Introduction
Juvenile idiopathic arthritis (JIA) is a
heterogeneous group of diseases characterized by arthritis of
unknown origin and disease onset before the age of 16 years; thus
JIA causes childhood disability (1,2). As a
result of chronic inflammation, patients with JIA suffer from joint
pain, swelling, restricted range of motion and joint deformity
(1). Severe extra-articular
abnormalities may also accompany this disease, including uveitis,
which occurs more frequently in antinuclear antibody (ANA)-positive
patients and can lead to blindness, and macrophage activation
syndrome (MAS), a complication of sJIA that can lead to multi-organ
insufficiency and even mortality (3,4)
Previous studies indicated that vitamin D played an
important role in maintaining both the skeletal and immune systems
(5,6). Vitamin D regulates the innate and
adaptive immune systems by activating the vitamin D receptor, which
is distributed widely on immune cells (7,8).
Consequently, vitamin D deficiency is associated with autoimmune
diseases such as diabetes, rheumatoid arthritis, systemic lupus
erythematosus, and JIA (9–20). A previous study reported suboptimal
vitamin D levels in JIA patients (13). Moreover, studies suggest that
25-hydroxyvitamin D (25OHD) is negatively associated with JIA
disease activity (14,15). Studies in mouse models of rheumatoid
arthritis confirmed that the vitamin D receptor plays an important
role in limiting the inflammatory phenotype (21), although other studies refute this
(16).
Previous work showed that patients with JIA,
especially those with higher disease activity, have low bone mass
(18). However, although several
studies have examined the role of vitamin D in JIA (18–22), the
immunomodulatory effects of supplementation with vitamin D have not
been previously investigated. The aim of the present study was to
examine the effect of cholecalciferol supplementation on serum
vitamin D levels, disease activity scores, and bone mineral density
in patients with JIA.
Patients and methods
The present study was a randomized, comparative,
monocentric trial (Chinese Clinical Trial Registry number:
ChiCTR-INR-16009235) conducted at the Children's Hospital of
Chongqing Medical University (Chongqing, China). From October 20,
2016 to February 15, 2018, 42 treatment-naive JIA patients
diagnosed at the Rheumatology and Immunology Inpatient Department
and followed at the Outpatient Clinic, Children's Hospital of
Chongqing Medical University, were enrolled. All participants
(6.9±3.1 years), or their guardians, provided written informed
consent. The study was approved by the Institutional Review Board
at Children's Hospital of Chongqing Medical University. Patients
were selected using a table of random numbers. All patients met the
2001 International League of Associations for Rheumatology
classification criteria (23).
Participants were randomized into two parallel groups using a
random number table: i) Vitamin D (Xiamen Lipin Pharmaceutical Co.,
Ltd.) supplementation [2000 IU per day; experimental group (EG)];
and ii) no treatment [control group (CG)]. Both groups received
standard therapy [glucocorticoids (0.5–1 mg/kg/d), non-steroidal
anti-inflammatory drugs (30–40 mg/kg/d), methotrexate (10–15
mg/m2/w), or sulfasalazine (30–50 mg/kd/d)]. None of the patients
had taken vitamin D for at least 3 months prior to entry into the
study. Exclusion criteria were as follows: A history of kidney
stones, hypercalciuria, intestinal malabsorption, primary
cardiovascular disease, lung disease, blood disease, liver disease,
a history of using drugs that inhibit bone resorption, a history of
allergy to vitamin D, and refusal to participate in the study.
Patients treated with methylprednisolone, biological agents, or
cyclophosphamide were also excluded. Demographic data, disease
duration, findings upon physical examination, erythrocyte
sedimentation rates, parathyroid hormone levels, disease activity,
bone mineral density (BMD), and serum 25OHD levels were evaluated.
