Introduction
Bisphenol A [BPA; 2,2-bis (4-hydroxyphenyl)], an
environmental contaminant, is an increasingly produced worldwide
chemical (1). BPA is used in the
manufacture of polycarbonate plastics, epoxy resins and hard
plastic bottles, which are commonly consumed products (2). Epoxy resins are used in food-contact
surface lacquer coatings for cans, automobile parts and as a
coating for PVC pipes (3). The
increased use of these products has led to a BPA content of >90%
in various human biological fluids, including human breast milk,
amniotic fluid and neonatal blood (4). The primary route of BPA exposure is via
ingestion (1), but transdermal
absorption and inhalation are probable secondary exposure routes,
particularly in individuals who work in companies that produce
BPA-based products (3,5). BPA is an endocrine-disrupting substance
with estrogenic and thyroid hormone-like effects (6). Several studies have reported that BPA
exposure promotes hepatotoxicity and induces oxidative damage via
different mechanisms (7,8). Other studies have determined an
association between BPA exposure and an increased risk of
reproductive dysfunction, cardiovascular disease, obesity, type II
diabetes and thyroid dysfunction (6,9). Sesame
(Sesamum indicum L.) is a crop that is produced worldwide.
It has been cultivated in Asia and Africa for its high content of
edible oil and protein (10) and has
been used as a traditional food source in the countries of East
Asia (11). Sesame oil is considered
to be a superior vegetable oil, as it has a high nutritional value
(12) that is therapeutically
effective within 3–12 h after oral administration (13). Sesame oil decreases blood pressure,
lipid profiles and lipid peroxidation, and increases enzymatic and
nonenzymatic antioxidants (14). The
potent antioxidant activity of sesame oil is due to its high
content of polyunsaturated fatty acids and lignans (15). Sesame lignans include sesamin,
sesamolin, sesamol, sesaminol, sesamolinol, pinoresinol,
matairesinol, lariciresinol and episesamin, which all exhibit a
broad spectrum of biological properties (16). Sesame lignans individually or in
combination, exhibit different biological activities. Sesamin has
been revealed to serve an important role in lipid and glucose
metabolism, hypertension, anti-inflammation and free radical
scavenge (17). Sesamolin promotes
apoptosis, suppresses neuronal reactive oxygen species (ROS)
generation and attenuates mutagenesis induced by
H2O2 (18,19).
Furthermore, sesaminol inhibits DNA oxidative damage, decreases
low-density lipoprotein (LDL) oxidation induced by copper and
inhibits membrane lipid peroxidation (20,21).
Yashaswini et al (22)
reported that the oral administration of sesamol and sesamin
modulates inflammatory and oxidative damage markers in
lipopolysaccharide (LPS)-injected rats. However, to the best of our
knowledge, there are no in vivo studies assessing the
protective effect of sesame lignans against BPA-induced
hyperlipidemia and oxidative damage. Accordingly, the aim of the
current study was to investigate the modulative effects of sesame
lignans against BPA-induced hepatic and cardiac toxicity in rats.
This was achieved by performing liver and heart function tests,
determining lipid profiles and assessing the levels of certain
oxidants and antioxidants. Biochemical parameters were also
confirmed via histopathological examinations of liver and heart
tissue. In addition, the dorsal aorta was quantitatively
analyzed.
Materials and methods
Chemicals
BPA, glutathione reductase (GR), glutathione (GSH),
pyrogallol, NADPH, 2-thiobarbituric acid,
1,1,3,3-tetraethoxypropane, 5,5′-dithiobis-(2-nitrobenzoic acid)
(DTNB) and bovine serum albumin were purchased from Sigma-Aldrich
(Merck KGaA). All other general chemicals and kits were of
analytical grade.
Extraction and purification of sesame
lignans
Sesame lignans were extracted and purified as
described by Reshma et al (23). Sesame oil (100 g) was mixed with
methanol (1:1 w/v) in an extraction vessel. The mixture was then
stirred for 10 min at 70°C and transferred into a separating
funnel. Following 15 min of settling time, the methanolic extract
was separated from the residual oil, which was subsequently
stripped of solvent and subjected to 9 additional sequential
extractions with fresh batches of methanol performed exactly as
described above. The 10 methanolic extracts were pooled and
concentrated via evaporation at 40°C using a rotary evaporator
(Model 4622; BUCHI Rotavapor™ R-100; Thermo Fisher Scientific,
Inc.). These extracts were then dispersed in petroleum ether at a
ratio of 1:0.5 (w/v). The mixture was left to stand for 24–48 h at
4°C to facilitate the lignan crystallization. Lignan crystals were
separated from the mixture via filtration, washed with cold
petroleum ether, dried in a vacuum oven at a temperature of 40°C
for 1 h and weighed.
Experimental design
A total of 40 adult male Wister albino rats (weight,
150–160 g) were obtained from the animal house of Faculty of
Medicine, Alexandria University, Egypt. The design and experimental
techniques of the current study were approved by the Institutional
Animal Care and Use Committee (IACUC) of Alexandria University in
accordance with the guidelines of the National Institutes of Health
Guide for the Care and Use of Laboratory Animals. All efforts were
made to minimise the suffering of rats during the experimental
period. Rats were housed in stainless steel cages and provided with
a basal diet and tap water ad libitum. Animals were
maintained under 25°C and a 12 h light/dark cycle with relative
humidity 50–60% during the experimental period. Rats were equally
and randomly divided into the following four groups (each, n=10): A
control group (C-group), a BPA-treated group (B-group) that
received 30 mg/kg (24), a sesame
lignans-treated group (S-group) that were treated with sesame
lignans (20 mg/kg) (25) and a BPA
plus sesame lignans-treated group (BS-group) that received BPA (30
mg/kg) and sesame lignans (20 mg/kg). Rats were orally administered
their respective doses daily for 6 weeks.
Sampling and tissue preparation
At the end of experimental period, rats were starved
for 12 h and then deeply euthanized via an intraperitoneal
injection of 100 mg/kg ketamine and 20 mg/kg xylazine. The 12 h
fasting period prior to sampling did not cause remarkable loss of
body weight or any observed adverse effects on rats. After
euthanasia, 4 ml of blood was obtained through cardiac puncture
using a sterile syringe. Death was subsequently confirmed by the
inhalation of CO2 from a pressurized tank at a flow rate
of 25% per min followed by cervical dislocation. Serum was obtained
by centrifugation of clotted blood at 1,000 × g at 25°C for 10 min
and kept at −20°C for lipid profiling and liver and heart function
tests. Liver, heart and dorsal aorta were immediately isolated and
washed with cold saline solution. Pieces of tissue were then fixed
for 12 h with 10% formalin at 25°C for histological examination.
Liver tissue (0.25 g) was subsequently homogenized individually in
5 ml of 5% trichloroacetic acid, containing 0.003 M EDTA. The
homogenate was centrifuged at 500 × g for 15 min at 4°C and the
supernatants were stored at −20°C for reduced GSH determination.
Additionally, 0.5 g liver tissue was isolated, washed and
homogenized in PBS (0.1 M; pH 7.4). The homogenate was centrifuged
at 25,000 × g for 10 min at 4°C (Hitachi Ltd.; model, EBA 12R).
Supernatants were stored in aliquots of 1ml at −20°C for subsequent
biochemical analysis.
Extraction of hepatic total
lipids
Total liver lipid content was measured via
gravimetric determination according to a method previously
described by Folch et al (26). Half gram of liver tissue was
homogenized in 5 ml of chloroform-methanol mixture (2:1, v/v) using
a Polytron homogenizer (model, TR-10; Tekmar). The solvent obtained
from the extract was evaporated and the extracted lipids were
reconstituted in 0.2 ml petroleum ether and used for the
determination of hepatic triglycerides and total cholesterol
concentrations.
