Introduction
Periodontitis is an inflammatory disease caused by
specific microorganisms and their products are main pathogens,
characterized by progressive periodontal ligaments and proximal
alveolar bone damage and a final loss of teeth (1). Periodontal diseases have been
recognized as potential risk factors for many systemic diseases,
such as diabetes mellitus and cardiovascular diseases (2). Hyperlipidemia, one of the modern
concerns of society, generates increasingly adverse effects on
general health. Increased emphasis has been placed on the influence
of hyperlipidemia on general inflammation and cardiovascular
disease, especially atherosclerosis (3).
A relationship between hyperlipidemia and
periodontitis has been noted in recent years (4–7).
Numerous animal experiments have further illustrated the existence
of the bi-directional relationship between hyperlipidemia and
periodontitis (8,9). The vicious circle formed between
inflammation and lipid metabolism is considered to be the
pathological basis between periodontitis and hyperlipidemia. On one
hand, inflammation leads to abnormal blood lipid metabolism;
periodontal pathogenic bacteria and their metabolites and
pro-inflammatory cytokines from periodontal infection cause lipid
metabolism disorder, lipid peroxidation, and elevated blood lipid
levels, which promote the formation of hyperlipidemia (10). On the other hand, elevated blood
lipid levels and lipid peroxidation lead to disorders of immune
regulation, stimulate the expression of pro-inflammatory cytokines,
cause oxidative stress, delay wound healing, and increase the
body's susceptibility to periodontitis (11,12).
In recent years, gingival mesenchymal stem cells
(GMSCs) have been found to possess properties of mesenchymal stem
cells (MSCs), including self-renewal, clonogenicity,
multi-differentiation, and expression of MSC-associated surface
markers (13). As newly discovered
MSCs, GMSCs have received increased attention due to their various
distinct properties: Accessible tissue source, ease of isolation,
uniformly homogenous property, rapid proliferation capacity, stable
morphology, and ability to maintain the characteristics of MSCs
over long-term culture time (14,15).
Local transplantation of GFP-labeled GMSC sheets in a dog model
with class III furcation defects was found to significantly
increase periodontal regeneration (16). In our previous study, we demonstrated
that GFP-labeled GMSCs transplanted by tail vein into mice with
mandibular bone defects could home to the defect sites and
accelerate bone regeneration (17).
In contrast to other MSCs, GMSCs displayed more
powerful anti-inflammatory and immunomodulatory functions. Recent
experimental studies have reported that GMSCs suppress T-lymphocyte
proliferation and activation, and possess powerful immunomodulatory
function on several innate immune cells, particularly macrophages,
mast cells, and dendritic cells (14,18). The
immunomodulatory properties of GMSCs have been applied in the
therapy of a few inflammatory-related diseases in animal models,
including immunological rejection, contact hypersensitivity, and
experimental colitis. Furthermore, these properties exhibited
unique advantages compared to other seed cells such as bone
marrow-derived stem cells (BMSCs) and periodontal ligament-derived
stem cells (PDLSCs) (19–21). Our previous study demonstrated that
hyperlipidemia can reduce homing efficiency of systemically
transplanted BMSCs and suppress bone regeneration (22). To date, however, the regulatory
effect of systematically transplanted GMSCs on lipid metabolism and
periodontal inflammation has not been reported. As a recent report
demonstrated, systematically transplanted BMSCs possess the
capacity to attenuate serum lipid profile, blood glucose, and
oxidative stress in rats with diabetes (19). As such, we speculated that GMSCs can
rectify lipid metabolism disorders and reduce inflammation,
therefore contributing to periodontal tissue reconstruction. In the
present study, we transplanted human GMSCs into experimental
hyperlipidemic mice with periodontitis via the tail vein to explore
the role of GMSCs in the regulation of lipid metabolism and
inflammation.
Materials and methods
Isolation, culture and
characterization of GMSCs
Human gingiva samples were extracted from discarded
tissues from healthy volunteers (18–25 years of age), who underwent
routine dental procedures at the Department of Stomatology,
Affiliated Hospital of Qingdao University. All procedures were
approved by the Clinical Research Ethics Committee of Qingdao
University (Qingdao, China) with informed consent provided by the
participants. The gingival tissues were washed several times with
PBS, containing 400 µg/ml streptomycin and 400 U/ml penicillin. The
tissues were then incubated overnight with α-MEM (Hyclone; GE
Healthcare) which contained 2 mg/ml dispase II (Sigma-Aldrich;
Merck KGaA) at 4°C. Following separation of the epithelial layer,
the connective tissue was minced into fragments and digested with 2
mg/ml collagenase I (Sigma-Aldrich; Merck KGaA) at 37°C for 40 min.
The tissue explants were placed into 25 mm2 culture
flask containing α-MEM medium with 15% FBS (Hyclone; GE Healthcare)
at 37°C in 5% CO2. The second to fourth generation cells
were used for further experiments.
A colony-forming unit-fibroblast (CFU-F) assay was
conducted to determine the colony forming efficiency of the GMSCs.
Adipogenic and osteogenic differentiation were performed to
determine the multipotent differentiation trait of the GMSCs. GMSCs
(1×106) at passage 4 were collected and washed twice
with PBS, and then the cells were incubated with antibodies against
human CD73 (1:20; cat. no. 344015; BioLegend, Inc.), CD90 (1:20;
cat. no. 328107; BioLegend, Inc.), CD105 (1:20; cat. no. 323205;
Biolegend, USA), STRO-1 (1:100; cat. no. 398401; eBioscience;
Thermo Fisher Scientific, Inc.), CD45 (1:20; cat. no. 368507;
BioLegend, Inc.) and CD31 (1:20; cat. no. 303103; BioLegend, Inc.)
for flow cytometry analysis with the use of a flow cytometer
(Beckman Coulter) (17,23).
Green fluorescent protein (GFP)
transfection of GMSCs
Lentiviral vectors with GFP (Genechem) were used to
label GMSCs to trace the fate of the GMSCs in the mice.
First-passage GMSCs (2×103 cells/well) were seeded in
6-well plates for 24 h. Then, the culture medium was replaced with
the virus solution diluted by serum-free α-MEM (50 µg/ml). The
viral solution was replaced with complete culture medium after
being transfected for 8 h. The GFP+ GMSCs were expanded
and cells from third to fifth passage were transplanted into the
mice.