The 27-joint juvenile arthritis disease activity score (JADAS-27)
(24) was used as a measure of
disease activity. Venous blood was collected (5 ml) at room
temperature and centrifuged for 10 min at 1409 × g, and the serum
was separated from the hemocytes. A blood sample of 150 µl was
taken. Deproteinization was performed with saturated zinc sulfate
solution (CAS: 7733-02-0; Shanghai Jingke Scientific Instrument
Co., Ltd.), acetonitrile (CAS: 75-05-8; Tokyo Chemical Industry UK
Ltd.) and dehydrated alcohol. 25OHD extraction from the serum was
conducted with hexane, and the hexane was evaporated using nitrogen
gas, then it was dissolved by methanol solution and detected using
high-performance liquid chromatography apparatus (APS80-16D;
AUPOS), with a flow rate of 0.5 ml/min. The Z-score, calculated
from dual-energy X-ray absorptiometry, was used as a measure of
bone mineral density (BMD; Delphi-A system; Hologic, Inc.). The
normal vitamin D range is 75 to 250 nmol/l. Vitamin D insufficiency
was defined as serum levels between <75 nmol/l and 50 nmol/l.
Levels lower than 50 nmol/l were classified as vitamin D deficiency
(25). The cumulative doses of
glucocorticoids were also compared in 6 months of each group to
identify differences. The primary outcomes were evaluated at weeks
0, 12, and 24. Safety and tolerability were also assessed at every
visit.
Statistical analysis
Student's t-test was used to analyze parametric data
and the Mann-Whitney U test was used for non-parametric data. The
one-sample Kolmogorov-Smirnov test was used to check data
distribution. Differences within each group were compared using
one-way analysis of variance (parametric data) or related-samples
Friedman's two-way analysis of variance by rank (non-parametric
data). Multiple comparison between the groups was performed using
the Friedman two-way analysis of variance (ANOVA) by Ranks Test.
Statistical analysis was performed using SPSS version 23.0 for Mac
OS (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference. Continuous variables with a
normal distribution are reported as the mean ± standard deviation
(SD) and non-normally distributed variables are reported as the
median (interquartile range).
Results
Forty-four subjects met the inclusion
criteria
Two of these refused assignment to the EG. The
remaining patients were assigned randomly to the CG (n=22) or the
EG (n=20). A total of six patients withdrew for personal reasons or
were lost to follow-up. Finally, 36 subjects completed the trial
and were included in the analysis (n=18 per group).
Data were collected at baseline and at week 12 and
24. All outcome measures, except the Z-score for BMD, showed a
non-normal distribution (Table I);
therefore, the Mann-Whitney U test was the main tool for data
analysis.
 | Table I.One-sample Kolmogorov-Smirnov test
parameters at baseline, week 12, and week 24. |
Table I.
One-sample Kolmogorov-Smirnov test
parameters at baseline, week 12, and week 24.
| A, Baseline |
|---|
|
|---|
| Parameter | Mean | SD | P-value |
|---|
| Experimental group
(n=18) |
| 25OHD,
nmol/l | 33.29 | 12.87 | 0.200 |
|
JADAS-27 | 16.19 | 9.57 | 0.048 |
|
Z-score | −1.10 | 1.48 | 0.200 |
| Control group
(n=18) |
| 25OHD,
nmol/l | 51.60 | 34.77 | 0.041 |
|
JADAS-27 | 14.31 | 6.22 | 0.200 |
|
Z-score | −1.15 | 0.96 | 0.200 |
|
| B, Week
12 |
|
| Parameter | Mean | SD | P-value |
|
| Experimental group
(n=18) |
| 25OHD,
nmol/l | 65.19 | 15.52 | 0.200 |
|
JADAS-27 | 4.56 | 5.89 | 0.022 |
|
Z-score | −0.68 | 1.47 | 0.200 |
| Control group
(n=18) |
| 25OHD,
nmol/l | 43.46 | 15.60 | 0.200 |
|
JADAS-27 | 5.51 | 7.51 | 0.012 |
|
Z-score | −0.95 | 0.77 | 0.200 |
|
| C, Week
24 |
|
| Parameter | Mean | SD | P-value |
|
| Experimental group
(n=18) |
| 25OHD,
nmol/l | 69.25 | 15.52 | 0.200 |
|
JADAS-27 | 0.94 | 2.31 | 0.000 |
|
Z-score | −0.61 | 1.29 | 0.200 |
| Control Group
(n=18) |
| 25OHD,
nmol/l | 38.83 | 13.12 | 0.040 |
|
JADAS-27 | 1.06 | 2.82 | 0.000 |
|
Z-score | −0.97 | 0.84 | 0.200 |
25OHD
The baseline data for both groups are presented in
Table II. The anthropometric,
clinical disease parameters were similar for both groups (all
P>0.05). The mean serum level of 25OHD in the EG at baseline was
33.29 nmol/l vs. 51.60 nmol/l in the CG (P=0.15). Overall, the
prevalence of vitamin D insufficiency/deficiency in all JIA
patients at baseline was 89.9%.
 | Table II.Baseline parameters of JIA patients
according to treatment group. |
Table II.