Biochemical analysis
Serum aspartate aminotransferase (AST) (27), serum alanine aminotransferase (ALT)
(27) and serum total bilirubin
(28) were assayed according to
their respective previously described methods using commercially
available diagnostic kits (Biosystems S.A.). Liver lipid
peroxidation end product (malondialdehyde; MDA) was measured by
2-thiobarbituric acid and expressed as nmol MDA/g tissue (29). The concentration of MDA in each
sample was obtained from a standard curve prepared from a serial
dilution of 1,1,3,3-tetraethoxypropane. Reduced GSH was determined
in liver homogenate by enzymatic method using NADPH as a reducing
agent and dependent on oxidation of GSH by DTNB, as previously
described (30). GR activity was
assayed in the liver tissues as previously described (31). Activity of glutathione peroxidase
(GPx) was assessed using cumene hydroperoxide as a substrate
(32). Assay of superoxide dismutase
(SOD) based on the ability of SOD to inhibit pyrogallol
self-oxidation in alkaline conditions (33). Creatine kinase MB (CK-MB) activity
was determined as previously described (34). Lactate dehydrogenase (LDH) catalyzes
the reduction of pyruvate by NADH to form lactate and
NAD+. This catalytic activity was assayed
spectrophotometrically at 340 nm (35). All biochemical assays were repeated
at least three times. The levels of triglycerides (TG), total
cholesterol (TC), low density lipoprotein cholesterol (LDL) and
high-density lipoprotein cholesterol (HDL-C) were determined using
commercially available diagnostic kits (Biosystems S.A.). Very
low-density lipoprotein cholesterol (VLDL-C) was calculated from
the values of TG, TC and HDL-C using Friedwald and Fredrickson's
formula (36).
Histopathological examination
For histological examination, pieces of liver, heart
and aorta were fixed in 10% neutral formalin for 12 h at 25°C. The
tissue pieces were dehydrated using ascending concentrations of
ethyl alcohol and then embedded in paraffin for 2 h to form blocks.
The blocks were trimmed and cut into 6-µm-thick sections. The
sections were de-waxed, hydrated and stained with hematoxylin and
eosin, as previously described (37). Stained sections of liver and heart
tissue were examined under a light microscope to assess the
histological changes. Quantitative analysis of aorta sections was
performed using analysis FIVE digital imaging software (Olympus
Corporation) and ImageJ (version 1.46r; National Institutes of
Health)
Statistical analysis
Statistical analysis was performed using SPSS
software (Version 16.0; SPSS, Inc.). Data were presented as the
mean ± standard error. There were 10 rats in each group and each
measurement was repeated three times. Comparisons among group mean
differences were assessed via one-way ANOVA. Means were then
statistically compared using the Least Significant Difference test
(LSD). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of BPA-treatment
As presented in Table
I, BPA-treatment caused a significant increase in the activity
of serum ALT, AST and total bilirubin compared with the control
group. Oral ingestion of BPA (B-group) significantly increased
serum levels of TG, TC, LDL-C and VLDL-C compared with the control
group (P<0.05). However, HDL-C levels were significantly
decreased (P<0.05; Table II).
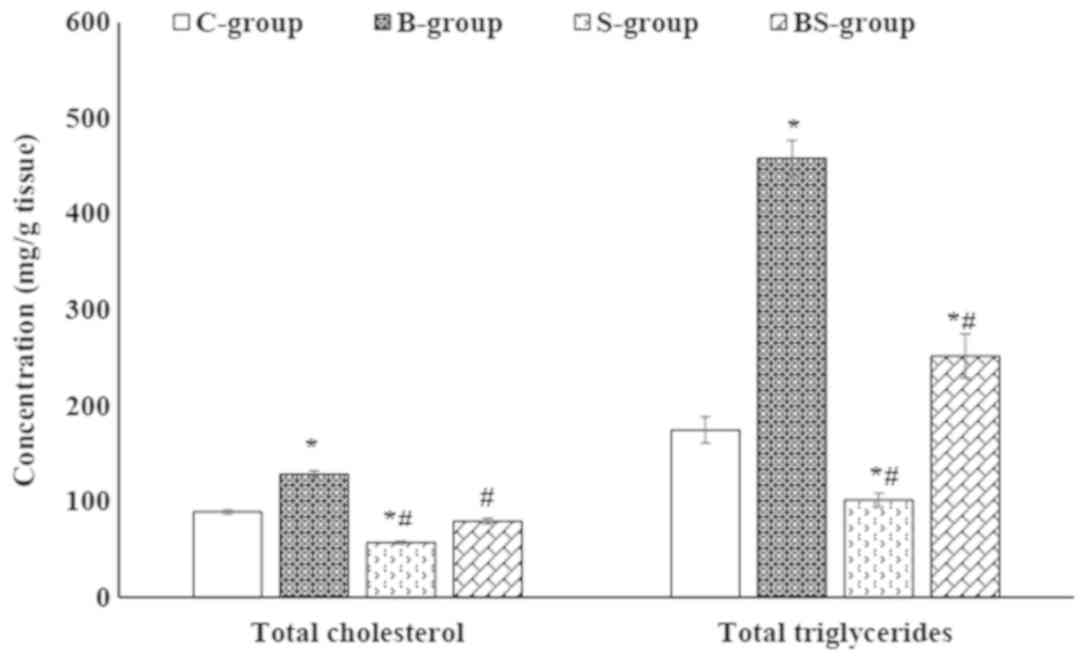
The accumulation of lipids in hepatocytes was observed in the
BPA-treated group via the significant elevation of TG and TC in
liver tissue compared with the control group (P<0.05; Fig. 1). Furthermore, BPA treatment caused a
significant (P<0.05) reduction in hepatic GPx, GR and SOD
activity, and a decrease in GSH compared with the control group.
BPA-treatment also resulted in a significant (P<0.05) increase
in hepatic malondialdehyde (MDA) levels compared with the control
group (Table III). In addition,
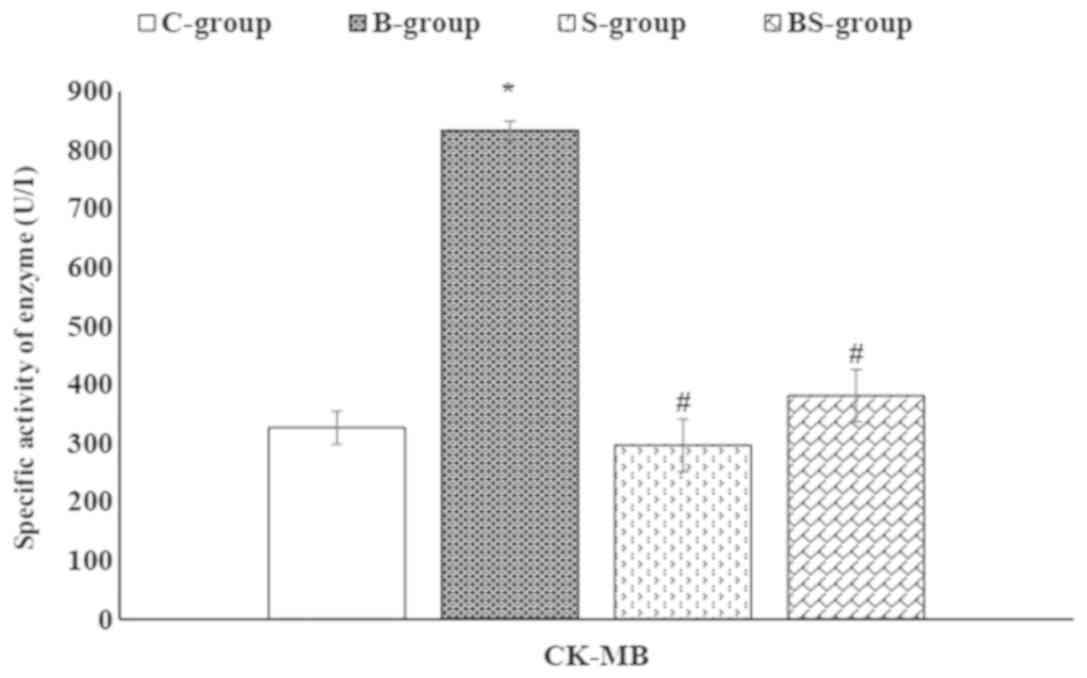
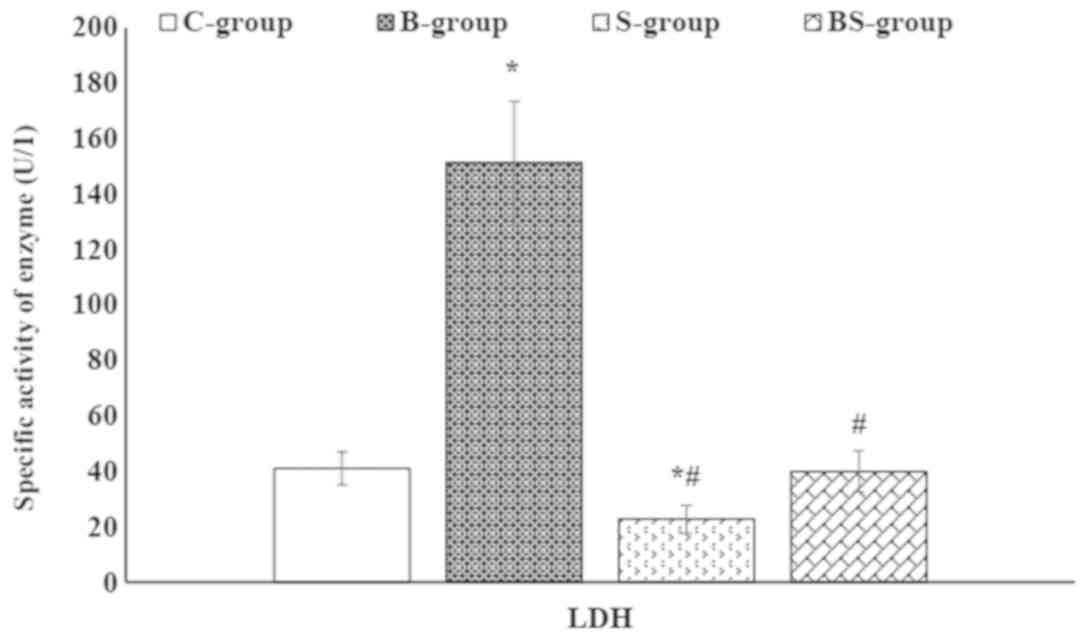
BPA significantly (P<0.05) increased the activities of serum
CK-MB and LDH compared with the control group (Figs. 2 and 3).
