Introduction
As a bone-destructive disease that is caused by a
disorder of the coagulation and fibrinolysis system and
insufficient blood supply, osteonecrosis of the femoral head (ONFH)
is a rare but disabling condition that usually leads to progressive
femoral head collapse and secondary arthritis necessitating total
hip arthroplasty (1). ONFH can be
categorized into traumatic and non-traumatic types. As a subtype of
ONFH, non-traumatic ONFH often occurs after the treatment of
inflammatory diseases by corticosteroid therapy (2). It has been reported that the onset and
development of non-traumatic ONFH is closely correlated with
various factors including human immunodeficiency virus infection,
autoimmune diseases, alcohol abuse, use of glucocorticoids and
coagulopathies (3). However, up to
now, the pathogenesis of this disease remains unclear.
In addition, messenger RNA (mRNA) that encode
protein products of the human genome also transcribe large sets of
non-coding RNAs that have no protein-coding ability (4). It has been reported that the
development of non-traumatic ONFH is usually accompanied by changes
in the expression of different types of non-coding RNA, such as
microRNAs (miRNAs/miR) (5). However,
the involvement of long non-coding (lnc)RNAs, which is a subgroup
of non-coding RNAs composed of >200 nucleotides and with pivotal
roles in both normal physiological and pathological processes
(6) is largely unknown. AWPPH is a
newly discovered lncRNA that plays an oncogenic role in the
development of hepatocellular carcinoma (7) and bladder cancer (8), whiles its involvement in non-traumatic
osteonecrosis of femoral head (ONFH) is unknown. Significantly
downregulated expression of AWPPH in ONFH patients compared with in
healthy controls was observed in the authors' preliminary
microarray analysis (data not shown), indicating the possible
involvement of AWPPH in ONFH. Therefore, a systemic investigation
on the functionality of AWPPH in non-traumatic ONFH was carried
out. It was demonstrated that lncRNA AWPPH can inhibit the
development of non-traumatic ONFH by upregulating Runx2 expression.
The present study provided new insights for the diagnosis and
treatment of non-traumatic osteonecrosis.
Materials and methods
Subjects
A total of 36 patients with ONFH were enrolled at
the Luoyang Orthopedic Hospital of Henan Province from January 2015
to January 2017. Those patients included 20 males and 16 females,
and were aged between 30 to 67 years, with a mean age of 48.3±7.9
years. The duration of disease ranged from 1 year to 12.5 years,
with a mean duration of 5.8±2.1 years. At the same time, 30 healthy
people with similar age and gender distributions were also included
to serve as the control group. The control group included 16 males
and 14 females, and were aged between 27 to 70 years, with a mean
age of 49.1±6.6 years. All participants signed informed consent.
This study has been approved by the ethics committee of Luoyang
Orthopedic Hospital of Henan Province.
Specimen collection
EDTA-PBS (2 mM) was used to dilute bone marrow
aspirates and Ficoll-Hypaque density gradient centrifugation was
performed to isolate mononuclear cells. Cells were cultured in
low-glucose Dulbecco's modified Eagle's medium (DMEM-LG,
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 1% antibiotic-antimycotic solution (Invitrogen; Thermo
Fisher Scientific, Inc.) and 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) in an incubator (37°C, 5%
CO2). Cells were harvested when mesenchymal stem cells
(MSCs) reached 80–90% confluence. Then cells were incubated with
0.25% trypsin for passage. Whole blood (20 ml) was extracted from
each participant on the day of admission. Serum samples were
prepared by keeping whole blood at room temperature for 90 min,
followed by centrifugation at 1,250 × g at room temperature for 20
min.
Cell line and cell culture
Human MSCs from bone marrow (hMSC-BM) was provided
by ScienCell Research Laboratories. Cells were cultured in
high-glucose DMEM containing 1% antibiotic
(streptomycin)-antimycotic solution (Invitrogen; Thermo Fisher
Scientific, Inc.) and 10% FBS in an incubator (37°C, 5%
CO2). MSCs were harvested when 80–90% confluence was
reached. Cells were then incubated with 0.25% trypsin for passage.
Serum was not added in case of drug treatment. After transfection,
cells were culture for 48 h before subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
MSCs and serum derived from patients were mixed with
TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.) to
extract total RNA. In cases of bone morphogenetic protein-2 (BMP-2)
treatment, MSCs were treated with BMP-2 (0, 25, 100 and 200 ng/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 12 h before use.
