Introduction
AF affects 1–2% of the general population and is the
most common type of persistent arrhythmia (1). AF is associated with an increased risk
of thromboembolism and heart failure (2).
Homeobox protein Nxk-2.5 (Nkx2.5) is a homeobox
transcription factor that promotes chamber-like myocardial gene
expression (3). Loss of Nkx2.5
function has been revealed to promote a pacemaker-like phenotype in
the pulmonary vein, which may increase ectopic pacemaker activity.
Results from a previous genome-wide association study (GWAS)
suggested that Nkx2.5 may be associated with the genetic variation
that underlies AF (4). Additionally,
a previous study demonstrated that Nkx2.5 loss of function was
associated with an increased susceptibility to familial AF
(5). However, this study did not
include an assessment of the association between Nkx2.5 silencing
and atrial electrical remodeling.
The results of the present study demonstrated that
Nkx2.5 mRNA and protein expression levels were decreased in a HF
rat model. These data revealed an association between Nkx2.5 and
AF. In addition, the effect of Nkx2.5 silencing on potassium/sodium
hyperpolarization-activated cyclic nucleotide-gated channel 4
(HCN4), gap junction alpha-5 protein (Cx40), calcium handling
proteins and protein Wnt-11 (Wnt11) signal expression levels were
assessed in HL-1 cells. Nkx2.5 silencing increased HCN4 expression,
decreased Cx40 expression and caused disturbance in the expression
of calcium handling proteins. Furthermore, Wnt11 signal protein
expression was decreased following Nkx2.5 silencing. These data
suggested that Nkx2.5 is a transcriptional regulator associated
with electrophysiological substrates.
Materials and methods
Animal model of MI
All experimental protocols were approved by The
Experimental Animal Care and Institutional Animal Ethical Committee
of Guizhou Medical University (Guizhou, China). All experiments
performed conformed to The Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
publication No. 86-23, revised 1985) (6). A total of 24 Male Sprague-Dawley rats
(weight 200–250 g) which were purchased from Hunan Silaike Jingda
Laboratory Animal Co., Ltd., (animal license no. 43004700021663).
The rats were acclimatized at 25°C with a 12-h light/dark cycle and
relative humidity of between 40 and 70%, and had free access to
food and water. The rats were anaesthetized with intraperitoneal
sodium pentobarbital (40 mg/kg; Sigma Aldrich; Merck KGaA).
Subsequent to ventilation with oxygen, a thoracotomy was performed.
The left descending (LAD) coronary artery was ligated with 7-0 silk
suture to produce an ischemic area consisting of 40–50% of the left
ventricle. Sham group rats underwent a thoracotomy without ligation
of LAD. A total of 4 weeks after surgery, echocardiography was
performed to assess left ventricular function. MI group rats with a
left ventricular ejection fraction of >70% were excluded from
the MI group. Rats were sacrificed and the left atrium (LA) tissues
were frozen at −80°C.
RNA interference
Adenoviral vectors carrying short hairpin RNA
(shRNA) that targeted rat Nkx2.5 and green fluorescent protein
(GFP) genes were designed and synthesized by Hanheng Biological
Technology Co., Ltd. The adenoviral vectors with GFP only and
without the knockdown sequence were used as the negative control
(NC) group. The sequences of the shRNA-NKX2.5 duplexes were as
follows: Sense,
5-CCGGGCCCTTCTCAGTCAAAGACATCTCGAGATGTCTTTGACTGAGAAGGGCTTTTTTG-3 and
anti-sense,
5-GATCCAAAAAAGCCCTTCTCAGTCAAAGACATCTCGAGATGTCTTTGACTGAGAAGGGC-3′.
The adenoviral particles encoding the shRNA-NKX2.5 and negative
control were measured as 1×1010 PFU/ml and were
preserved at −80°C.
Cell culture and NKX2.5 shRNA
transfection assays
HL-1 cells are a cardiac cell line from the AT-1
mouse atrial cardiomyocyte tumor lineage that has retained the
differentiated cardiac morphological, biochemical and
electrophysiological properties of adult atrial myocytes (7), and they have been used previously for
the study of atrial myocytes electrophysiology (8). HL-1 cells were used to perform the
Nkx2.5 loss of function experiments. HL-1 cells were cultured under
appropriate conditions and plated onto 6-well plates. HL-1 cells
were transfected with shRNA-Nkx2.5 at different multiplicities of
infection (MOI; 0, 5, 10, 15, 20 and 50), to identify the
appropriate MOI. Sh-Nkx2.5 were transfected into HL-1 cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturers protocol. NC HL-1 cells were transfected
with adenoviral vectors carrying GFP only. A period of 4 h
subsequent to transfection, HL-1 cells were cultured in a
humidified atmosphere of 5% CO2 at 37°C in Claycomb
medium (Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal
bovine serum, 2 mM l-glutamine (Invitrogen; Thermo Fisher
Scientific) and 0.1 mM norepinephrine (Sigma-Aldrich; Merck KGaA)
as previously described (6).