 | Table I.Effect of orally administered BPA
and/or sesame lignans on the liver function of male rats. |
Table I.
Effect of orally administered BPA
and/or sesame lignans on the liver function of male rats.
|
| Animal
treatment |
|---|
|
|
|
|---|
| Parameters | C-group | B-group | S-group | BS-group |
|---|
| Activity of AST
(U/l) | 24.60±1.42 |
39.43±1.05a |
17.73±0.69a,b |
23.09±2.77b |
| Activity of ALT
(U/l) | 14.66±0.78 |
34.92±0.96a |
5.56±0.78a,b |
10.13±1.12a,b |
| Total bilirubin
(mg/dl) | 0.35±0.03 |
0.79±0.05a |
0.09±0.01a,b |
0.27±0.02b |
 | Table II.Effect of BPA and/or sesame lignans
on the lipid profile of male rats. |
Table II.
Effect of BPA and/or sesame lignans
on the lipid profile of male rats.
|
| Animal
treatment |
|---|
|
|
|
|---|
| Parameters
(mg/dl) | C-group | B-group | S-group | BS-group |
|---|
| Cholesterol | 186.70±1.58 |
271.38±1.00a |
153.33±1.13a,b |
212.91±8.19a,b |
| LDL-C | 102.68±3.02 |
159.04±5.71a |
72.05±2.96a,b |
95.31±2.46b |
| HDL-C | 39.72±1.06 |
12.22±1.87a |
46.41±1.79a,b |
34.79±1.12a,b |
| VLDL-C | 32.68±2.20 |
73.02±3.60a |
15.40±1.24a,b |
29.82±1.57b |
| Triglyceride | 163.43±11.01 |
365.14±18.03a |
73.14±6.13a,b |
150.28±7.52b |
 | Table III.Effect of orally administered BPA
and/or sesame lignans on the liver oxidant and antioxidant status
of rats. |
Table III.
Effect of orally administered BPA
and/or sesame lignans on the liver oxidant and antioxidant status
of rats.
|
| Animal
treatment |
|---|
|
|
|
|---|
| Parameters | C-group | B-group | S-group | BS-group |
|---|
| MDA (nmol/gm
tissue) | 438.80±11.73 |
626.80±35.62a |
223.80±16.03a,b |
325.20±8.96a,b |
| GSH (nmol/mg
protein) | 19.04±0.96 |
8.76±1.12a |
36.57±2.66a,b |
23.35±0.43b |
| GR (mu/mg
protein) | 15.01±0.93 |
8.72±0.39a |
17.75±0.92a,b |
12.44±0.76a,b |
| GPx (mu/mg
protein) | 644.75±20.19 |
403.72±11.35a |
838.40±27.72a,b |
658.40±13.41b |
| SOD (U/mg
protein) | 4.44±0.19 |
2.28±0.27a |
5.31±0.32a,b |
3.68±0.35b |
The histological analyses performed in the current
study confirmed the deteriorating effect of BPA on both the liver
and heart tissues. The control group exhibited normal hepatocyte
architecture, central vein, blood sinusoids and hepatocytes
(Fig. 4). BPA treatment caused
hemorrhage in the central vein, loss of the normal hepatocyte
architecture, degenerated and vacuolized hepatocytes with pyknotic
nuclei, dilation of the hepatic sinusoids and lymphocyte
aggregation (Fig. 4Ba and Bb).
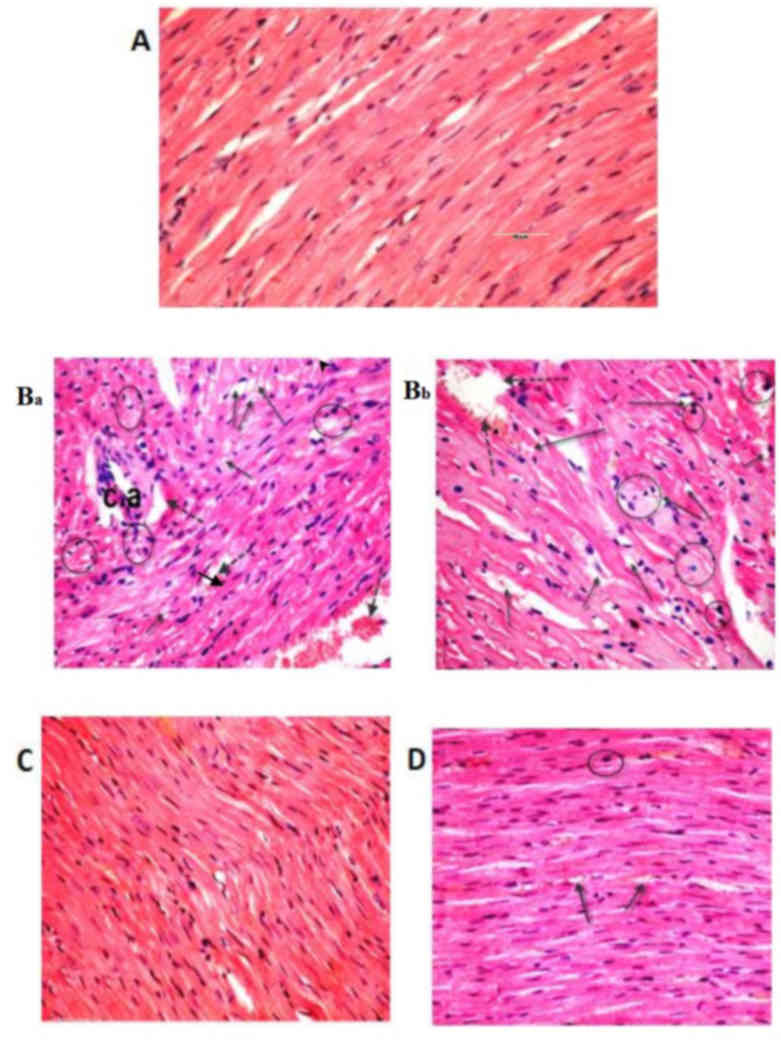
Histological examinations of heart tissues of the control group
revealed normal architecture of the myocardial fibers and branching
muscle fibers with centrally located oval nuclei (Fig. 5A). The examination of the B-group
revealed the loss of the normal muscle fiber architecture, loss of
cross striations and fragmentation of sarcoplasm, cardiac muscle
cell cytoplasmic vacuolization, connective tissue edema,
degenerative changes in myocardial fibers with congested blood
vessels and the thickening of coronary branches (Fig. 5Ba and Bb). Examination of the dorsal
aorta of the control group revealed a normal tunica intima with a
single irregular layer of endothelial cells, tunica media with
elastic fibers and a normal tunica adventitia (Fig. 6A). BPA-treated rats demonstrated
sclerotic changes in its walls, atrophy of elastic fibers, loss of
tunica intimal endothelial cells, disorganized tunica media and
adventitia, and vacuolation of the tunica media and adventitia
compared with normal layers of the dorsal aorta of the control
group (Fig. 6Ba and Bb).