NanoDrop™ 2000 Spectrophotometers (Thermo Fisher Scientific, Inc.)
was used to measure RNA concentration. RNA samples with a A260/A280
ratio between 1.8 and 2.0 were subjected to reverse transcription
to synthesize cDNA using PrimeScript RT Reagent Kit (Takara Bio,
Inc., Tokoyo, Japan) through following thermal conditions: 25°C for
5 min, 55°C for 20 min and 75°C for 5 min. PCR mixtures were
prepared using SYBR® Green Realtime PCR Master Mix
(Toyobo Life Science, Tokoyo, Japan) PCR reactions were performed
using primers listed below: 5′-CTGGATGGTCGCTGCTTTTTA-3′ (forward)
and 5′-AGGGGGATGAGTCGTGATTT-3′ (reverse) for human lncRNA AWPPH;
5′-CGGCCCTCCCTGAACTCT-3′ (forward) and 5′-TGCCTGCCTGGGGTCTGTA-3′
(reverse) for human Runx2; 5′-GACCTCTATGCCAACACAGT-3′ (forward) and
5′-AGTACTTGCGCTCAGGAGGA-3′ (reverse) for human β-actin. PCR
reaction conditions were: 95°C for 35 sec, followed by 40 cycles of
95°C for 15 sec and 60°C for 42 sec. Data were processed using
2−ΔΔCq method (9) and
expression of AWPPH and Runx2 was normalized to β-actin.
Establishment of AWPPH overexpression
and short hairpin (sh)RNA silencing cell lines
AWPPH cDNA was inserted into pIRSE2-EGFP vector
(Clontech Laboratories, Inc., Mountainview, Palo Alto, CA, USA) to
construct AWPPH expression vector. AWPPH shRNA expression vector
and scrambled shControl were provided by Shanghai GenePharma Co.,
Ltd., (Shanghai, China). The target site for AWPPH shRNA was
GGTCTGGTCGGTTTCCCATTT. hMSC-BM cells were cultured overnight to
reach 80–90% confluence and transfection was performed using
Lipofectamine 2000 reagent (11668-019; Invitrogen; Thermo Fisher
Scientific Inc.) to transfect 10 nM vectors into 5×105
cells. Empty pIRSE2-EGFP vector and scrambled shControl
(5′-CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG-3′) were as
negative controls. Cells without transfection were used as
control.
Western-blot
RIPA buffer (Cell Signaling Technology, Inc.) was
mixed with in vitro cultured hMSC-BM cells to extract total
protein. Protein concentration was measured using bicinchoninic
acid method. Protein samples were denatured and subjected to 10%
SDS-PAGE gel electrophoresis (20 µg per lane), followed by