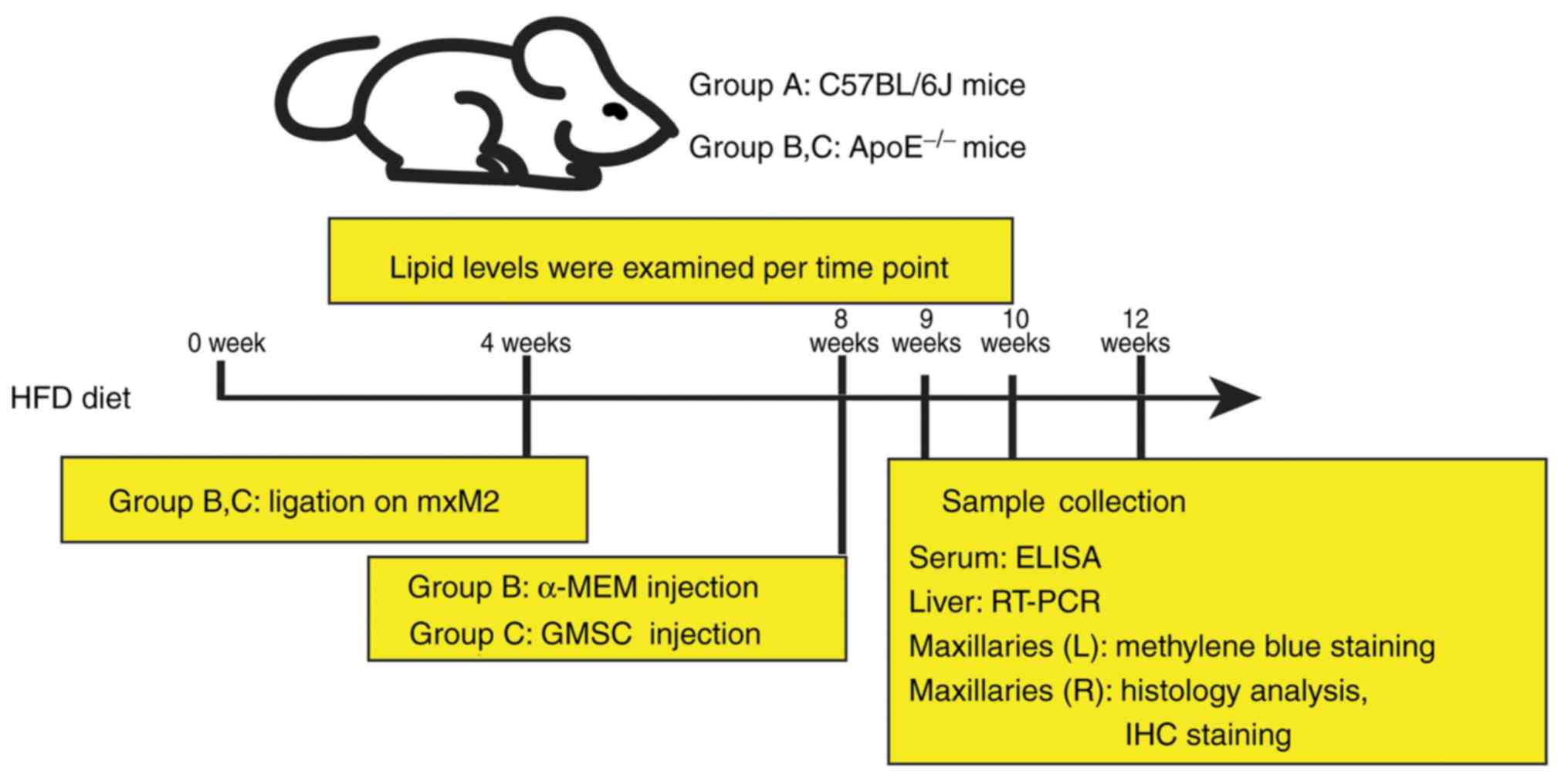
Establishment of the experimental
hyperlipidemia model with periodontitis in mice
Male, 8-week-old ApoE−/− mice with a
C57BL/6J background and wild-type C57BL/6J mice (total number of
mice: 60; weight, 20.59±1.42 g; Peking University Health Science
Center) were all fed a cholate/high-cholesterol/high-fat diet
(0.5%/1.25%/15%, respectively) throughout the experiments. Animals
were maintained under a temperature controlled (24±1.0°C)
environment with a 12-h dark/light cycle and fed a high fat diet
(HFD) and aseptic water ad libitum. This study was
consistent with the Institutional Review Board and the Ethics
Committee of the Affiliated Hospital of Qingdao University
(approval no. QYFYKYLL-2017-01-2).
After 4 weeks of HFD, a 5-0 silk ligature was placed
on the maxillary second molars (mxM2) of the ApoE−/−
mice for 4 weeks. ApoE−/− mice were divided into two
groups, Group B and Group C (n=20 per group), while wild-type
C57BL/6J mice without any treatment were assigned to Group A
(n=20). To confirm the establishment of periodontitis, 2 mice in
every group were sacrificed and the maxillary bones were removed
and treated using the following procedure.
The isolated samples were boiled for 10 min under a
bar pressure to remove soft tissue and then soaked in a hydrogen
peroxide solution for 12 h. Afterwards, the samples were stained
with methylene blue and washed with PBS. Images were captured using
a stereomicroscope (Olympus Corporation) at ×200 magnification and
assessed with Image-Pro-Plus 6.0 software program (Media
Cybernetics, Inc.). Alveolar bone loss was determined as the sum of
distances from the alveolar bone crest (ABC) to the cementoenamel
junction (CEJ) at 12 sites as previously described (24): 4 sites for mxM1 (disto-buccal groove
and disto-palatal groove, buccal of distal cusp and palatal of
distal cusp); 6 sites for mxM2 (mesio-buccal cusp and mesio-palatal
cusp, buccal groove and palatal groove, disto-buccal cusp and
disto-palatal cusp) and 2 sites for mxM3 (buccal cusp and palatal
cusp).
GMSC transplantation
After 4 weeks of ligation on mxM2, the suture was
removed, followed by GMSC transplantation. Briefly, Group C was
administered 500 µl α-MEM containing 1×106 GMSCs; Group
B was injected with 500 µl α-MEM as control. Mice were anesthetized
using 1.5% pentobarbital and sacrificed with carbon dioxide at 1, 2
and 4 weeks after transplantation (Fig.
1).
Blood sample preparation and reverse
transcription-polymerase chain reaction (RT-PCR) of liver
tissues
Blood samples were obtained from the angular vein at
0, 4, 8, 9, 10 and 12 weeks after HFD to test serum lipid levels,
including triglyceride (TG), total cholesterol (TC), low density
lipoprotein cholesterol (LDL) and high density lipoprotein
cholesterol (HDL) using an autoanalyzer (Hitachi). The serum levels
of interleukin (IL)-6, tumor necrosis factor (TNF)-α and IL-10 were
determined by ELISA kits (Elabscience), according to the protocol
of the manufacturer.
TRIzol reagent (Takara) was used to extract the RNA
from liver tissues on the basis of the protocol of the
manufacturer. High-capacity cDNA reverse transcription kits
(Takara) were used to synthesize the complementary DNA in
accordance with the protocol of the manufacturer. cDNA was then
subjected to quantitative real-time PCR amplification using
PowerUp™ SYBR Green Master Mix (Takara). Reactions were run on a
LightCycler 480 Real-Time PCR System (Roche Applied Science).
Relative mRNA expression of the peroxisome proliferator-activated
receptor α (PPARα) and sterol regulatory element binding
protein-1c (SREBP-1c) genes was calculated by the
2−ΔΔCq (25) method and
normalized to the gene GAPDH as an internal control. The
forward and reverse primers are listed in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Target genes | Primer | Sequence | Fragment length
(bp) |
|---|
| GAPDH | Forward |
5′-ATGGGTGTGAACCACGAGA-3′ | 229 |
|
| Reverse |
5′-CAGGGATGATGTTCTGGGCA-3′ |
|
| PPAR-α | Forward |
5′-CCAGGCTTTGCAAACTTGGA-3′ | 180 |
|
| Reverse |
5′-GATGTCACAGAACGGCTTCC-3′ |
|
|
SREBP-1c | Forward |
5′-CCCACCTCAAACCTGGATCT-3′ | 229 |
|
| Reverse |
5′-AAGCAGCAAGATGTCCTCCT-3′ |
|
Morphometric and histologic
analyses
The left maxillaries were stained with methylene
blue using the same methods as described above in the section
(establishment of experimental hyperlipidemia model with
periodontitis in mice.). The right maxillaries were isolated
after perfusion fixation with 4% paraformaldehyde. The tissue was
demineralized in 10% EDTA for 4 weeks and then embedded in
paraffin. Tissue sections, 5-µm thick, were cut in the
mesial-distal direction. Sections of the most central areas of the
second molars were selected, for which two sections were stained
with hematoxylin and eosin (H&E) and Masson Trichrome (MT)
staining. Images were captured under a light microscope at ×100
magnification (Olympus Corporation).
Fluorescence microscope observation
and immunohistochemical staining for GFP
To trace the fate of GMSCs in mice with
periodontitis, fluorescence microscope observation and
immunohistochemical staining were performed. The rehydrated
sections were washed with PBS for 5 min and stained with DAPI for 5
min. The slices were observed under a fluorescence microscope
(Olympus Corporation) at ×400 magnification after being washed with
PBS.
Immunohistochemical study was performed using a
rabbit antibody (GFP; 1:100; cat. no. AB183734; Abcam) against GFP.
Sections were treated with 3% H2O2 for 10 min
to block peroxidase after being dewaxed and hydrated. Then, the
sections were incubated with the primary antibody at 37°C for 90
min, washed with PBS, and incubated with anti-rabbit secondary
antibody (PV-6001; 1:10; Origene Technologies, Inc.) for 30 min at
37°C. Nuclear staining was performed with hematoxylin.