Furthermore, quantitative analysis of the aortic atherosclerotic
composition of the B-group revealed a significant (P<0.05)
increase in mean wall thickness compared with that of the control
group (Table IV).
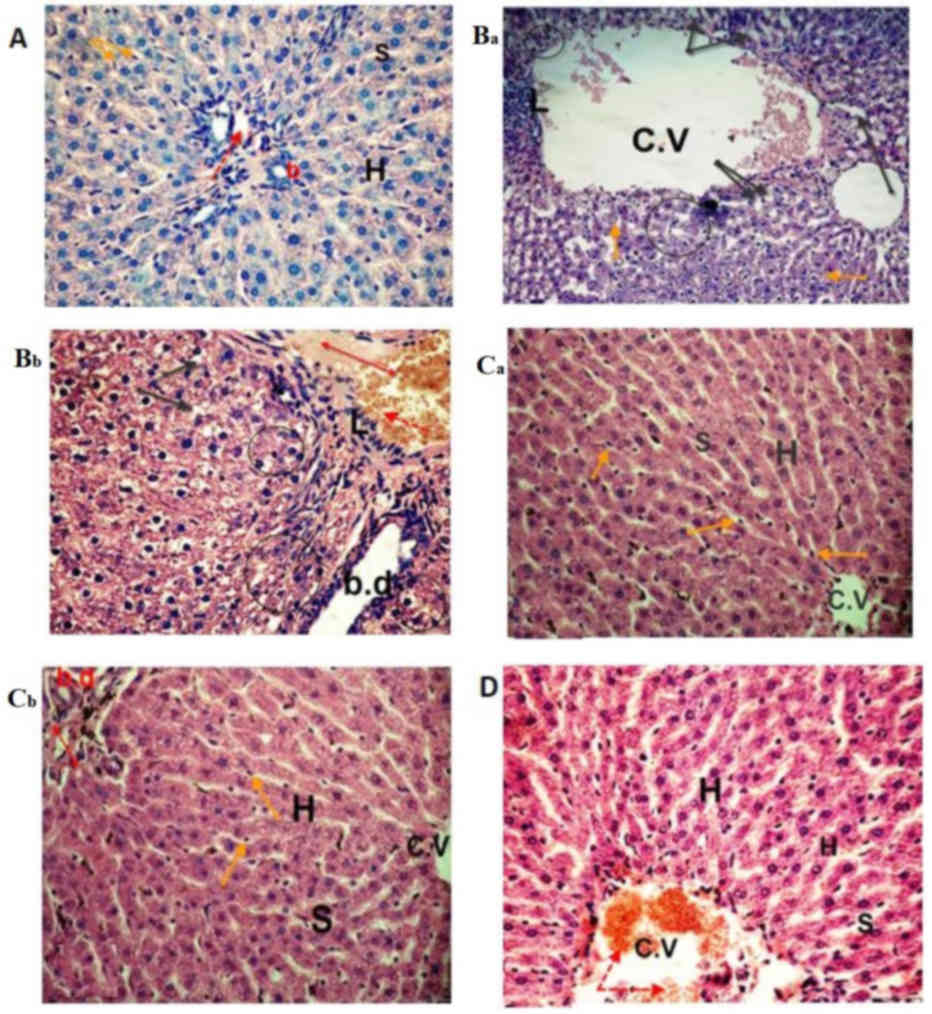 | Figure 4.Photomicrographs of liver sections.
The (A) control and (Ca and Cb) sesame lignans-treated group
exhibited normal hepatocyte architecture and central vein with
normal blood sinusoids and hepatocytes. (Ba and Bb) Liver sections
from the BPA-treated group exhibited distention and hemorrhage in
the central and portal vein (red dotted arrow and double headed red
arrow, respectively). L, loss of the normal architecture,
degenerated hepatocytes with pyknotic nuclei (black circle),
vacuolated hepatocytes (black arrows), dilation of blood sinusoids
and an increased number of kupffer cells (orange arrows) were also
identified. BPA-induced histological changes were markedly reduced
in the (D) BPA and sesame lignans-treated group, with moderate
improvement in the hepatic cells (H&E staining; magnification,
×400). C.V, central vein; S, sinusoids; H, hepatocytes; BPA,
bisphenol A; L, lymphocyte aggregation; b.d, bile duct. |
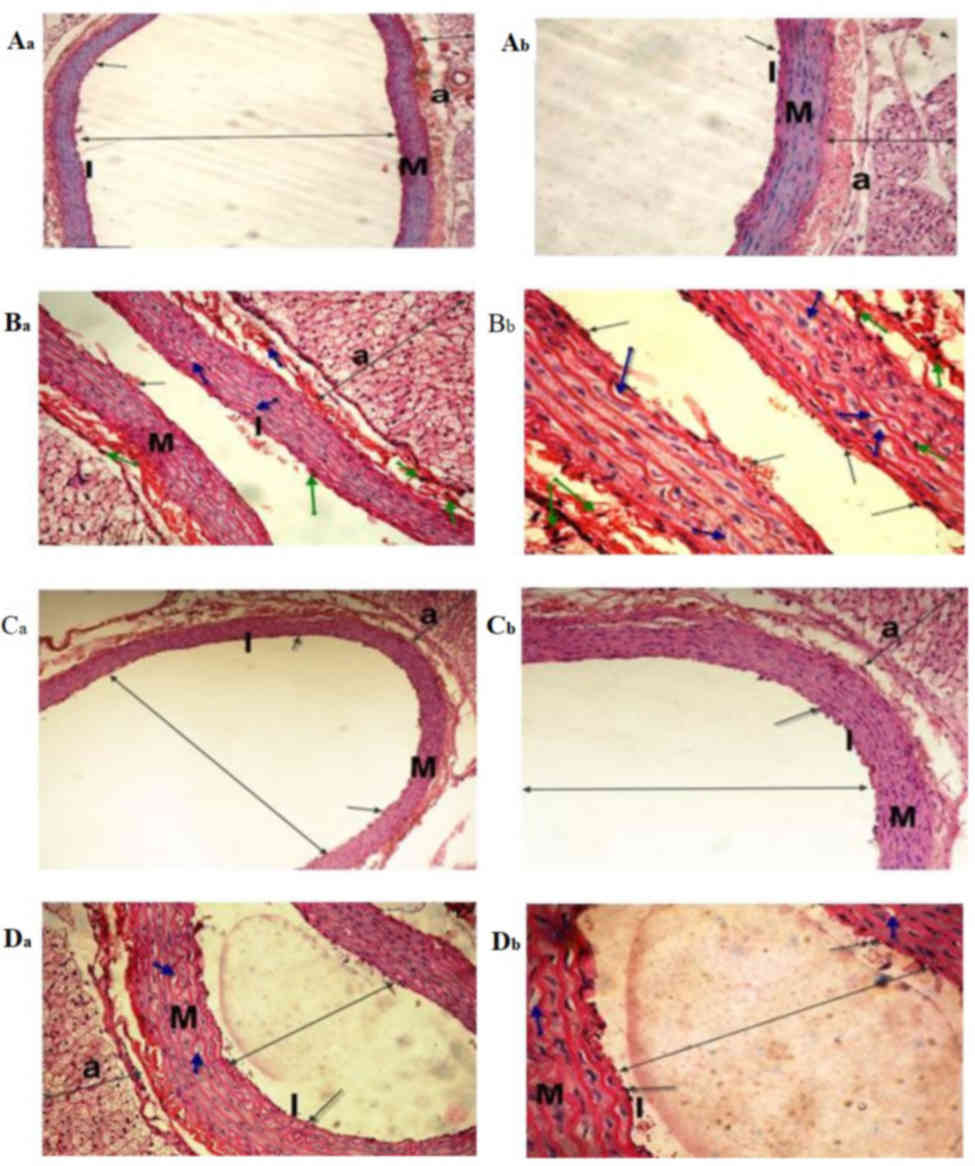 | Figure 6.Light micrographs of the dorsal
aorta. The (Aa and Ab) control and (Ca and Cb) sesame
lignans-treated group exhibited normal tunica intima with a single
irregular layer of endothelial cells (black arrow). The tunica
media, comprising elastic fibers and the tunica adventitia also
appeared normal. (Ba and Bb) The BPA-treated
group exhibited sclerotic changes in the walls and atrophy of
elastic fibers (green arrows), loss of the tunica intima
endothelial cells, disorganized and vacuolation of the tunica media
(blue-arrow), increased adventitial thickness and decreased lumen
size of the aorta (double-headed arrows) compared with the control
group. (Da and Db) Histological changes induced after BPA-treatment
were markedly reduced in the BPA and sesame lignans-treated group
(H&E staining; Aa, Ba, Ca and Da magnification, ×200; Ab, Bb,
Cb, and Db magnification, ×400). I, tunica intima; M, tunica media;
a, tunica adventitia. |
 | Table IV.Effect of BPA and/or sesame lignans
on the wall thickness of male rat dorsal aortas. |
Table IV.
