmol/l | 1 week
pre-operation | 304.67±55.58 | – |
|
|
| 3 months
post-operation | 271.17±31.90 | 0.115 |
|
|
| 6 month
post-operation |
213.00±44.85a | 0.028 |
|
|
| 1 year
post-operation |
197.33±33.34a | 0.027 |
|
|
| 2 years
post-operation |
194.33±27.12a | 0.028 |
|
|
| 3 years
post-operation |
181.50±15.51a | 0.026 |
Blood potassium was found to be higher 3 months
post-surgery compared with the preoperative levels, and was
statistically different from 6 months post-surgery (P<0.05), and
remained normal (between 3.5 and 5.5 mmol/l) over the follow-up
period of 1 to 2 years. Blood chlorine recovered slowly and
decreased significantly over the 3 years post-operation
(P<0.05). However, improvement in renal function was not ideal.
The results of urea nitrogen improved 6 months post-operatively
compared with pre-operative levels (P<0.05), but concentration
at the 2 years follow-up was slightly higher than at the 1 year's
follow-up, though still significantly lower than that
pre-operatively. Serum creatinine was found to be statistically
different from 6 months post-operatively compared with
pre-operatively (P<0.05), but did not normalize (53–106 µmol/l)
until 3 years post-surgery (Table I
and Fig. 3).
The catheter was replaced once per month. All
patients had normal defecation. No severe acidosis or electrolyte
disturbances occurred during the 3 years of follow-up and the serum
creatinine levels remained stable between 162 and 203 µmol/l. All
patients acquired satisfactory health status according to the
results of EORTC QLQ C-30 survey (data not shown). Though one
patient had poor life quality, most patients were satisfied with
their quality of life post-surgery.
Discussion
Fisch et al (5) described a variation of
ureterosigmoidostomy in 1990s. This surgery, termed the
sigma-rectum or the Mainz II pouch, creates a low-pressure
rectosigmoid reservoir of increased capacity. Its major advantages
are the simplicity and reproducibility of the operation. Several
studies have demonstrated that sigma-rectum pouch is a safe and
acceptable procedure for urinary diversion, with high continence
rates and a low incidence of complications (10,11).
Nevertheless, nocturnal urinary incontinence, retrograde renal
pelvis infection, ureteral-intestinal anastomotic stenosis,
hyperchloremic metabolic acidosis, hypokalemia and impaired renal
function are common complications occurring as a result of this
procedure (12–15).
Oral administration of sodium bicarbonate has been
revealed to significantly reduce the incidence of metabolic
acidosis after sigma-rectum pouch surgery (14,16).
Most patients exhibit mild symptoms post-surgery. Severe symptoms,
manifesting as fatigue, anorexia, weight loss, polydipsia or
lethargy, are rare. If these symptoms persist, they can lead to
severe metabolic disorders and may even be life-threatening
(16). The absorption of urine in
the intestine and damage to renal function are the main causes of
metabolic disturbance after sigma-rectum pouch surgery. The
mechanism of hyperchloremic metabolic acidosis has been revealed to
be the ionized transport of ammonium, which substitutes for sodium
in the Na+-H+ antiport (12). The exchange of the weak acid
NH4 for a proton is coupled with the exchange of
bicarbonate for chloride. Thus, NH4Cl is absorbed across
the lumen into the blood in exchange for
H2CO3 (CO2 and water). Ammonium
may also enter the blood from the bowel lumen through potassium
channels (12). Hypokalemia or the
total-body depletion of potassium is found to be more common in
patients with ureterosigmoidostomy than in patients who undergo
other types of urinary intestinal diversion (14). Patients most susceptible to
total-body potassium depletion have been shown to be those with
long-standing uncorrected metabolic acidosis (17). Potassium depletion may be due to
renal potassium wasting, as a result of renal damage, osmotic
diuresis and gut loss through intestinal secretion. Postoperative
renal damage is thought to be related to hydronephrosis, caused by
the lack of ureteral motility and the stenosis of ureterointestinal
anastomosis (12).
The metabolite content of urine absorbed by the
intestinal mucosa is determined by several factors, including the
selected intestinal segment and its area, the residence time of
urine in the pouch, urine osmotic pressure, urine pH value and
liver function, in conjunction with overlong voiding intervals,
particularly the long-term contact of urine with the intestinal
mucosa due to urine reflux and storage in the descending colon at
night (12).