transmembrane to PVDF membrane. After blocking in PBS containing 5%
non-fat milk for 2 h at room temperature, incubation with primary
antibodies including rabbit anti-Runx2 antibody (1:2,000; cat. no.
ab23981; Abcam) and anti-GAPDH (1:2,000; cat. no. ab8245; Abcam)
was performed overnight at 4°C. After washing, membranes were
further incubated with anti-rabbit IgG-HRP secondary antibody
(1:1,000; cat. no. MBS435036; MyBioSource, Inc.) at room
temperature for 1 h. After washing, Amersham™ ECL™ Western Blotting
Reagent (Sigma-Aldrich, Merck KGaA) method was used to develop
signal. Image J v1.46 software (National Institutes of Health,
Bethesda, MD, USA) was used to normalize relative expression of
Runx2 to GAPDH.
Statistical analysis
SPSS19.0 (IBM Corps., Armonk, NY, USA) was used for
all statistical analyses in this study. Count data (basic clinical
data) were processed using a Chi-square test. Comparisons of
measurement data between two groups and among multiple groups were
performed by unpaired Student's t-test and one-way analysis of
variance followed by least significant difference test,
respectively. Receiver operating characteristic (ROC) curve
analysis was performed using the default parameters and the
software automatically output images. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of lncRNA AWPPH and Runx2
mRNA in MSCs and serum derived from non-traumatic ONFH patients and
healthy people
Expression of lncRNA AWPPH and mRNA in MSCs and
serum collected from non-traumatic ONFH patients and healthy people
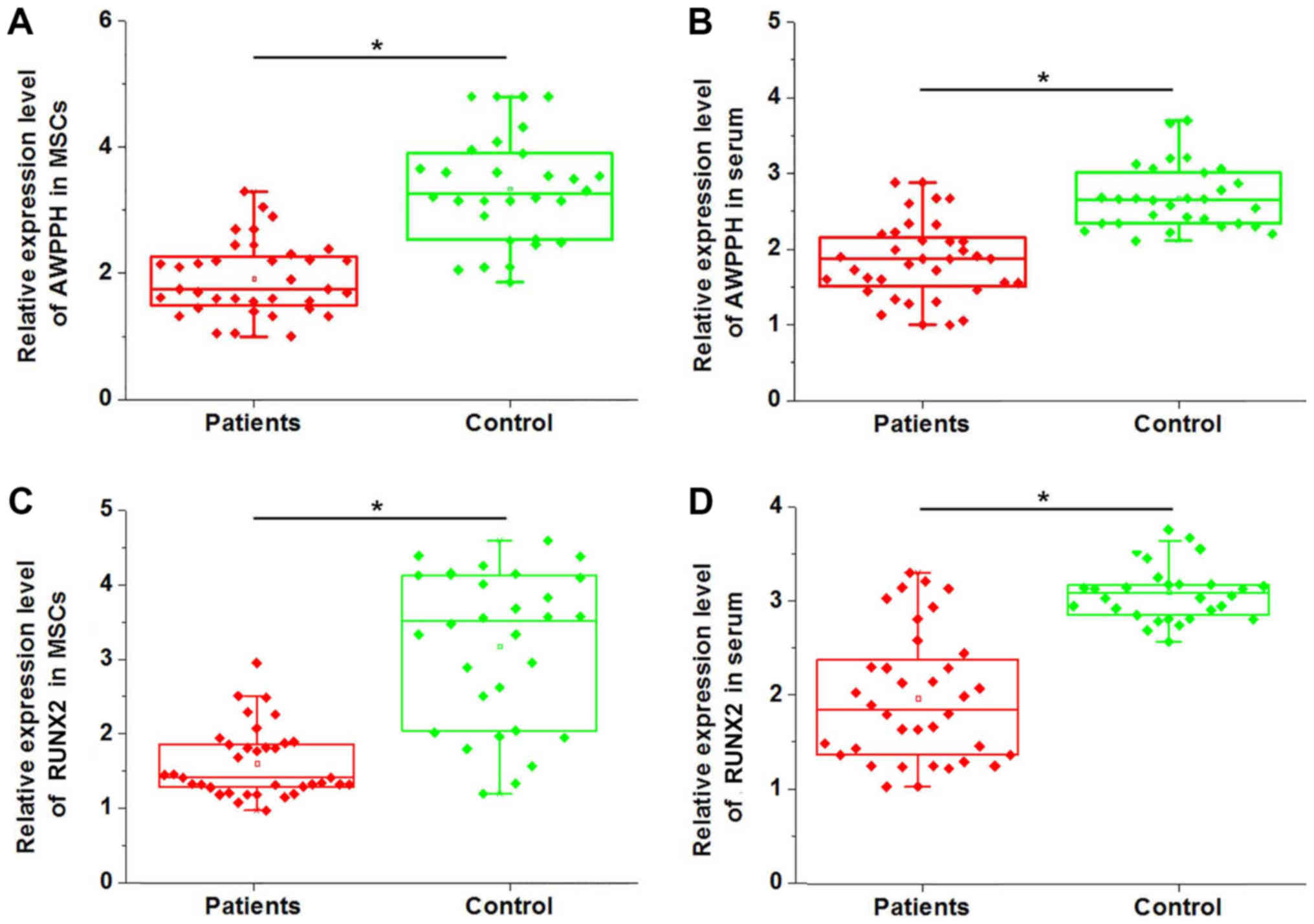
was detected by RT-qPCR. As presented in Fig. 1A, expression of lncRNA AWPPH in MSCs
was significantly decreased in patients with non-traumatic ONFH
compared with in healthy people (P<0.05). Similarly, expression
of lncRNA AWPPH in serum was also significantly downregulated in
patients with non-traumatic ONFH compared with in healthy people
(P<0.05; Fig. 1B). In addition,
expression of Runx2 in MSCs (Fig.
1C) and serum (Fig. 1D) was also
downregulated in ONFH patients compared with in the controls. Those
data suggest that downregulation of lncRNA AWPPH and Runx2 is
likely to be involved in the pathogenesis of non-traumatic
ONFH.