Effect of BPA and/or sesame lignans
on the wall thickness of male rat dorsal aortas.
|
| Animal
treatment |
|---|
|
|
|
|---|
| Parameter | C-group | B-group | S-group | BS-group |
|---|
| Wall thickness
(µm) | 165.85±0.34 |
293.65±0.26a |
161.50±0.57b |
171.95±0.68b |
Sesame lignans attenuates liver
function tests
S-group treatment was revealed to improve rat liver
function as indicated by the significant (P<0.05) reduction in
the activities of serum AST, ALT and total bilirubin level compared
with the B-group. Furthermore, BS-group treatment significantly
(P<0.05) decreased the aforementioned serum parameters compared
with the B-group, returning them to levels similar to that of the
control (Table I).
Sesame lignans improves lipid
profiles
As presented in Table
II, it was revealed that S-group treatment significantly
(P<0.05) reduced the levels of serum TG, TC, LDL-C and VLDL-C,
and significantly (P<0.05) elevated HDL-C compared with the C-
and B-group. The co-administration of sesame lignans and BPA
(BS-group) improved levels of the aforementioned lipid profile
parameters compared with the B-group. Furthermore, S-group
treatment significantly (P<0.05) reduced hepatic TG and TC
levels compared with the control group. The results of Fig. 1 also demonstrated a significant
(P<0.05) reduction in hepatic TG and TC contents in the BS-group
compared with the B-group.
Sesame lignans ameliorates hepatic
oxidative status
As presented in Table
III, the results revealed significant (P<0.05) increases in
the activities of hepatic GR, GPx and SOD, and increased levels of
GSH in the S-group compared with the control group. Additionally,
significant (P<0.05) increases in the aforementioned antioxidant
parameters were observed in the BS-group compared with the B-group.
However, MDA levels were significantly (P<0.05) reduced in the
S- and BS-groups compared with the control group and B-group.
Sesame lignans attenuates cardiac
function tests
As presented in Figs.
2 and 3, a non-significant
(P>0.05) change in the serum activity of CK-MB (a
cardiac-specific enzyme) was observed in the S-group compared with
the control group. However, a significant (P<0.05) reduction was
demonstrated in the serum activity of CK-MB in the BS-group
compared with the B-group. In addition, S-group treatment decreased
the serum LDH levels to less than that of the control. Furthermore,
the activity of serum LDH was significantly (P<0.05) decreased
in the BS-group compared with the B-group.
Sesame lignans improves hepatic and
cardiac histological changes
Photomicrographs of control and sesame
lignans-treated liver tissue revealed normal cellular architecture
with distinct hepatic cells, sinusoidal spaces and central veins
(Fig. 4A, Ca and Cb). Furthermore,
liver sections of rats treated with sesame lignans in combination
with BPA revealed that most of the histological alterations induced
by BPA were markedly reduced. The histological changes observed
after BPA-treatment were also attenuated from severe to moderate
after treatment with sesame lignans (Fig. 4D). Heart sections of the control and
sesame lignans-treated groups also demonstrated normal myofibrillar
architecture with striations, a branched appearance and continuity
with adjacent myofibrils (Fig. 5A and
C). Consistent with this result, sesame lignans treatment in
the BS-group markedly reduced heart tissue histopathological
alterations following BPA exposure (Fig.
5D). Histopathological examination of the dorsal aorta in the
control and sesame lignans-treated groups revealed a smooth and
continuous intimal surface without lumen irregularities (Fig. 6A and C). Examination of the BS-group
dorsal aortas demonstrated a marked reduction in the histological
alterations induced by BPA (Fig.
6D). The quantitative analysis of S- and BS-group aortic
compositions revealed a significant (P<0.05) reduction in the
mean wall thickness of the dorsal aorta compared with the B-group
(Table IV).
Discussion
Previous studies have focused on the impact of BPA
on human health and have determined that the toxic effects of BPA
may be due to enhanced oxidative stress (7,8). The
present study revealed that BPA induced hepatic oxidative stress,
steatosis and affects the secretory function and integrity of the
liver. Increased levels of hepatic MDA, the decreased activities of
GPx, GR and SOD, and the decreased levels of GSH indicated that
there were increased levels of oxidative stress in liver cells.
These results are consistent with that of Maćczak et al
(38) who reported that BPA
treatment induces oxidative damage. Another previous study
demonstrated that BPA increased lipid peroxidation and decreased
the activity of antioxidant defense enzymes produced in rat livers
(39). The BPA-mediated reduction of
GSH levels may be due to its conjugation with BPA-toxic metabolites
and its oxidation to oxidized glutathione (40). Furthermore, certain lipid
peroxidation end products [including malondialdehyde (MDA) and
4-hydroxynonenal] are able to alter the activity of mitochondrial
enzymes and deplete the glutathione pool (41). Decreased GSH levels may therefore
lead to decreased GPx activities (42). Decreased GPx activity is associated
with an increased level of the hepatic H2O2,
as well as the direct inhibition of SOD activity (43). It has been reported that BPA reacts
with oxygen radicals, decomposing them to various reactive
metabolites that have potent oxidant activity (44). These metabolites increase ROS
production, inhibit the activity of antioxidative enzymes and
increase H2O2 and thiobarbituric acid
reactive substance levels (44). The
BPA-mediated increase of ROS may enhance the cleavage of peptide
chains and the cross-linking of amino acids in enzymes, leading to
the change or loss of enzyme activity (45). Therefore, the mechanism of
BPA-induced oxidative damage may be primarily caused by the
inhibition of the antioxidant enzyme system, increasing ROS
content.
In the present study, BPA induced a dyslipidemic
state and enhanced the accumulation of triglycerides (steatosis)
and cholesterol in rat liver tissue. These results are consistent
with those of Lin et al (46), who demonstrated that BPA induced the
intracellular accumulation of fat droplets in a dose-dependent
manner. Furthermore, it has been reported that BPA exposure results
in hypertriglyceridemia, hypercholesterolemia, changes in the
composition of fatty acids and the upregulation of genes associated
with de novo lipogenesis and cholesterol synthesis in rat
livers (47). BPA-induced hepatic
lipid accumulation may arise from the increased expression of the
transcription factor, sterol regulatory element binding protein-1,
which increases the quantity and activity of the enzymes that
catalyze lipogenesis, triggering hepatic lipid accumulation
(46). The BPA-induced increase of
VLDL-C may be due to the increased synthesis and excretion of
VLDL-C from liver tissue as a result of the increased hepatic mRNA
expression of apolipoprotein B (48). The increased hepatic output of VLDL-C
may therefore be a consequence of increased hepatic TG.
BPA-induced oxidative stress and lipid peroxidation
has been demonstrated to increase hepatic damage and disrupt the
integrity of cellular membranes, leading to leakage of cytoplasmic
liver enzymes (49). The present
study revealed that BPA caused significant increases in the
activities of ALT and AST, and in the level of total bilirubin.
These results are congruent with the results of a previous study by
Korkmaz et al (50). The
results of the aforementioned study revealed that BPA-treatment
elevated the activities of ALT, AST and LDH, and caused marked
defects in liver morphology. Additionally, Nicolucci et al
(51) reported that patients with
liver disease exhibit higher BPA levels compared with normal
individuals, suggesting an association between BPA-exposure and the
health status of the liver. The results of histopathological
examination performed in the current study supports those of
biochemical experiments. When compared with control livers, BPA
treatment induced the severe disruption of the liver's
architecture, resulting in cellular infiltration, the formation of
large cytoplasmic vacuoles and hepatic sinusoids, and an increased
number of Kupffer cells. Hepatic lipid accumulation and oxidative
stress, followed by liver injury and inflammation, are pathogenic
events for non-alcoholic steatohepatitis (52).