Transfection efficiency was assessed using light and fluorescent
microscopes (Olympus Corporation) 24 h after transfection
(magnification, ×100). A total of 48 h after transfection, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis were performed to assess the efficiency of
Nkx2.5 silencing at the mRNA and protein levels, and HL-1 cells
were harvested for subsequent experiments.
RT-qPCR analysis
RT-qPCR analysis was performed to assess Nkx2.5,
HCN4 and Cx40 mRNA levels present in HL-1 cells and Nkx2.5
expression in the rat LA tissues. Total RNA was extracted from
snap-frozen tissues and HL-1 cells using TRIzol® (Thermo
Fisher Scientific, Inc.). RNA (2 µg each sample) was subsequently
reverse transcribed from cDNA using a Transcriptor First Strand
cDNA Synthesis kit (Cat. No. 11483188001; Roche Diagnostics GmbH)
according to the manufacturers protocol. PCR amplification was
quantified using SYBR Green Master Mix (Vazyme). The primers
sequences were as follows: NKX2.5 forward, 5-ACGCCCTTCTCAGTCAAAGA-3
and reverse, 5-TAAAATGTAGGGGCGGTTGG-3; Cx40 forward,
5-TGGGCCAGTACCTCCTCTAT-3 and reverse, 5-GATCTTCTTCCAGCCCAGGT-3;
HCN4 forward, 5-AACCTGGGGGCTGGACAGA-3 and reverse,
5′-CTGGGCAGCCTGTGGAGAG-3′; GAPDH forward,
5′-ACAGCAACAGGGTGGTGGAC-3′ and reverse, 5′-TTTGAGGGTGCAGCGAACTT-3′.
The expression of the target genes was normalized to that of GAPDH
by applying the 2−ΔΔCq method (9).
Western blot analysis
Western blot analysis was performed to assess the
levels of Nkx2.5, HCN4, Cx40, sarcoplasmic reticulum
Ca2+-ATPase (SERCA2a), total ryanodine receptor (RyR)
and phosphorylated RyR at the serine-2814 site (p2814-RyR),
voltage-dependent L-type calcium channel subunit alpha-1C
(Cav1.2), total phospholamban (PLB), phosphorylated
phospholamban at Thr17 (P-PLB) and Wnt11 protein expression in HL-1
cells. A total of 4 weeks after surgery, rat hearts were harvested
following euthanasia. The LA tissue was used for detecting Nkx2.5
protein expression. Total HL-1 cell and LA tissue proteins were
extracted separately using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein concentration was examined using a BCA
protein assay kit (cat. no., AS1086; Aspen Biological). A total of
40 µg protein was separated by 8–12% SDS-PAGE and transferred onto
equilibrated polyvinylidene difluoride (PVDF) membranes.
Subsequently, the PVDF membranes were blocked with 5% skim milk for
1 h at room temperature and incubated with primary antibodies
overnight at 4°C against Nkx2.5 (1:1,000; cat. no., ab106923;
Abcam), HCN4 (1:1,000; cat. no., ab32675; Abcam), Cx40 (1:1,000;
cat. no., ab16585; Abcam), SERCA2a (1:500; cat. no., ab2861;
Abcam), ryanodine receptor (RyR; 1:1,000; cat. no., ab2868; Abcam),
RyR2 phosphorylated at serine (S)2814 (P2814-RyR2; 1:500; cat. no.,
A010-31; Badrilla Ltd.), Cav1.2 (1:500; cat. no.,
ab84814; Abcam), PLB (1:1,000; cat. no., ab85146; Abcam),
phosphorylated (p)-PLB (1:500; cat. no., ab62170; Abcam) and Wnt11
(1:1,000; cat. no., ab31962; Abcam). After washing, membranes were
subsequently treated with horseradish peroxidase (HRP)-conjugated
rabbit anti-goat IgG (1:10,000; cat. no. AS1108; Aspen Biological)
or HRP-conjugated goat anti-rabbit IgG (1:10,000; cat. no. AS1107;
Aspen Biological) secondary antibodies for 1 h at room temperature.