Diagnostic value of lncRNA AWPPH
expression in MSCs and serum for patients with non-traumatic
ONFH
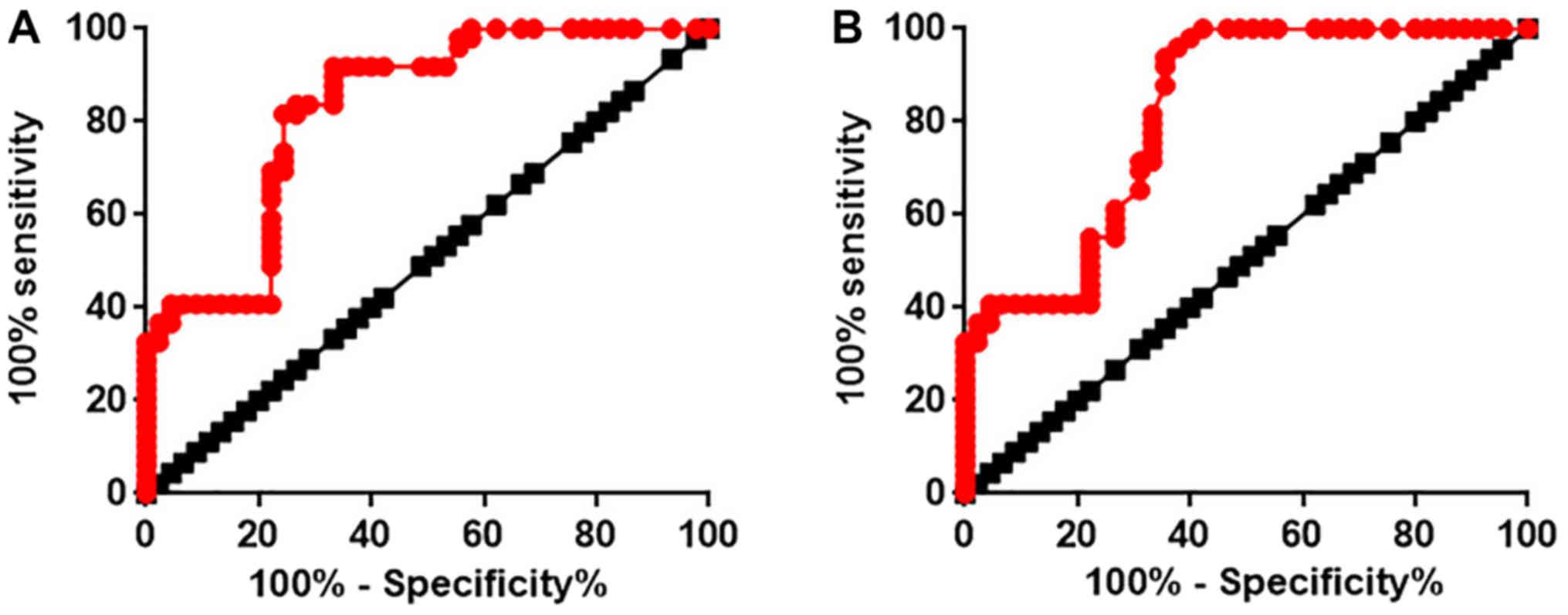
ROC curve analysis was performed to analyze the
diagnostic value of lncRNA AWPPH expression in MSCs and serum for
patients with ONFH. As presented in Fig.
2A, the area under the curve (AUC) of the use of lncRNA AWPPH
expression in MSCs for the diagnosis of non-traumatic ONFH was
0.8259 with 95% confidence interval of 0.7417 to 0.9100
(P<0.0001; data not shown). In addition, AUC of the use of
lncRNA AWPPH expression in serum for the diagnosis of non-traumatic
ONFH was 0.8177 with 95% confidence interval of 0.7310 to 0.9044
(P<0.0001).
Association between lncRNA AWPPH
expression in MSCs and serum with the clinicopathological data of
patients with non-traumatic ONFH
Chi-square analysis was performed to analyze the
association between lncRNA AWPPH expression in MSCs and serum and
the clinicopathological data of patients with non-traumatic ONFH.
As presented in Tables I and
II, lncRNA AWPPH expression in MSCs
and serum exhibited no significant association with age, gender and
living habits of patients with non-traumatic ONFH (P>0.05).
However, a significant association between AWPPH expression and
course of disease was observed (P<0.05).
 | Table I.Correlation between long noncoding RNA
AWPPH expression in mesenchymal stem cells and the
clinicopathological data of patients with non-traumatic
osteonecrosis of femoral head. |
Table I.
Correlation between long noncoding RNA
AWPPH expression in mesenchymal stem cells and the
clinicopathological data of patients with non-traumatic
osteonecrosis of femoral head.
| Variables | Groups | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Gender | Male | 20 | 11 | 9 | 0.45 | 0.5 |
|
| Female | 16 | 7 | 9 |
|
|
| Age | >45 (years) | 21 | 12 | 9 | 1.03 | 0.31 |
|
| <45 (years) | 15 | 6 | 9 |
|
|
| Course of
disease | >5 years | 17 | 12 | 5 | 5.45 | 0.02 |
|
| <5 years | 19 | 6 | 13 |
|
|
| Smoking | Yes | 16 | 9 | 7 | 0.45 | 0.5 |
|
| No | 20 | 9 | 11 |
|
|
| Drinking | Yes | 14 | 6 | 8 | 0.47 | 0.49 |
|
| No | 22 | 12 | 10 |
|
|
 | Table II.Correlation between long noncoding RNA
AWPPH expression in serum and the clinicopathological data of
patients with non-traumatic osteonecrosis of femoral head. |
Table II.