The results of the present study revealed
significant decreases in the activities of CK-MB, LDH and AST in
the BPA-treated group. This indicated that damage or inflammation
was present in the heart tissue. Ljunggren et al (53) demonstrated that BPA alters a number
of proteins involved in the structural integrity of the myocardial
left ventricle. Furthermore, Aboul et al (54) reported that BPA generated ROS and
reduced the level of antioxidants in rat heart tissue, which may
lead to cardiovascular diseases. A further study demonstrated that
BPA inhibited the ventricular function of the heart by elevating
ROS in myositis, inducing damage (55). In addition to inducing oxidative
stress, BPA increases VLDL-C and LDL-C, and decreases HDL-C, which
are considered to be atherogenic indicators. The estrogen-like
effect of BPA has a significant effect on cholesterol, which may be
the reason for the development of myocardial infarction and other
complications such as arteriosclerotic vascular diseases (56). The histopathological examination of
rat heart tissue performed in the current study revealed that BPA
caused the severe disorganization and degeneration of myocardial
fibers, cytoplasmic vacuolization in cardiac muscle and hemorrhages
between myofibrils, confirming the presence of damage or
inflammation. BPA treatment also induced sclerotic changes in the
walls of the dorsal aorta and atrophy of elastic fibers. This was
demonstrated by various changes in the tunica intima and media,
including fragmentation, disintegration, vacuolation and a marked
increase in thickness. In addition, quantitative analysis of the
aortic atherosclerotic composition of the BPA-treated group
demonstrated a significant increase in its mean wall thickness,
indicating atherosclerotic changes.
The results of the current study indicated that
sesame lignans attenuated BPA-induced oxidative stress by
decreasing MDA, increasing the specific activities of GPx, GR and
SOD, and increasing the level of GSH in the livers of S- and
BS-group rats. The present study also revealed that sesame lignans
supplementation attenuated measured parameters to a level similar
to that of the control. This may be due to its free radical
scavenging properties. These results are consistent with those of
Yashaswini et al (22). The
study revealed that sesame lignans increased the activities of GPx
and GR, and decreased MDA levels in LPS-treated rats. Furthermore,
Ma et al (57) reported that
sesamin decreased ROS and MDA production in the liver extract of
CCL4-treated mice. The augmented SOD activity induced by
sesame lignans enhances the ability of hepatic cells to decompose
superoxide anions produced by BPA into H2O2,
preventing further generation of free radicals.
H2O2 is subsequently broken down by GPx,
which uses GSH as a reducing agent. Therefore, increasing GPx
activity and GSH levels via sesame lignans should protect liver
tissue against oxidative damage (58).
In the present study, BPA-induced dyslipidemia and
hepatic lipid accumulation were partially or completely attenuated
by the co-administration of sesame lignans. Suwimol et al
(59) confirmed the results of the
current study by reporting that sesame seed powder reduces hepatic
total lipid, plasma cholesterol and LDL-C levels in rats. Sesame
lignans decreases cholesterol levels by inhibiting its intestinal
absorption, increasing its fecal and biliary excretion, and
downregulating hepatic 3-hydroxy-3-methyl gluteryl coenzyme A
reductase activity (60). The
hypotriglyceridemic effect of sesame lignans may be due to the
inhibition of lipogenesis, the promotion of fatty acid oxidation
and ketogenesis at the expense of its esterification into TG
(61). Additionally, sesamin
increases fat burning and decreases fat storage by activating
peroxisome proliferator-activator receptor alpha (PPARα), which
induces the expression of β-oxidation enzymes and represses
lipogenic enzymes (62).
Furthermore, PPARα activation increases the level of uncoupling
proteins, resulting in the expenditure of more calories and fat
loss (63). In addition, sesamin has
been associated with a reduction of LDL-C and the elevation of
HDL-C levels (64). The reduction of
leptin is another possible mechanism for the sesame lignans-induced
inhibition of hepatic lipid accumulation (65).
The present study revealed that sesame lignans
administration exhibits potent hepatoprotective effects against
BPA-induced hepatic damage in rats. It improved the secretory
function and structural integrity of liver cells by reducing the
activities of serum ALT and AST, and reducing the total bilirubin
level, producing results that were lower than the control and
BPA-treated groups. These results are consistent with those
emphasized by Ma et al (57)
who demonstrated that sesamin effectively reduced serum ALT and
AST, and alleviated hepatic histological changes. The
hepatoprotective effects exerted by sesame lignans may be due to
their anti-inflammatory effects, as sesamin has been reported to
inhibit arachidonic acid synthesis and reduce the production of
pro-inflammatory cytokines (66).
Furthermore, sesame lignans possess general anti-inflammatory
effects as indicated by their ability to reduce C-reactive protein
levels (67). The antioxidative
effect of sesame lignans and their lipid peroxidation lowering
effect contributes to their hepatoprotective effect (17). The hepatoprotective effects exerted
by sesame lignans were confirmed following histopathological
examination, which revealed that sesame lignans attenuated
BPA-induced hepatic alterations. The attenuation of hepatic lipid
accumulation (steatosis) and peroxidation by sesame lignans may
therefore prevent or decrease the pathogenic events of BPA-induced
steatohepatitis.
Sesame lignans protected against myocardial tissue
damage by lowering the activity of serum AST, LDH and CK-MB when
compared with the control. The protective effect of sesame lignans
against myocardial injury may be attributed to the enhancement of
certain endogenous antioxidants, including GSH, SOD and GPx
(68). Antioxidants have been
revealed to attenuate the progression of cardiovascular diseases in
clinical trials (69). Furthermore,
sesame lignans decreases the risk of cardiovascular diseases by
lowering total cholesterol and VLDL-C levels, and elevating HDL-C
(70). The histopathological
examinations performed in the current study demonstrated that
sesame lignans treatment decreased the degeneration and
vacuolization of myocardial fibers that were induced by BPA. These
results are in line with those by Li et al (71) who demonstrated that sesamin reversed
the abnormal changes of the heart and cardiac hypertrophy, and
improved myocardial fibrosis. In addition, the morphometric
analysis of the dorsal aorta performed in the current study
revealed that the histological alterations induced by BPA were
markedly reduced by the co-administration of sesame lignans. This
was confirmed by the significant reduction of its mean wall
thickness, which may further confirm the protective effect of
sesame lignans against atherosclerotic plaque deposition in the
dorsal aorta. Sesame lignans may be helpful to improve endothelial
function and prevent the developments of cardiovascular diseases.
The present study had some limitations. The effect of BPA exposure
on the degree of liver tissue collagen deposition and on serum
levels of troponin I and T should be investigated in future study
to confirm its fibrogenic and heart dysfunction effects,
respectively. Furthermore, N-acetyl cysteine or vitamin C should be
used as a positive control in future studies and the NF-kB
signaling pathway should be tested to confirm the protective and
antioxidant effects of sesame lignans on hepatic and cardiac
tissues.
The results of the current study indicated that
sesame lignans attenuated hepatic oxidative stress and protected
liver tissue against BPA-induced oxidative damage. Furthermore,
BPA-induced increases in serum and liver TC and TG were attenuated
by the oral administration of sesame lignans. In addition, sesame
lignans restored the liver and heart integrity, as indicated by its
ability to attenuate function tests. These results were confirmed
by the histopathological examinations. The oral administration of
sesame lignans ameliorated BPA-induced histopathological changes in
heart and hepatic tissues.
Acknowledgements
The authors would like to thank the late Professor
Ahmed Sobhey El-Sharaky (professor of Biochemistry, Biochemistry
Department, Faculty of Science, Alexandria University) for
suggesting the idea for this study. The authors would also like the
acknowledge Mrs Salma A. Rezk (Pathology Department, School of
Medicine, University of North Carolina, USA) for her professional
revision of the manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed in the present study
are included in this published article.