The chemiluminescence of the blots was detected using an ECL kit
(Beyotime Institute of Biotechnology) and protein expression was
normalized to GAPDH. Densitometric analysis was subsequently
performed using the Image J software (version 15.0; National
Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS v.19.0
(IBM Corp). Data was expressed as the mean ± standard error of the
mean. Comparisons between the data were performed using an unpaired
Students t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
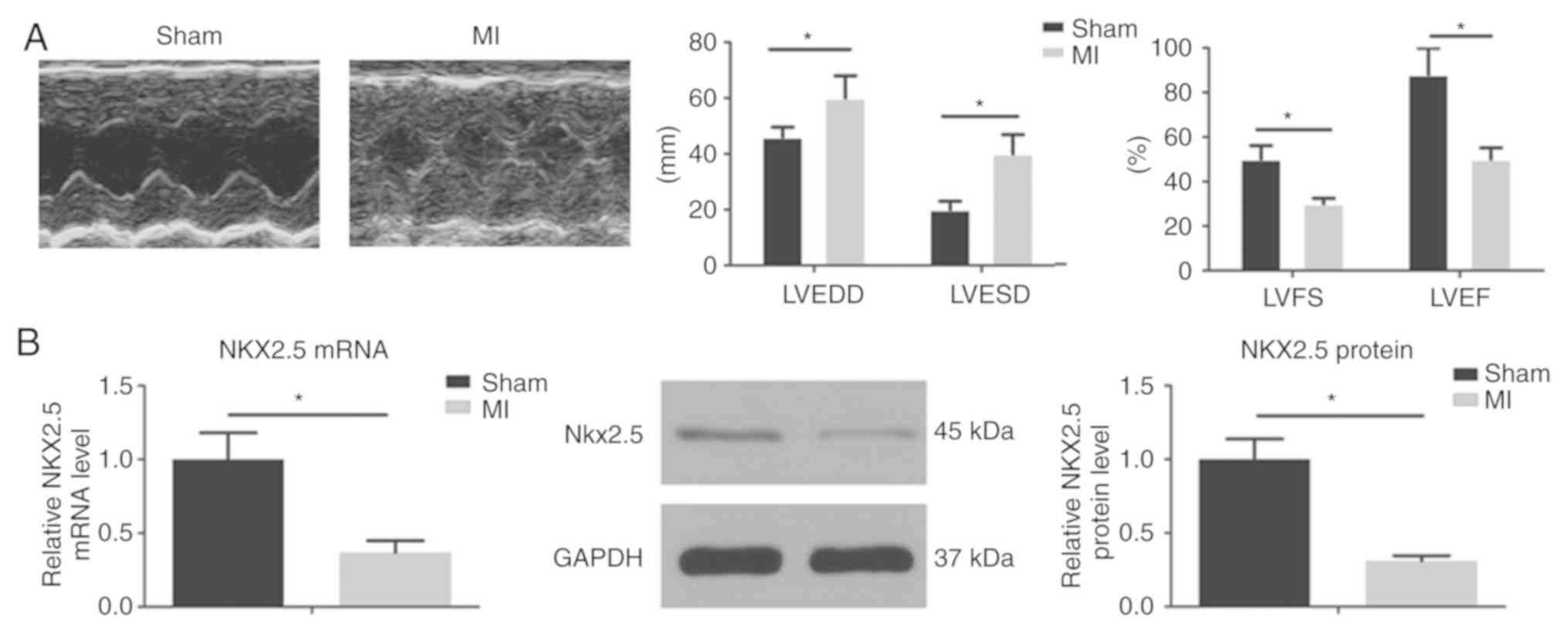
Nkx2.5 expression is impaired in a
myocardial infarction-heart failure rat model
Heart failure is one of the most common causes of
AF. A previous study has demonstrated that MI-induced heart failure
leads to atrial remodeling and promotes AF (10). In the present study, the size of left
ventricle was enlarged and the LV contraction function was
decreased 4 weeks after LAD ligation. To assess whether AF was
associated with changes in Nkx2.5 expression, Nkx2.5 mRNA and
protein levels were measured in the rats LA tissues. Nkx2.5 mRNA
and protein expression levels were decreased in the LA of the AF
model rats when compared with the sham rats (Fig. 1).