Hyperchloremic metabolic acidosis can be treated by
administering a potassium supplement, alkalizing agents and
blockers of chloride transport (18). However, in cases of severe dysbolism
that do not respond to conventional drug treatment, further damage
to renal function can occur, forming a vicious cycle. The results
of this case report suggested that surgical intervention to reduce
urine absorption in the intestine and improve renal function might
represent the best option to save the lives of these patients.
Although the average serum creatinine level did not decrease to
normal level (53–106 µmol/l) following surgery, and patients who
had undergone unilateral nephrectomy due to retrograde infection
may also be one of the reasons why the serum creatinine did not
reach normal level.
Urinary undiversion from a sigma-rectum pouch to a
cutaneous urinary stoma separates the pouch from the intestinal
tract and creates a stoma on the abdominal wall for continuous
urine discharge, without interfering with normal defecation
(similar to colon conduit). This procedure reduces the surface
contact area of the intestinal mucosa with urine and the residence
time of urine in the intestinal tract. The metabolites from urine
are then not so easily absorbed by the intestinal mucosa, so
acidosis and electrolyte disturbances can be corrected. During the
surgery, the stenotic ureter-pouch anastomosis was expanded, to
decrease hydronephrosis, thereby improving renal function. This
urinary undiversion procedure is an alternative treatment for
severe complications following sigma-rectum pouch surgery.
The major preoperative preparations were correcting
acidosis and electrolyte disorders and improving the general
condition of patients. Bowel preparation was performed as for
routine intestinal surgery. Bleeding and surrounding visceral
injury can easily be caused during the urinary undiversion
operation, due to adhesion and changes in local anatomical
structure. However, no massive hemorrhage or other surgery-related
complications were observed in the six patients. To ensure the best
outcomes it was recommended that attention was paid to the
following points during surgery: i) The posterior rectus sheath, if
directly cut from the original incision, could easily damage the
adherent intestinal canal below; therefore the rectus abdominis was
pulled outward to expose the posterior rectus sheath following the
opening of the anterior sheath, then the abdominal cavity was
entered from the upper part of the incision without adhesion and
the adherent posterior sheath and small intestine were isolated.
ii) The descending colon was identified and dissected downward to
the pouch, and the small intestine adhering to the pouch was
separated; ~5 cm of the distal part of the pouch was then freed in
order to ease the tension-free anastomosis of the sigmoid colon
with the rectum. During the separation process the blood supply of
the pouch was protected. iii) The mesentery supplying blood to the
pouch was moderately freed so that the pouch could be pulled out of
the abdominal stoma. iv) The ureters were not isolated outside of
the pouch to avoid injury and influencing the blood supply due to
severe adhesion around the anastomotic stomas. v) The pouch was
opened and the ureteric orifice located. Injection of 10 ml
methylene blue intravenously was performed if it was difficult to
identify the orifice. The anastomotic stoma was dilated in patients
with stricture and a 7 Fr single J tube implanted for 2 weeks to
relieve hydronephrosis. vi) The cutaneous diversion was
incontinent. Urine retention and infection are the main causes of
stone formation (19). Therefore, a
26 Fr mushroom catheter was inserted in the stoma for constant
drainage to reduce the residence time of urine in the pouch.
In conclusion, urinary undiversion from sigma-rectum
pouch to cutaneous urinary stoma exhibited a therapeutic effect in
six patients with severe metabolic disorders after sigma-rectum
pouch surgery. However, the long-term effectiveness of this
procedure is yet to be confirmed and more data are needed to
establish its benefit. Of the hundreds of patients who have
undergone sigma-rectum pouch surgery at BenQ Medical Center, only
very few have been observed to suffer from serious metabolic
disorders, which are complications that need attention and timely
correction. Surgical treatment is recommended to improve patient
quality of life, and urinary undiversion surgery may be an
effective option.
Acknowledgements
Not applicable.
Funding
Funding was obtained from Nanjing Medical Science
and Technique Development Foundation (grant no. QRX17099).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HS and HW had full access to all the data in the
study and were responsible for the integrity and the accuracy of
the data analysis. KL and WW were involved in data analysis. HY was
involved in the implementation of surgery and the design of
research methods.