Statistical analysis
Statistical analysis was performed using a
statistical package (SPSS 19.0, SPSS Inc.). Data are expressed as
the mean ± SD. Differences for all groups were analyzed by one-way
analysis of variance with LSD-t test. The level of statistical
significance chosen was P<0.05.
Results
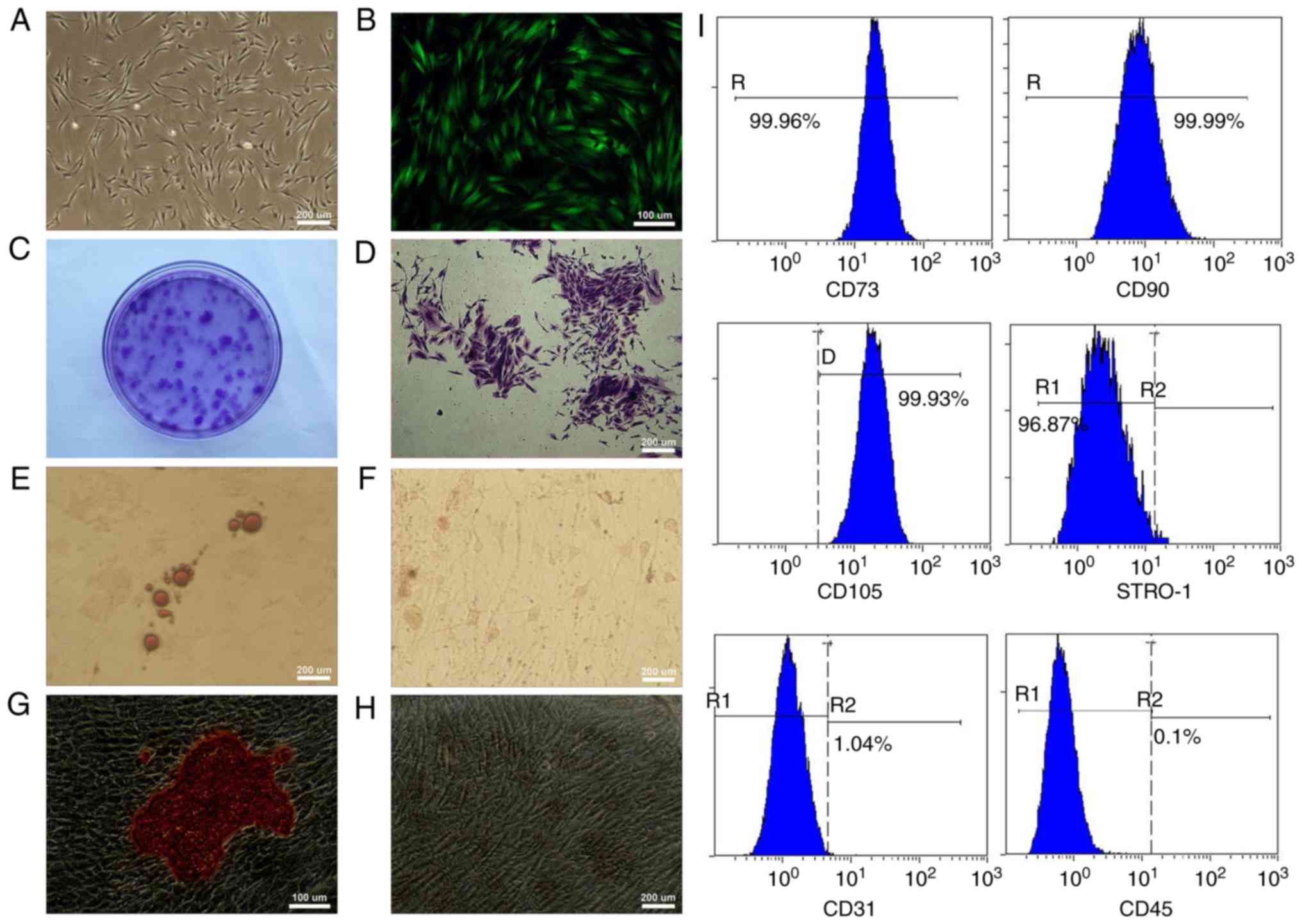
Characterization, multiple
differentiation potential and transfection of GMSCs
The adherent GMSCs were observed 5–7 days after
initiation of the primary culture and reached 80% confluence by the
14–21 day. Under the optical microscope, the primary GMSCs
displayed a fibroblast-like spindle form (Fig. 2A). At 72 h post-transfection, strong
expression of GFP could be observed under the fluorescence
microscope, and stable GFP expression was obtained during the
process of subculturing (Fig. 2B).
The cell colonies were easily identified using crystal violet
staining (Fig. 2C and D). After the
GMSCs had been cultured in the adipogenic medium for 2 weeks,
lipid-rich globules were confirmed by Oil Red O staining in
cytoplasm of the differentiated cells (Fig. 2E). No positive cell was found in the
control group (Fig. 2F). After the
GMSCs were cultured in the osteogenic medium for 4 weeks,
mineralized nodules were observed using Alizarin Red S staining
(Fig. 2G), and no positive cell was
found in the control group (Fig.
2H). Flow cytometric analysis exhibited that GMSCs were
positive for MSC markers CD73, CD90, CD105 and STRO-1 and
expression percentages were >95%. Additionally, GMSCs were
negative for hematopoietic cell markers CD45 and CD31 and
expression percentages were <2% (Fig.
2I).
An experimental hyperlipidemia model
with periodontitis in mice is successfully established by ligation
in ApoE−/− mice
Serum lipid levels of the ApoE−/− mice
were significantly increased after the 4-week HFD.
ApoE−/− mice displayed an 11.69-fold increase in LDL
levels and an 8.31-fold increase in TC levels compared to those of
the C57BL/6J mice. Moreover, a 2.04-fold increase in serum HDL
levels and a 1.89-fold increase in serum TG levels were found in
the ApoE−/− mice compared to those of the C57BL/6J mice
(Table II). The results
demonstrated that a hyperlipidemic model induced by diet was
successfully constructed in the ApoE−/− mice after the
4-week HFD. The distances from CEJ to ABC at 12 sites bilaterally
of the isolated maxillary bones were measured and calculated
together, which represented the overall alveolar bone resorption
level. After a 4-week placement of ligatures, the alveolar bone
resorption level of ApoE−/− mice was 4.76±0.55 mm, which
was significantly higher than that of the C57BL/6J mice with
1.99±0.25 mm (P<0.05). This finding demonstrated that a
periodontitis model was successfully constructed as alveolar bone
resorption is an important pathological change of periodontitis.
These results indicated that an experimental hyperlipidemia model
with periodontitis was constructed in ApoE−/− mice fed
with a 4-week HFD and a 4-week placement of ligatures.
 | Table II.Comparison of the plasma lipid levels
(mean ± SD, mmol/l) between ApoE−/− mice and C57BL/6J
mice at week 0 and 4 following the HFD (n=6). |
Table II.
Comparison of the plasma lipid levels
(mean ± SD, mmol/l) between ApoE−/− mice and C57BL/6J
mice at week 0 and 4 following the HFD (n=6).
|
| C57BL/6J mice | ApoE−/−
mice |
|---|
|
|
|
|
|---|
| Plasma lipid
levels | Week 0 | Week 4 | Week 0 | Week 4 |
|---|
| TG | 0.82±0.21 | 0.92±0.22 | 1.18±0.21 |
1.74±0.35a |
| TC | 1.99±0.14 | 3.65±0.52 | 8.67±1.39 |
30.33±7.71a,b |
| HDL | 1.51±0.17 | 2.4±0.20 | 2.54±0.47 |
4.89±0.62a,b |
| LDL | 0.35±0.07 | 0.84±0.16 | 2.19±0.31 |
9.82±3.03a,b |
GMSCs attenuate serum lipid
profiles
At week 8, 9, 10 and 12, there was a significant
increase in TG, TC, LDL and HDL levels in Group B and C compared to
those in Group A (P<0.05). At week 9, 10 and 12, Group C
exhibited a significant decrease in TG, TC, and LDL levels and a
significant increase in HDL levels compared to those in Group B
(P<0.05; Fig. 3). These results
showed that GMSCs can attenuate serum lipid profiles.