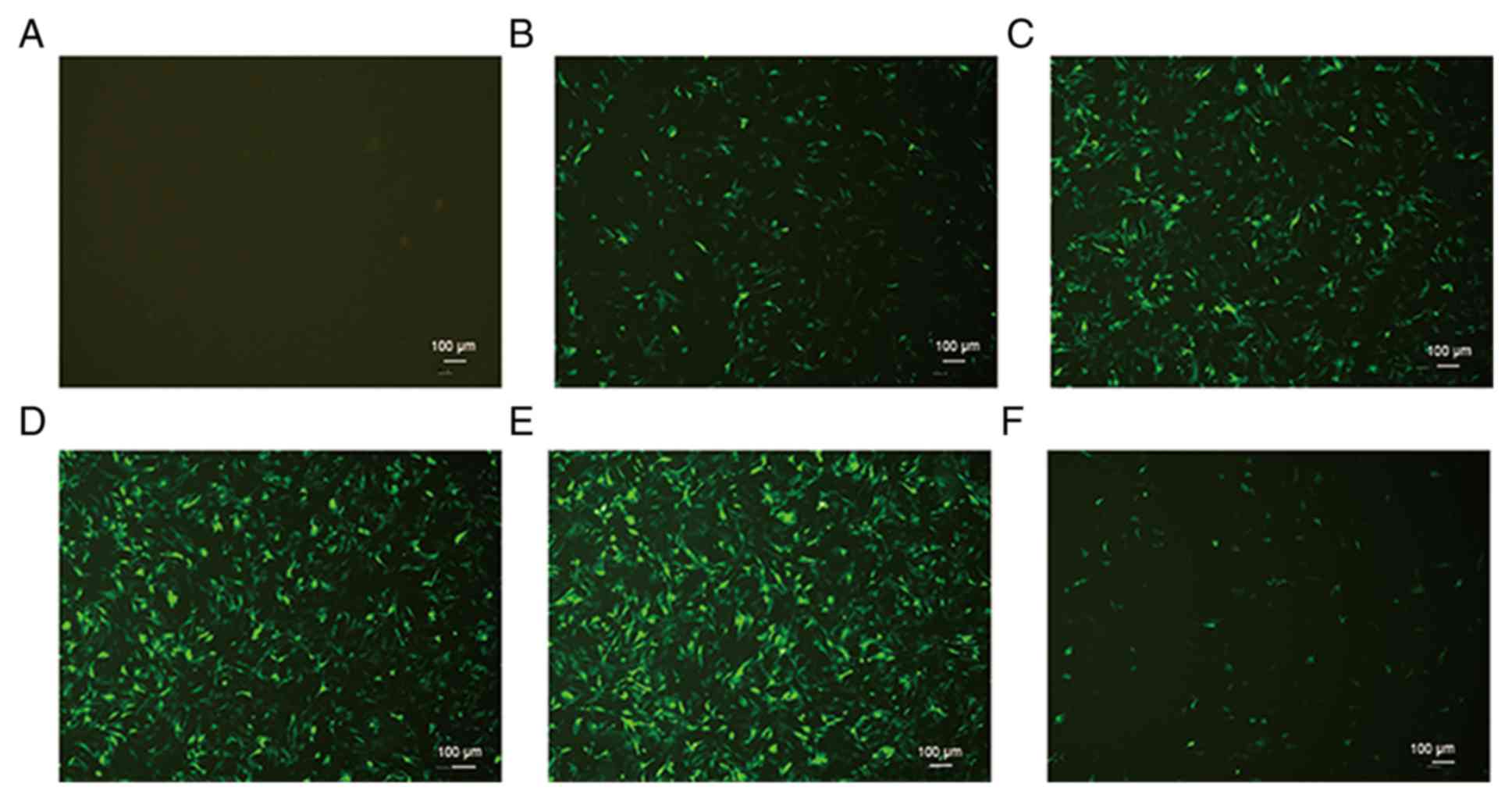
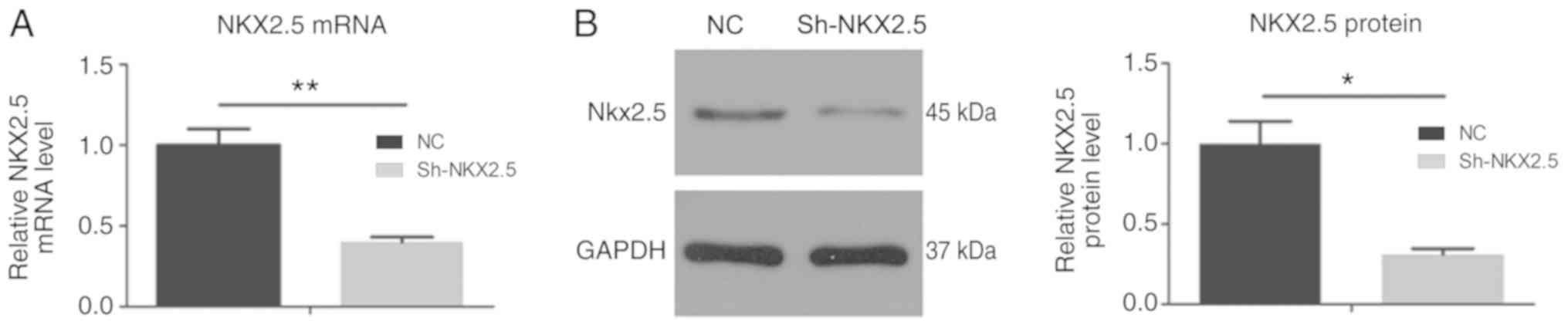
Optimum MOI value of transfection and
Nkx2.5 gene silencing efficiency in HL-1 cells
HL-1 cells were transfected with GFP and the Nkx2.5
gene at a range of MOI values. HL-1 cells exhibited green
fluorescence following viral transfection. As MOI increased to 20,
the transfection efficiency was concomitantly increased. At MOI 20,
the transfection efficiency was >80% (Fig. 2). The optimum MOI value of
transfection was 20 and was used for all subsequent analyses in the
present study. Nkx2.5 mRNA and protein levels were significantly
decreased following Nkx2.5 interference 48 h following transfection
(Fig. 3).