Ethics approval and consent to
participate
All procedures performed in this study involving
human participants were in accordance with the ethical standards of
the institutional and/or national research committee and with the
1964 Helsinki declaration (20) and
its later amendments or comparable ethical standards. Written
informed consent was obtained from all patients.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Health Organization (WHO) Consensus
Conference in Bladder Cancer, ; Hautmann RE, Abol-Enein H, Hafez K,
Haro I, Mansson W, Mills RD, Montie JD, Sagalowsky AI, Stein JP,
Stenzl A, Studer UE and Volkmer BG: Urinary diversion. Urology. 69
(Suppl 1):17–49. 2007. View Article : Google Scholar
|
|
3
|
Lee RK, Abol-Enein H, Artibani W, Bochner
B, Dalbagni G, Daneshmand S, Fradet Y, Hautmann RE, Lee CT, Lerner
SP, et al: Urinary diversion after radical cystectomy for bladder
cancer: Options, patient selection, and outcomes. BJU Int.
113:11–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee DJ, Tyson MD and Chang SS: Conduit
Urinary Diversion. Urol Clin North Am. 45:25–36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fisch M, Wammack R, Müller SC and
Hohenfellner R: The Mainz pouch II (sigma rectum pouch). J Urol.
149:258–263. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Djokić JH, Milojević B, Pejčić T, Aćimović
M, Stamenković V and Džamić Z: Sigma-rectum pouch (Mainz pouch II).
Acta Chir Iugosl. 61:29–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hadzi-Djokic JB and Basic DT: A modified
sigma-rectum pouch (Mainz pouch II) technique: Analysis of outcomes
and complications on 220 patients. BJU Int. 97:587–591. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhvania G, Mshvildadze Sh, Managadze G and
Khvadagiani G: Results of radical cystectomy with Mainz pouch II
diversion (single institution experience). Georgian Med News.
211:7–13. 2012.
|
|
9
|
Fayers PM, Aaronson NK, Bjordal K,
Groenvold M, Curran D and Bottomley A: The EORTC QLQ-C30 Scoring
Manual (3rd edition)European Organisation for Research and
Treatment of Cancer; Brussels: 2001
|
|
10
|
Atta MA: Detubularized isolated
ureterosigmoidostomy: Description of a new technique and
preliminary results. J Urol. 156:915–919. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gilja I, Kovacić M, Radej M, Kosuta D,
Bakula B and Goles L: The sigmoidorectal pouch (Mainz pouch II).
Eur Urol. 29:210–215. 1996.PubMed/NCBI
|
|
12
|
Dahl DM and McDougal WS: Use of Intestinal
Segments in Urinary DiversionCampbell Walsh-Urology. Wein AJ,
Kavoussi LR, Novick AC, Partin AW and Peters CA: 10th. Saunders
Elsevier; Philadelphia: pp. 2441–2449. 2012
|
|
13
|
McDougal WS, Stampfer DS, Kirley S,
Bennett PM and Lin CW: Intestinal ammonium transport by ammonium
and hydrogen exchange. J Am Coll Surg. 181:241–248. 1995.PubMed/NCBI
|
|
14
|
Sun B, Yan JM, Li JY, Guo HQ, Hong Q, Yao
ZY, Zhou GB, Pan GX and Li XC: Long-term follow-up on the effects
of sigmoid-rectal pouch for urinary diversion. Urol J.
11:1629–1635. 2014.PubMed/NCBI
|
|
15
|
Obek C, Kural AR, Ataus S, Coşkuner E,
Demirkesen O, Citçi A, Onder AU and Solok V: Complications of the
Mainz pouch II (sigma rectum pouch). Eur Urol. 39:204–211. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geist RW and Ansell JS: Total body
potassium in patients after ureteroileostomy. Surg Gynecol Obstet.
113:585–590. 1961.PubMed/NCBI
|
|
17
|
Stein R, Fisch M, Andreas J, Bockisch A,
Hohenfellner R and Thüroff JW: Whole-body potassium and bone
mineral density up to 30 years after urinary diversion. Br J Urol.
82:798–803. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kraut JA and Madias NE: Metabolic
acidosis: Pathophysiology, diagnosis and management. Nat Rev
Nephrol. 6:274–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferriero M, Guaglianone S, Papalia R, Muto
GL, Gallucci M and Simone G: Risk assessment of stone formation in
stapled orthotopic ileal neobladder. J Urol. 193:891–896. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Medical Association Declaration of
Helsinki, . World Medical Association Declaration of Helsinki.
JAMA. 310:21912013. View Article : Google Scholar : PubMed/NCBI
|