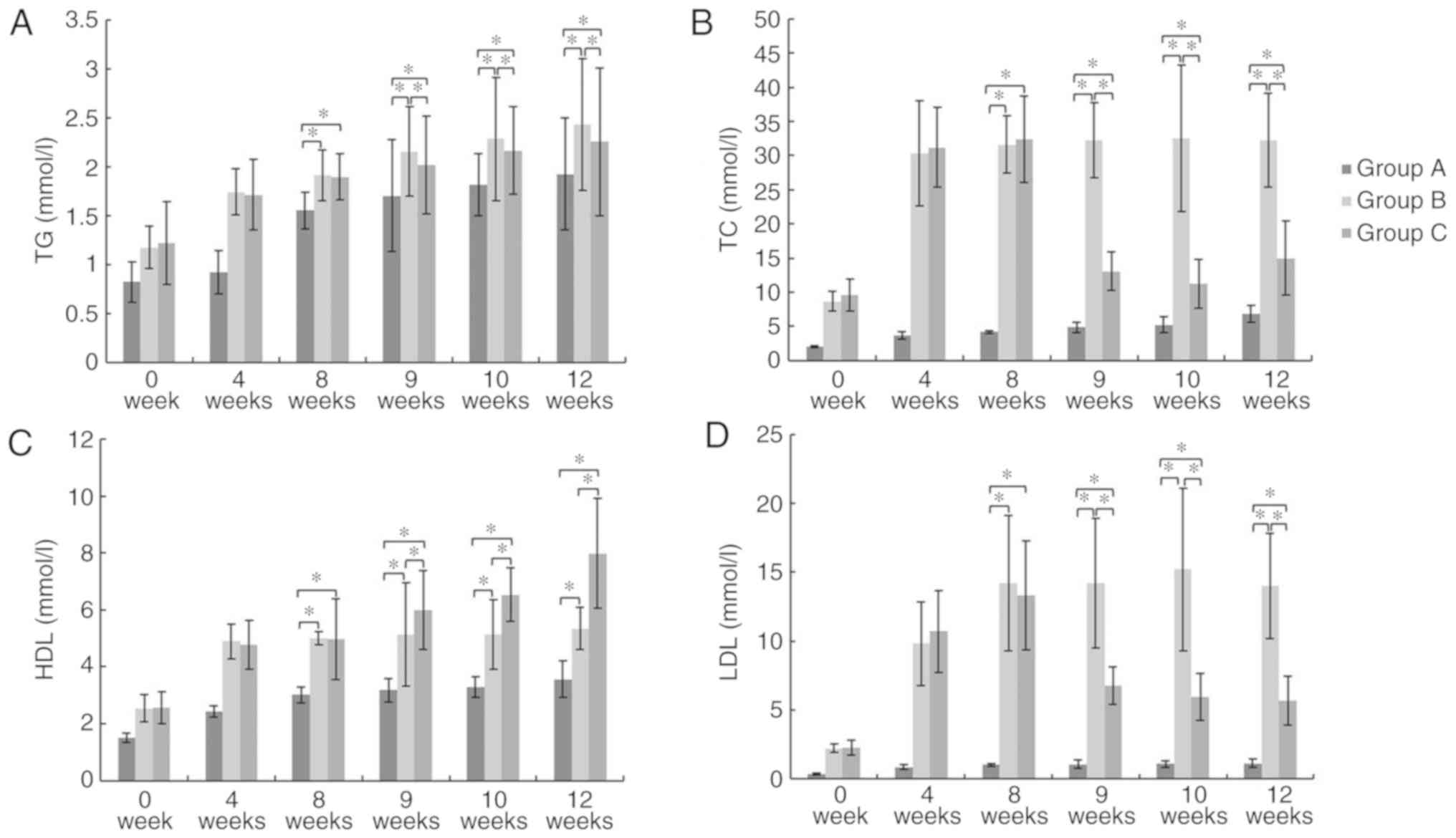 | Figure 3.Serum lipid levels. (A) TG, (B) TC,
(C), HDL and (D) LDL. *P<0.05. At week 9, 10, and 12, Group C
exhibited a significant decrease in TG, TC, and LDL levels and a
significant increase in HDL levels compared to those in Group B.
TG, triglyceride; TC, total cholesterol; HDL, high density
lipoprotein cholesterol; LDL, low density lipoprotein
cholesterol. |
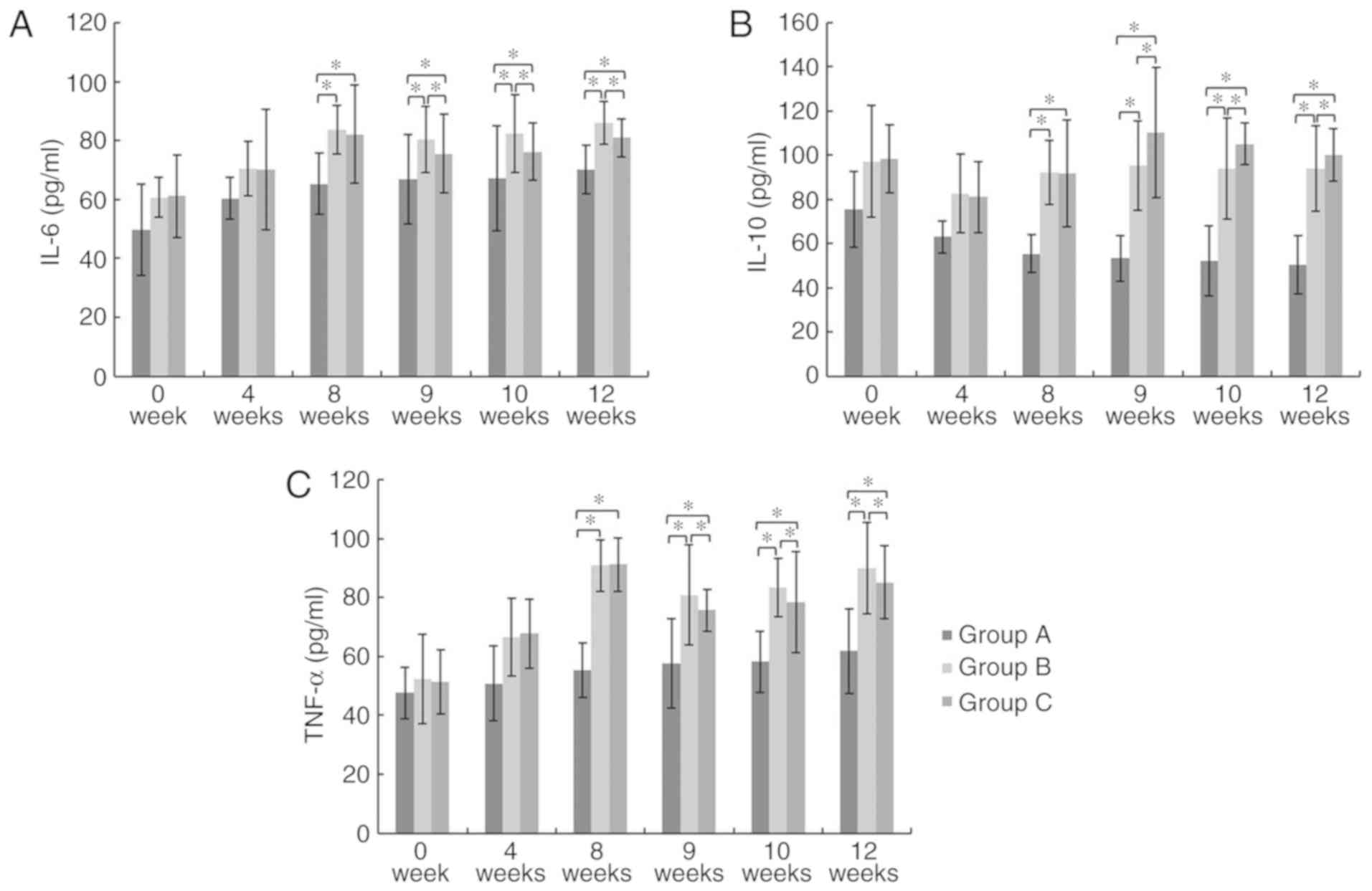
GMSCs display anti-inflammatory
functions
At week 8, 9, 10 and 12, the serum IL-6, IL-10 and
TNF-α levels in Group B and C were significantly higher than those
in Group A. At week 9, 10 and 12, the serum IL-6 and TNF-α
(pro-inflammatory cytokines) levels in group C were significantly
decreased compared to those in Group B, while the serum IL-10
(anti-inflammatory cytokine) level in Group C was significantly
higher than that of Group B (P<0.05; Fig. 4). These results suggest that GMSCs
display anti-inflammatory functions.