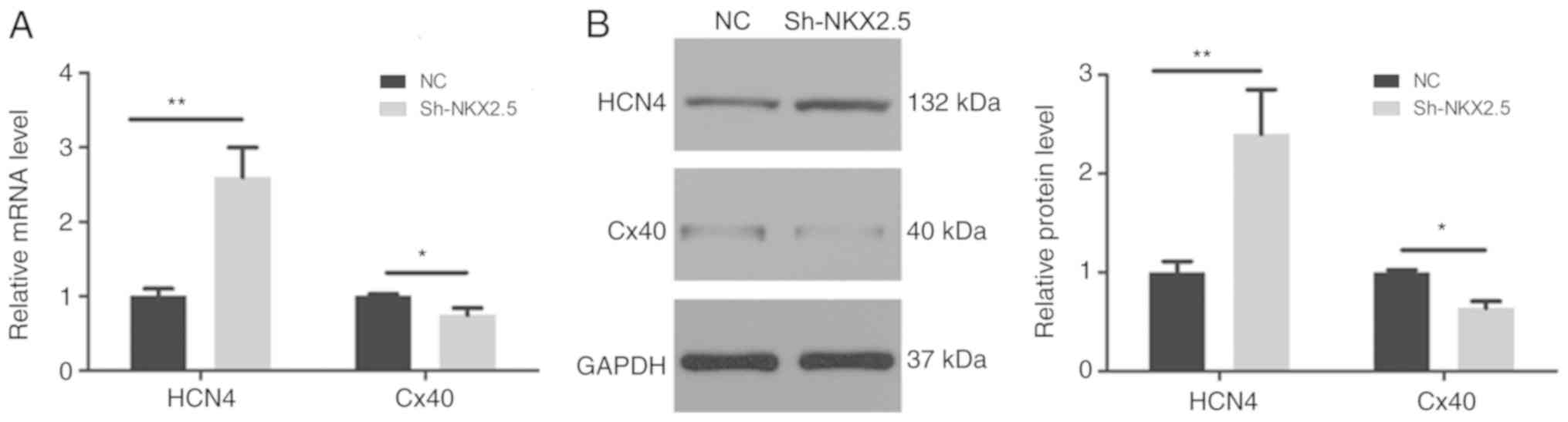
Effect of Nkx2.5 silencing on the
expression of HCN4 and Cx40
To further investigate the underling effect of
Nkx2.5 on electrophysiology, the mRNA and protein expression levels
of HCN4 and Cx40 were analyzed. As presented in Fig. 4, HCN4 expression increased
significantly following Nkx2.5 silencing; by contrast, Cx40
expression was severely decreased.
Effect of Nkx2.5 silencing on the
expression of calcium handling and Wnt11 signaling
To investigate the regulatory role of Nkx2.5 on the
expression of calcium handling proteins, the present study assessed
whether Nkx2.5 silencing impaired calcium handling protein
expression levels. Western blot analysis revealed significantly
decreased protein expression levels of SERCA2a, Cav1.2
and P-PLB, whereas the phosphorylated RyR2 expression level was
increased. No change in total RyR and total PLB expression levels
was exhibited in the sh-Nkx2.5 HL-1 cells. Wnt11 expression was
decreased following transfection with Nkx2.5 (Fig. 5).