Authors' contributions
SME designed the current study, analyzed and
interpreted the data for biochemical analysis in plasma and
tissues, and wrote the manuscript. ASAN deigned the experiments and
wrote the manuscript. HMA performed and interpreted histological
examinations of liver and heart tissues. ASG followed-up animals
during the experimental period and performed biochemical analysis.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Animal Care and Use Committee (IACUC) of Alexandria University
(Alexandria, Egypt) in accordance with the guidelines of National
Institutes of Health guide for the care and use of laboratory
animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vandenberg LN, Hauser R, Marcus M, Olea N
and Welshons WV: Human exposure to bisphenol A (BPA). Reprod
Toxicol. 24:139–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bushnik T, Haines D, Levallois P, Levesque
J, Van Oostdam J and Viau C: Lead and bisphenol A concentration in
the Canadian population. Health Rep. 21:7–18. 2010.PubMed/NCBI
|
|
3
|
Kang JH, Kondo F and Katayama Y: Human
exposure to bisphenol A. Toxicology. 226:79–89. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vandenberg LN, Chahoud I, Heindel JJ,
Padmanabhan V, Paumgartten FJ and Schoenfelder G: Urinary,
circulating, and tissue biomonitoring studies indicate widespread
exposure to bisphenol A. Cien Saude Colet. 17:407–434. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zalko D, Jacques C, Duplan H, Bruel S and
Perdu E: Viable skin efficiently absorbs and metabolizes bisphenol
A. Chemosphere. 82:424–430. 2009. View Article : Google Scholar
|
|
6
|
Khalil N, Ebert JR, Wang L, Belcher S, Lee
M, Czerwinski SA and Kannan K: Bisphenol A and cardiometabolic risk
factors in obese children. Sci Total Environ. 470-471:726–732.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hassan ZK, Elobeid MA, Virk P, Omer SA,
ElAmin M, Daghestani MH and AlOlayan EM: Bisphenol A induces
hepatotoxicity through oxidative stress in rat model. Oxid Med Cell
Longev. 2012:1948292012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JH, Lee MR and Hong YC: Modification
of the association of bisphenol A with abnormal liver function by
polymorphisms of oxidative stress-related genes. Environ Res.
147:324–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santangeli S, Maradonna F, Gioacchini G,
Cobellis G, Piccinetti CC, Dalla Valle L and Carnevali O:
BPA-induced deregulation of epigenetic patterns: Effects on female
zebrafish reproduction. Sci Rep. 6:219822016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohammed F, Abdulwali N, Guillaume D,
Tenyang N, Ponka R, Al-Gadabi K, Bchitou R, Abdullah AH and Naji
KM: Chemical composition and mineralogical residence of sesame oil
from plants grown in different Yemeni environments. Micochem J.
140:269–277. 2018. View Article : Google Scholar
|
|
11
|
Hung WL, Lu CH, Liao CD and Hwang LS:
Safety evaluation of nano/sub-microsized lignin glycosides from
sesame meal [2013]. Food Control. 30:129–136. 2013. View Article : Google Scholar
|
|
12
|
Khier MKSE, Ishag KEA and Yagoub AEGA:
Chemical composition and oil characteristics of sesame seed
cultivars grown in Sudan. J Agric Biol Sci. 4:761–766. 2008.
|
|
13
|
Hsu DZ, Chen KT, Chien SP, Li YH, Huang BM
and Chuang YC: Sesame oil attenuates acute iron-induced lipid
peroxidation-associated hepatic damage in mice. Shock. 26:625–630.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sankar D, Sambandam G, Ramakrishna Rao M
and Pugalendi KV: Modulation of blood pressure, lipid profiles and
redox status in hypertensive patients taking different edible oils.
Clin Chim Acta. 355:97–104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu CT and Liu MY: Daily sesame oil
supplementation attenuates local renin-angiotensin system via
inhibiting MAPK activation and oxidative stress in cardiac
hypertrophy. J Nutr Biochem. 42:108–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan Y, Li H, Fu G, Chen X, Chen F and Xie
M: The relationship of antioxidant components and antioxidant
activity of sesame seed oil. J Sci Food Agric. 95:2571–2578. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dar AA and Arumugam N: Lignans of sesame:
Purification methods, biological activities and biosynthesis-A
review. Bioorg Chem. 50:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park SH, Ryu SN, Bu Y, Kim H, Simon JE and
Kim KS: Antioxidant components as potential neuroprotective agents
in sesame (sesamum indicam L). Food Rev Int. 26:103–121.
2010. View Article : Google Scholar
|
|
19
|
Grougnet R, Magiatis P, Laborie H, Lazarou
D, Papadopoulos A and Skaltsounis AL: Sesamolinol glucoside,
disaminyl ether, and other lignans from sesame seeds. J Agric Food
Chem. 60:108–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Periasamy S, Liu CT, Chien SP, Chen YC and
Liu MY: Daily sesame oil supplementation mitigates
ketoconazole-induced oxidative stress-mediated apoptosis and
hepatic injury. J Nutr Biochem. 37:67–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H and Tsao R: Dietary polyphenols,
oxidative stress and antioxidant and anti-inflammatory effects.
Curr Opin Food Sci. 8:33–42. 2016. View Article : Google Scholar
|
|
22
|
Yashaswini PS, Sadashivaiah B, Ramaprasad
TR and Singh SA: In vivo modulation of LPS induced leukotrienes
generation and oxidative stress by sesame lignans. J Nutr Biochem.
41:151–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reshma MV, Balachandran C, Arumughan C,
Sunderasan A, Sukumaran D, Thomas S and Saritha SS: Extraction,
separation and characterisation of sesame oil lignan for
nutraceutical applications. Food Chem. 120:1041–1046. 2010.
View Article : Google Scholar
|
|
24
|
Morgan AM, El-Ballal SS, El-Bialy BE and
EL-Borai NB: Studies on the potential protective effect of cinnamon
against bisphenol A- and octylphenol-induced oxidative stress in
male albino rats. Toxicol Rep. 1:92–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Baluchnejadmojarad T, Roghani M, Jalali
Nadoushan MR, Vaez Mahdavi MR, Kalalian-Moghaddam H,
Roghani-Dehkordi F, Dariani S and Raoufi S: The sesame lignin
sesamin attenuates vascular dysfunction in streptozotocin diabetic
rats: Involvement of nitric oxide and oxidative stress. Eur J
Pharmacol. 698:316–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Folch J, Lees M and Sloane GH: A simple
method for the isolation and purification of total lipids from
animal tissues. J Biol Chem. 226:497–509. 1957.PubMed/NCBI
|
|
27
|
Bergmeyer HU, Herder M and Red R: Approved
recommendation (1985) on IFCC methods for the measurement of
catalytic concentration of enzymes. Part3. FCC method of alanine
aminotransferase. J Clin Chem Clin Biochem. 24:481–489.
1986.PubMed/NCBI
|
|
28
|
Jendrassik L and Gróf P: Vereinfachte
photometrische. Methoden zur Bestimmung des Blutbilirubins.
Biochemische Zeitschrift. 297:82–89. 1938.
|
|
29
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissue by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Griffith OW: Determination of glutathione
and glutathione disulfide using glutathione reductase and 2-vinyl
pyridine. Anal Biochem. 106:207–212. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smith JK, Vierheller TL and Thorne CA:
Assay of glutathione reductase in crude tissue homogenate using 5–5
dithio bis-(2-nitrobenzoic acid). Anal Biochem. 175:408–413. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paglia E and Valentine N: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
33
|
Marklund S and Marklund G: Involvement of
superoxide anion radical in auto-oxidation of pyrogallol and
convenient assay for SOD. Eur J Biochem. 47:469–474. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
IFCC methods for the measurement of
catalytic concentration of enzymes. Part 7, . IFCC method for
creatine kinase. JIFCC. 1:130–139. 1989.
|
|
35
|
Dito WR: Lactate dehydrogenase: A brief
reviewClinical Enzymology. Griffiths JC: Masson Publishing USA; New
York: pp. pp181979
|
|
36
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
37
|
Drury RAB and Wallington EA: Carleton's
histological techniqueOxford University Press; New York: 1981
|
|
38
|
Maćczak A, Cyrkler M, Bukowska B and
Michałowicz J: Bisphenol A, bisphenol S, bisphenol F and bisphenol
AF induce different oxidative stress and damage in human red blood
cells (in vitro study). Toxicol In Vitro. 41:143–149. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abdel-Wahab WM: Thymoquinone attenuates
toxicity and oxidative stress induced by bisphenol A in liver of
male rats. Pak J Biol Sci. 17:1152–1160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bukowska B: Glutathione: Its biosynthesis,
induction agents and concentrations in selected diseases. Med Pr.