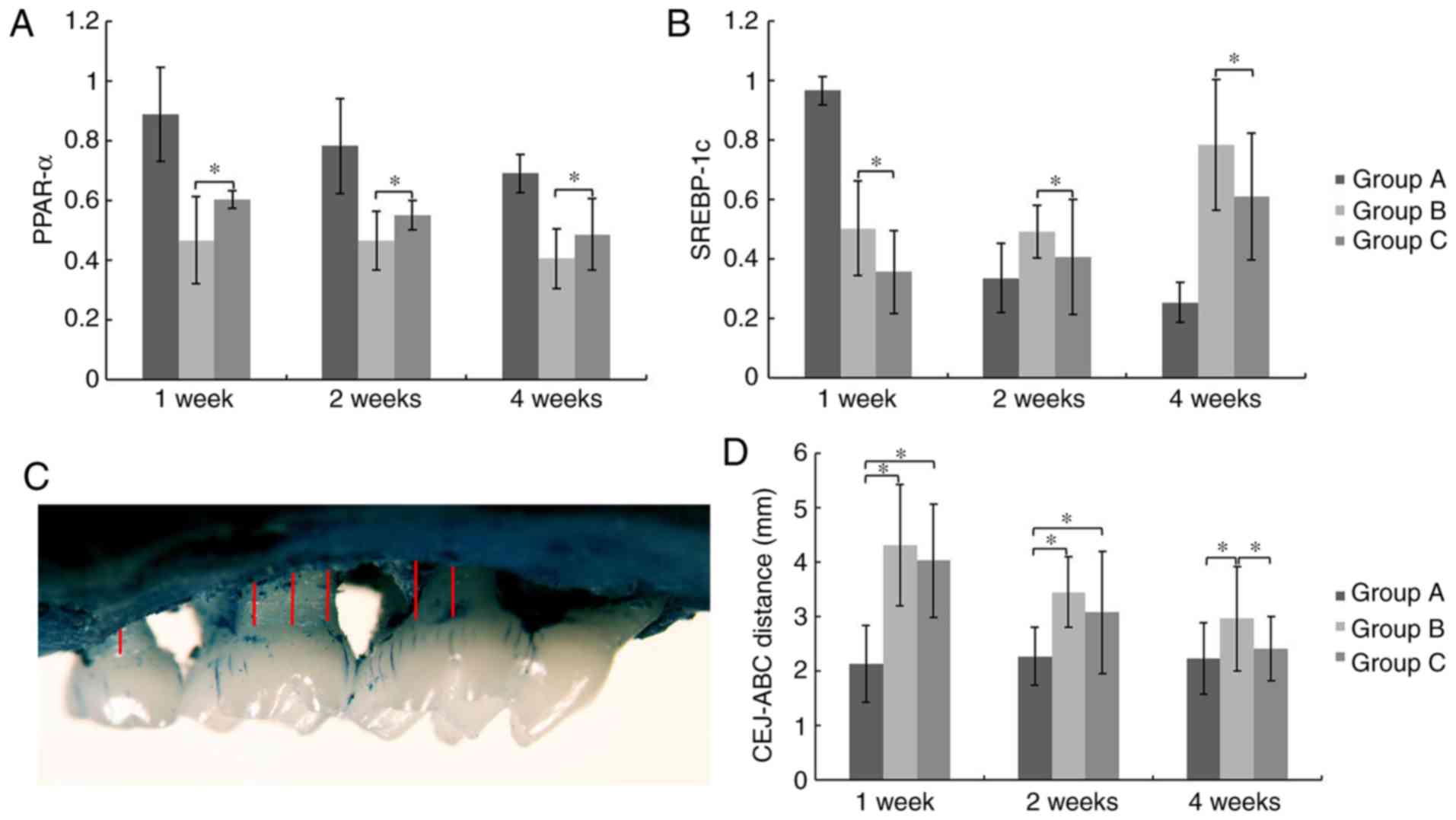
GMSCs affect expression of hepatic
genes associated with lipid metabolism
At week 1, 2 and 4 after cell transplantation,
levels of PPAR-α mRNA expression in the liver of Group C were
significantly higher compared to that of Group B, while the levels
of SREBP-1c mRNA were significantly reduced (P<0.05; Fig. 5A and B). These results indicate that
GMSCs affect expression of hepatic genes associated with lipid
metabolism.
GMSCs promote alveolar bone
regeneration
Alveolar bone loss was determined as the distance
from ABC to CEJ (Fig. 5C). At week 1
and 2 post-transplantation, there were insignificant differences
between Group B and C. However, alveolar bone loss in Group C was
significantly less than that of Group B and no significant
difference was detected between Group A and C at the 4th week,
which indicated the promotion of alveolar bone regeneration by
systemically transplanted GMSCs (P<0.05; Fig. 5D).
GMSCs improve the pathologic features
of periodontitis
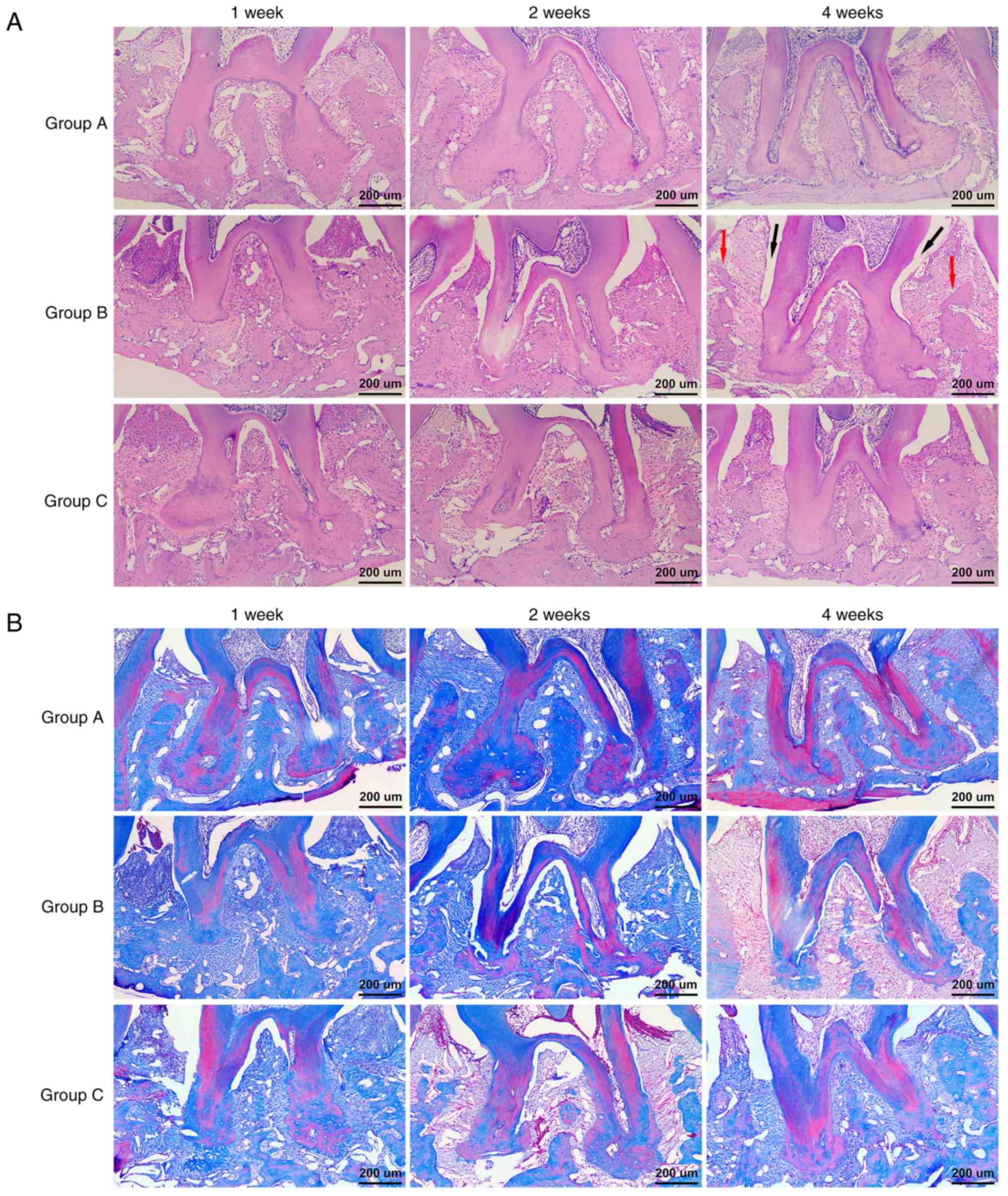
Histological changes in the interradicular region
were observed by H&E and MT staining. At week 1 and 2 after
transplantation, deep periodontal pockets, attachment loss,
inflammatory cell infiltration, and severe alveolar bone
destruction were detected in Group B and Group C by H&E
staining. At week 4 post-transplantation, less periodontal pocket
depth, attachment loss and inflammatory cell infiltration and
higher alveolar bone heights were observed in Group C compared to
those of Group B, which were consistent with the methylene blue
staining results (Fig. 6A).