Baseline parameters of JIA patients
according to treatment group.
| Parameter | Experimental group
n=18 | Control group
n=18 | P-value |
|---|
| Age, years | 7.6 (4.3) | 6.3 (2.9) | 0.31 |
| Season |
|
| 0.66 |
| Summer
(%) | 22 | 11 |
|
| Other
(%) | 78 | 89 |
|
| Type |
|
| 0.71 |
| SJIA
(%) | 27 | 33 |
|
|
Non-SJIA (%) | 73 | 67 |
|
| Sex |
|
| 0.49 |
| Male
(%) | 44 | 28 |
|
| Female
(%) | 56 | 62 |
|
| 25OHD, nmol/l | 33.29 (12.87) | 51.60 (34.77) | 0.15 |
| JADAS-27 | 16.19 (9.57) | 14.31 (6.22) | 0.84 |
| Z-score | −1.10 (1.48) | −1.15 (0.96) | 0.94 |
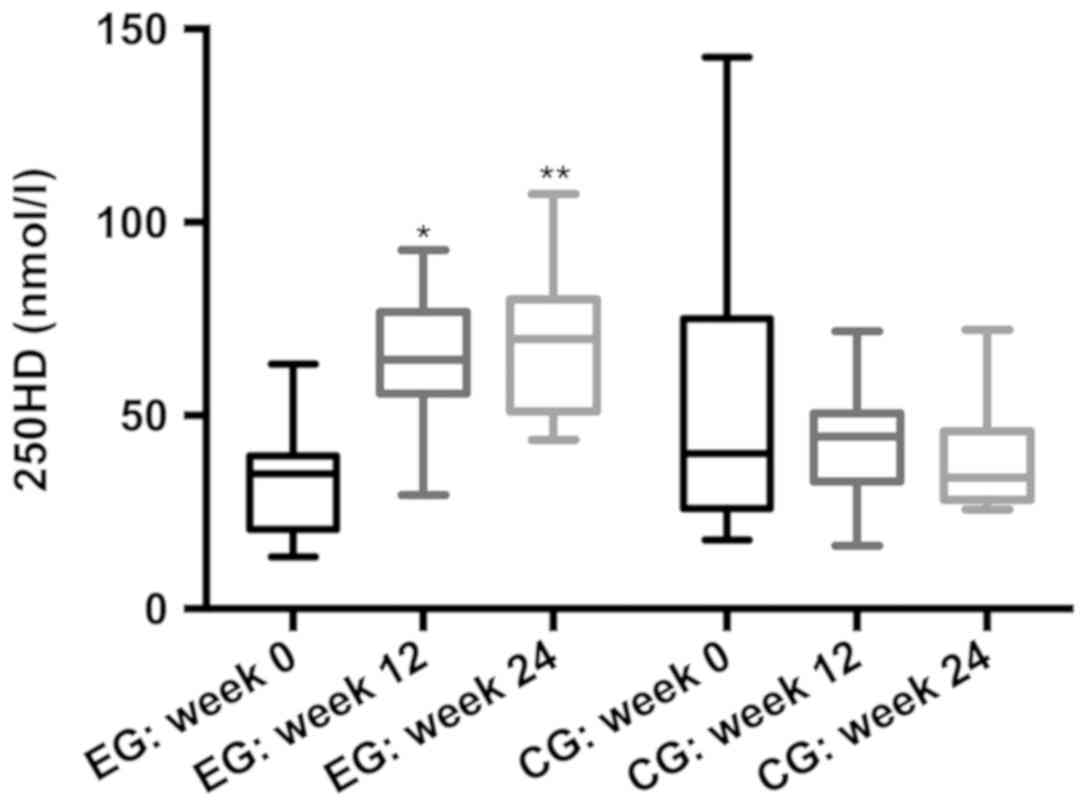
After 12 and 24 weeks of supplementation, serum
25OHD levels in EG patients were higher than those in CG patients
(week 12: 65.19 vs. 43.46, respectively; P<0.05; week 24: 69.25
vs. 38.83, respectively; P<0.05). After 24 weeks, 39.9% of
patients in the EG had serum 25OHD levels >75 nmol/l; by
contrast, 0% of patients in the CG (P<0.05) reached these
levels. Moreover, only 18.75% of patients in the CG reached 25OHD
levels >50 nmol/l, compared with 88.89% in the EG after 24 weeks
(Fig. 1).