Correlation between long noncoding RNA
AWPPH expression in serum and the clinicopathological data of
patients with non-traumatic osteonecrosis of femoral head.
| Items | Groups | Cases | High-expression | Low-expression | χ2 | P-value |
|---|
| Gender | Male | 20 | 12 | 8 | 1.8 | 0.18 |
|
| Female | 16 | 6 | 10 |
|
|
| Age | >45 (years) | 21 | 12 | 9 | 1.03 | 0.31 |
|
| <45 (years) | 15 | 6 | 9 |
|
|
| Course of
disease | >5 years | 17 | 12 | 5 | 5.45 | 0.02 |
|
| <5 years | 19 | 6 | 13 |
|
|
| Smoking | Yes | 16 | 10 | 6 | 1.8 | 0.18 |
|
| No | 20 | 8 | 12 |
|
|
| Drinking | Yes | 14 | 6 | 8 | 0.47 | 0.49 |
|
| No | 22 | 12 | 10 |
|
|
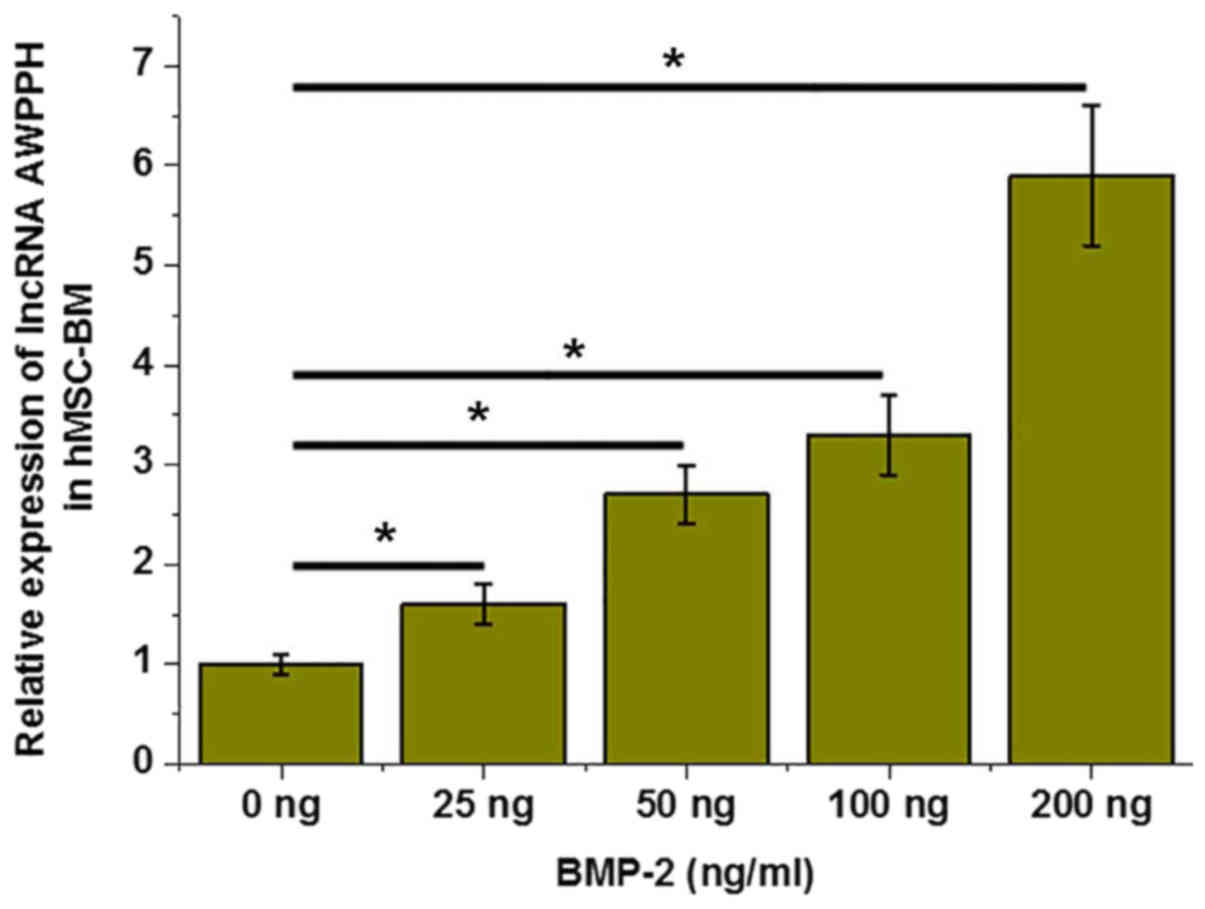
BMP-2 induces the expression of lncRNA
AWPPH in hMSC-BM cells
BMP-2 is an inducer of osteoblastic differentiation.
In the present study, different concentrations of BMP-2 (0, 25, 100
and 200 ng/ml) were used to treat hMSC-BM cells for 12 h and
expression of lncRNA was detected by RT-qPCR. As presented in
Fig. 3, BMP-2 significantly
upregulated the expression of lncRNA AWPPH in a dose-dependent
manner (P<0.05).