Additionally, MT staining indicated that almost all of the alveolar
bones were stained blue and there were more reddish stained mature
bones in Group C than in Group B at week 4 (Fig. 6B). These results showed that GMSCs
can improve the pathologic features of periodontitis and promote
alveolar bone regeneration.
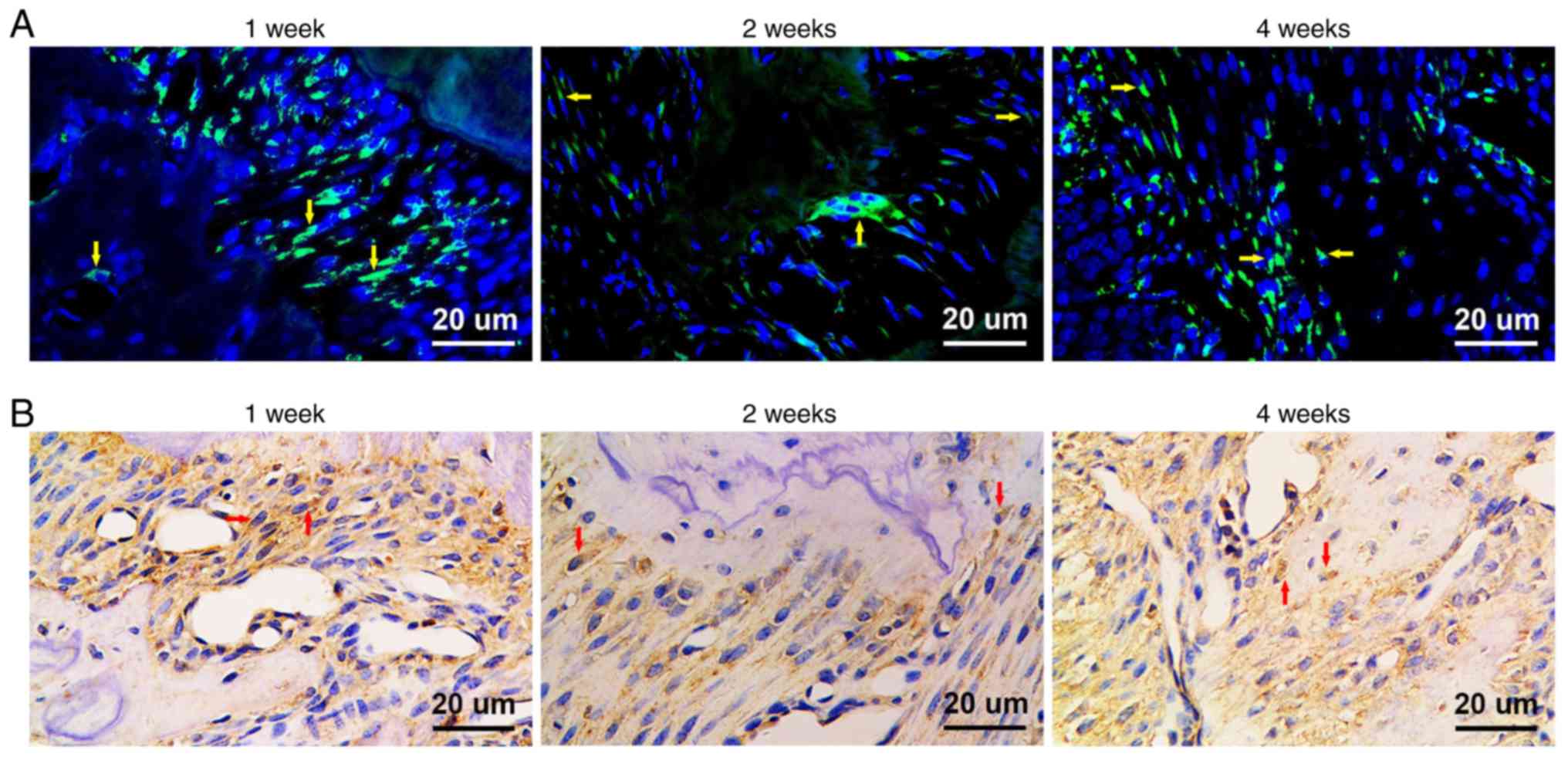
GMSCs home to periodontal injury
sites
GFP-positive (GFP+) cells were detected
in both fluorescence microscope observation and immunohistochemical
staining. At week 1 post-transplantation, GFP+
fibroblast-like cells were detected in the periodontal ligament. At
week 2 post-transplantation, GFP+ fibroblast-like cells
were found in the periodontal ligament and GFP+
osteoblasts were detected close to the alveolar bone. Furthermore,
GFP+ osteoblasts were identified in the area of newly
formed alveolar bone at week 4 post-transplantation. These results
revealed that GMSCs can home to periodontal injury sites and
promote tissue regeneration (Fig. 7A and
B).
Discussion
Stem cell-based therapy has been considered to be a
hopeful alternative for regenerative medicine, but various
drawbacks and limitations hinder the popularization and application
of these mesenchymal stem cells (MSCs). Bone marrow has been
regarded as the best source of MSCs despite various sources to
date. However, bone marrow is a limited source and a large amount
of pain is caused to the patient when samples are harvested
(26–28). By comparison, the source of
gingiva-derived mesenchymal stem cells (GMSCs) is discarded gingiva
tissue from patients undergoing tooth extraction or periodontal
surgery. In addition, it is reported that most of the biological
properties of GMSCs are superior to those of bone marrow-derived
mesenchymal stem cells (BMSCs) (23).
To confirm our assumption that GMSCs possess the
abilities of lipid metabolism regulation and anti-inflammation, we
isolated GMSCs from gingival tissue, where the harvested cells
displayed self-renewal capabilities and multilineage
differentiation potential toward the adipogenic and osteogenic
conditions in vitro when GMSCs were cultured with built
lineage-specific differentiation factors. This was in conformity
with previous findings (23,29). Moreover, the results of flow
cytometric analysis showed that GMSCs express the MSC markers CD73,
CD90, CD105 and STRO-1, but lack the hematopoietic stem marker CD45
and endothelial cell marker CD31. These results were consistent
with the criteria for mesenchymal stromal cells suggested by the
International Society for Cellular Therapy (26). As the integration and expression of
GFP genes in MSCs have no significant effect on stem cell traits
(30), we transduced the GFP
gene into GMSCs using lentiviral vectors to trace the fate of GMSCs
in vivo.
In the 1990s, ApoE−/− mouse models were
developed by two independent research groups, and both groups
reported that ApoE gene deficiency resulted in severe
hyperlipidemia and spontaneous atherosclerotic lesions (31,32). In
our research, a hyperlipidemia model was constructed using
ApoE−/− mice that were fed a high fat diet (HFD). Our
findings exhibited that ApoE−/− mice displayed dramatic
increases in serum total cholesterol (TC) and low density
lipoprotein cholesterol (LDL) levels when compared to those in the
C57 BL/6J mice after a 4-week HFD. In effect, the existence of
ligatures disturbed periodontal homeostasis via accelerating local
accumulation of bacteria (24).