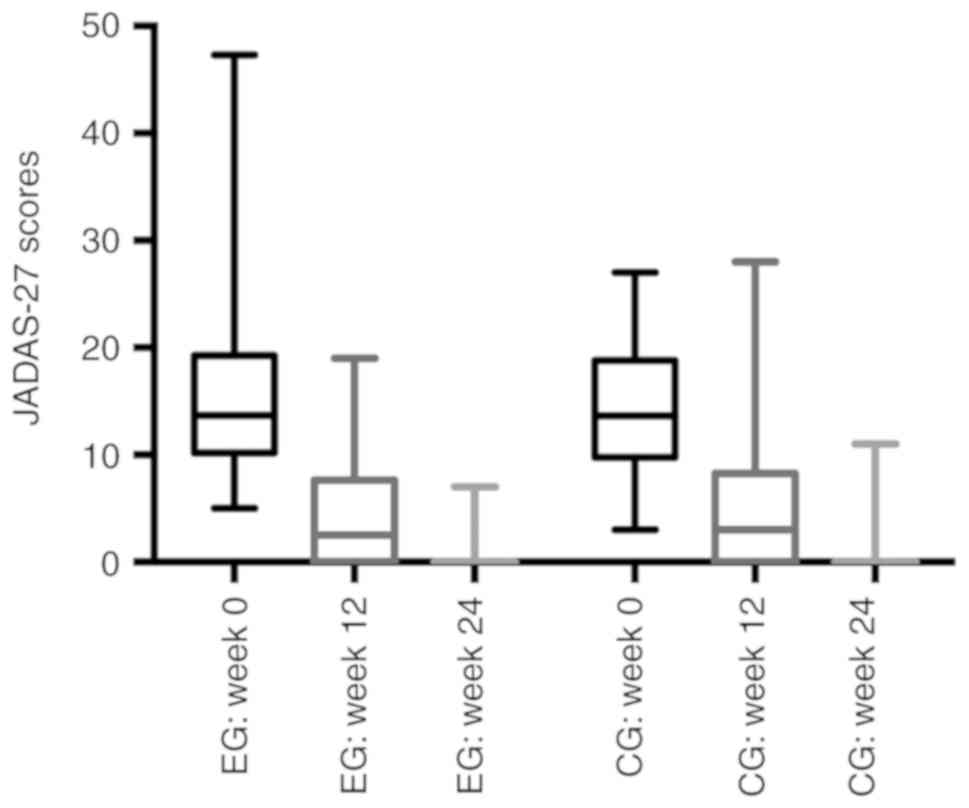
JADAS-27 score
The JADAS-27 score for 18 patients who received
vitamin D supplementation was no better than that in the CG at week
12 or at the end of the study [week 12: 4.56 vs. 4.56,
respectively, P>0.05; week 24: 0.94 vs. 1.06, respectively;
P>0.05]. They all exhibited good outcomes in terms of clinical
characteristics as 38.89% of the patients in the EG recorded a
JADAS-17 score of 0 at week 12, whereas 83.33% achieved this score
at week 24; and percentages in the CG were 37.50 and 83.33%,
respectively (Fig. 2).