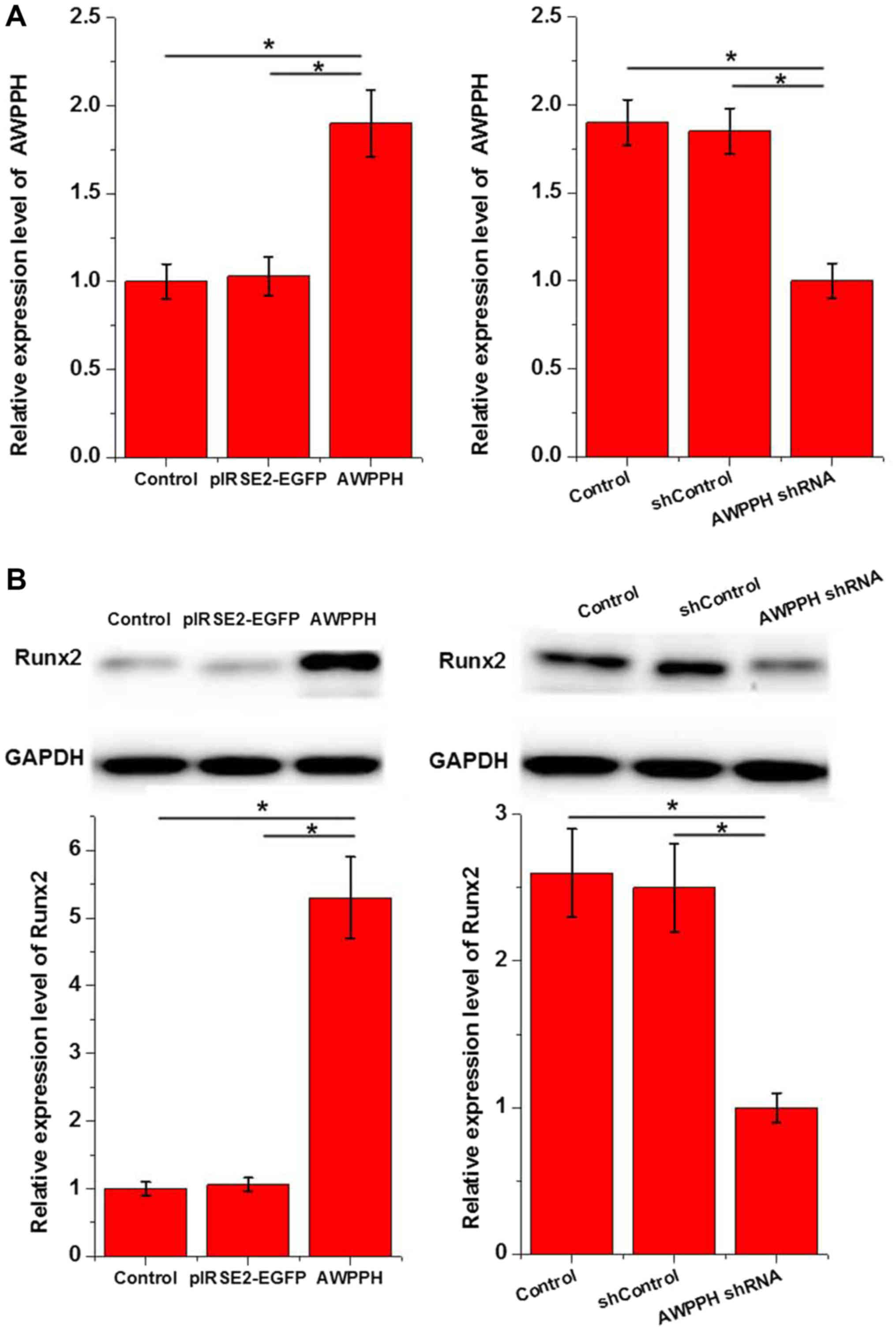
Effects of AWPPH overexpression and
shRNA silencing on expression of Runx2 in hMSC-BM cells
Effects of AWPPH overexpression and shRNA silencing
hMSC-BM cell lines were constructed and confirmed by measuring the
expression level of AWPPH by RT-qPCR. Runx2 is a marker of
osteoblastic differentiation. Therefore, the effects of altered
AWPPH overexpression on Runx2 expression were investigated. The
results demonstrated that AWPPH overexpression significantly
promoted (P<0.05; Fig. 4A) and
shRNA silencing significantly inhibited (P<0.05; Fig. 4B) the expression of Runx2 in hMSC-BM
cells.