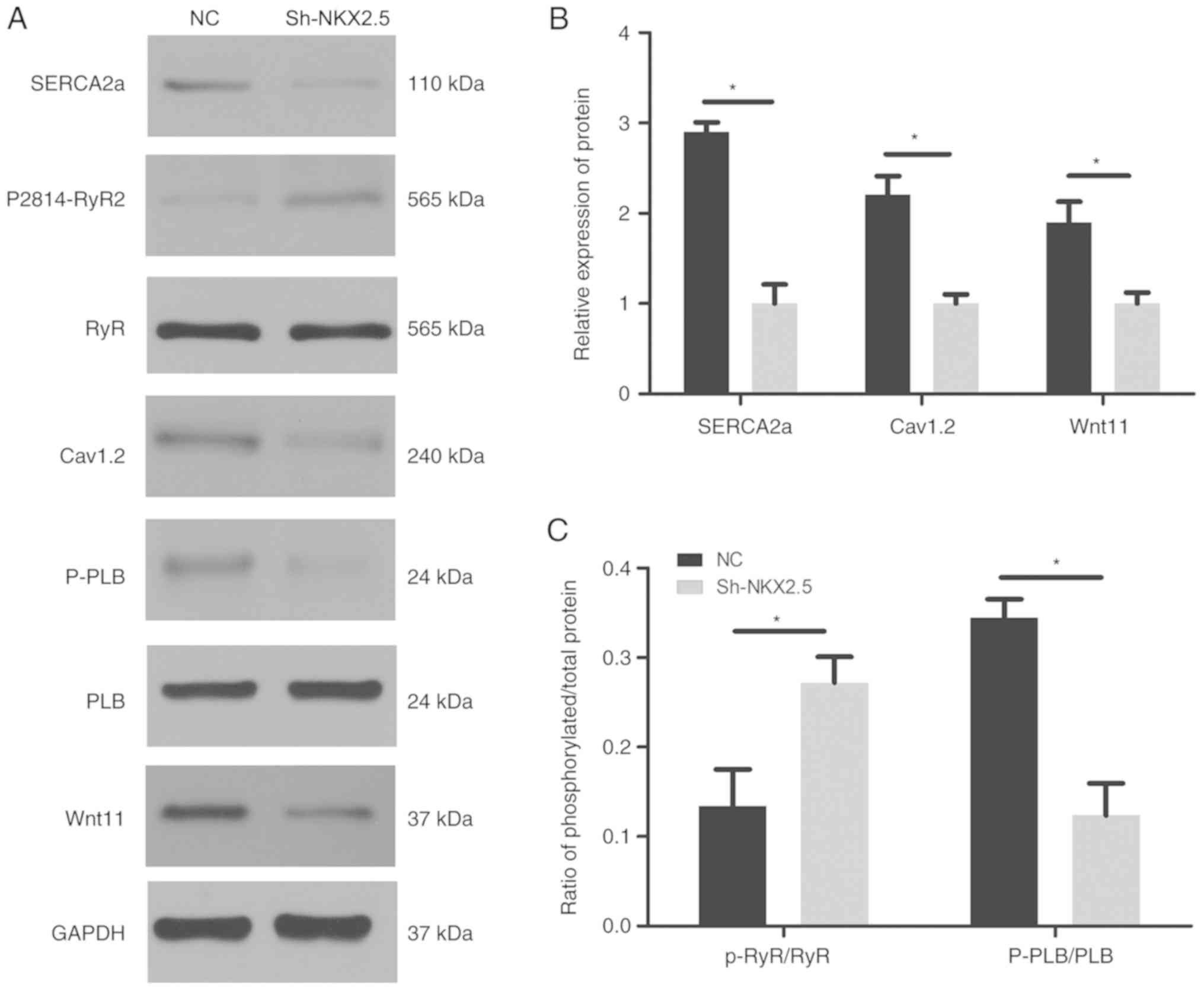 | Figure 5.Nkx2.5 downregulation disrupted the
expression of calcium handling proteins and Wnt11 signaling. (A)
Representative western blot analysis blots and summary analysis of
(B) SERCA2a, Cav1.2, Wnt11 and (C) p-RyR2/RyR, p-PLB/PLB protein
expression levels. (n=5 animals/group). *P<0.05. Nkx2.5,
homeobox protein Nxk-2.5; SERCA2a, sarcoplasmic reticulum
Ca2+-ATPase; PS2814-RyR2, ryanodine receptor 2 phosphorylated at
serine 2814; Cav1.2, voltage-dependent L-type calcium channel
subunit alpha-1C; PLB, total phospholamban; P-PLB, phosphorylated
phospholamban at Thr17; Wnt11, protein Wnt-11; sh, short hairpin
RNA; NC, negative control.. |
Discussion
Nkx2.5 is a homeobox transcription factor that
serves an important role in embryogenesis. A recent study found
that GWAS identified risk variants near Nkx2.5, which are
associated with AF, and it has been suggested that Nkx2.5 may be a
causative connection (4); however,
it did not provide experimental results regarding the regulation of
Nkx2.5 and its association with electrophysiology.