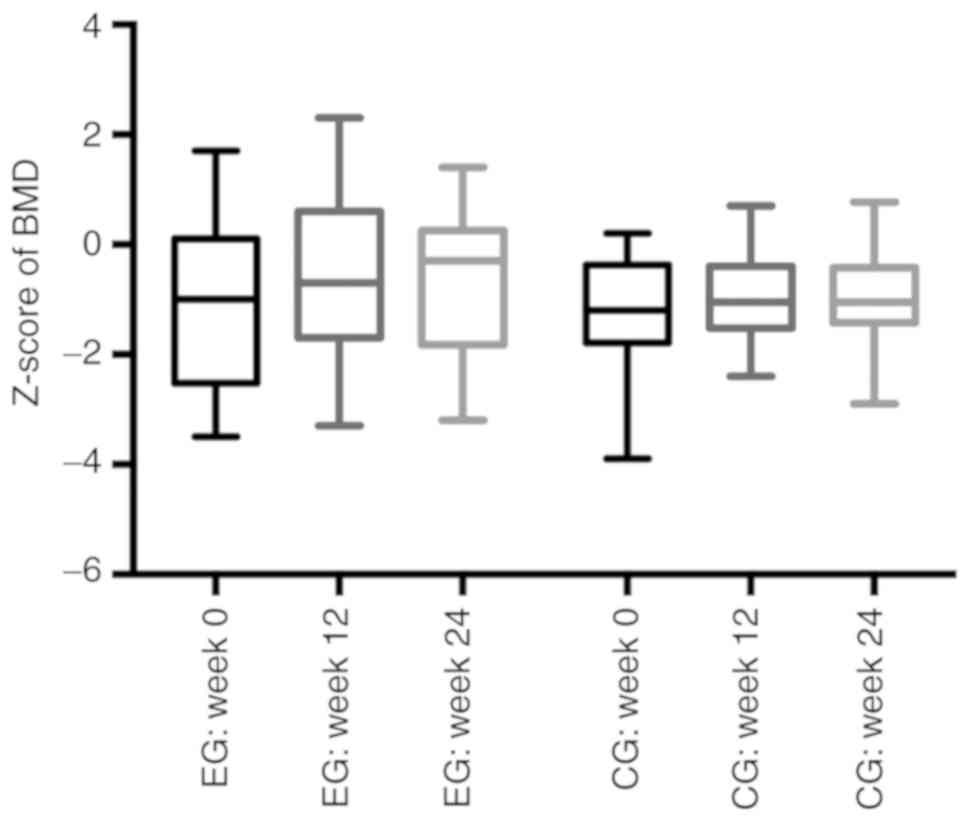
BMD
The one-sample Kolmogorov-Smirnov test revealed that
BMD data showed a normal distribution. The one-way analysis of
variance and related-samples Friedman's two-way ANOVA by rank tests
showed no significant differences in the Z-score for BMD in either
group at week 24 (P>0.05). There were no significant differences
between the EG and CG at 12 or 24 weeks (−0.68 vs. −0.95,
respectively; P>0.05, at week 12; −0.61 vs. −0.97, respectively;
P>0.05, at week 24; Fig. 3).
In the present study, five patients with systemic
JIA (nEG=3, nCG=4) were treated with glucocorticoids (Table III). The cumulative doses of
glucocorticoids in 6 months showed no significant differences
between the two groups (2249.17 vs. 2117.50, respectively;
P>0.05)
 | Table III.Glucocorticoid therapy of JIA
patients according to treatment group. |
Table III.
Glucocorticoid therapy of JIA
patients according to treatment group.
| Number | Group | Cumulative dose mg,
(6 months) |
|---|
| 1 | EG | 1617.5 |
| 2 | EG | 2850 |
| 3 | EG | 2280 |
| 4 | CG | 3132.5 |
| 5 | CG | 2585 |
| 6 | CG | 1215 |
| 7 | CG | 1537.5 |
Above all the patients, only 4 patients' serum 25OHD
concentrations of <80 nmol/l; half of these (n=2) showed a
significant benefit in terms of BMD (P<0.05).
Supplementation was well tolerated, with no safety
issues for either group. No serious adverse events were recorded,
and no patients had kidney calculi, liver damage, hypercalcemia, or
gastrointestinal symptoms.
Discussion
Taken together, the findings presented above suggest
that cholecalciferol supplementation (2,000 IU per day) for 24
weeks led to a significant increase in serum 25OHD levels in JIA
patients but did not reduce disease activity or improve BMD.