After 4 weeks of ligatures, we observed obvious alveolar bone loss
in the ApoE−/− mice, which indicated that periodontitis
was established. Most of the previous studies constructed the
animal model of periodontitis using a silk ligature soaked with
live Porphyromonas gingivalis suspension (33,34),
however our ligation method without bacteria was more
economical.
Group C showed a significant increase in HDL levels
and a significant reduction in TC, triglycerides (TG) and LDL
levels when compared to those of Group B post-transplantation,
which were in line with former findings confirming that MSCs have
the capacity to attenuate serum lipid profiles and oxidative stress
(19). MSCs derived from induced
pluripotent stem cells were found to restore cigarette
smoke-induced cardiac dysfunction through regulation of fatty acid,
triglyceride and cholesterol metabolism, which led to alleviation
of cardiac inflammation and oxidative stress in a rat model of
passive smoking (35).
Adipose-derived mesenchymal stem cell infusion was found to
significantly repress the growth in body weight and dramatically
improve serum lipid profiles (TG, TC, LDL) in diet-induced obese
mice and db/db mice respectively as obesity and hyperlipidemia
models (36). A significant 33%
reduction in serum TC levels in BMSC-treated mice was found after 8
weeks of a Western-type diet (37).
Similar to BMSCs, GMSCs display powerful
immunomodulatory impacts on innate immune cells, particularly
macrophages, dendritic cells (DCs) and mast cells (MCs) (14,38–40). In
a mouse model of skin wound healing, treatment using GMSCs
exhibited a dynamic downregulation in the expression of
M1-cytokines (IL-6 and TNF-α) and an upregulation in the amount of
M2 macrophages and the level of anti-inflammatory cytokine IL-10.
This then mitigated local inflammation and significantly enhanced
wound repair during the wound healing process (40). Through a PGE2-mediated
activation of the system E prostanoid (EP)
receptor/cAMP/protein-kinase-A (PKA) which created a docking site
for the initiation of IL-10 transactivation, GMSCs significantly
repressed the maturation and activation of DCs, reducing their
antigen presentation capacity and ameliorating the inflammatory
response (14). GMSCs have been
shown to exhibit a suppressive effect on the acquired immune system
via an increase in IL-10 and decrease in tryptophan secretion in a
cell-cell contact dependent and independent manner, to repress
PHA-dependent T-lymphocyte proliferation and activation (13,21). The
present study showed a downregulation in serum pro-inflammatory
cytokine (TNF-α and IL-6) levels and an upregulation in serum
anti-inflammatory cytokine (IL-10) level after GMSC
transplantation, which are in line with previous research studies
that have confirmed that GMSCs display marked immunomodulatory
properties.
In a previous study, BMSCs displayed significant
upregulation of the PPAR-α pathway and downregulation of fatty acid
biosynthesis when systematically transplanted 7 days before renal
ischemia/reperfusion in rats (41).
Nevertheless, a recent study using a rat model indicated that
systematically transplanted BMSCs in radiation-induced heart injury
decreased the expression of PPAR-α (42). In the present study, PPAR-α mRNA
expression in the liver of Group C was significantly higher
compared to that of Group B at week 1, 2 and 4 after
transplantation. Furthermore, the infusion of MSCs activated the
PPAR-α pathway. PPAR-α controls the expression of several types of
hepatic genes encoding for enzymes/proteins involved in fatty acid
metabolism (uptake, intracellular transport, activation and
oxidation), lipogenesis, ketogenesis and lipoprotein/cholesterol
metabolism. Numerous studies have shown that PPAR-α plays a key
role in hepatic lipoprotein and fatty acid metabolism (43,44).
Liver mRNA expression of SREBP-1c had an 18% reduction in
BMSC-treated atherosclerosis mice (37). However, transplantation of BMSCs had
no effect on SREBP-1c mRNA expression in diet-induced obese mice
(45). Our results displayed that
SREBP-1c mRNA expression in the liver of Group C was significantly
decreased compared to that of Group B at week 1, 2 and 4 after
transplantation of GMSCs. SREBP-1c induces the expression of a
family of genes which have an effect on fatty acid synthesis and
glucose utilization (46).
In this study, alveolar bone loss in Group B was
significantly greater than that of Group C at week 4 post
transplantation, which indicated promotion of systemically
transplanted GMSCs on alveolar bone regeneration. Results of
H&E and MT staining showed more newly formed bone and higher
alveolar bone heights in Group C compared with those of Group B at
week 4 post-transplantation. GFP+ gingival fibroblasts,
periodontal ligament cells and osteoblasts were detected after
transplantation. These results forcefully supported our hypothesis
that systemically transplanted GMSCs can home to periodontitis
sites and differentiate into periodontal tissue cells.
Overall, the findings of the present study showed
that systematically transplanted GMSCs regulate lipid metabolism
and inflammation in hyperlipidemic mice with periodontitis. Further
studies are needed to clarify the cellular and molecular mechanisms
underlying the improvement of hyperlipidemia and inflammatory
responses. The present study provides a novel strategy with which
to clinically treat periodontitis patients with hyperlipidemia and
other systemic diseases and offers experimental evidence for
application of GMSCs in cell replacement therapy.
Acknowledgements
Not applicable.
Funding
This work was supported by Shandong Province Key
Research Plan (grant no. 2018GSF118150) to QX and Shandong
Provincial National Science Foundation (grant no. ZR2017MH083) to
ZW.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors aided in the proposal of the research
idea and participated in the design of the experiments. XL
performed the animal experiments and wrote the manuscript. ZW and
WBS collected and analyzed the data. WDS and RH performed the cell
experiment. AP and QX interpreted the data and critically revised
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in studies involving
animals were approved by the Institutional Review Board and The
Ethics Committee of The Affiliated Hospital of Qingdao University
(approval no. QYFYKYLL-2017-01-2).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
1999 International International Workshop
for a Classification of Periodontal Diseases and Conditions.
Papers; Oak Brook, illinois: October 30-November 2, 1999. Ann
Periodontol. 4(i): pp. 1–112. 1999, PubMed/NCBI
|
|
2
|
Jain N, Jain GK, Javed S, Iqbal Z,
Talegaonkar S, Ahmad FJ and Khar RK: Recent approaches for the
treatment of periodontitis. Drug Discov Today. 13:932–943. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nelson RH: Hyperlipidemia as a risk factor
for cardiovascular disease. Prim Care. 40:195–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Awartani F and Atassi F: Evaluation of
periodontal status in subjects with hyperlipidemia. J Contemp Dent
Pract. 11:033–040. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sangwan A, Tewari S, Singh H, Sharma RK
and Narula SC: Periodontal status and hyperlipidemia: Statin users
versus non-users. J Periodontol. 84:3–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sandi RM, Pol KG, Basavaraj P, Khuller N
and Singh S: Association of serum cholesterol, triglyceride, high
and low density lipoprotein (HDL and LDL) levels in chronic
periodontitis subjects with risk for cardiovascular disease (CVD):
A cross sectional study. J Clin Diagn Res. 8:214–216.
2014.PubMed/NCBI
|
|
7
|
D'Aiuto F, Nibali L, Parkar M, Suvan J and
Tonetti MS: Short-term effects of intensive periodontal therapy on
serum inflammatory markers and cholesterol. J Dent Res. 84:269–273.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maglakelidze N, Galogre A and Tsagareli Z:
Functional-morphologic aspects of changes of mucosal gingiva
microcirculatory bed vessels in experimental gingivitis against the
background of hypercholesterolemia. Georgian Med News. 121:71–74.