The results of the present study demonstrated that
Nkx2.5 mRNA and protein expression levels were decreased in the AF
rat model, suggesting a potential association between Nkx2.5 loss
of function and atrial electrophysiology substrates. Specifically,
in HL-1 cells, Nkx2.5 silencing led to ion channel remodeling. HCN4
channels, the pore-forming α-subunits of funny channels, have been
demonstrated to be closely associated with spontaneous activity
(11). The increased expression of
HCN4 in the atrial and pulmonary vein, and the decreased expression
in the sinoatrial node, were revealed to be associated with the
onset and maintenance of AF (12).
In the present study, Nkx2.5 silencing increased HCN4 mRNA and
protein expression levels in HL-1 cells, which may generate
abnormal automaticity and pacemaker activity in the atrium in
vivo. In patients with AF, a variety of mutations in the coding
region of Cx40 have been previously identified (13,14). In
a Cx40 loss of function mouse model, atrial conduction velocity was
decreased with the increased incidence of AF episodes (15). In the present study, Nkx2.5 silencing
was demonstrated to downregulate Cx40.
Abnormal calcium handling has been indicated to
contribute to AF. In the present study, phosphorylated RyR at S2814
was increased following Nkx2.5 silencing. RyR2 is the primary SR
calcium release channel located in cardiomyocytes and it has been
revealed that increased phosphorylation of RyR2 increases the
incidence of arrhythmia due to diastolic SR calcium leakage
(16). SERCA2a is responsible for
pumping calcium back to the SR during diastole to avoid an increase
in intracytoplasmic calcium concentration, which may increase the
sodium-calcium exchange and triggered activity. PLB has been
demonstrated to bind to SERCA2a and inhibit its activity; following
phosphorylation, this inhibition of SERCA2a is lost (17). In the present study, SECA2a and P-PLB
expression was impaired during Nkx2.5 silencing. These data support
the assumption that Nkx2.5 gene silencing may lead to electrical
remodeling, which may be the mechanism by which Nkx2.5 variation is
associated with the onset of arrhythmia events.
The Wnt11 signaling pathway has been previously
demonstrated to be crucial for myocardial cell differentiation
(18,19) and is in parallel with Nkx2.5
expression in differentiating embryoid bodies (20). Wnt11 signaling is important in
ventricle organization. The expression of ventricular genes and the
ventricular structure are dysregulated during Wnt11 downregulation
(21). Furthermore, the Wnt11
signaling pathway is a critical regulator of calcium handling
(8,22). In the present study, the association
between Nkx2.5 and Wnt11 was assessed. The results revealed that
Wnt11 protein expression was significantly decreased following
Nkx2.5 silencing. These results provide evidence for a potential
association between Nkx2.5 and the Wnt11 signaling pathway.
In conclusion, to the best of our knowledge, the
present study revealed for the first time that Nkx2.5 expression is
significantly decreased in a HF rat model. The present study
provides evidence that impaired Nk2.5 function is associated with
calcium homeostasis and Cx40 and HCN4 expression levels, which
leads to atrial electric remodeling. This is associated with
arrhythmogenesis, which may act via the Wnt11 signaling
pathway.
Although Nkx2.5 gene silencing was demonstrated to
be associated with the alteration of calcium handling proteins,
HCN4, Cx40 and Wnt11 expression levels, further in vivo and
in vitro experiments assessing the effect of Nkx2.5 on
atrial electrophysiology, including action potential duration,
effective refractory period and calcium leak, are required. Further
experiments examining Nkx2.5 overexpression in an AF animal model
are required to explore the role of NKX2.5 in the pathogenesis of
AF.
Acknowledgements
Not applicable.
Funding
The present study was supported The Science and
Technology Foundation of Guizhou Province [grant no.,
LH-(2017)-7199].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
WZ conceived, designed and supervised the present
study. JC and SX developed the methodology, reviewed the manuscript
and analyzed and interpreted the data. WL, LRW, LW and YL were
responsible for statistical analysis and data interpretation. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
All experimental protocols were approved by The
Experimental Animal Care and Institutional Animal Ethical Committee
of Guizhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilke T, Groth A, Mueller S, Pfannkuche M,
Verheyen F, Linder R, Maywald U, Bauersachs R and Breithardt G:
Incidence and prevalence of atrial fibrillation: An analysis based
on 8.3 million patients. Europace. 15:486–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andrade J, Khairy P, Dobrev D and Nattel
S: The clinical profile and pathophysiology of atrial fibrillation:
Relationships among clinical features, epidemiology, and
mechanisms. Circ Res. 114:1453–1468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye W, Wang J, Song Y, Yu D, Sun C, Liu C,
Chen F, Zhang Y, Wang F, Harvey RP, et al: A common Shox2-Nkx2-5
antagonistic mechanism primes the pacemaker cell fate in the
pulmonary vein myocardium and sinoatrial node. Development.