The number of participants was small. Only 60
patients with JIA are diagnosed at the Children's Hospital of
Chongqing Medical University each year, and the number of
treatment-naive patients is even lower than this. A single-center
study may also suffer from selection bias. Despite these
limitations, to the best of our knowledge, the present work is the
first to examine the outcomes of cholecalciferol supplementation
(2,000 IU per day) in Southwestern China. The results of the
present study revealed that 2,000 IU per day of vitamin D
supplementation was safe. Future work examining the effects of high
dose supplementation will be needed to clarify the relationship
between JIA and vitamin D.
A previous study showed that the immunomodulatory
effects of cholecalciferol were only observed in individuals for
whom the 25OHD increased to more than 100 nmol/l and any beneficial
effect disappeared when serum levels dropped below 100 nmol/l
(26). Other reports revealed that
the anti-inflammatory benefits of vitamin D were due to increased
expression of toll-like receptor 2 (TLR2) by peripheral blood
mononuclear cells and reduced TLR2-mediated production of tumor
necrosis factor-α, interleukin-6, and interferon-γ. These cytokines
promoted local inflammation, leading to tissue damage and
expression of auto-antigens, which then triggered autoreactive
immune responses in JIA (1,27,28).
In China, the daily amount of vitamin D recommended
by the Chinese Nutrition Society is 400 IU/d (10 µg/d) (29); the tolerable upper intake level is
1800 IU/d (45 µg/d). In the present study a safe oral dose was
defined as 2,000 IU/d (30).
Therefore, after week 24 of supplementation, serum 25OHD reached
100 nmol/l only in one patient. This result may be due to
insufficient vitamin D supplementation. This is supported by a
study of systemic lupus erythematosus patients who received 50,000
IU/week (19).
The lack of change in BMD noted between the groups
may be because serum 25OHD levels were below the functional
threshold. According to some reports, serum 25OHD concentrations of
more than 80 nmol/l facilitate calcium absorption in the intestine
(31,32). In the present study, only four
patients reached this level; half of these (n=2) showed a
significant benefit in terms of BMD (P<0.05).
Disease activity in each group decreased
significantly after 12 weeks, but differences of JADAS-27 between
and within the two groups were not clear. The plasma half-life of
25OHD is 3 weeks (33). Plasma
levels of 25OHD stabilize after three to four half-lives (about 12
weeks); however, it was not possible to reduce the follow-up period
in the present study to detect potential differences before this
time. A tool that can measure disease activity more accurately may
be needed for future studies.
It is important not to ignore the fact that
Glucocorticoids/methylprednisolone used in systemic JIA may affect
the outcome of the present study. Because JIA is a heterogeneous
group of diseases, usually only glucocorticoids/methylprednisolone
are used for the treatment of systemic JIA, which resembles more an
auto-inflammatory disease than an autoimmune disease (34). Treatment may have a strong effect on
BMD and JIA disease activity indexes. However, in the present
study, five patients with systemic JIA (nEG=3, nCG=4) were treated
with glucocorticoids still showed no statistically significant
difference (2249.17 vs. 2117.50, respectively; P>0.05)
High dose, randomized, double-blinded, controlled
studies with a greater number of subjects are required to fully
examine the benefits of vitamin D and it's in vivo role in
JIA patients. It would be interesting to examine serum levels of
25OHD and disease activity changes in patients with JIA during and
after pregnancy (35).
Acknowledgements
The authors would like to thank Dr Zhang Zhiyong, Dr
An Yunfei and Dr Ding Yuan for aiding in the selection of
participants (all, Department of Rheumatology and Immunology,
Children's Hospital of Chongqing Medical University).
Funding
The project was supported by Children's Hospital of
Chongqing Medical University and Chongqing City Health and Family
Planning Committee (grant no. 2016MSXM033).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TX, TT, ZY and LC designed and supervised the study.
TT, LM, XL and TX performed the experiments, with help from all the
other authors. TT, ZY and LC analyzed the data, while TT and TX
wrote the manuscript. All authors read and approved the final form
of manuscript.
Ethics approval and consent to
participate
The experimental protocol was established, according
to the ethical guidelines of the Helsinki Declaration and was
approved by the Institutional Review Board of Children's Hospital
of Chongqing Medical University. Written informed consent was
obtained from individual or guardian participants.