55:501–509. 2004.(In Polish). PubMed/NCBI
|
|
41
|
Guéraud F, Atalay M, Bresgen N, Cipak A,
Eckl PM, Huc L, Jouanin I, Siems W and Uchida K: Chemistry and
biochemistry of lipid peroxidation products. Free Radic Res.
44:1098–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wojnar W, Zych M and Kaczmarczyk-Sedlak I:
Antioxidative effect of flavonoid naringenin in the lenses of type
1 diabetic rats. Biomed Pharmacother. 108:974–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Soumya R and Vani R: Vitamin C as a
modulator of oxidative stress in erythrocytes of stored blood. Acta
Haematologica Polonica. 48:350–356. 2017. View Article : Google Scholar
|
|
44
|
Vahdati Hassani F, Abnous K, Mehri S,
Jafarian A, Birner-Gruenberger R, Yazdian Robati R and Hosseinzadeh
H: Proteomics and phosphoproteomics analysis of liver in male rats
exposed to bisphenol A: Mechanism of hepatotoxicity and biomarker
discovery. Food Chem Toxicol. 112:26–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ke C, Liu X, Zuo H, Zhao J, Yang X and
Yuan J: The oxidative damage of bisphenol A on the organs of the
mice. Sci Res. 5:1190–1194. 2013.
|
|
46
|
Lin Y, Ding D, Huang Q, Liu Q, Lu H, Lu Y,
Chi Y, Sun X, Ye G, Zhu H, et al: Downregulation of miR-192 Causes
Hepatic Steatosis and Lipid Accumulation by Inducing SREBF1: Novel
mechanism for bisphenol A-triggered non-alcoholic fatty liver
disease. Biochim Biophys Acta Mol Cell Biol Lipids. 1862:869–882.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marmugi A, Ducheix S, Lasserre F, Polizzi
A, Paris A, Priymenko N, Bertrand-Michel J, Pineau T, Guillou H,
Martin PG and Mselli-Lakhal L: Low doses of bisphenol A induce gene
expression related to lipid synthesis and trigger triglyceride
accumulation in adult mouse liver. Hepatology. 55:395–407. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Marmugi A, Lasserre F, Beuzelin D, Ducheix
S, Huc L, Polizzi A, Chetivaux M, Pineau T, Martin P, Guillou H and
Mselli-Lakhal L: Adverse effects of longterm exposure to bisphenol
A during adulthood leading to hyperglycaemia and
hypercholesterolemia in mice. Toxicology. 325:133–143. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rönn M, Kullberg J, Karlsson H, Berglund
J, Malmberg F, Orberg J, Lind L, Ahlström H and Lind MP: Bisphenol
A exposure increases liver fat in juvenile fructose-fed Fischer 344
rats. Toxicology. 303:125–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Korkmaz A, Ahbab MA, Kolankaya D and
Barlas N: Influence of vitamin C on bisphenol A, nonylphenol and
octylphenol induced oxidative damages in liver of male rats. Food
Chem Toxicol. 48:2865–2871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nicolucci C, Errico S, Federico A, Dallio
M, Loguercio C and Diano N: Human exposure to Bisphenol A and liver
health status: Quantification of urinary and circulating levels by
LC-MS/MS. J Pharm Biomed Anal. 140:105–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Periasamy S, Chien SP, Chang PC, Hsu DZ
and Liu MY: Sesame oil mitigates nutritional steatohepatitis via
attenuation of oxidative stress and inflammation: A tale of two-hit
hypothesis. J Nutr Biochem. 25:232–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ljunggrena SA, Igglandb M, Rönnc M, Lindd
L, Lindc PM and Karlsson H: Altered heart proteome in fructose-fed
Fisher 344 rats exposed to bisphenol A. Toxicology. 347-349:6–16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aboul Ezz A, Khadrawy Y and Mourad I: The
effect of bisphenol A on some oxidative stress parameters and
acetylcholinesterase activity in the heart of male albino rats.
Cytotechnol. 67:145–155. 2015. View Article : Google Scholar
|
|
55
|
Suthar H, Verma RJ, Patel S and Jasrai YT:
Green tea potentially ameliorates bisphenol A-induced oxidative
stress: An in vitro and in silico study. Biochem Res Inter.
2014:2597632014. View Article : Google Scholar
|
|
56
|
Helal EG, Badawi MM, Soliman G, Abdel-Kawi
AN, Fadel EA and Abozaid GM: Physiological and Histopathological
studies on Bisphenol-A compound as xenoestrogen in male albino
rats. Egypt J Hospit Med. 50:127–136. 2013. View Article : Google Scholar
|
|
57
|
Ma JQ, Ding J, Zhang L and Liu CM:
Hepatoprotective properties of sesamin against CCl4
induced oxidative stress-mediated apoptosis in mice via JNK
pathway. Food Chem Toxicol. 64:41–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Makni M, Fetoui H, Garoui EM, Gargouri NK,
Jaber H, Makni J, Boudawara T and Zeghal N: Hypolipidemic and
hepatoprotective seeds mixture diet rich in omega-3 and omega-6
fatty acids. Food Chem Toxicol. 48:2239–2246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Suwimol S, Wiroj J, Kewalin W, Natchapon
J, Pathamaporn H and Auranun S: Effects of sesame seeds consumption
on serum cholesterol and oxidative status in hypercholesterolemia.
Food Public Health. 2:193–196. 2012.
|
|
60
|
Asgary S, Kopaei RM, Najafi S, Heidarian E
and Sahebkar A: Antihyperlipidemic effects of Sesamum
indicum L. in rabbits fed a high-fat diet.
ScientificWorldJournal. 2013:3658922013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim M, Woo M, Noh JS, Choe E and Song YO:
Sesame oil lignans inhibit hepatic endoplasmic reticulum stress and
apoptosis in high-fat diet-fed mice. J Functional Foods.
37:658–665. 2017. View Article : Google Scholar
|
|
62
|
Penalvo JL, Hopia A and Adlercreutz H:
Effect of sesamin on serum cholesterol and triglycerides levels in
LDL receptor-deficient mice. Eur J Nutr. 45:439–444. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kushiro M, Masaokab T, Hageshitab S,
Takahashia Y, Idea T and Suganoc M: Comparative effect of sesamin
and episesamin on the activity and gene expression of enzymes in
fatty acid oxidation and synthesis in rat liver. J Nutr Biochem.
13:289–295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ide T, Kushiro M, Takahashi Y, Shinohara
K, Fukuda N and Yasumoto S: Sesamin, a sesame lignan, as a potent
serum lipid-lowering food component. JARQ. 37:151–158. 2003.
View Article : Google Scholar
|
|
65
|
Fernández-Formoso G, Pérez-Sieira S,
González-Touceda D, Dieguez C and Tovar S: Leptin, 20 years of
searching for glucose homeostasis. Life Sci. 140:4–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chavali SR, Zhong WW and Forse RA: Dietary
alpha-linolenic acid increases TNF-alpha, and decreases IL-6, IL-10
in response to LPS: Effects of sesamin on the delta-5 desaturation
of omega6 and omega3 fatty acids in mice. Prostaglandins Leukot
Essent Fatty Acids. 58:185–191. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Haider DG, Leuchten N, Schaller G, Gouya
G, Kolodjaschna J, Schmetterer L, Kapiotis S and Wolzt M:
C-reactive protein is expressed and secreted by peripheral blood
mononuclear cells. Clin Exp Immunol. 146:533–539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Saleem MT, Chetty MC and Kavimani S:
Sesame oil enhances endogenous antioxidants in ischemic myocardium
of rat. Brazilian J Pharmacogn. 22:669–675. 2012. View Article : Google Scholar
|
|
69
|
Conti V, Izzo V, Corbi G, Russomanno G,
Manzo V, De Lise F, Donato A and Filippelli A: Antioxidant
supplementation in the treatment of aging-associated diseases.
Front Pharmacol. 7:242016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ragavendran P, Sophia D, Arulraj A and
Gopalakrishnan VK: Cardioprotective effect of aqueous, ethanol and
aqueous ethanol extract of Aerva lanata (Linn.) against
doxorubicin-induced cardiomyopathy in rats. Asian Pac J Trop
Biomed. 2 (Suppl):S212–S218. 2012. View Article : Google Scholar
|
|
71
|
Li WX, Kong X, Zhanga JX and Yang JR:
Long-term intake of sesamin improves left ventricular remodelling
in spontaneously hypertensive rats. Food Funct. 4:453–460. 2013.
View Article : Google Scholar : PubMed/NCBI
|