142:2521–2532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nielsen JB, Thorolfsdottir RB, Fritsche
LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM,
Sveinbjornsson G, et al: Biobank-driven genomic discovery yields
new insight into atrial fibrillation biology. Nat Genet.
50:1234–1239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang RT, Xue S, Xu YJ, Zhou M and Yang
YQ: A novel NKX2.5 loss-of-function mutation responsible for
familial atrial fibrillation. Int J Mol Med. 31:1119–1126. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
National Institutes of Health, . Guide for
the Care and Use of Laboratory Animals. 8th. National Academies
Press; Washington: pp. 86–23. 2011
|
|
7
|
Claycomb WC, Lanson NA Jr, Stallworth BS,
Egeland DB, Delcarpio JB, Bahinski A and Izzo NJ Jr: HL-1 cells: A
cardiac muscle cell line that contracts and retains phenotypic
characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA.
95:2979–2984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lozano-Velasco E, Hernandez-Torres F,
Daimi H, Serra SA, Herraiz A, Hove-Madsen L, Aránega A and Franco
D: Pitx2 impairs calcium handling in a dose-dependent manner by
modulating Wnt signalling. Cardiovasc Res. 109:55–66. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cardin S, Guasch E, Luo X, Naud P, Le
Quang K, Shi Y, Tardif JC, Comtois P and Nattel S: Role for
MicroRNA-21 in atrial profibrillatory fibrotic remodeling
associated with experimental postinfarction heart failure. Circ
Arrhythm Electrophysiol. 5:1027–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DiFrancesco D: HCN4, sinus bradycardia and
atrial fibrillation. Arrhythm Electrophysiol Rev. 4:9–13. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YD, Hong YF, Zhang Y, Zhou XH, Ji YT,
Li HL, Hu GJ, Li JX, Sun L, Zhang JH, et al: Association between
reversal in the expression of hyperpolarization-activated cyclic
nucleotide-gated (HCN) channel and age-related atrial fibrillation.
Med Sci Monit. 20:2292–2297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang YQ, Liu X, Zhang XL, Wang XH, Tan HW,
Shi HF, Jiang WF and Fang WY: Novel connexin40 missense mutations
in patients with familial atrial fibrillation. Europace.
12:1421–1427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Yang YQ, Gong XQ, Wang XH, Li RG,
Tan HW, Liu X, Fang WY and Bai D: Novel germline GJA5/connexin40
mutations associated with lone atrial fibrillation impair gap
junctional intercellular communication. Hum Mutat. 34:603–609.
2013.PubMed/NCBI
|
|
15
|
Lübkemeier I, Andrié R, Lickfett L, Bosen
F, Stöckigt F, Dobrowolski R, Draffehn AM, Fregeac J, Schultze JL,
Bukauskas FF, et al: The Connexin40A96S mutation from a patient
with atrial fibrillation causes decreased atrial conduction
velocities and sustained episodes of induced atrial fibrillation in
mice. J Mol Cell Cardiol. 65:19–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Houser SR: Role of RyR2 phosphorylation in
heart failure and arrhythmias: Protein kinase A-mediated
hyperphosphorylation of the ryanodine receptor at serine 2808 does
not alter cardiac contractility or cause heart failure and
arrhythmias. Circ Res. 114:1320–1327; discussion 1327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samuel TJ, Rosenberry RP, Lee S and Pan Z:
Correcting calcium dysregulation in chronic heart failure using
SERCA2a gene therapy. Int J Mol Sci. 19:2018. View Article : Google Scholar
|
|
18
|
Bisson JA, Mills B, Paul Helt JC, Zwaka TP
and Cohen ED: Wnt5a and Wnt11 inhibit the canonical Wnt pathway and
promote cardiac progenitor development via the Caspase-dependent
degradation of AKT. Dev Biol. 398:80–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cohen ED, Miller MF, Wang Z, Moon RT and
Morrisey EE: Wnt5a and Wnt11 are essential for second heart field
progenitor development. Development. 139:1931–1940. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terami H, Hidaka K, Katsumata T, Iio A and
Morisaki T: Wnt11 facilitates embryonic stem cell differentiation
to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun.
325:968–975. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagy II, Railo A, Rapila R, Hast T,
Sormunen R, Tavi P, Räsänen J and Vainio SJ: Wnt-11 signalling
controls ventricular myocardium development by patterning
N-cadherin and beta-catenin expression. Cardiovasc Res. 85:100–109.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Panáková D, Werdich AA and Macrae CA:
Wnt11 patterns a myocardial electrical gradient through regulation
of the L-type Ca(2+) channel. Nature. 466:874–878. 2010. View Article : Google Scholar : PubMed/NCBI
|