Patient consent for publication
In the present clinical trial, the patient's
guardian signed a written informed consent to publish any relevant
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prakken B, Albani S and Martini A:
Juvenile idiopathic arthritis. Lancet. 377:2138–2149. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martini A and Lovell DJ: Juvenile
idiopathic arthritis: State of the art and future perspectives. Ann
Rheum Dis. 69:1260–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bracaglia C, Prencipe G and Benedetti FD:
Macrophage Activation Syndrome: Different mechanisms leading to a
one clinical syndrome. Pediatr Rheumatol Online J. 15:52017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tappeiner C, Klotsche J, Sengler C, et al:
Risk factors and biomarkers for the occurrence of uveitis in JIA:
Data from the Inception Cohort of Newly diagnosed patients with
Juvenile Idiopathic Arthritis (ICON-JIA) study. Arthritis
Rheumatol. 2018:[J]. https://doi.org/10.1002/art.40544
|
|
5
|
DeLuca HF: Overview of general physiologic
features and functions of vitamin D. Am J Clin Nutr. 80
(Suppl):1689S–1696S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White JH: Vitamin D metabolism and
signaling in the immune system. Rev Endocr Metab Disord. 13:21–29.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamen DL and Tangpricha V: Vitamin D and
molecular actions on the immune system: Modulation of innate and
autoimmunity. J Mol Med (Berl). 88:441–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White JH: Vitamin D metabolism and
signaling in the immune system. Rev Endocr Metab Disord. 13:21–29.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maddaloni E, Cavallari I, Napoli N and
Conte C: Vitamin D and diabetes mellitus. Front Horm Res.
50:161–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeffery LE, Raza K and Hewison M: Vitamin
D in rheumatoid arthritis-towards clinical application. Rheumatol.
12:201–210. 2016.
|
|
11
|
Iruretagoyena M, Hirigoyen D, Naves R and
Burgos PI: Immune response modulation by vitamin D: Role in
systemic lupus erythematosus. Front Immunol. 6:5132015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lima GL, Paupitz J, Aikawa NE, Takayama L,
Bonfa E and Pereira RM: Vitamin D supplementation in adolescents
and young adults with juvenile systemic lupus erythematosus for
improvement in disease activity and fatigue scores: A randomized,
double-blind, placebo-controlled trial. Arthritis Care Res
(Hoboken). 68:91–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Finch SL, Rosenberg AM and Vatanparast H:
Vitamin D and juvenile idiopathic arthritis. Pediatr Rheumatol
Online J. 16:342018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bouaddi I, Rostom S, El Badri D, Hassani
A, Chkirate B, Abouqal R, Amine B and Hajjaj-Hassouni N: Vitamin D
concentrations and disease activity in Moroccan children with
juvenile idiopathic arthritis. BMC Musculoskelet Disord.
15:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Çomak E, Doğan ÇS, Uslugökçeoğlu A, et al:
Association between vitamin D deficiency and disease activity in
juvenile idiopathic arthritis. Turk J Pediatr. 71 (Suppl 3):702.
2013.
|
|
16
|
Pelajo CF, Lopez-Benitez JM, Kent DM,
Price LL, Miller LC and Dawson-Hughes B: 25-hydroxyvitamin D levels
and juvenile idiopathic arthritis: Is there an association with
disease activity? Rheumatol Int. 32:3923–3929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang T, Tang X, Xu L, Huang Y, Zeng J and
Li Q: Evaluation of bone mass in children and young adults with
juvenile idiopathic arthritis. Clin Exp Rheumatol. 33:758–764.