2005.(In Russian).
|
|
9
|
Li L, Messas E, Batista EL Jr, Levine RA
and Amar S: Porphyromonas gingivalis infection accelerates
the progression of atherosclerosis in a heterozygous apolipoprotein
E-deficient murine model. Circulation. 105:861–867. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buhlin K, Gustafsson A, Pockley AG,
Frostegård J and Klinge B: Risk factors for cardiovascular disease
in patients with periodontitis. Eur Heart J. 24:2099–2107. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seijkens T, Hoeksema MA, Beckers L, Smeets
E, Meiler S, Levels J, Tjwa M, de Winther MP and Lutgens E:
Hypercholesterolemia-induced priming of hematopoietic stem and
progenitor cells aggravates atherosclerosis. FASEB J. 28:2202–2213.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fentoğlu Ö, Kırzıoğlu FY, Bulut MT, Kumbul
Doğuç D, Kulaç E, Önder C and Günhan M: Evaluation of lipid
peroxidation and oxidative DNA damage in patients with
periodontitis and hyperlipidemia. J Periodontol. 86:682–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y,
Shi S and Le AD: Mesenchymal stem cells derived from human gingiva
are capable of immunomodulatory functions and ameliorate
inflammation-related tissue destruction in experimental colitis. J
Immunol. 183:7787–7798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su WR, Zhang QZ, Shi SH, Nguyen AL and Le
AD: Human gingiva-derived mesenchymal stromal cells attenuate
contact hypersensitivity via prostaglandin E2-dependent mechanisms.
Stem Cells. 29:1849–1860. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wada N, Wang B, Lin NH, Laslett AL,
Gronthos S and Bartold PM: Induced pluripotent stem cell lines
derived from human gingival fibroblasts and periodontal ligament
fibroblasts. J Periodontal Res. 46:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu X, Ge S, Chen S, Xu Q, Zhang J, Guo H
and Yang P: Human gingiva-derived mesenchymal stromal cells
contribute to periodontal regeneration in beagle dogs. Cells
Tissues Organs. 198:428–437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu QC, Wang ZG, Ji QX, Yu XB, Xu XY, Yuan
CQ, Deng J and Yang PS: Systemically transplanted human
gingiva-derived mesenchymal stem cells contributing to bone tissue
regeneration. Int J Clin Exp Pathol. 7:4922–4929. 2014.PubMed/NCBI
|
|
18
|
Xu X, Chen C, Akiyama K, Chai Y, Le AD,
Wang Z and Shi S: Gingivae contain neural-crest- and
mesoderm-derived mesenchymal stem cells. J Dent Res. 92:825–832.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
EI-Tantawy WH and Haleem EN: Therapeutic
effects of stem cell on hyperglycemia, hyperlipidemia, and
oxidative stress in alloxan-treated rats. Mol Cell Biochem.
391:193–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang L, Li N, Xie H and Jin Y:
Characterization of mesenchymal stem cells from human normal and
hyperplastic gingiva. J Cell Physiol. 226:832–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang QZ, Nguyen AL, Yu WH and Le AD:
Human oral mucosa and gingiva: A unique reservoir for mesenchymal
stem cells. J Dent Res. 91:1011–1018. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu QC, Hao PJ, Yu XB, Chen SL, Yu MJ,
Zhang J and Yang PS: Hyperlipidemia compromises homing efficiency
of systemically transplanted BMSCs and inhibits bone regeneration.
Int J Clin Exp Pathol. 7:1580–1587. 2014.PubMed/NCBI
|
|
23
|
Tomar GB, Srivastava RK, Gupta N,
Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC and Wani MR: Human
gingiva-derived mesenchymal stem cells are superior to bone
marrow-derived mesenchymal stem cells for cell therapy in
regenerative medicine. Biochem Biophys Res Commun. 393:377–383.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abe T and Hajishengallis G: Optimization
of the ligature-induced periodontitis model in mice. J Immunol
Methods. 394:49–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren G, Zhang L, Zhao X, Xu G, Zhang Y,
Roberts AI, Zhao RC and Shi Y: Mesenchymal stem cell-mediated
immunosuppression occurs via concerted action of chemokines and
nitric oxide. Cell Stem Cell. 2:141–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W,
Yang P and Pei X: Gingiva-derived mesenchymal stem cell-mediated
therapeutic approach for bone tissue regeneration. Stem Cells Dev.
20:2093–2102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye Z, Yu X and Cheng L: Lentiviral gene
transduction of mouse and human stem cells. Methods Mol Biol.
430:243–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Plump AS, Smith JD, Hayek T, Aalto-Setälä
K, Walsh A, Verstuyft JG, Rubin EM and Breslow JL: Severe
hypercholesterolemia and atherosclerosis in apolipoprotein
E-deficient mice created by homologous recombination in ES cells.
Cell. 71:343–353. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang SH, Reddick RL, Piedrahita JA and
Maeda N: Spontaneous hypercholesterolemia and arterial lesions in
mice lacking apolipoprotein E. Science. 258:468–471. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meulman T, Peruzzo DC, Stipp RN, Gonçalves
PF, Sallum EA, Casati MZ, Goncalves RB and Nociti FH Jr: Impact of
Porphyromonas gingivalis inoculation on ligature-induced
alveolar bone loss. A pilot study in rats. J Periodontal Res.
46:629–636. 2011.PubMed/NCBI
|
|
34
|
Lin J, Bi L, Yu X, Kawai T, Taubman MA,
Shen B and Han X: Porphyromonas gingivalis exacerbates
ligature-induced, RANKL-dependent alveolar bone resorption via
differential regulation of Toll-like receptor 2 (TLR2) and TLR4.
Infect Immun. 82:4127–4134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang Y, Li X, Zhang Y, Yeung SC, Zhen Z,
Ip MSM, Tse HF, Lian Q and Mak JCW: Induced pluripotent stem
cells-derived mesenchymal stem cells attenuate cigarette
smoke-induced cardiac remodeling and dysfunction. Front Pharmacol.
8:5012017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu GY, Liu J, Wang YL, Liu Y, Shao Y, Han
Y, Qin YR, Xiao FJ, Li PF, Zhao LJ, et al: Adipose-derived
mesenchymal stem cells ameliorate lipid metabolic disturbance in
mice. Stem Cells Transl Med. 5:1162–1170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frodermann V, van Duijn J, van Pel M, van
Santbrink PJ, Bot I, Kuiper J and de Jager SC: Mesenchymal stem
cells reduce murine atherosclerosis development. Sci Rep.
5:155592015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
English K and Mahon BP: Allogeneic
mesenchymal stem cells: Agents of immune modulation. J Cell
Biochem. 112:1963–1968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee RH, Oh JY, Choi H and Bazhanov N:
Therapeutic factors secreted by mesenchymal stromal cells and
tissue repair. J Cell Biochem. 112:3073–3078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang QZ, Su WR, Shi SH, Wilder-Smith P,
Xiang AP, Wong A, Nguyen AL, Kwon CW and Le AD: Human
gingiva-derived mesenchymal stem cells elicit polarization of m2
macrophages and enhance cutaneous wound healing. Stem Cells.
28:1856–1868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Erpicum P, Rowart P, Poma L, Krzesinski
JM, Detry O and Jouret F: Administration of mesenchymal stromal
cells before renal ischemia/reperfusion attenuates kidney injury
and may modulate renal lipid metabolism in rats. Sci Rep.
7:86872017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao S, Zhao Z, Wu R, Zeng Y, Zhang Z, Miao
J and Yuan Z: Bone marrow mesenchymal stem cell transplantation
improves radiation-induced heart injury through DNA damage repair
in rat model. Radiat Environ Biophys. 56:63–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pyper SR, Viswakarma N, Yu S and Reddy JK:
PPARalpha: Energy combustion, hypolipidemia, inflammation and
cancer. Nucl Recept Signal. 8:e0022010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rakhshandehroo M, Knoch B, Müller M and
Kersten S: Peroxisome proliferator-activated receptor alpha target
genes. PPAR Res. 2010:6120892010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lin YY, Chen CY, Lin Y, Chiu YP, Chen CC,
Liu BH, Mersmann HJ, Wu SC and Ding ST: Modulation of glucose and
lipid metabolism by porcine adiponectin receptor 1-transgenic
mesenchymal stromal cells in diet-induced obese mice. Cytotherapy.
15:971–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ferré P and Foufelle F: SREBP-1c
transcription factor and lipid homeostasis: Clinical perspective.
Horm Res. 68:72–82. 2007.PubMed/NCBI
|