Discussion
The onset of non-traumatic ONFH requires the
involvement of multiple non-coding RNAs. In a study on
corticosteroid-induced non-traumatic ONFH, Li et al
(10) identified 11 differentially
expressed miRNAs, indicating the involvement of miRNAs in the
development of this disease. In another study, Wei et al
(11) found that miR-17-5p was
downregulated and lncRNA HOTAIR was upregulated in non-traumatic
ONFH, and lncRNA HOTAIR overexpression inhibited the expression
miR-17-5p to participate in the development of non-traumatic ONDH
by regulating osteogenic differentiation and proliferation. AWPPH
is a novel lncRNA with significantly upregulated expression in the
development of hepatocellular carcinoma (7) and bladder cancer (8). In the present study, expression levels
of lncRNA AWPPH in MSCs and serum were identified to be increased
in non-traumatic ONFH patients compared with healthy people. The
results of the present study suggest that, in addition to cancer,
lncRNA AWPPH may also participate in the pathogenesis of other
human diseases, such as non-traumatic ONFH.
As a rare bone-destructive disease, non-traumatic
ONFH is sometimes misdiagnosed by physicians using traditional
diagnostic methods, such as preoperative radiograph images,
magnetic resonance images (12).
Therefore, highly sensitive biomarkers are needed to improve the
diagnosis of this disease. It has been reported that serum levels
of α-melanocyte stimulating factor (MSH) were significantly
decreased in non-traumatic ONFH patients compared with in healthy
people and were further decreased with the progression of disease,
and the reduced expression level of α-MSH is a sensitive diagnostic
marker for non-traumatic ONFH (13).
In the present study, ROC curve analysis demonstrated that
expression of lncRNA AWPPH in both MSCs and serum can be used to
effectively distinguish non-traumatic ONFH patients from healthy
people, indicating that lncRNA AWPPH expression may serve as a
promising diagnostic marker for non-traumatic ONFH. In addition, as
a less invasive technique, detection of lncRNA AWPPH in serum
through blood extraction should be a preferred method. Expression
of lncRNAs can be altered under certain conditions, such as aging
(14), alcohol abuse (15) and tobacco consumption (16), which may affect the accuracy of the
use of lncRNAs in the diagnosis of diseases. In the present study,
lncRNA AWPPH expression in MSCs and serum demonstrated no
significant correlations with the age, gender and smoking and
drinking habits of patients with non-traumatic ONFH, indicating the
high accuracy of the use of AWPPH in the diagnosis of non-traumatic
ONFH. However, it is worth noting that altered expression of lncRNA
AWPPH has been observed in different types of human diseases
(7,8). There, multiple markers should be used
to improve the specificity of the diagnosis of non-traumatic
ONFH.
Altered osteoblastic differentiation is a key
pathological change during the development of non-traumatic ONFH
(17). BMP-2 induces osteoblastic
differentiation (18). In this
study, BMP-2 induces the expression of lncRNA AWPPH in hMSC-BM
cells in a dose-dependent manner, indicating the involvement of
lncRNA AWPPH in the regulation of osteoblastic differentiation.
Runx2 is marker of osteoblastic differentiation (19) and increased expression level of Runx2
promotes osteoblastic differentiation (20), while downregulation of Runx2 may
promote the progression of non-traumatic ONFH (17). In this study, AWPPH overexpression
promoted and shRNA silencing inhibited the expression of Runx2 in
hMSC-BM cells. The results of the present study suggest that AWPPH
may inhibit the development of non-traumatic ONFH by promoting
osteoblastic differentiation through the upregulation of Runx2
expression.
In conclusion, AWPPH was downregulated in
non-traumatic ONFH patients compared with in healthy controls.
Expression of AWPPH is a sensitive diagnostic marker for
non-traumatic ONFH, especially for patients with longer duration of
disease. AWPPH overexpression promoted, while AWPPH shRNA silencing
inhibited the expression of Runx2 expression in hMSC-BM cells.
Therefore, it was concluded that lncRNA AWPPH may participate in
the development of non-traumatic osteonecrosis of femoral head by
upregulating Runx2.
Acknowledgements
Not applicable.
Funding
The authors would like to thank the financial
support from the Special Subject of TCM Science Research in Henan
Province (grant no. 2017ZY2032).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
XC designed experiments. XC, JL and DL performed
experiments. LZ and QW analyzed data. XC drafted the manuscript.
All authors approved the final version of the manuscript.
Ethics approval and consent to
participate
This study has been approved by the ethics committee
of Luoyang Orthopedic Hospital of Henan Province. All participants
signed informed consent.