2015.PubMed/NCBI
|
|
18
|
Lima GL, Paupitz J, Aikawa NE, et al: A
randomized double-blind placebo-controlled trial of vitamin D
supplementation in adolescents and young adults with Juvenile-onset
SLE: Improvement in disease activity and fatigue scores. Arthritis
Care Res (Hoboken). 68:912015. View Article : Google Scholar
|
|
19
|
Ellis JA, Scurrah KJ, Li YR, Ponsonby AL,
Chavez RA, Pezic A, Dwyer T, Akikusa JD, Allen RC, Becker ML, et
al: Epistasis amongst PTPN2 and genes of the vitamin D pathway
contributes to risk of juvenile idiopathic arthritis. J Steroid
Biochem Mol Biol. 145:113–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Çomak E, Doǧan CS, Gökçeoglu AU, et al:
Evaluation of vitamin D levels in children with juvenile idiopathic
arthritis. Çocuk Sagligi Hastalik Derg. 55:191–196. 2012.
|
|
21
|
Zwerina K, Baum W, Axmann R, Heiland GR,
Distler JH, Smolen J, Hayer S, Zwerina J and Schett G: Vitamin D
receptor regulates TNF-mediated arthritis. Ann Rheum Dis.
70:1122–1129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stagi S, Bertini F, Cavalli L,
Matucci-Cerinic M, Brandi ML and Falcini F: Determinants of vitamin
D levels in children, adolescents, and young adults with juvenile
idiopathic arthritis. J Rheumatol. 41:1884–1892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petty RE, Southwood TR, Manners P, Baum J,
Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J,
Prieur AM, et al International League of Associations for
Rheumatology, : International League of Associations for
Rheumatology classification of juvenile idiopathic arthritis:
Second revision, Edmonton, 2001. J Rheumatol. 31:390–392.
2004.PubMed/NCBI
|
|
24
|
Consolaro A, Ruperto N, Bazso A, Pistorio
A, Magni-Manzoni S, Filocamo G, Malattia C, Viola S, Martini A and
Ravelli A; Paediatric Rheumatology International Trials
Organisation, : Development and validation of a composite disease
activity score for juvenile idiopathic arthritis. Arthritis Rheum.
61:658–666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holick MF, Binkley NC, Bischoff-Ferrari
HA, Gordon CM, Hanley DA, Heaney RP, Murad MH and Weaver CM;
Endocrine Society, : Evaluation, treatment, and prevention of
vitamin D deficiency: An Endocrine Society clinical practice
guideline. J Clin Endocrinol Metab. 96:1911–1930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ojaimi S, Skinner NA, Strauss BJ,
Sundararajan V, Woolley I and Visvanathan K: Vitamin D deficiency
impacts on expression of toll-like receptor-2 and cytokine profile:
A pilot study. J Transl Med. 11:1762013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jarvis JN, Jiang K, Frank MB, Knowlton N,
Aggarwal A, Wallace CA, McKee R, Chaser B, Tung C, Smith LB, et al:
Gene expression profiling in neutrophils from children with
polyarticular juvenile idiopathic arthritis. Arthritis Rheum.
60:1488–1495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gregorio A, Gambini C, Gerloni V,
Parafioriti A, Sormani MP, Gregorio S, De Marco G, Rossi F, Martini
A, et al: Lymphoid neogenesis in juvenile idiopathic arthritis
correlates with ANA positivity and plasma cells infiltration.
Rheumatology (Oxford). 46:308–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chinese Nutrition Society. Dietary
nutrient intake for Chinese residents (M)(in Chinese). China light
industry press; 2010
|
|
30
|
John N: Hathcock. (3rd). Vitamin and
Mineral Safety. 33–36. 2014.
|
|
31
|
Heaney RP, Dowell MS, Hale CA and Bendich
A: Calcium absorption varies within the reference range for serum
25-hydroxyvitamin D. J Am Coll Nutr. 22:142–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abrams SA, Hicks PD and Hawthorne KM:
Higher serum 25-hydroxyvitamin D levels in school-age children are
inconsistently associated with increased calcium absorption. J Clin
Endocrinol Metab. 94:2421–2427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zerwekh JE: Blood biomarkers of vitamin D
status. Am J Clin Nutr. 87:1087S–1091S. 2008.[J]. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mellins ED, Macaubas C and Grom AA:
Pathogenesis of systemic juvenile idiopathic arthritis: Some
answers, more questions. Nat Rev Rheumatol. 7:416–426. 2011.[J].
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ursin K, Lydersen S, Skomsvoll JF and
Wallenius M: Disease Activity of Juvenile Idiopathic Arthritis
during and after Pregnancy: A Prospective Multicenter Study. J
Rheumatol. 45:257–265. 2018. View Article : Google Scholar : PubMed/NCBI
|