Patient consent for publication
All patients signed informed consent for the
publication of data in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Choi HR, Steinberg ME and Y Cheng E:
Osteonecrosis of the femoral head: Diagnosis and classification
systems. Curr Rev Musculoskelet Med. 8:210–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okazaki S, Nagoya S, Matsumoto H, Mizuo K,
Sasaki M, Watanabe S, Yamashita T and Inoue H: Development of
non-traumatic osteonecrosis of the femoral head requires toll-like
receptor 7 and 9 stimulations and is boosted by repression on
nuclear factor kappa B in rats. Lab Invest. 95:92–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Assouline-Dayan Y, Chang C, Greenspan A,
Shoenfeld Y and Gershwin ME: Pathogenesis and natural history of
osteonecrosis. Semin Arthritis Rheum. 32:94–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet 15 Spec No. 1:R17–R29. 2006. View Article : Google Scholar
|
|
5
|
Li Z, Yang B, Weng X, Tse G, Chan MTV and
Wu WKK: Emerging roles of MicroRNAs in osteonecrosis of the femoral
head. Cell Prolif. 51:Feb;2018.doi: 10.1111/cpr.12405. View Article : Google Scholar
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao X, Liu Y and Yu S: Long noncoding RNA
AWPPH promotes hepatocellular carcinoma progression through YBX1
and serves as a prognostic biomarker. Biochim Biophys Acta Mol
Basis Dis. 1863:1805–1816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu F, Zhang X, Yu Q, Han G, Diao F, Wu C
and Zhang Y: LncRNA AWPPH inhibits SMAD4 via EZH2 to regulate
bladder cancer progression. J Cell Biochem. 119:4496–4505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Jiang C, Li X, Wu WKK, Chen X, Zhu
S, Ye C, Chan MTV and Qian W: Circulating microRNA signature of
steroid-induced osteonecrosis of the femoral head. Cell Prolif.
51:e124182018. View Article : Google Scholar
|
|
11
|
Wei B, Wei W, Zhao B, Guo X and Liu S:
Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate
osteogenic differentiation and proliferation in non-traumatic
osteonecrosis of femoral head. PLoS One. 12:e01690972017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takahashi S, Fukushima W, Yamamoto T,
Iwamoto Y, Kubo T, Sugano N and Hirota Y; Japanese Sentinel
Monitoring Study Group for Idiopathic Osteonecrosis of the Femoral
Head, : Temporal trends in characteristics of newly diagnosed
nontraumatic osteonecrosis of the femoral head from 1997 to 2011: A
hospital-based sentinel monitoring system in Japan. J Epidemiol.
25:437–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mao Z, Liu G, Chen JJ, Liu D, Xu MP, Zhao
C, Yang HT and Yue YB: Serum α-melanocyte-stimulating hormone may
act as a protective biomarker for non-traumatic osteonecrosis of
the femoral head. Ann Clin Biochem. 55:453–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bianchessi V, Badi I, Bertolotti M, Nigro
P, D'Alessandra Y, Capogrossi MC, Zanobini M, Pompilio G, Raucci A
and Lauri A: The mitochondrial lncRNA ASncmtRNA-2 is induced in
aging and replicative senescence in endothelial cells. J Mol Appl
Cardiol. 81:62–70. 2015. View Article : Google Scholar
|
|
15
|
Zheng H, Li P, Kwok JG, Korrapati A, Li
WT, Qu Y, Wang XQ, Kisseleva T, Wang-Rodriguez J and Ongkeko WM:
Alcohol and hepatitis virus-dysregulated lncRNAs as potential
biomarkers for hepatocellular carcinoma. Oncotarget. 9:224–235.
2017.PubMed/NCBI
|
|
16
|
Lu L, Xu H, Luo F, Liu X, Lu X, Yang Q,
Xue J, Chen C, Shi L and Liu Q: Epigenetic silencing of miR-218 by
the lncRNA CCAT1, acting via BMI1, promotes an altered cell cycle
transition in the malignant transformation of HBE cells induced by
cigarette smoke extract. Toxicol Appl Pharmacol. 304:30–41. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pengde K, Fuxing P, Bin S, Jing Y and
Jingqiu C: Lovastatin inhibits adipogenesis and prevents
osteonecrosis in steroid-treated rabbits. Joint Bone Spine.
75:696–701. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishimura R, Kato Y, Chen D, Harris SE,
Mundy GR and Yoneda T: Smad5 and DPC4 are key molecules in
mediating BMP-2-induced osteoblastic differentiation of the
pluripotent mesenchymal precursor cell line C2C12. J Biol Chem.
273:1872–1879. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Yang M, Lin L, Chen P, Ma KT,
Zhou CY and Ao YF: Runx2 overexpression enhances osteoblastic
differentiation and mineralization in adipose-derived stem cells in
vitro and in vivo. Calcif Tissue Int. 79:169–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsubara T, Kida K, Yamaguchi A, Hata K,
Ichida F, Meguro H, Aburatani H, Nishimura R and Yoneda T: BMP2
regulates osterix through Msx2 and Runx2 during osteoblast
differentiation. J Biol Chem. 283:29119–29125. 2008. View Article : Google Scholar : PubMed/NCBI
|